Abstract
Alzheimer’s disease (AD) is a progressive, neurodegenerative disease characterized by progressive decline of memory and cognitive functions. Despite tremendous progress that has been made in understanding disease progression and therapeutics of AD, we still do not have drugs that are capable of slowing its progression. The purpose of our study was to investigate the effects of the mitochondria-targeted antioxidants (MTAs) MitoQ and SS31, and the anti-aging agent resveratrol on neurons from a mouse model of Alzheimer’s disease (AD) (Tg2576 line) and on mouse neuroblastoma (N2a) cells incubated with the amyloid beta (Aβ) peptide. Using electron and confocal microscopy, gene expression analysis, and biochemical methods, we studied mitochondrial structure and function, and neurite outgrowth in N2a cells treated with MitoQ, SS31, and resveratrol, and then incubated with Aβ. In N2a cells only incubated with the Aβ, we found increased expressions of mitochondrial fission genes and decreased expression of fusion genes, and also decreased expression of peroxiredoxins, endogenous cytoprotective antioxidant enzymes. Electron microscopy of the N2a cells incubated with Aβ revealed a significantly increased number of mitochondria, indicating that Aβ fragments mitochondria. Biochemical analysis revealed that function is defective in mitochondria. Neurite outgrowth was significantly decreased in Aβ-incubated N2a cells, indicating that Aβ affects neurite outgrowth. However, in N2a cells treated with MitoQ, SS31, and resveratrol, and then incubated with Aβ, abnormal expression of peroxiredoxins and mitochondrial structural genes were prevented and mitochondrial function was normal; intact mitochondria were present and neurite outgrowth was significantly increased. In primary neurons from amyloid beta precursor protein (AβPP) transgenic mice that were treated with MitoQ and SS31, neurite outgrowth was significantly increased and cyclophilin D expression was significantly decreased. These findings suggest that the MTAs, MitoQ and SS31 prevent Aβ toxicity in mitochondria, which would warrant the study of MitoQ and SS31 as potential drugs to treat patients with AD.
Keywords: Amyloid beta, Amyloid precursor protein 1, Cyclophilin D, Mitochondrial-targeted antioxidants, Peroxiredoxins
INTRODUCTION
Alzheimer’s disease (AD) is a late-onset neurodegenerative disorder that is characterized by the progressive decline of memory, cognitive functions, and changes in behavior and personality [1,2]. In addition to the presence of extracellular amyloid beta (Aβ) protein deposits and intracellular neurofibrillary tangles (3). AD is also associated with the loss of synapses, loss of synaptic function [4,5], mitochondrial structural and functional abnormalities [6–16], inflammatory responses [17,18], and neuronal loss [2,3].
In a previous study, to determine cellular changes associated with the mutant amyloid precursor protein (AβPP) and Aβ in AD progression, we conducted a global, time-course gene expression study using an AD transgenic mouse model (APP transgenic mice; Tg2576 mouse line) [7]. We found an up-regulation of mitochondrial genes in the 2-, 5-, and 18-month-old AβPP mice, suggesting that mitochondrial function is impaired by mutant APP and Aβ and that the up-regulation of mitochondrial genes may be a compensatory response to mutant AβPP and mutant Aβ. Further, recently, we [10] and several other researchers [19,20] have recently found Aβ monomers and oligomers in mitochondria in the cortex and hippocampus of AβPP transgenic mouse lines and mouse neuroblastoma (N2a) neurons expressing mutant APP [10], and postmortem AD brains. Further, using AβPP transgenic mice [10,19,21], AβPP/PS1 transgenic mice [22] and AβPP/PS1/Tau mice [16,20] to study mitochondrial function, researchers have reported that mitochondrial Aβ decreases cytochrome oxidase activity, and increases H2O2 production and protein carbonyls [10,16,19,20]. These findings suggest that early interventions targeted at mitochondria may be effective in delaying AD development and slowing its progression.
Given the significant involvement of mitochondrial Aβ in synaptic damage, a diet of antioxidants in AD patients may decrease mitochondrial toxicity and boost cognitive function. However, recent studies in which AD patients consumed antioxidants gave mixed results. Some studies found a reduced risk of AD in elderly persons treated with natural antioxidants, such as vitamins C and E [23–26]. Other studies found that a diet of antioxidants in elderly persons with and without AD did not delay AD onset [27–30]. One possible reason for these mixed findings is that vitamins C and E, which are naturally occurring antioxidants, might not cross the blood-brain barrier effectively and so cannot reach the brain. Therefore, they cannot reach neuronal mitochondria in order to decrease mitochondrial toxicity.
Recently, several MTAs – including MitoQ, MitoVitE, and cell-permeable, small peptide antioxidants – have been developed [31,32]. Reports suggest that these MTAs are capable of entering mitochondria: MitoQ reaches the mitochondrial matrix [31] and SS31 concentrates in the inner mitochondrial membrane [32] several hundred folds more than do natural antioxidants, and decrease mitochondrial toxicity [31,32].
Further, studies of SS31 treatment using experimental MPTP mice for Parkinson’s disease found that SS31 decreased mitochondrial swelling and toxicity in these mice, and prevented dopaminergic cell death [33]. However, SS31 has not been tested in neurons and in AD transgenic mouse models. Recent studies have demonstrated that resveratrol, an anti-aging agent with anti-aging properties, reduces mitochondrial dysfunction, suggesting that resveratrol may have neuroprotection properties for AD [34,35].
In the study reported here, we sought to determine whether MitoQ and SS31 can prevent or reduce mitochondrial damage in N2a cells incubated with Aβ peptide 25–35, and primary neurons in brains from APP mice. We also studied resveratrol to determine its efficacy in reducing mitochondrial dysfunction induced by Aβ in N2a cells and in the primary neurons of APP mice and non-transgenic, wild-type mice [34,35]. We studied: 1) mRNA expression and protein levels of the neuroprotective genes PGC1α and FOXO1, and the NMDA receptor; a family of cytoprotective, antioxidant enzyme proteins - peroxiredoxins 1–6, mitochondrial-encoded electron transport chain (ETC) genes, and mitochondrial structural genes; the fission genes Drp1 and Fis1 and the fusion genes Mfn1, Mfn2, Opa1, and Tomm40; and the mitochondrial matrix proteins HSP60, Bcl2, and CypD; 2) ultrastructural changes in N2a cells, including changes in the number of mitochondria and their morphology; 3) neurite outgrowth, 4) mitochondrial function (H2O2 production, cytochrome oxidase activity, lipid peroxidation, ATP production, cell viability, and mitochondrial membrane potential), and 5) neurite outgrowth and CypD levels in primary neurons from APP mice and wild-type mice treated with MitoQ, SS31, and resveratrol.
MATERIALS AND METHODS
Tissue culture work
N2a cells (ATCC, Manassas, VA) were grown for 7 days in a serum-free medium (1:1 mixture of DMEM and OptiMEM, plus penicillin and streptomycin [Invitrogen, Carlsbad, CA]) or until the cells developed neuronal processes. The N2a cells that developed neuronal processes were used for 7 experimental treatments and one control: 1) N2a cells were incubated for 48 hrs with the Aβ peptide 25–35 (in-frame and reverse 35-25 – 30 μM); 2) N2a cells were treated with MitoQ for 48 hrs (0.1 μM; the original MitoQ was dissolved in DMSO and further diluted in PBS); 3) N2a cells were treated with SS31 for 48 hrs (0.1 nM; the original SS31 was dissolved in DMSO and further diluted in PBS); 4) N2a cells were treated with resveratrol for 48 hrs (5 μM dissolved in PBS; Sigma-Aldrich, St. Louis, MO); 5) N2a cells were treated with MitoQ (0.1 μM) for 6 hrs and then incubated with Aβ 25–35 for 48 hrs, 6) N2a cells were treated with SS31 (1 nM) for 6 hrs and then incubated with Aβ 25–35 for 48 hrs, 7) N2a cells were treated with resveratrol (5 μM) for 6 hrs and then incubated with Aβ 25–35 for 48 hrs, and 8) N2a cells were untreated and not incubated (the negative control). The cells from all the treatments were then harvested, and the cell pellet was collected and used for quantitative real-time RT-PCR; assays for ATP production, lipid peroxidation, and MTT determination. Isolated mitochondria from cells of all the treatments were used to determine cytochrome oxidase activity and H2O2.
Quantification of mRNA expression of peroxiredoxins, ETC, and neuroprotective genes using real-time RT-PCR
Using the reagent TriZol (Invitrogen), we isolated total RNA from 3 independent treatments of N2a cells (n=3) from control (untreated N2a cells) and experimental treatments (N2a cells treated with Aβ; N2a cells treated with MitoQ, SS31, and resveratrol; N2a cells pretreated with MitoQ and then incubated with Aβ; N2a cells pretreated with SS31 and then incubated with Aβ; and N2a cells pretreated with resveratrol and then incubated with Aβ). Using primer express Software (Applied Biosystems), we designed the oligonucleotide primers for the housekeeping genes β-actin; GAPDH; PGC1α [36], FOXO1 [37] and NMDA receptor [38], a family of cytoprotective, antioxidant enzyme proteins (peroxiredoxins 1, 2, 3, 4, 5 and 6) [39,40], mitochondrially encoded ETC genes (Complex I – subunit 1 & 5, Complex III – CytB, Complex IV - Cox1, and Complex V – ATPase); mitochondrial structural genes; fission (Drp1 and Fis1); fusion genes (MFN1, MFN2, Opa1, Tomm40); and mitochondrial matrix proteins (HSP60, Bcl2 and CypD). The primer sequences and amplicon sizes are listed in Table 1. With SYBR-Green chemistry-based quantitative real-time RT-PCR, we measured mRNA expression of the genes mentioned above as described by Manczak et al. [41]. Briefly, 2 μg of DNAse-treated total RNA was used as starting material, to which we added 1 μl of oligo (dT), 1 μl of 10 mM dNTPs, 4 μl of 5× first strand buffer, 2 μl of 0.1 M DTT, and 1 μl RNAse outout. The reagents RNA, dT), and dNTPs were mixed first, then heated at 65°C for 5 min, and finally chilled on ice until the remaining components were added. The samples were incubated at 42°C for 2 min, and then 1 μl of Superscript II (40 U/μl) was added. The samples were then incubated at 42°C for 50 min, at which time the reaction was inactivated by heating at 70°C for 15 min.
Table 1.
Summary of real-time RT-PCR oligonucleotide primers used in measuring mRNA expression in mitochondrial structural genes, ETC-encoded genes, peroxiredoxins and neuroprotective genes of N2a cells treated with Aβ and MTAs, and N2a cells treated with MTA followed by incubation with Aβ.
| Gene | DNA Sequence (5′-3′) | PCR Product Size |
|---|---|---|
| Mitochondrial Structural Genes | ||
| Drp1 | Forward Primer GCGCTGATCCCGCGTCAT | 54 |
| Reverse Primer CCGCACCCACTGTGTTGA | ||
| Fis1 | Forward Primer GCCCCTGCTACTGGACCAT | 62 |
| Reverse Primer CCCTGAAAGCCTCACACTAAGG | ||
| MFN1 | Forward Primer TCTCCAAGCCCAACATCTTCA | 62 |
| Reverse Primer ACTCCGGCTCCGAAGCA | ||
| MFN2 | Forward Primer ACAGCCTCAGCCGACAGCAT | 56 |
| Reverse Primer TGCCGAAGGAGCAGACCTT | ||
| Opa1 | Forward Primer TGGGCTGCAGAGGATGGT | 59 |
| Reverse Primer CCTGATGTCACGGTGTTGATG | ||
| TOMM40 | Forward Primer CCTGCCCTCTGACCTTTCC | 60 |
| Reverse Primer GAGAGCTGGCAGCCAACAC | ||
| Cyclophilin D | Forward Primer CAGCCAAGCCCTCCAACTC | 58 |
| Reverse Primer GCCGATGTCCACGTCAAAG | ||
| BCL2 | Forward Primer TGAGACTCCCAGCATGAAGGA | 60 |
| Reverse Primer GCCTTCCACGCACTCCAT | ||
| HSP60 | Forward Primer GAAATGCTTCGACTACCCACAGT | 57 |
| Reverse Primer CCAGTGCCCGGGACACT | ||
| Mitochondrial-encoded Electron Transport Chain Genes | ||
| NADH (sub1) | Forward Primer CGGGCCCCCTTCGAC | 72 |
| Reverse Primer GGCCGGCTGCGTATTCT | ||
| NADH (sub3) | Forward Primer TGTACTCAGAAAAAGCAAATCCATATG | 75 |
| Reverse Primer AATAATAGAAATGTAATTGCTACCAAGAAAAA | ||
| NADH (sub5) | Forward Primer CGGACGAACAGAACGCAAATA | 70 |
| Reverse Primer TAAAATGAATCCGATGTCTCCGA | ||
| CYT B | Forward Primer TTATCGCGGCCCTAGCAA | 75 |
| Reverse Primer TAATCCTGTTGGGTTGTTTGATCC | ||
| COX 1 | Forward Primer GAAGAGACAGTGTTTCATGTGGTGT | 73 |
| Reverse Primer TCCTGGGCCTTTCAGGAATA | ||
| COX3 | Forward Primer CAGGATTCTTCTGAGCGTTCTATCA | 75 |
| Reverse Primer AATTCCTGTTGGAGGTCAGCA | ||
| ATP6 | Forward Primer TGTGGAAGGAAGTGGGCAA | 72 |
| Reverse Primer CCACTATGAGCTGGAGCCGT | ||
| Cytoprotective Antioxidant Enzyme Genes | ||
| Prx1 | Forward Primer TGGCTCGACCCTGCTGATAG | 61 |
| Reverse Primer GGAGCAGGATACCCAATTTTTG | ||
| Prx2 | Forward Primer CCCCTGAATATCCCTCTGCTT | 57 |
| Reverse Primer CGCCGTAATTCTGGGACAA | ||
| Prx3 | Forward Primer GGCCCCATTTCTTGGAT | 60 |
| Reverse Primer CAGGGCAGGCTAAGGGAAAG | ||
| Prx4 | Forward Primer CCTGTTGCGGACCGAATCT | 55 |
| Reverse Primer GGGTCCGGAACCGTTCAT | ||
| Prx5 | Forward Primer CCCGATCAAGGTGGGAGAT | 56 |
| Reverse Primer CCCGGTTCCCCTTCAAATA | ||
| Prx6 | Forward Primer TCTGGCAAAAAATACCTCCGTTA | 58 |
| Reverse Primer GCCCCAATTTCCGCAAAG | ||
| Neuroprotective Genes | ||
| FOXO1 | Forward Primer CCCGTCCTAGGCACGAACT | 56 |
| Reverse Primer ACGCGCCCAGAACTTAACTTC | ||
| PGC1α | Forward Primer GGACAGTCTCCCCGTGGAT | 57 |
| Reverse Primer TCCATCTGTCAGTGCATCAAATG | ||
| NMDA receptor | Forward Primer GGTCAGTTCTGTCCTGCACATC | 62 |
| Reverse Primer TGACTCTCCCGCGGAAAC | ||
| Housekeeping Genes | ||
| Beta Actin | Forward Primer ACGGCCAGGTCATCACTATTC | 65 |
| Reverse Primer AGGAAGGCTGGAAAAGAGCC | ||
| GAPDH | Forward Primer TTCCCGTTCAGCTCTGGG | 59 |
| Reverse Primer CCCTGCATCCACTGGTGC | ||
Quantitative real-time PCR amplification reactions were performed in N2a cells in an ABI Prism 7900 sequence detection system (Applied Biosystems, Foster City, CA) in a 25-μl volume of total reaction mixture. The reaction mixture consisted of 1× PCR buffer containing SYBR-Green; 3 mM MgCl2; 100 nm of each primer; 200 nm of dATP, dGTP, and dCTP each; 400 nm of dUTP; 0.01 U/μl of AmpErase UNG; and 0.05 U/μl of AmpliTaq Gold. A 20 ng cDNA template was added to each reaction mixture.
The CT-values of β-actin and the GAPDH were tested to determine the unregulated endogenous reference gene in N2a cells that were treated with Aβ and protective molecules, and in untreated N2a cells. In the latter case, the CT-value was similar in untreated and N2a cells treated with MTAs and Aβ. The CT-value is an important quantitative parameter in real-time PCR analysis as described in Manczak et al [41], and Gutala & Reddy [42]. All RT-PCR reactions were carried out in triplicate, with no template control. The PCR conditions were: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The fluorescent spectra were recorded during the elongation phase of each PCR cycle. To distinguish specific amplicons from non-specific amplifications, a dissociation curve was generated. The CT-values were calculated with sequence-detection system (SDS) software V1.7 (Applied Biosystems) and an automatic setting of base line, which was the average value of PCR, cycles 3–15, plus CT generated 10 times its standard deviation. The amplification plots and CT-values were exported from the exponential phase of PCR directly into a Microsoft Excel worksheet for further analysis.
The mRNA transcript level was normalized against β-actin and the GAPDH at each dilution. The standard curve was the normalized mRNA transcript level, plotted against the log-value of the input cDNA concentration at each dilution. To compare β-actin, GAPDH, and neuroprotective markers, relative quantification was performed according to the CT method (Applied Biosystems; [41,42]. Briefly, the comparative CT method involved averaging triplicate samples, which were taken as the CT values for β-actin, GAPDH, and neuroprotective markers. β-actin normalization was used in the present study because β-actin CT values were similar for the N2a cells treated with Aβ, for the neuroprotective molecules, mitochondrial ETC genes, mitochondrial structural genes and for the untreated N2a cells. The ΔCT-value was obtained by subtracting the average β-actin CT value from the average CT-value of for the neuroprotective genes, peroxiredoxins, mitochondrial ETC genes, mitochondrial structural genes. The ΔCT of N2a cells was used as the calibrator. The fold change was calculated according to the formula 2−(Δ ΔCT), where ΔΔCT is the difference between ΔCT and the ΔCT calibrator value.
To determine the statistical significance of mRNA expression in untreated N2a cells and N2a cells treated with Aβ, MitoQ, Resveratrol, SS31, and/or a combination of MitoQ+Aβ, resveratrol+Aβ, and SS31+Aβ, the CT value difference between untreated N2a cells and treated N2a cells was used in relation to β-actin normalization, and statistical significance was calculated using one-way ANOVA.
Immunoblotting analysis
To determine whether MitoQ, SS31, resveratrol, or Aβ alters the protein levels of mitochondrial structural and neuroprotective genes that showed a decreased in mRNA expression, in our real-time RT-PCR, we performed immunoblotting analyses of protein lysates from N2a neurons of control and experimental treatments in independent N2a neurons treatments (n=3). Fifty μg protein lysates from control N2a neurons and N2a neurons that were treated with MitoQ, SS31, or resveratrol, or that were incubated with Aβ were resolved on a 4–12% Nu-PAGE gel (Invitrogen). The resolved proteins were transferred to nylon membranes (Novax Inc., San Diego, CA) and then were incubated for 1 hr at room temperature with a blocking buffer (5% dry milk dissolved in a TBST buffer). The nylon membranes were incubated overnight with the primary antibodies Prx1 (1:200; rabbit polyclonal Abcam, Cambridge, MA), Prx2 (1:200, rabbit polyclonal Abcam), Prx3 (1:300; mouse-monoclonal Abcam), Prx4 (1:500, rabbit polyclonal), Prx5 (1:500; mouse-monoclonal Abcam), Prx6 (1:500, rabbit polyclonal Abcam, NMDAR (1:400 rabbit polyclonal Abcam), PGC1α (1:500 rabbit polyclonal Abcam, FOXO1 (1:1000 rabbit polyclonal Abcam), Drp1 (1:200 rabbit polyclonal Novus Biological. Inc, Littleton, CO), Fis1 (1:200, Protein Tech Group Inc., Chicago, IL), Mfn1 (1:300, rabbit polyclonal Santa Cruz Biotechnology Inc. Santa Cruz, CA, Mfn2 (1:300, rabbit polyclonal Abcam), Opa1 (1:500; mouse-monoclonal BD Biosciences, San Jose, CA), and β-actin (1:500; mouse-monoclonal Chemicon-Millipore, Temicula, CA).
The membranes were washed with a TBST buffer3 times at 10-min intervals and then incubated for 2 hr with appropriate secondary antibodies, followed by 3 additional washes at 10-min intervals. Microglial proteins were detected with chemiluminescent reagents (Pierce Biotechnology, Rockford, IL), and the bands from immunoblots were quantified on a Kodak Scanner (ID Image Analysis Software, Kodak Digital Science, Kennesaw, GA). Briefly, image analysis was used to analyze gel images captured with a Kodak Digital Science CD camera. The lanes were marked to define the positions and specific regions of the bands. An ID fine-band command was used to locate and scan the bands in each lane and to record the readings.
H2O2 production
Using an Amplex® Red H2O2 Assay Kit (Molecular Probes, Eugene, OR), we measured the production of H2O2 in independent experiments (n=4) of N2a cells treated with MitoQ and SS31, and then incubated with Aβ, as described in Manczak et al. [10]. Briefly, the production of H2O2 was measured in the mitochondria isolated from N2a cells, from the control and the experimental treatments. A BCA Protein Assay Kit (Pierce Biotechnology) was used to estimate protein concentration. The reaction mixture contained mitochondrial proteins (μg/μl), Amplex Red reagents (50 μM), horseradish peroxidase (0.1 U/ml), and a reaction buffer (1X). The mixture was incubated at room temperature for 30 min, followed by spectrophotometer readings of fluorescence (570 nm). Finally, H2O2 production was determined, using a standard curve equation expressed in nmol/μg mitochondrial protein.
Cytochrome oxidase activity
Cytochrome oxidase activity was measured in the mitochondria isolated from N2a cells of control and experimental treatments (n=4), as described in Manczak et al. [10]. Enzyme activity was assayed spectrophotometrically using a Sigma Kit (Sigma–Aldrich) following manufacturer’s instructions. Briefly, 2 μg mitochondrial protein was added to 1.1 ml of a reaction solution containing 50 μl 0.22 mM ferricytochrome c fully reduced by sodium hydrosulphide, Tris–HCl (pH 7.0), and 120 mM potassium chloride. The decrease in absorbance at 550 mM was recorded for 1-min reactions at 10-sec intervals. Cytochrome c oxidase activity was measured according to the following formula: mU/mg total mitochondrial protein = (A/min sample – (A/min blank) × 1.1 mg protein × 21.84). The protein concentrations were determined following the BCA method.
ATP levels
ATP levels were measured in mitochondria isolated from N2a cells of control and experimental treatments (n=4) using the ATP determination kit (Molecular Probes) [43]. The bioluminescence assay is based on the reaction of ATP with recombinant firefly luciferase and its substract luciferin. Luciferase catalyzes the formation of light from ATP and luciferin. It is the emitted light that is linearly related to the ATP concentration, which was measured using a luminometer. We measured ATP from mitochondrial pellets using a standard curve method.
Lipid peroxidation assay
Lipid peroxidates are unstable indicators of oxidative stress in neurons [44]. 4-hydroxy-2-nonenol (HNE) is the final product of lipid peroxidation that was measured in the cell lysates from N2a cells of control and experimental treatments (n=4), using an HNE-His ELISA Kit (Cell BioLabs, Inc., San Diego, CA). Briefly, freshly prepared protein was added to a 96-well protein binding plate and incubated overnight at 4°C. It was then washed 3 times with wash buffer. After the last wash, the anti-HNE-His antibody was added to the wells and incubated for 2 hr at room temperature, and then washed the wells 3 times. Next, the samples were incubated with a secondary antibody conjugated with peroxidase for 2 hr at room temperature, followed by an incubation with enzyme substrate. Optical density was measured to quantify the level of HNE.
Cell viability test (MTT assay)
Mitochondrial respiration, an indicator of cell viability, was assessed in the N2a cells from control and experimental treatments (n=4), using the mitochondrial-dependent reduction of 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) to formazan [45]. Briefly, in this reduction, N2a cells were seeded in 12-well plates at a density of ~105 neurons per well. After treatments, MTT (5 mg/ml in PBS) was added to the plates, and the cells (control and experimental) were incubated for 3 hrs. The medium was then replaced with an SDS/DMSO lysis buffer, and MTT absorption was measured at 570 nm. Results were expressed as the percentage of MTT reduction, assuming that the absorbance of the control N2a cells was 100%.
Mitochondrial membrane potential
To determine the effect of Aβ on the mitochondrial membrane potential, the potential was measured in N2a cells from control and experimental treatments (n=4) using live-cell imaging and JC-1 (fluorochrome (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide) staining, as described in Wagner et al. [46]. In JC-1 staining, the JC1 dye accumulates in the mitochondria of healthy cells as aggregates and are fluorescent red. When cells are under stress or exposed to toxic chemicals, such as Aβ, the mitochondrial potential collapses, and the JC1 dye can no longer accumulate in the mitochondria. Consequently, the JC1 aggregates remain in the cytoplasm, in a monomeric form, which is green. In this experiment, the N2a cells were stained for 10 min at 37°C, until a final concentration of 5 μg/ml JC1 was reached. The cells were then washed 3 times with PBS and analyzed for mitochondrial membrane potential by measuring the red:green ratio with confocal microscopy.
Electron microscopy
To determine the effects of Aβ on the number and morphology of mitochondria and the rescue effects of MitoQ, SS31, and resveratrol on mitochondria, we conducted transmission electron microscopy (TEM) of N2a cells from control and experimental treatments (n=4). Treated and untreated N2a cells were fixed in 100 mM sodium cacodylate (pH 7.2), 2.5% glutaraldehyde, 1.6% paraformaldehyde, 0.064% picric acid, and 0.1% ruthenium red. They were gently washed and post-fixed for 1 hr in 1% osmium tetroxide plus 08% potassium ferricyanide, in 100 mM sodium cacodylate, pH 7.2. After a thorough rinsing in water, the N2a cells were dehydrated, infiltrated overnight in 1:1 acetone:Epon 812, infiltrated for 1 hr with 100% Epon 812 resin, and embedded in the resin. After polymerization, 60- to 80-nm thin sections were cut on a Reichert ultramicrotome and stained for 5 min in lead citrate. They were then rinsed and post-stained for 30 min in uranyl acetate, and then rinsed again and dried. EM was performed at 60 kV on a Philips Morgagne TEM, equipped with a CCD, and images were collected at original magnifications of 1,000–37,000x. The numbers of mitochondria were counted in the control N2a cells (number of cells = 50) and experimental groups (number of cells per group=40), and statistical significance was determined, using one-way ANOVA.
Immunofluorescence analysis and quantification of neurite outgrowth
To study neurite outgrowth, N2a cells were grown in a serum-free medium for 7 days or until the neurons developed neuronal processes on gridded microscopic slides (BD Biosciences). We performed immunofluorescence analysis using the Drp1 antibody (a synaptic branching protein) in control N2a cells and N2a cells from the 7 experimental treatments.
N2a cells were washed with warm PBS, fixed in freshly prepared 4% paraformaldehyde in PBS for 10 min, and then washed with PBS and permeabilized with 0.1% Triton-X100 in PBS. They were blocked with 1% blocking solution (Invitrogen) for 1 hr at room temperature. All neurons were incubated overnight with a rabbit polyclonal anti-Drp1 primary antibody (Novus Biologicals) at a concentration of 1:400, at 4°C. After being incubated with the primary antibody, the cells were washed 3 times with PBS, for 10 min each. The neurons were incubated with a secondary antibody conjugated with Fluor 488 (Invitrogen) for 1 hr at room temperature. The cells were washed neurons 3 times with PBS, and slides were mounted. Photographs were taken with a multiphoton laser scanning microscope system (ZeissMeta LSM510).
To quantify the immunoreactivity of the Drp1 antibody, for each treatment 10–15 photographs were taken at ×20 magnification. For the measurement of neurite outgrowth, the immunoreactivity of Drp1 and neurite length were quantified using the ‘neurite tracer’ method published by Pool et al. [47]. The signal intensity indicating the immunoreactivity of the cell body and neurite length was quantified for several randomly selected images, and statistical significance was assessed, using one-way ANOVA for Drp1 in N2a cells from control N2a cells versus N2a cells treated with MitoQ, SS31, and resveratrol, and then incubated with Aβ.
AβPP mice and primary neuronal culture
The AβPP transgenic mice (Tg2576 line) and age-matched wild-type littermates were housed at the Oregon National Primate Research Center of Oregon Health & Science University (OHSU). The mice were a gift from Dr Karen Ashe, University of Minnesota [48]. The OHSU Institutional Animal Care and Use Committee approved all procedures for animal care according to guidelines set forth by the National Institutes of Health.
Primary neuronal cultures of APP mice and wild-type mice were prepared using methods described by Brewer and Torricelli [49], with slight modifications. Briefly, mice postnatal day 1 were decapitated and the brains removed into a room-temperature Hibernate®-A medium (Brain Bits, Springfield IL) supplemented with B-27 (Invitrogen) and 0.5mM GlutaMAX™ (Invitrogen). The hippocampus was then dissected and reserved for culturing, and the cerebellum was removed for genotyping. Hippocampus pairs from individual mice were minced into pieces less than 1 mm3 and transferred to a solution of papain (2 mg/ml; Worthington Biochemical Corp, Lakewood, NJ) that was dissolved in Hibernate®-A without calcium, but supplemented with 0.5mM GlutaMAX™. The tissue was digested for 30 min in a shaking water bath at 30°C. Digested tissue was then removed to 2 ml HABG and triturated 10 times with a fire-polished, siliconized (Sigmacote; Sigma, St. Louis MO), 9″ glass pipette. Samples were allowed to settle by gravity for about 1 min. Then the supernatant containing dissociated neurons was removed to a fresh tube. An additional 2 ml HABG was added to the pellet, and the process was repeated until 6 ml dissociated neurons were collected. Neurons were centrifuged at 200×g for 2 min and then washed with 2 ml HABG. The pellets were resuspended in 2 ml Neurobasal™ (Invitrogen) supplemented with B-27 and 0.5 mM GlutaMAX™ (growth medium). The neurons were counted, plated at 500 neurons/mm2 on poly-D-lysine-coated coverslips, and placed into a 37°C incubator at 5% CO2. One hour after plating, the growth medium was completely replaced. After 3 days in vitro and every 2 days thereafter for 10 days, one-half of the growth medium was changed. The in vitro hippocampal neurons were treated with MitoQ (20 nM), SS31 (1 nM), and resveratrol (5 μM), and were used for experiments.
Treatment with mitochondrial-targeted antioxidants and quantification of neurite outgrowth
One group of postnatal day 1 hippocampal neurons from AβPP transgenic mice and wild-type mice remained untreated, and one group was treated with MitoQ, SS31, and resveratrol for 48 hrs. All neurons were washed with warm PBS, then fixed in a freshly prepared 4% paraformaldehyde for 10–15 min, and washed with PBS and permeabilized with 0.1% Triton-X100 in PBS. All incubations were performed in a humidified environment. All neurons were blocked with 1% blocking solution (Invitrogen) for 1 hr at room temperature. Primary antibodies were diluted in the blocking solution and used under the following 2 conditions: CypD mouse monoclonal (EMD, Gibbbstown, NJ), 1:200, room temperature, 1 hr; and Drp1 rabbit polyclonal (Novus Biologicals, Littleton, CO), 1:500, 4°C, overnight. After being incubated with an appropriate primary antibody, the neurons were washed 3 times for 10 min with PBS. A fluorophore-labeled appropriate secondary antibody was then applied for 1 hr at room temperature. The secondary antibody (1:500 in blocking solution) was selected from Alexa Fluor® 488 goat anti-rabbit or Alexa Fluor® 568 goat anti-mouse antibodies (Invitrogen) as appropriate. For double-labeling studies, antigens were labeled sequentially, meaning that the primary antibody was applied singly and then the appropriate secondary antibody was applied. Then the second primary antibody was applied, followed by the appropriate secondary antibody.
Ten to 15 pictures of hippocampal neurons from AβPP transgenic mice and wild-type mice treated with MitoQ, SS31, and resveratrol each were taken at 20× the original magnification. For the measurement of neurite outgrowth (Drp1) and CypD expression, the immunoreactivities of Drp1 and CypD were quantified using the method published by Pool et al. [47].
Statistical considerations
Statistical analyses were conducted for mitochondrial structural and functional parameters in N2a cells from control and experimental treatments using one-way ANOVA with Dunnett correction. The parameters included H2O2, cytochrome oxidase activity, lipid peroxidation, ATP production, cell viability, mitochondrial membrane potential, Drp1 levels (neurite outgrowth), and electron microscopy values. To determine the effect of MitoQ, SS31 and resveratrol in the absence and presence of Aβ we analyzed/compared the data 2 ways: 1. untreated N2a cells (control) versus N2a cells treated with MitoQ, SS31, resveratrol and incubated with Aβ; 2. N2a cells incubated with Aβ versus N2a cells pretreated with MitoQ and then incubated Aβ, N2a cells pretreated with SS31 and then incubated with Aβ and N2a cells pretreated with resveratrol and then incubated with Aβ.
We also assessed the statistical significance for Drp1 and CypD immunoreactivities in primary neurons from APP mice and wild-type mice between untreated primary neurons and primary neurons treated with MitoQ, untreated primary neurons and primary neurons treated with SS31, and untreated primary neurons and primary neurons treated with resveratrol using one-way ANOVA.
RESULTS
mRNA expression of mitochondrial structural genes
We determined the effects of Aβ on mitochondrial structural genes and the protective effects of MTAs on mRNA expression of the mitochondrial genes Drp1 and Fis1 (fission); Mfn1, Mfn2, Opa1 and Tomm40 (fusion); and CypD, Hsp60, and Bcl2 (matrix genes) (Table 2A). We analyzed the data 2 ways: 1) to determine the effect of Aβ, MitoQ, SS31, and resveratrol (Table 2A) in N2a cells, and 2) to determine the preventive effects of MitoQ, SS31 and resveratrol, if any, in the presence of Aβ (Table 2B).
Table 2.
Fold changes of mRNA expression in mitochondrial structural genes, electron transport chain genes, and neuroprotective genes in Aβ-treated N2a cells, treated with mitochondrial targeted antioxidants, and treated with mitochondrial targeted antioxidants+ Aβ peptide.
| Gene | Untreated N2a cells Vs Aβ-Treated N2a Cells, and MTAs-Treated N2a Cells. Comparison A | AβTreated N2a Cells Vs MTAs+Aβ-Treated N2a Cells. Comparison B | |||||
|---|---|---|---|---|---|---|---|
| Aβ | MitoQ | SS31 | Resv | MitoQ+ Aβ | SS31+ Aβ | Resv+ Aβ | |
| Mitochondrial structural genes | |||||||
| DRP1 | 1.7* | 1.1 | 1.5* | 1.1 | −1.2* | −1.2* | −1.4* |
| FIS1 | 3.4* | 1.9* | 1.6* | 1.0 | −1.4* | −2.7* | −2.9* |
| MFN1 | −1.4* | 1.8* | 1.4 | 1.3 | 1.0 | 1.1 | 1.2 |
| MFN2 | −1.8* | 1.5* | 1.3 | 1.0 | 1.0 | 1.0 | 1.7* |
| OPA1 | −2.5* | 1.9* | 2.0* | 1.0 | 2.0* | 2.1* | 2.8* |
| TOMM40 | −1.7* | 1.2 | 2.3 | 1.1 | 1.4 | 1.5 | 1.5 |
| Cyclophilin D | 2.2** | −1.6* | −1.3 | 1.0 | 1.6 | 1.3 | 1.1 |
| BCL-2 | 5.1** | 3.5** | 5.0** | 2.2* | −3.0* | −2.6* | −1.6* |
| HSP60 | 5.7* | 2.8* | 5.5** | 1.3 | 4.9** | 3.4* | 1.5 |
| Electron transport chain genes | |||||||
| NADH (sub1) | 2.2* | 1.6 | 7.4** | 1.6 | 1.5 | 1.0 | −1.6* |
| NADH (sub3) | 2.5 | 1.5 | 8.2** | 2.1* | 1.2 | 6.6** | 1.7* |
| NADH (sub5) | 2.5* | 1.4 | 6.6** | 2.0* | 1.2 | −1.6* | −1.3* |
| Cyt B | 2.1* | 1.8* | 9.5** | 1.9* | 1.6 | 1.3 | −1.2* |
| COX1 | 2.7* | 1.5 | 9.4** | 2.1* | 1.2 | 1.1 | −1.4* |
| COX3 | 2.1* | 2.5* | 8.9** | 1.5 | 2.0* | 7.3** | 1.2 |
| ATP-6 | 2.2* | 1.8* | 8.2** | 1.8* | 1.0 | 1.1 | −1.3* |
| Antioxidant enzymes - Peroxiredoxins | |||||||
| PRX1 | −5** | 1.6 | 1.5 | 1.0 | 5.5** | 4.4** | 3.5** |
| PRX2 | −2.3** | 1.3 | 2.8* | 2.3* | 4.3** | 3.3** | 3** |
| PRX3 | −1.3* | 1.3 | 4.5** | 5.3** | 1.5 | 4.2** | 2.2* |
| PRX4 | −1.7* | 1.9* | 3.4** | 1.8* | 2.3* | 4.1** | 3.7** |
| PRX5 | −2.8** | 1.5 | 2.6* | 1.7* | 4.2** | 3.7** | 5.0** |
| PRX6 | −4.5** | 4.4** | 3** | 6** | 2.9** | 3.0** | 3.1** |
| Neuroprotective genes | |||||||
| FOX01 | 7.5** | 3.1* | 14.9** | 3.7* | 1.6* | 1.6* | 1.2 |
| NMDA receptor | 3.1* | 8.2** | 8.8** | 9.4** | 4.3* | 3.7** | 1.3 |
| PGC-1α | 2.4* | 4.8** | 2.5* | 1.8* | 2.5* | 1.4 | −1.4* |
indicates statistical significance level P<0.05
indicates statistical significance level P<0.005
In N2a cells treated with Aβ compared to untreated N2a cells, mRNA expression levels significantly increased in Drp1, by 1.7 fold (P<0.05); and in Fis1, by 3.4 fold (P<0.05). In contrast, the levels of mRNA expression of mitochondrial fusion genes were significantly decreased (Table 2A), indicating that the presence of abnormal mitochondrial dynamics in N2a cells treated with Aβ. Interestingly, mRNA expression of matrix genes were significantly increased (CypD by a 2.2-fold, P<0.005; Bcl2 by a 5.1-fold, P<0.005; and HSP by a 5.7 fold, P<0.05) in N2a cells treated with Aβ, indicating that mitochondrial matrix genes were activated.
mRNA levels were significantly decreased for Drp1 (1.2 fold decrease, P<0.05 in MitoQ treated cells; 1.2 fold decrease, P<0.05 in SS31; 1.4 fold decrease for resveratrol treated cells) and Fis1 (1.4 fold decrease, P<0.05 in MitoQ; 2.7 fold decrease, P<0.05 in SS31 and 2.9 fold in resveratrol treated) in cells pretreated with MitoQ, SS31, and resveratrol and then treated with Aβ compared with cells treated with Aβ alone (Table 2B). These observations indicate that MTAs and resveratrol prevent abnormal fission and fusion gene expressions.
mRNA expression of ETC genes
To determine the effects of Aβ, MitoQ, SS31, and resveratrol on mitochondrial oxidative phosphorylation, mRNA expression levels of ETC genes were measured. Significantly increased mRNA expressions were found in all ETC genes except NADH subunit3 studied in the N2a cells treated with Aβ (Table 2A), indicating a compensatory response by mitochondrial-encoded genes. Interestingly, in N2a cells treated with SS31 relative to untreated N2a cells, significantly increased levels of mRNA expression were found in ETC genes (NADH subunit1 increased by 7.4 fold, P<0.005; subunit3, by 8.2 fold, P<0.005; subunit5, by 6.6 fold, P<0.005; CytB increased by 9.5 fold, P<0.005; COX1 increased by 9.4 fold, P<0.005; COX3, by 8.9 fold, P<0.005; and ATPase6 increased by 8.2 fold (P<0.005) (Table 2A). MitoQ and resveratrol treated N2a cells showed increased of ETC genes but mRNA levels were not significant for all genes studied (Table 2A).
As shown Table 2B, mRNA expression levels were increased for ETC all genes but not significant in N2a cells pretreated with MitoQ, SS31 and incubated with Aβ compared to N2a cells treated with Aβ alone. Overall, these findings suggest that SS31 enhance mitochondrial gene expressions in the presence and absence of Aβ.
mRNA expression of peroxiredoxins
To determine the effects of Aβ, MitoQ, SS31 and resveratrol in the antioxidant enzymes peroxiredoxins (Prxs 1–6), mRNA expression levels of Prxs were measured. As shown in Table 2A, levels of mRNA expression were found significantly decreased in all Prxs (1–6) in N2a cells treated with Aβ. A 5-fold decrease was found for Prx1 (P<0.005); for Prx6, a 4.5-fold decrease (P<0.005); for Prx5, a 2.8-fold decrease (P<0.005); for Prx2, a 2.3-fold decrease (P<0.005); for Prx4, a 1.7-fold decrease (P<0.05); and for Prx3, a 1.3-fold decrease (P<0.05). Interestingly, Prxs were significantly increased in N2a cells treated with SS31 (a 4.5-fold increase for Prx3 with P<0.005; a 3.4-fold increase for Prx4 with P<0.005; a 2.6-fold increase for Prx6 with P<0.005), resveratrol (a 6-fold increase for Prx6 with P<0.005; a 5.5-fold increase for Prx3 with P<0.005) and MitoQ (a 4.4-fold increase for Prx6 with P<0.005).
As shown in Table 2B, the levels of mRNA expression of Prxs were significantly increased in N2a cells pretreated with SS31, MitoQ and resveratrol and then incubated with Aβ compared to cells treated with Aβ alone, indicating that MTAs and resveratrol prevent the decreased mRNA expressions of antioxidant enzymes (Prxs) caused by Aβ.
mRNA expression in neuroprotective genes
mRNA expressions of neuroprotective genes PGC1α and FOXO1, and the NMDA receptor (NMDAR) were measured in N2a cells treated with Aβ, MitoQ, SS31 and resveratrol (Table 2A). The levels of mRNA expression were significantly increased for PGC1α (2.4 fold with P<0.05); for FOXO1 (7.5 fold with P<0.05); and for NMDAR (3.1 fold with P<0.05) in N2a cells treated with Aβ. Interestingly, neuroprotective genes were significantly increased in N2a cells treated with SS31 (a 14.9-fold increase for FOXO1 with P<0.005; a 8.8 fold increase for NMDAR with P<0.005; a 2.5 fold increase for PGC1α with P<0.05), MitoQ (a 8.2-fold increase for NMDAR with P<0.005; a 4.8-fold increase for PGC1α with P<0.005; a 3.1-fold increase for FOXO1 and resveratrol (a 9.4-fold increase for NMDAR with P<0.005; a 3.7-fold increase for FOXO1 (Table 2A). These increased expressions of neuroprotective genes, indicate MitoQ, SS31 and resveratrol are protective to N2a cells.
The levels of mRNA expression of NMDAR, FOXO1 and PGC1α were significantly increased in N2a cells pretreated with SS31, MitoQ and then incubated with Aβ compared to N2a cells treated with Aβ alone (Table 2B) but not for resveratrol pretreated cells, indicating that MTAs enhance mRNA expressions of neuroprotective genes in the presence of Aβ.
Immunoblotting analysis
Peroxiredoxins
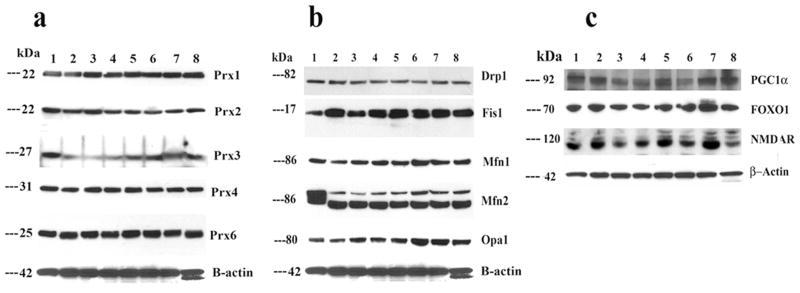
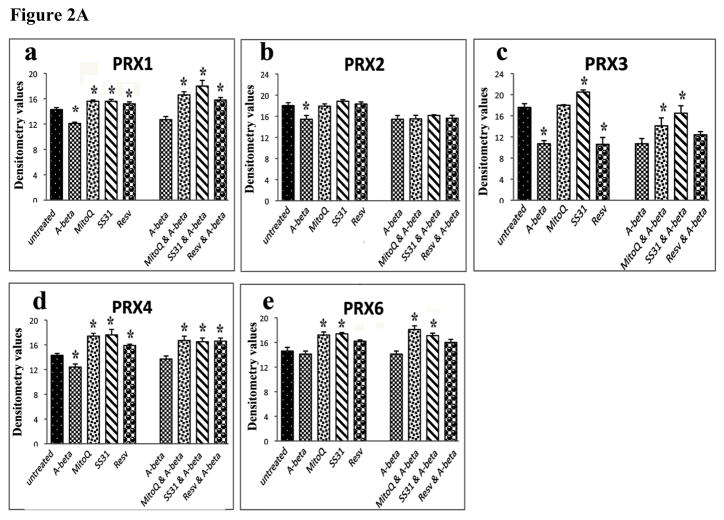
In N2a cells treated with Aβ, protein levels were significantly decreased for Prx1 (P<0.004), Prx2 (P<0.04), Prx3 (P<0.001) (Figure 1A – western blot, and Figure 2A - quantitative densitometry values). These findings agreed with our real-time RT-PCR data. However, protein levels were significantly increased for Prx1 (P<0.03), Prx3 (P<0.02), Prx4 (P<0.01) and Prx6 (P<0.01) for SS31 treated cells; followed by significantly increased for Prx1 (P<0.001), Prx4 (P<0.01) and Prx6 (P<0.01) in MitoQ treated cells. However, in resveratrol treated cells, significantly increased protein levels were found only Prx1. These observations indicate that SS31 increased peroxiredoxins at RNA and protein levels.
Figure 1.
Representative immunoblots of peroxiredoxins, mitochondrial proteins, and neuroprotective proteins in control N2a neurons, Aβ-incubated N2a cells, and N2a neurons treated with MitoQ and SS31 and then incubated with Aβ (n=3). Image A. Fifty μg of protein lysate was used from each cell sample; immunoblotting analysis was performed using antibodies of peroxiredoxins (1, 2, 3, 4, and 6). Image B. Drp1, Fis1, Mfn1, Mfn2, and Opa1. Image C. PGC1α, FOXO1, and NMDAR. Bottom panel shows immunoblotting of β-actin for equal loading. Lane 1 represents control N2a cells; lane 2, Aβ-incubated N2a cells; lane 3, resveratrol-treated N2a neurons; lane 4, N2a cells treated with resveratrol and then incubated with Aβ; lane 5, MitoQ-treated N2a cells; lane 6, N2a cells treated with MitoQ and then incubated with Aβ; lane 7, SS31-treated N2a cells; and lane 8, N2a cells treated with SS31 and then incubated with Aβ.
Figure 2.
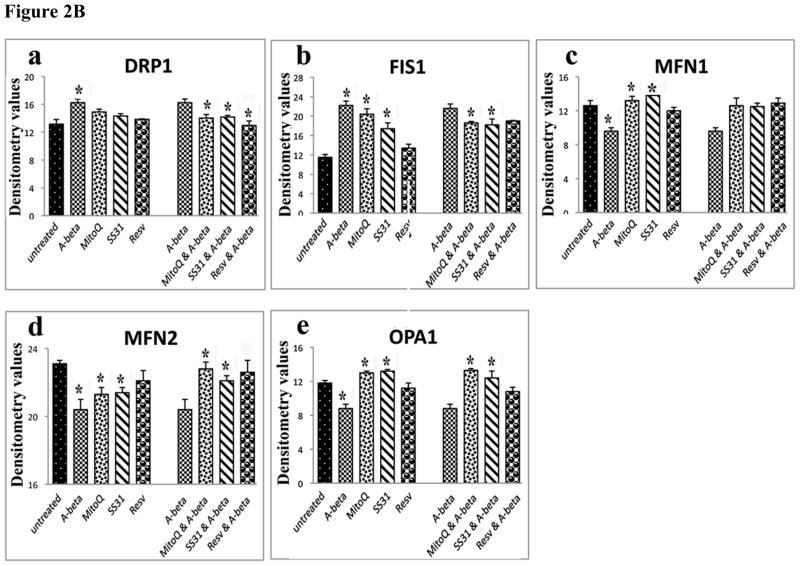
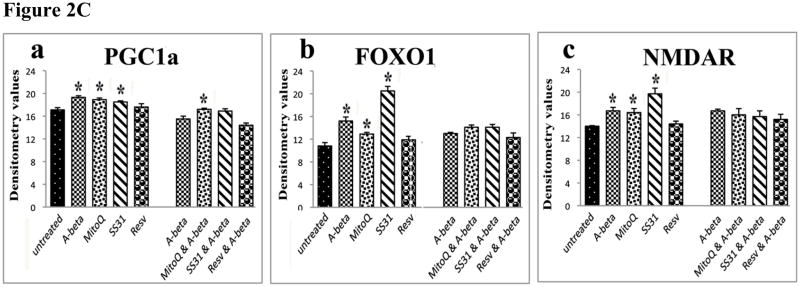
Quantitative immunoblotting analyses of mitochondrial structural proteins, peroxiredoxins, neuroprotective proteins (n=4). Figure 2A shows the quantitative protein data on mitochondrial structural proteins; 2B, represents quantitative protein data on peroxiredoxins; and 2C, quantitative protein data on neuroprotective proteins. * denotes statistical significance.
The protein levels of Prxs were significantly increased in N2a cells pretreated with SS31, MitoQ and then incubated with Aβ compared to cells treated with Aβ alone (Figure 1A – western blot, and Figure 2A -quantitative densitometry values), indicating that MTAs enhance protein levels of Prxs in the presence of Aβ. No significant changes were found in cells pretreated with resveratrol and incubated with Aβ.
Mitochondrial structural proteins
To determine the effects of Aβ on mitochondrial structural proteins and the useful effects of MitoQ, SS31 and resveratrol at protein level, we quantified mitochondrial proteins in 3 independent treatments of N2a cells with Aβ, MitoQ, SS31 and resveratrol. In N2a cells treated with Aβ compared to untreated N2a cells, significantly increased proteins for Drp1 (P<0.02) and Fis1 (P<0.001) (Figure 1B – western blot, and Figure 2B - quantitative densitometry values). In contrast, we found decreased levels of mitochondrial fusion proteins, Mfn1 (P<0.02) Mfn2 (P<0.004) and Opa1 (P<0.04) (Figure 1B – western blot, and Figure 2B quantitative densitometry values). in N2a cells incubated with Aβ compared to compared untreated cells, indicating the presence of abnormal mitochondrial dynamics even at protein level in N2a cells treated with Aβ.
Neuroprotective proteins
Significantly increased levels of proteins in NMDAR (P<0.01) and FOXO1 (P<0.01) were found in N2a cells treated with Aβ. However, in N2a cells treated with MitoQ and SS31, protein levels significantly increased for NMDAR (MitoQ, P<0.04; SS31, P<0.01), FOXO1 (MitoQ, P<0.03; SS31, P<0.001) and PGC1α (MitoQ, P<0.02; SS31, P<0.03). However, in those cells treated with only resveratrol, protein levels did not increase (Figure 1C – western blot and Figure 2C - quantitative densitometry values).
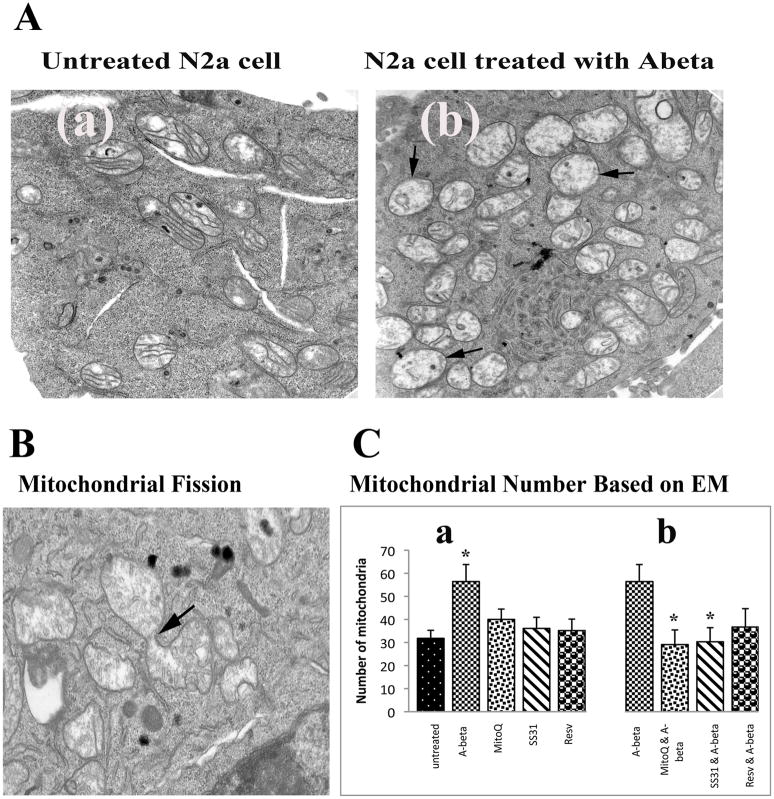
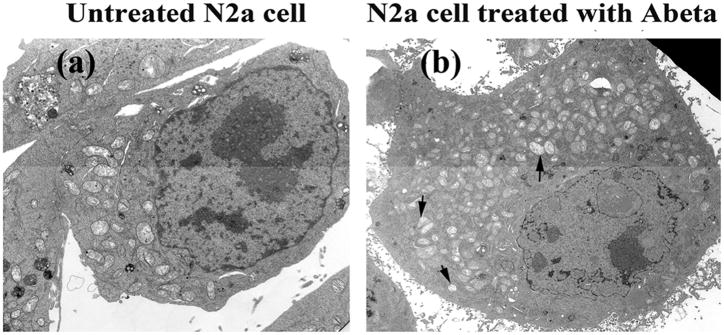
Electron microscopy
To determine the effects of Aβ on mitochondrial morphology and number, N2a cells were incubated with Aβ, and the number and morphology of mitochondria were studied using electron microscopy (EM). The number of mitochondria significantly increased (P<0.05) (Figure 3C, image a, and Figure 4, image b) in Aβ-incubated N2a cells compared to N2a cells not incubated with Aβ. Most mitochondria were swollen, most of the inner mitochondrial membrane and cristae were not visible, and those that were visible were damaged (Figure 4A, image b). Mitochondrial fragmentation was evident in most of the Aβ-treated N2a cells (Figure 4, image b).
Figure 3.
Electron microscopy in control N2a neurons, Aβ-incubated N2a cells, and N2a cells treated with MitoQ and SS31, and then incubated with Aβ (n=4). Image A shows (a) a control N2a cell compared to (b) a representative Aβ-incubated N2a cell. Mitochondria increased in the Aβ-incubated N2a cell, and they exhibited morphological changes, including swollen and damaged inner mitochondrial membrane and cristae. Image B shows high magnification of mitochondrial fragmentation of an Aβ-incubated N2a cell. Image C shows results from quantitative analysis of mitochondria: (a) in control N2a cell compared to N2a cells treated with MitoQ, SS31, and resveratrol, and then incubated with Aβ. Significantly increased mitochondria were found in Aβ-incubated N2a neurons (P<0.05) compared to the control N2a cells, and (b) in Aβ-incubated N2a cells compared to N2a cells treated with MitoQ, SS31, and resveratrol, and then incubated with Aβ. Significantly decreased mitochondria were found in the N2a cells treated with MitoQ and then incubated with Aβ (P<0.05), and in N2a neurons treated with SS31 and then incubated with Aβ (P<0.05), compared to Aβ-treated N2a cells.
Figure 4.
Mitochondrial fragmentation in Aβ-treated N2a cell. Increased mitochondria were found in Aβ-treated N2a cell (image b).
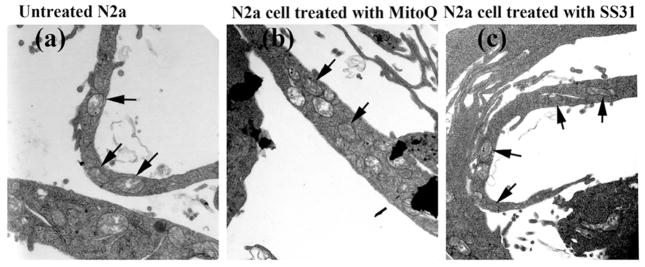
In the untreated N2a cells, elongated mitochondria were found in the neuronal processes, and even at the terminals (Figure 5, image a) of the N2a cells, indicating that mitochondria travel along neuronal processes.
Figure 5.
Electron microscopy of N2a cells, with a focus on neuronal processes. (a) Elongated mitochondria were found in neuronal processes and even in terminals of neurons. Black arrows indicate intact mitochondria in neuronal processes of (b) MitoQ- and (c) SS3-treated N2a cells.
Intact mitochondria were found in the neuronal processes of N2a neurons treated with MitoQ (Figure 5, image b) and SS31 (Figure 5, image c) but not then incubated with Aβ, indicating that mitochondrial trafficking is not affected in MitoQ- and SS31-treated N2a cells. However, in N2a cells treated with MitoQ, SS31 and resveratrol, and then incubated with Aβ, mitochondrial swelling and fragmentation was not found, indicating protective effects of MitoQ, SS31, and resveratrol.
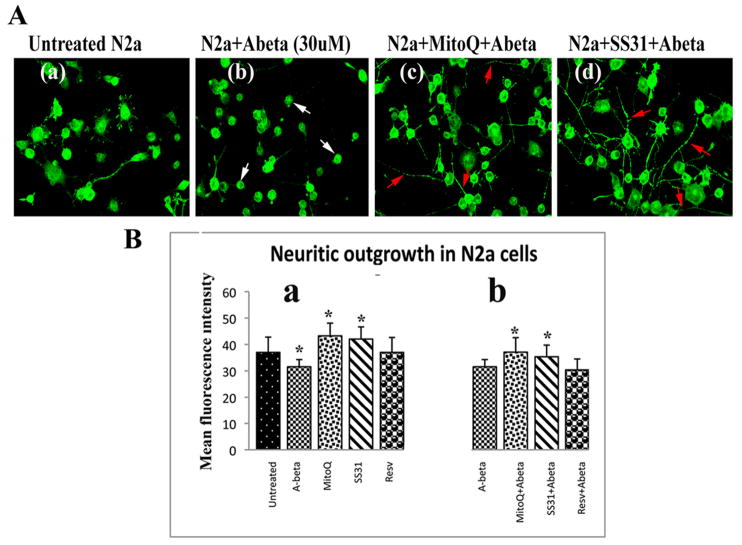
Neurite outgrowth
Neurite outgrowth was measured in N2a cells incubated with Aβ to determine whether Aβ affects neurite length. We found neurite growth significantly decreased (marked by immunoreactivity of Drp1) in the N2a cells incubated with Aβ compared to the N2a cells not incubated with Aβ (P<0.01) (Figure 6B, image a – quantitative data; and Figure 6A, image b - cells), indicating that Aβ does affect neurite length. Significantly increased neurite outgrowth was found in N2a cells treated with MitoQ (P<0.01) and SS31 (P<0.03) (Figure 6B, image a – quantitative data) compared to N2a cells not treated with MitoQ or SS31 and also not incubated with Aβ. However, neurite outgrowth did not significantly change in the resveratrol-treated N2a cells that were then incubated with Aβ.
Figure 6.
Neurite outgrowth in control N2a cells, Aβ-incubated N2a cells, N2a cells treated with MitoQ and SS31 and then incubated with Aβ (n=4). Image A presents representative images of (a) Drp1 immunostaining of control N2a cells, (b) Aβ-incubated N2a cells, (c) N2a cells treated with MitoQ and then incubated with Aβ, and (d) N2a cells treated with SS31 and then incubated with Aβ. White arrows indicate the loss of neuronal processes in the Aβ-incubated N2a cells, and red arrows indicate an increase in neuronal processes in N2a cells treated with MitoQ and SS31. Image B shows quantitative analysis of Drp1 immunostaining in N2a cells. (a) Significantly decreased Drp1 immunostaining in neuronal processes was found in Aβ-incubated N2a cells (P<0.01) compared to control N2a cells, and significantly increased Drp1 immunostaining (neurite outgrowth) was found in neurons treated with MitoQ (P<0.01) and SS31 (P<0.03) compared to control N2a cells. (b) Significantly increased Drp1 immunostaining (neurite outgrowth) was found in N2a cells treated with MitoQ and SS31 and then incubated with Aβ (P<0.01 and P<0.03 respectively), compared to Aβ-incubated N2a cells.
Significant neurite outgrowth was found in N2a cells treated MitoQ and then incubated with Aβ (P<0.01; Figure 6B, image b – quantitative data), and in N2a cells treated with SS31 and then incubated with Aβ (P<0.03; Figure 6B, image b – quantitative data), compared to the N2a cells that were only incubated with Aβ, indicating that MitoQ and SS31 prevent neurite outgrowth from Aβ toxicity.
Mitochondrial dysfunction
To determine whether Aβ affects mitochondrial function and the preventive effects of MitoQ and SS31, parameters of mitochondrial function were studied in N2a cells (n=4). The parameters included H2O2 production, cytochrome oxidase activity, lipid peroxidation, ATP production, cell viability, and mitochondrial membrane potential.
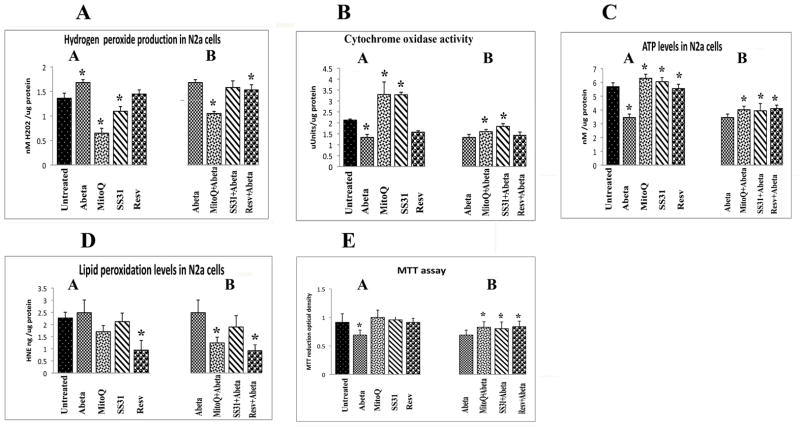
H2O2production
Significantly increased levels of H2O2 were found in mitochondria from N2a cells incubated with Aβ (P<0.002) but no changes in H2O2 were found in N2a cells incubated with Aβ 35-25 (data not shown). In measurements taken of H2O2 from mitochondria isolated from cells treated with SS31, MitoQ, and resveratrol, but not incubated with Aβ, significantly decreased levels of H2O2 were found in N2a cells treated with MitoQ (P<0.01) and SS31 (P<0.04) (Figure 7A, image a). These findings suggest that antioxidants that are targeted to mitochondria decrease H2O2 more effectively than do antioxidants that are not targeted to mitochondria, such as resveratrol. Significantly decreased levels of H2O2 were found in the N2a cells treated with MitoQ and then incubated with Aβ (P<0.01; Figure 7A, image b).
Figure 7.
Mitochondrial functional parameters in control N2a cells, in Aβ-incubated N2a cells, in N2a neurons treated with MitoQ and SS31, and in N2a cells treated with MitoQ and SS31 and then incubated with Aβ (n=4). We analyzed mitochondrial functional data in 2 ways: (1) the control N2a cells were compared to the N2a cells treated MitoQ, SS31 and resveratrol, and incubated with Aβ (image a); and (2) Aβ-incubated N2a cells were compared to (i) N2a cells treated with MitoQ and then incubated with Aβ, (ii) N2a cells treated with SS31 and then incubated with Aβ, and (iii) N2a cells treated with resveratrol and then incubated with Aβ (image b). We performed statistical analysis using ANOVA following the Dunnett correction, for: (A) cytochrome oxidase activity, (B) H2O2 production. (C) ATP levels, (D) lipid peroxidation, and (E) cell viability.
Cytochrome oxidase activity
Significantly decreased levels of cytochrome oxidase activity were found in N2a cells that were incubated with Aβ (P<0.001; Figure 7B, image a). However, significantly increased levels were found in the MitoQ-treated N2a cells (P<0.04; Figure 7B, image a) and in the SS31-treated N2a cells (P<0.02) that were not then incubated with Aβ, whereas resveratrol-treated cells that were not then incubated with Aβ exhibited significantly decreased cytochrome oxidase activity (P<0.02). As shown in Figure 7B, image b, significantly increased activity was found in the N2a cells treated with MitoQ and incubated with Aβ (P<0. 03) and in those treated with SS31 and then incubated with Aβ (P<0.02), compared cells incubated with Aβ alone, indicating that MitoQ and SS31 prevent toxic effects of Aβ on cytochrome oxidase activity.
ATP production
Significantly decreased levels of ATP were found in N2a cells that were incubated with Aβ (P<0.0005; Figure 7C, image a) but were not first treated with MitoQ, SS31, or resveratrol. As shown in Figure 7C, image a, significantly increased levels of ATP were found in N2a cells treated with MitoQ (P<0.01) and SS31 (P<0.02) but not then incubated with Aβ, compared to untreated N2a cells. Increased ATP levels were found at significant levels in the N2a cells treated MitoQ, SS31, and resveratrol, and then incubated with Aβ (P<0.02, P<0.04, and P<0.04, respectively), indicating that MitoQ, SS31, and resveratrol enhance ATP levels in the presence of Aβ (Figure 7C, image b).
Lipid peroxidation
To determine whether Aβ affects lipid peroxidation in N2a cells that underwent Aβ incubation, we measured 4-hydroxy-2-nonenol, an indicator of lipid peroxidation. Increased levels of lipid peroxidation were found, but not in significant amounts (P=0.54; Figure 7D, image a). However, significantly decreased levels were found in the resveratrol-treated N2a cells (P<0.001) relative to cells that were not incubated with Aβ, and in those treated with resveratrol and then incubated with Aβ (P<0.002). Also, significantly decreased levels were found in N2a cells that were treated with MitoQ and then incubated with Aβ (P<0.005; Figure 7D, image b).
Cell viability
Significantly decreased levels of cell viability were found in Aβ-treated N2a cells (P<0.001; Figure 7E, image a). Cell viability was also significantly increased in N2a cells treated with MitoQ and SS31 and then incubated with Aβ, compared to N2a cells only incubated with Aβ, indicating that MitoQ and SS31 prevent a decrease in cell viability that Aβ causes.
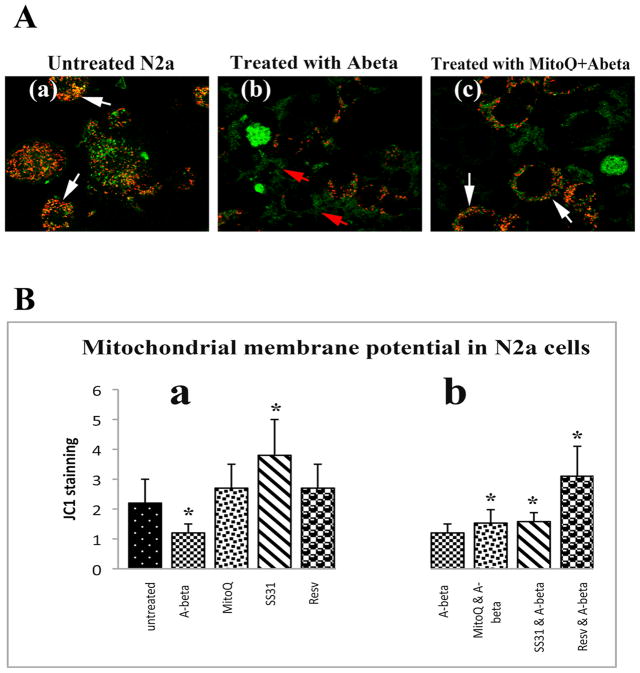
Mitochondrial membrane potential
Significantly decreased mitochondrial membrane potential was found in N2a cells that underwent incubation with Aβ (P<0.001; Figure 8B, image a). Increased levels of the mitochondrial membrane potential were found in the N2a cells treated with SS31 (P<0.002; Figure 8B, image a) but not then incubated with Aβ. In N2a cells treated with MitoQ, SS31, and resveratrol, and then incubated with Aβ, the membrane potential significantly increased in all 3 treatments (MitoQ+Aβ, P<0.04; SS31+Aβ-P<0.02; and resveratrol+Aβ, P<0.001; Figure 8B, image b), compared to N2a cells only incubated with Aβ. These results indicate that MitoQ, SS31, and resveratrol rescued membrane potential from Aβ toxicity.
Figure 8.
Mitochondrial membrane potential using live-cell imaging and JC1 staining. Image A is a representative image of N2a cells with an accumulation of JC1 in the mitochondria (in red): (a) in control N2a cells, (b) in Aβ-incubated N2a cells, where JC1 aggregates remain in the cytosol (in green), and (c) in N2a cells treated with MitoQ and then incubated with Aβ, where JC1 aggregates are in the mitochondria (in red). Image B, from quantitative analysis of mitochondrial membrane potential in N2a cells (n=4): (a) in Aβ-incubated N2a cells, in MitoQ-treated N2a cells, in SS31-treated N2a cells, and in resveratrol-treated N2a cells compared to control N2a cells, and (b) in N2a cells treated with Mito, SS31, and resveratrol, and then incubated with Aβ, compared to Aβ-incubated N2a cells. Significantly decreased mitochondrial membrane potential was found in Aβ-incubated N2a cells (P<0.001). Increased levels of the mitochondrial membrane potential were found in the SS31-treated N2a cells (P<0.002). In N2a cells treated with MitoQ, SS31, and resveratrol, and then incubated with Aβ, the membrane potential significantly increased in all 3 treatments (MitoQ+Aβ, P<0.04; SS31+Aβ-P<0.02; and resveratrol+Aβ, P<0.001) compared to Aβ-incubated N2a neurons, indicating that MitoQ, SS31, and resveratrol prevented the membrane potential from Aβ toxicity.
AβPP transgenic mice primary neurons and mitochondrial-targeted antioxidants treatments
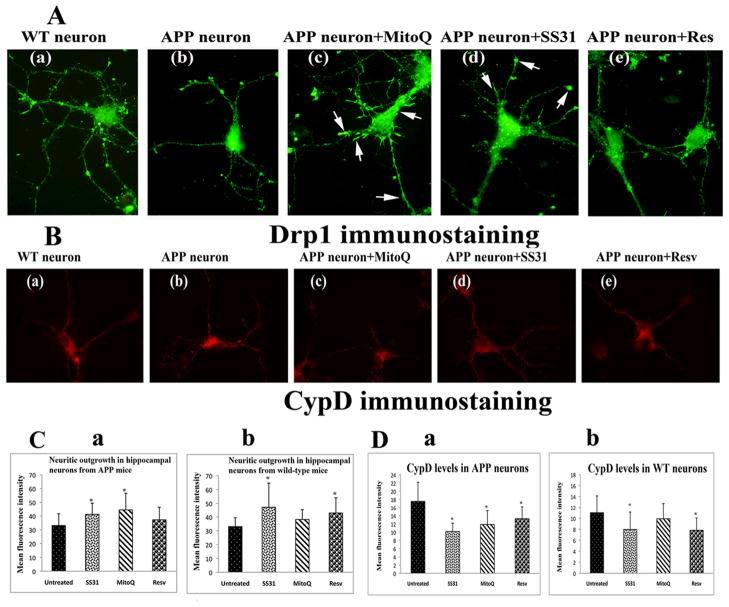
Using Drp1 immunostaining, neurite outgrowth was measured in untreated primary neurons from AβPP transgenic mice and non-transgenic littermates and in those treated with MitoQ, SS31 and resveratrol. Significantly increased immunoreactivity of Drp1 was found in cultured hippocampal neurons from AβPP transgenic mice treated with SS31 (P<0.044; Figure 9C-image a) and MitoQ (P<0.024; Figure 9C-image a), but not in those treated with resveratrol (P=0.1). Enhanced synaptic branching was found in hippocampal neurons treated with MitoQ (Figure 9A-image c) and SS31 (Figure 9A-image d) compared to untreated primary neurons, indicating that MitoQ and SS31 are capable of increasing synaptic connectivity in AD neurons. In the hippocampal neurons from wild-type mice, immunoreactivity of Drp1 was significantly increased in the neurons treated with SS31 (P<0.03) (Figure 9C-image b).
Figure 9.
Neurite outgrowth in primary hippocampal neurons from AβPP transgenic mice and wild-type mice with its hippocampal neurons treated with MitoQ, SS31, and resveratrol. Image A shows representative Drp1-stained hippocampal neurons: (a) wild-type, (b) AβPP transgenic, (c) MitoQ-treated APP, (d) SS31-treated APP, and (e) resveratrol-treated AβPP transgenic. Immunostaining was conducted using a Drp1 antibody (a marker for synaptic branching). White arrows indicate increased synaptic branching in hippocampal neurons treated with MitoQ and SS31. Fig. 9B. Cyclophilin D levels in hippocampal neurons from AβPP transgenic mice and wild-type mice. Image B shows representative CypD-stained hippocampal neurons: (a) wild-type, (b) AβPP transgenic, (c) MitoQ-treated AβPP transgenic, (d) SS31-treated AβPP transgenic, and (e) resveratrol-treated AβPP transgenic. Immunostaining was performed using the CypD antibody. Fig. 9C shows quantitative analysis of Drp1 immunostaining in hippocampal neurons from APP and wild-type mice. (a) Significantly increased Drp1 was found in hippocampal neurons from APP mice treated with MitoQ (P<0.024) and with SS31 (P<0.044), compared to control hippocampal neurons from AβPP transgenic mice. (b) Significantly increased Drp1 was found in hippocampal neurons from wild-type mice treated with SS31 (P<0.03) and resveratrol (P<0.038), compared to control hippocampal neurons from wild-type mice. Figure 9D presents quantitative analysis of CypD in hippocampal neurons from AβPP transgenic mice and wild-type mice. (a) Significantly decreased CypD was found in hippocampal neurons from AβPP transgenic mice treated with SS31 (P<0.0004), MitoQ (P<0.006), and resveratrol (P<0.013), compared to control hippocampal neurons from AβPP transgenic mice, and (b) Hippocampal neurons from wild-type mice treated with SS31 and resveratrol showed significantly decreased levels of CypD, compared to control hippocampal neurons.
In the hippocampal neurons from the APP mice, immunoreactivity of CypD was found significantly decreased in the neurons treated with SS31 (P<0.0004; Figure 9D-image a), MitoQ (P<0.006; Figure 9D-image a), and resveratrol (P<0.013; Figure 9D-image a).
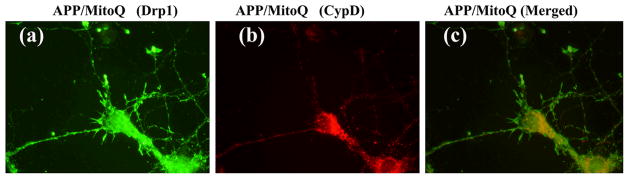
Our double labeling analysis of Drp1 and CypD in primary neurons concur with Drp1 and CypD quantification data (Figure 10). Overall, these findings indicate that MTAs can decrease CypD levels, inhibit mitochondrial pore openings, and protect mitochondria from AβPP transgenic mice carrying mutant APP and Aβ.
Figure 10.
Double-labeling analysis of (a) Drp1 (synaptic branching protein), (b) CypD (mitochondrial matrix protein), and (c) the proteins Drp1 and CypD merged in MitoQ-treated AβPP transgenic mice hippocampal neurons.
DISCUSSION
We investigated the toxicity of the Aβ peptide and the protective effects of the MTAs, MitoQ, and SS31, and resveratrol on mitochondrial structure and function, and neurite outgrowth in AD neurons. Our study concludes that Aβ damages mitochondrial structural and function in cells, and MTAs can prevent this damage caused by Aβ.
mRNA expression
Decreased mRNA levels of mitochondrial fusion genes and increased expression of fission genes were found in N2a cells incubated with Aβ. These results suggest that an imbalance in mitochondrial dynamics in these Aβ-incubated N2a cells. Our findings are in agreement with earlier studies by Wang and colleagues [13,50,51] who found abnormal mitochondrial dynamics in neurons affected by Aβ.
Interestingly, in our study, increased expression of CypD was also found in the N2a cells incubated with Aβ, suggesting that an increase in CypD damages mitochondria, possibly by inducing the opening of mitochondrial permeability pores [12,52], making them susceptible to structural damage. In contrast, mRNA expression of CypD was found decreased in N2a cells treated with MitoQ and SS31. CypD is a mitochondrial member of the cyclophilin family of peptidyl prolyl-cis, trans-isomerases and has a crucial role in protein folding [12,52]. CypD is located in the matrix of mitochondria and is involved in regulating the mitochondrial permeable transition pore. The pore opening raises the permeability of the mitochondrial inner membrane, allows an influx of cytosolic molecules into the mitochondrial matrix, increases the matrix volume, disrupts the mitochondrial outer membrane, and ultimately damages mitochondrial structure and function. Overall, findings from this study, together with others [12,52], indicate that Aβ increases CypD expression, may promote the opening of mitochondrial inner membrane, and may be involved in damaging mitochondrial structure.
In our study, increased levels of mRNA expression in the mitochondrial fission genes did not occur in N2a cells treated with either MitoQ or SS31, and then incubated with Aβ, suggesting that MitoQ and SS31 may prevent mitochondrial structural genes from expressing abnormally. Overall, MitoQ and SS31 appear to protect mitochondrial structure by regulating mitochondrial fission, fusion, and matrix genes.
In N2a cells not treated with MitoQ, SS31, or resveratrol, but incubated with Aβ, mRNA expressions significantly decreased in all peroxiredoxins (1–6), particularly Prx1 (with a 5-fold decrease) and Prx1 (a 4.5-fold decrease). Our immunoblotting findings agreed with these mRNA expression results. The decrease of Prxs in N2a cells incubated with Aβ was prevented in those cells that were first treated with MitoQ and SS31, suggesting that these MTAs preserve endogenous antioxidant enzymes in cells. Further, Prxs increased in N2a cells treated with MitoQ, SS31, and resveratrol. These findings suggest that Aβ decreases antioxidant enzyme activities in N2a cells, and MitoQ, SS31, increase Prxs expression in N2a cells.
Peroxiredoxins are a group of antioxidant enzymes present in all organisms. They protect neurons by regulating redox, phosphorylation, and oligomerization in cells [39]. Peroxiredoxins neutralize H2O2 and participate in decreasing oxidative damage, which protects cells from various toxic insults, including Aβ toxicity. The expression of Prxs is a strong indicator of antioxidant activity in the brain. The literature on Prxs expression and activity in the brains from AD patients is limited [53–55]. In postmortem AD brains, Prx1 and Prx6 were found to be greater than control brains, and this increase may be a protective compensatory response against Aβ toxicity [53,55]. Our study is the first to investigate the preventive effects of MTAs against Aβ toxicity in N2a cells, and the rescue effects of Prxs in protecting AD mice neurons against Aβ toxicity.
The increase of the neuroprotective genes PGC1α, FOXO1, and NMDAR may be a compensatory response against Aβ toxicity in N2a cells treated with Aβ. Recent literature suggests that increased mRNA expression of genes PGC1α, FOXO1 and NMDA receptor reported to decrease oxidative damage in brain tissues in patients with neurodegenerative diseases and oxidative insults [36,56–63]. Interestingly, in N2a cells pretreated with MitoQ and SS31, and then incubated with Aβ, the expression of mRNA further increased, indicating that MTAs protects cells from Aβ toxicity. However, the protective effect of resveratrol was lower than that of SS31 or MitoQ, suggesting that targeted antioxidants protect cells against Aβ much more effectively than do untargeted antioxidants, such as resveratrol.
PGC1α is a transcription co-activator that interacts with a range of transcription factors involved in a wide variety of biological responses, including adaptive thermogenesis and mitochondrial biogenesis of several tissues, such as brain tissues [36]. The overexpression of PGC1α was found to protect mammalian cells, including neurons, from mitochondrial oxidative damage by mediating gene expressions of transcription factors. Recently, lower-than-normal levels of PGC-1α expression were found in elderly persons and elderly persons with neurodegenerative diseases such as Alzheimer’s [56] and Huntington’s [57–59]. Our present findings of increased PGC1αexpression in cells treated with MitoQ and SS31 suggest that these MTAs can play a key role in decreasing oxidative damage.
FOXO1, a fork head transcription factor of the FOXO subfamily, which plays an important role in cellular differentiation, proliferation, and energy metabolism [60–62]. FOXO1 has been implicated with oxidative stress and elevated glucose concentrations. The chronic exposure of cells to elevated glucose concentrations causes a deterioration of β cell function. Glucose toxicity is thought to arise as a consequence of chronic oxidative stress, when intracellular glucose concentrations exceed the glycolytic capacity of the β cell. The over-expression of FOXO1 suppresses oxidative stress under hyperglycemia or abnormal glucose metabolism [62]. The increase in FOXO1 expression that we found in N2a cells treated with MitoQ and SS31 indicate that these MTAs may play a protective role via the FOXO1 pathway in cells.
Recently, NMDAR activity in synapses was found to have protective effects by stimulating the antioxidant enzymes peroxiredoxins [40,63]. In the present study, we measured mRNA expression of PGC1α, FOXO1, and NMDAR to determine the toxic effects of Aβ and the rescue effects of MitoQ, SS31, and resveratrol in relation to peroxiredoxins.
Electron microscopy
Given the greater number of damaged mitochondria that we found in N2a cells incubated with Aβ than in N2a cells not incubated with Aβ, Aβ may likely increase mitochondrial fragmentation and damage. Altered and damaged mitochondria have been identified as extensive in AD patients, and they may be responsible for cognitive changes in these patients [6,7,10,15,19,20,51,52,64]. In our study, MitoQ and SS31 reduced Aβ toxicity. N2a cells that were treated with these MTAs – whether or not the treatment was followed by Aβ incubation – exhibited less mitochondrial damage than N2a cells that were treated with Aβ. Further, MitoQ- and SS31-treated N2a neurons exhibited a more intact mitochondrial structure than N2a cells incubated with Aβ, suggesting that the MTAs balance fission and fusion machinery. These findings agree with real-time RT-PCR and protein data of fission and fusion genes and with mitochondrial functional data. Elongated mitochondria were found in the soma, axons, neuronal processes, and terminals of untreated N2a cells that were not incubated with Aβ, indicating that healthy elongated mitochondria travel all along neuronal processes to supply ATP at nerve terminals. The mitochondria found in the neuronal processes of the MitoQ- and SS31-treated N2a cells were much healthier than the elongated mitochondria in the untreated cells, suggesting that the MTAs may also enhance the intact structure of mitochondria and may facilitate the supply of ATP at nerve terminals.
Neurite outgrowth
Recent studies revealed that the movement of mitochondria into dendritic spines correlates with development and morphological plasticity of the spines. Drp1 is a member of the dynamin superfamily of GTPases. Drp1 is primarily localized in the cell body, axons, and dendrites. Drp1 regulates mitochondrial morphology and distribution [65] and is good marker for synaptic branching. A decrease in the expression of Drp1 correlates with a reduction in dendritic mitochondrial content and the loss of synapses and dendritic spines, an indication that Drp1 is critical for synaptic growth in neurons [65]. In the present study, we found significantly decreased neurite outgrowth (marked by Drp1 immunostaining) in N2a cells that were incubated with Aβ. However, in N2a cells treated with MitoQ and SS31, neurite outgrowth significantly increased in comparison to N2a cells incubated with Aβ, indicating that these MTAs enhance neurite outgrowth. This association of MitoQ and SS31 with an increase in neurite outgrowth has implications for treating AD patients, particularly when the disease is actively progressing and fewer neurons remain in the AD brains. We also found increased neurite outgrowth in cells treated with MitoQ and SS31, in comparison to untreated N2a cells or those incubated with Aβ, indicating that these MTAs may be capable of increasing neurite outgrowth. The outcome from this increase in neurite outgrowth, in N2a cells treated with MitoQ and SS31 may have implications in terms of enhancing synaptic branching of neurons and cognitive functions of normal people.
Neurite outgrowth in AD transgenic primary neurons
In postnatal primary neurons from AD transgenic mice carrying human mutant APP, treatment of N2a neurons with MitoQ and SS31 increased neurite outgrowth, also indicating that these enhance synaptic branching in neurons with over-expressed mutant APP and Aβ. Further, antioxidants targeted to mitochondria, such as MitoQ and SS31, have been reported to cross the blood brain barrier, ultimately reaching neurons and mitochondria, decreasing mitochondrial dysfunction, and increasing ATP production [31,32]. This study is the first to demonstrate that MitoQ and SS31 increase synaptic branching in primary neurons from AD mice and wild-type mice, suggesting that antioxidants targeted to mitochondria boost neurite outgrowth. These results also suggest that MitoQ and SS31 play an important role in enhancing neuronal branching in neurons that carry APP mutations, and also in wild-type neurons.
Mitochondrial function
N2a cells that were treated with MitoQ and SS31 exhibited less abnormal mitochondrial function that did N2a cells incubated with Aβ (Figure 7). Similar to other researchers’ findings [66,67], our data indicated that mitochondrial function is abnormal in N2a cells incubated with Aβ, more specifically, Aβ negatively affected N2a cells in terms of increasing free radicals and lipid peroxidation; decreasing cytochrome oxidase activity; reducing the mitochondrial membrane potential and ATP production; and reducing cell viability. In N2a cells treated with MitoQ and SS31, and then incubated with Aβ, the neurons exhibited decreased mitochondrial dysfunction. Mitochondrial function – in terms of ATP production, cytochrome oxidase activity, and mitochondrial membrane potential – was significantly improved in N2a cells only incubated with the MTAs. These findings are consistent with gene-expression data and data indicating the reduction of neurite outgrowth in these neurons. In the presence of Aβ, MitoQ and SS31 prevented mitochondrial depolarization, a major parameter of mitochondrial function. Thus, all of our data point to MitoQ and SS31 protecting N2a cells from Aβ toxicity. Resveratrol, in particular, may protect N2a cells from lipid peroxidation generated by oxidative stress and may enhance the mitochondrial membrane potential. In our study, MitoQ- and SS31-treated N2a cells significantly scavenged free radicals and increased cytochrome oxidase activity (Figure 7), but it was the resveratrol-treated N2a cells that exhibited a significant decreased in lipid peroxidation levels and a significant increase in the mitochondrial membrane potential.
CONCUSIONS
The purpose of our study was to investigate the effects of the MTAs MitoQ and SS31, and the anti-aging agent resveratrol on N2a cells from an AD mouse model and on N2a cells from these mice, that were incubated with Aβ.
In N2a cells only incubated with the Aβ, we found increased mRNA levels of fission genes, decreased mRNA levels of fusion genes, and decreased mRNA expression of peroxiredoxins. Electron microscopy revealed a significantly increased number of fragmented mitochondria in these cells, indicating that Aβ damages mitochondria by activating fission genes. Our extensive biochemical analysis of mitochondrial function (H2O2 production, cytochrome oxidase activity, lipid peroxidation, ATP production, cell viability and mitochondrial membrane potential), revealed the dominance of dysfunctional mitochondria and a significant decrease in neurite outgrowth, also indicating negative effects from Aβ on mitochondria.
However, in N2a cells treated with MitoQ, SS31, and resveratrol, and then incubated with Aβ, abnormal expression of peroxiredoxins and mitochondrial with abnormal structure were prevented. Mitochondrial functional changes – including H2O2 production, cytochrome oxidase, ATP production, lipid peroxidation, mitochondrial membrane potential, and cell viability – were not affected in the N2a cells that were treated with the MitoQ, SS31 but were not incubated with Aβ. Neurite outgrowth significantly increased in these cells. This is the first study to report the positive effects of MitoQ, and SS31 against Aβ peptide toxicity.
In primary neurons from AβPP transgenic mice, which were treated with MitoQ and SS31, neurite outgrowth significantly increased and cyclophilin D expression significantly decreased. This is the first study to report that MitoQ and SS31 significantly increased neurite outgrowth and synaptic connectivity in neurons affected by AD. These findings indicate that MitoQ and SS31 prevent Aβ toxicity in mitochondria and that the study of these MitoQ, SS31 as potential drugs to treat AD patients is warranted.
Acknowledgments
This research presented was supported by NIH grants AG028072, AG026051, and RR00163, Alzheimer Association grant IIRG-09-92429, and Medivation, Inc. We thank Drs. Mike Webb and Eric Barklis (OHSU) for their assistance with EM of neurons. We also thank Dr. Karen Ashe (University of Minnesota for gifting the APP mice.
Footnotes
CONFLICT OF INTEREST STATEMENT
Patent applications have been filed by the Cornell Research Foundation Inc (CRF) for the technology (SS peptides) described in this article. Hazel H Szeto is the inventor. CRF, on behalf of Cornell University, has licensed the technology for further research and development to a commercial enterprise in which CRF and Dr. Szeto have financial interests. Patents in the MitoQ technology are being commercialized by Antipodean Pharmaceuticals in which M. Murphy has a financial interest.
References
- 1.Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 3.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 4.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 5.Terry RD. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 6.Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, et al. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy PH, McWeeney S, Park BS, Manczak M, Gutala RV, Partovi D, Jung Y, Yau V, Searles R, Mori M, Quinn J. Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: up-regulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer’s disease. Hum Mol Genet. 2004;13:1225–1240. doi: 10.1093/hmg/ddh140. [DOI] [PubMed] [Google Scholar]
- 8.Swerdlow RH, Khan SM. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med Hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 9.Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 10.Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 11.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 12.Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, et al. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat Med. 2008;14:1097–105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddy PH. Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer’s disease. Exp Neurol. 2009;218:286–292. doi: 10.1016/j.expneurol.2009.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massaad CA, Washington TM, Pautler RG, Klann E. Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U SA. 2009;106:13576–13581. doi: 10.1073/pnas.0902714106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, Ozmen L, Bluethmann H, Dröse S, Brandt U. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc Natl Acad Sci U S A. 2009;106:20057–20062. doi: 10.1073/pnas.0905529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 18.Manczak M, Mao P, Nakamura K, Bebbington C, Park B, Reddy PH. Neutralization of granulocyte macrophage colony-stimulating factor decreases amyloid beta 1–42 and suppresses microglial activity in a transgenic mouse model of Alzheimer’s disease. Hum Mol Genet. 2009;18:3876–3893. doi: 10.1093/hmg/ddp331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, Yan SD. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB J. 2005;19:2040–1. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 20.Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009;106:14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J Neurosci. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckert A, Hauptmann S, Scherping I, Rhein V, Müller-Spahn F, Götz J, Müller WE. Soluble beta-amyloid leads to mitochondrial defects in amyloid precursor protein and tau transgenic mice. Neurodegener Dis. 2008;5:157–159. doi: 10.1159/000113689. [DOI] [PubMed] [Google Scholar]
- 23.Masaki KH, Losonczy KG, Izmirlian G, Foley DJ, Ross GW, Petrovitch H, Havlik R, White LR. Association of vitamin E and C supplement use with cognitive function and dementia in elderly men. Neurology. 2000;54:1265–1272. doi: 10.1212/wnl.54.6.1265. [DOI] [PubMed] [Google Scholar]
- 24.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, Wilson RS, Scherr PA. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA. 2002;287:3230–3237. doi: 10.1001/jama.287.24.3230. [DOI] [PubMed] [Google Scholar]
- 25.Grodstein F, Chen J, Willett WC. High-dose antioxidant supplements and cognitive function in community-dwelling elderly women. Am J Clin Nutr. 2003;77:975–984. doi: 10.1093/ajcn/77.4.975. [DOI] [PubMed] [Google Scholar]
- 26.Zandi PP, Anthony JC, Khachaturian AS, Stone SV, Gustafson D, Tschanz JT, Norton MC, Welsh-Bohmer KA, Breitner JC Cache County Study Group. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol. 2004;61:82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- 27.Mendelsohn AB, Belle SH, Stoehr GP, Ganguli M. Use of antioxidant supplements and its association with cognitive function in a rural elderly cohort: the MoVIES Project. Monongahela Valley Independent Elders Survey. Am J Epidemiol. 1998;148:38–44. doi: 10.1093/oxfordjournals.aje.a009556. [DOI] [PubMed] [Google Scholar]
- 28.Luchsinger JA, Tang MX, Shea S, Mayeux R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch Neurol. 2003;60:203–208. doi: 10.1001/archneur.60.2.203. [DOI] [PubMed] [Google Scholar]
- 29.Fillenbaum GG, Kuchibhatla MN, Hanlon JT, Artz MB, Pieper CF, Schmader KE, Dysken MW, Gray SL. Dementia and Alzheimer’s disease in community-dwelling elders taking vitamin C and/or vitamin E. Ann Pharmacother. 2005;39:2009–2014. doi: 10.1345/aph.1G280. [DOI] [PubMed] [Google Scholar]
- 30.Gray SL, Anderson ML, Crane PK, Breitner JC, McCormick W, Bowen JD, Teri L, Larson E. Antioxidant vitamin supplement use and risk of dementia or Alzheimer’s disease in older adults. J Am Geriatr Soc. 2008;56:291–295. doi: 10.1111/j.1532-5415.2007.01531.x. [DOI] [PubMed] [Google Scholar]
- 31.Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 32.Szeto HH. Development of mitochondria-targeted aromatic-cationic peptides for neurodegenerative diseases. Ann N Y Acad Sci. 2008;1147:112–121. doi: 10.1196/annals.1427.013. [DOI] [PubMed] [Google Scholar]
- 33.Yang L, Zhao K, Calingasan NY, Luo G, Szeto HH, Beal MF. Mitochondria targeted peptides protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Antioxid Redox Signal. 2009;11:2095–2104. doi: 10.1089/ars.2009.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anekonda TS. Resveratrol--a boon for treating Alzheimer’s disease? Brain Res Rev. 2006;52:316–326. doi: 10.1016/j.brainresrev.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Anekonda TS, Reddy PH. Neuronal protection by sirtuins in Alzheimer’s disease. J Neurochem. 2006;96:305–313. doi: 10.1111/j.1471-4159.2005.03492.x. [DOI] [PubMed] [Google Scholar]
- 36.Liang H, Ward WF. PGC-1alpha: a key regulator of energy metabolism. Adv Physiol Educ. 2006;30:145–151. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- 37.Nakae J, Cao Y, Oki M, Orba Y, Sawa H, Kiyonari H, Iskandar K, Suga K, Lombes M, Hayashi Y. Forkhead transcription factor FoxO1 in adipose tissue regulates energy storage and expenditure. Diabetes. 2008;57:563–576. doi: 10.2337/db07-0698. [DOI] [PubMed] [Google Scholar]
- 38.Hardingham GE. Coupling of the NMDA receptor to neuroprotective and neurodestructive events. Biochem Soc Trans. 2009;37:1147–1160. doi: 10.1042/BST0371147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 40.Hattori F, Oikawa S. Peroxiredoxins in the central nervous system. Subcell Biochem. 2007;44:357–374. doi: 10.1007/978-1-4020-6051-9_17. [DOI] [PubMed] [Google Scholar]
- 41.Manczak M, Park BS, Jung Y, Reddy PH. Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular Med. 2004;5:147–162. doi: 10.1385/NMM:5:2:147. [DOI] [PubMed] [Google Scholar]
- 42.Gutala RV, Reddy PH. The use of real-time PCR analysis in a gene expression study of Alzheimer’s disease post-mortem brains. J Neurosci Methods. 2004;132:101–107. doi: 10.1016/j.jneumeth.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Drew B, Leeuwenburgh C. Method for measuring ATP production in isolated mitochondria: ATP production in brain and liver mitochondria of Fischer-344 rats with age and caloric restriction. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1259–R1267. doi: 10.1152/ajpregu.00264.2003. [DOI] [PubMed] [Google Scholar]
- 44.Hall ED, Bosken JM. Measurement of oxygen radicals and lipid peroxidation in neural tissues. Curr Protoc Neurosci. 2009;Chapter 7:1–51. doi: 10.1002/0471142301.ns0717s48. [DOI] [PubMed] [Google Scholar]
- 45.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 46.Wagner OI, Lifshitz J, Janmey PA, Linden M, McIntosh TK, Leterrier JF. Mechanisms of mitochondria-neurofilament interactions. J Neurosci. 2003;23:9046–9058. doi: 10.1523/JNEUROSCI.23-27-09046.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pool M, Thiemann J, Bar-Or A, Fournier AE. NeuriteTracer: a novel ImageJ plugin for automated quantification of neurite outgrowth. J Neurosci Methods. 2008;168:134–139. doi: 10.1016/j.jneumeth.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 48.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 49.Brewer GJ, Torricelli JR. Isolation and culture of adult neurons and neurospheres. Nat Protoc. 2007;2:1490–1498. doi: 10.1038/nprot.2007.207. [DOI] [PubMed] [Google Scholar]
- 50.Wang X, Su B, Fujioka H, Zhu X. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer’s disease patients. Am J Pathol. 2008;173:470–482. doi: 10.2353/ajpath.2008.071208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, Johnson N, Capano M, Edwards M, Crompton M. Cyclophilin-D promotes the mitochondrial permeability transition but has opposite effects on apoptosis and necrosis. Biochem J. 2004;383(Pt 1):101–109. doi: 10.1042/BJ20040669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Power JH, Asad S, Chataway TK, Chegini F, Manavis J, Temlett JA, Jensen PH, Blumbergs PC, Gai WP. Peroxiredoxin 6 in human brain: molecular forms, cellular distribution and association with Alzheimer’s disease pathology. Acta Neuropathol. 2008;115:611–622. doi: 10.1007/s00401-008-0373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cumming RC, Dargusch R, Fischer WH, Schubert D. Increase in expression levels and resistance to sulfhydryl oxidation of peroxiredoxin isoforms in amyloid beta-resistant nerve cells. J Biol Chem. 2007;282:30523–30534. doi: 10.1074/jbc.M700869200. [DOI] [PubMed] [Google Scholar]
- 55.Yao J, Taylor M, Davey F, Ren Y, Aiton J, Coote P, Fang F, Chen JX, Yan SD, Gunn-Moore FJ. Interaction of amyloid binding alcohol dehydrogenase/Abeta mediates up-regulation of peroxiredoxin II in the brains of Alzheimer’s disease patients and a transgenic Alzheimer’s disease mouse model. Mol Cell Neurosci. 2007;35:377–382. doi: 10.1016/j.mcn.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 56.Qin W, Haroutunian V, Katsel P, Cardozo CP, Ho L, Buxbaum JD, Pasinetti GM. PGC-1alpha expression decreases in the Alzheimer disease brain as a function of dementia. Arch Neurol. 2009;66:352–361. doi: 10.1001/archneurol.2008.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weydt P, Pineda VV, Torrence AE, Libby RT, Satterfield TF, Lazarowski ER, Gilbert ML, Morton GJ, Bammler TK, Strand AD, et al. Thermoregulatory and metabolic defects in Huntington’s disease transgenic mice implicate PGC-1alpha in Huntington’s disease neurodegeneration. Cell Metab. 2006;4:349–362. doi: 10.1016/j.cmet.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 58.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, Handschin C, Zheng K, Lin J, Yang W, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 59.Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 60.Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–146. [PubMed] [Google Scholar]
- 61.Birkenkamp KU, Coffer PJ. FOXO transcription factors as regulators of immune homeostasis: molecules to die for? J Immunol. 2003;171:1623–1629. doi: 10.4049/jimmunol.171.4.1623. [DOI] [PubMed] [Google Scholar]
- 62.Gross DN, van den Heuvel AP, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene. 2008;27:2320–2336. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- 63.Papadia S, Soriano FX, Léveillé F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008;11:476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crouch PJ, Blake R, Duce JA, Ciccotosto GD, Li QX, Barnham KJ, Curtain CC, Cherny RA, Cappai R, Dyrks T, et al. Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-beta1–42. J Neurosci. 2005;25:672–679. doi: 10.1523/JNEUROSCI.4276-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 66.Aleardi AM, Benard G, Augereau O, Malgat M, Talbot JC, Mazat JP, Letellier T, Dachary-Prigent J, Solaini GC, Rossignol R. Gradual alteration of mitochondrial structure and function by beta-amyloids: importance of membrane viscosity changes, energy deprivation, reactive oxygen species production, and cytochrome c release. J Bioenerg Biomembr. 2005;37:207–225. doi: 10.1007/s10863-005-6631-3. [DOI] [PubMed] [Google Scholar]
- 67.Casley CS, Canevari L, Land JM, Clark JB, Sharpe MA. Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J Neurochem. 2002;80:91–100. doi: 10.1046/j.0022-3042.2001.00681.x. [DOI] [PubMed] [Google Scholar]