Abstract
Background. Vaccinia virus keratitis (VACVK) is a complication of smallpox vaccination that can result in blindness. There are no Food and Drug Administration–approved treatments for VACVK, and vaccinia immunoglobulin (VIG) is contraindicated in isolated VACVK. We used a rabbit model of infection to compare several therapeutic options for VACVK.
Methods. Rabbit eyes were infected with 105 plaque-forming units of the Dryvax strain of vaccinia virus and scored daily for 28 days using a modified MacDonald-Shadduck scoring system. Animals were treated for 10 days after the onset of keratitis with albumin, VIG, prednisolone acetate, trifluridine, or combinations thereof. Ocular viral titers and vaccinia-specific antibody titers were determined by plaque assay and enzyme-linked immunosorbent assay, respectively.
Results. Treatment with intravenous VIG neither exacerbated nor ameliorated VACVK. Topical prednisolone acetate interfered with viral clearance, and ocular disease rebounded in prednisolone-treated groups. The most effective treatment was topical trifluridine alone.
Conclusions. We conclude that (1) VIG did not negatively affect the treatment of isolated keratitis, (2) topical corticosteroids should not be used for treating VACVK, and (3) treatment with topical trifluridine, with or without intravenous VIG, is the preferred therapeutic regimen for treating VACVK.
Ocular vaccinia is a common side effect of smallpox vaccination, usually the result of an accidental transfer of vaccinia virus (VACV) from the inoculation site to the eye. In a group of 40,000 primary vaccinees, ocular vaccinia occurred 1–4 times [1, 2], and manifestations included conjunctival disease, iritis, and keratitis [3, 4]. Vaccinia virus keratitis (VACVK) begins as a finely granular opacification of the cornea and can progress to ulceration, endothelial keratitis, and diffuse interstitial keratitis [3]. Corneal neovascularization, aqueous flare, and aqueous cells also commonly occur [3]. VACVK was estimated to occur in up to 30% of all ocular vaccinia cases [3].
The recent reinstitution of vaccination of military personnel, hospital staff, and first responders has led to a reevaluation of the available treatments for adverse reactions. The only compound currently approved by the Food and Drug Administration for use in treating complications of smallpox vaccination is vaccinia immunoglobulin (VIG) [5]. However, VIG is currently contraindicated for use in isolated cases of VACVK [5], based on the finding that VIG exacerbated corneal scarring in a rabbit model of VACVK [6]. Case studies in which patients with VACVK were treated with VIG did not find prolonged corneal scarring related to the treatment [2, 7–9]. These conflicting reports highlight the need for a comprehensive, controlled study to investigate the effect of VIG on VACVK.
Several therapies have been used clinically to treat VACVK. Topical administration of interferon from monkey kidney in patients appeared to reduce the severity of VACVK-related epithelial disease but not stromal disease [10]. The nucleoside analog idoxuridine (5-iodo-2'-deoxyuridine) has been used topically to treat VACVK, often in conjunction with VIG, in spite of toxicity concerns [2, 11, 12]. Corticosteroids have also been used on their own or in conjunction with antivirals [8, 13, 14]. Trifluridine (trifluorothymidine [TFT]), has been shown to inhibit VACV infection in vitro, is more effective than idoxuridine at reducing VACVK in rabbits [15, 16] and is currently used off-label to treat VACVK [4, 17]. The lack of Food and Drug Administration–approved treatment for VACVK highlights a pressing need for a controlled analysis of possible therapies. We therefore compared the effects of different therapeutic combinations of VIG, prednisolone acetate, and TFT in a rabbit model of VACVK. Our results identify the optimum treatment regimen and demonstrate that the VIG could be potentially used even in cases of isolated keratitis.
METHODS
Cells and Viruses
Vero cells (CCL-81; American Type Culture Collection [ATCC]) and HeLa cells (CCL-2; ATCC) were propagated in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and penicillin-streptomycin (30-002-CI; Mediatech). To propagate virus [18], Vero cells were infected with the New York City Department of Health Laboratories strain of VACV (VR-1536; ATCC) at a multiplicity of infection of .01 in Dulbecco's modified Eagle's medium supplemented with 2% fetal bovine serum. The cells and supernatants were harvested when the cytopathic effect reached 90%–100%, the cells were frozen and thawed 3 times to release bound virus, and cell debris was pelleted by centrifugation. The supernatants were then layered onto a cushion of 36% sucrose in HEPES-buffered Hank's balanced salt solution (H-HBSS) (CC-5024; Lonza) and centrifuged at 20,000 g for 80 min in an SW28 rotor (Beckman). The pelleted virus was then titered on HeLa cells, resuspended in H-HBSS at a concentration of 109 plaque-forming units [PFUs]/mL, and stored in 100-μL aliquots at −80°C until use. Endotoxin levels in the viral stocks were determined using the ToxinSensor Chromogenic LAL Endotoxin assay kit (L00350; GenScript). The concentration was .0159 units/mL, which translated to .08 ng/mL. Each eye received .004 ng of endotoxin.
Animals
Female rabbits Hra:(NZW)SPF, weighing 3–4 kg, were obtained from Harlan. Fifty-six naive animals were randomly assigned to 1 of 7 experimental groups. All procedures in this protocol were in compliance with the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, the Office of Laboratory Animal Welfare, Association for Research in Vision and Ophthalmology guidelines, and the University of Wisconsin Institutional Animal Care and Use Committee.
Animal Inoculation and Disease Scoring
On day 1 of the study, animals were anesthetized with ketamine hydrochloride (up to 30 mg/kg) (Lloyd Laboratories) and midazolam hydrochloride (1 mg/kg) (Abraxis Pharmaceutical Products) via intramuscular injection into the lateral gastrocnemius muscle. If needed, an additional dose of ketamine was given at (2–5 mg/kg). The right eye of each rabbit was trephinated to a depth of ∼.25 mm using a 7.5-mm-diameter Hessburg-Barron vacuum trephine (Jedmed). After trephination, 20 μL of the viral inoculum (105 PFUs) [19] was placed in the trephine wound. The eyes were examined twice daily until the onset of keratitis, when treatment commenced according to the schedule outlined in Table 1. Prednisolone acetate and TFT were applied in single drops to the infected eyes.
Table 1.
Vaccinia Keratitis Treatment Schedule
| Group and Treatmenta | Dose | Route | Frequency |
| Group 1 | |||
| Albumin | Protein matched to VIG | Intravenous | Repeat after 7 days (2 total doses) |
| Group 2 | |||
| VIG | 6000 U/kg | Intravenous | Single dose |
| Group 3 | |||
| VIG | 6000 U/kg | Intravenous | Repeat after 7 days (2 total doses) |
| Group 4 | |||
| VIG | 6000 U/kg | Intravenous | Single dose |
| 1% trifluridine | 9 doses ∼2 h apart | Topical | For 10 days |
| 1% prednisolone acetate | 4 doses ∼4 h apart | Topical | For 10 days |
| Group 5 | |||
| VIG | 6000 U/kg | Intravenous | Repeat after 7 days (2 total doses) |
| 1% trifluridine | 9 doses ∼2 h apart | Topical | For 10 days |
| 1% prednisolone acetate | 4 doses ∼4 h apart | Topical | For 10 days |
| Group 6 | |||
| 1% trifluridine | 9 doses ∼2 h apart | Topical | For 10 days |
| 1% prednisolone acetate | 4 doses ∼4 h apart | Topical | For 10 days |
| Group 7 | |||
| 1% trifluridine | 9 doses ∼2 h apart | Topical | For 10 days |
NOTE. Each group included 8 rabbits. VIG, vaccinia immunoglobulin.
Ocular irritation was scored according to a modified MacDonald-Shadduck scoring system, as described elsewhere [19]. Briefly, corneal opacity was scored on a scale of 0–6, with 6 corresponding to perforation; the percentage of corneal opacity was scored on a scale of 0–4, based on the percentage of involvement; and corneal neovascularization was scored on a scale of 0–2, based on invasion from the limbus. Conjunctival congestion and chemosis were scored on scales of 0–3 and 0–4, respectively. Discharge from the cornea was scored on a scale of 0–3, and fluorescein staining on a scale of 0–4, based on the percentage of corneal area involved. Slit-lamp evaluations were performed using a hand-held Kowa SL-15 biomicroscope (Kowa) at high (×15) magnification. Indirect ophthalmoscopy was performed on days 1 and 14 using a Keeler VantagePlus indirect headset (Keeler Instruments) with a Volk Pan Retinal 2.2 Lens (Volk Optical). An AccuPach V pachymeter (Accutome) was used to determine the thickness of infected corneas on days 21–28. Proparacaine hydrochloride sterile ophthalmic solution (Akorn) was placed in each eye as needed for evaluation.
Photography
Digital and fluorescein photographs were taken before inoculation of the virus (day 0) and on days 7, 14, and 28, as described elsewhere [19].
Virus Isolation
Corneal swab samples were collected from the right eye once before inoculation of the virus and on days 2, 3, 4, 6, 8, 10, 12, and 14 after infection. Samples were stored in H-HBSS at −80°C until they were titered. Titers were determined by plaque assay on HeLa cells.
Detection of Anti-VACV and Human Immunoglobulin G Antibodies by Enzyme-Linked Immunosorbent Assay
Blood samples were collected once before inoculation of the virus (baseline) and on days 7 and 14, from the jugular vein (baseline, day 7, and day 14) and by cardiac puncture (day 28). The enzyme-linked immunosorbent assay for VIG and rabbit anti-VACV antibodies were described elsewhere [19]. Samples were considered positive if the absorbance values were >3 standard deviations greater than the negative control.
Histology
Eyes, eyelids, and extraocular lesions were fixed in 4% paraformaldehyde and then embedded in paraffin, sectioned, stained with hematoxylin-eosin, and examined microscopically with a BX51 light microscope (Olympus America). Pathological changes were scored as described elsewhere [19].
Statistical Analysis
Statistical analyses included Kruskal-Wallis analysis of variance on ranks, and pairwise multiple comparisons and were performed using SigmaPlot software (version 11.0; Systat Software).
RESULTS
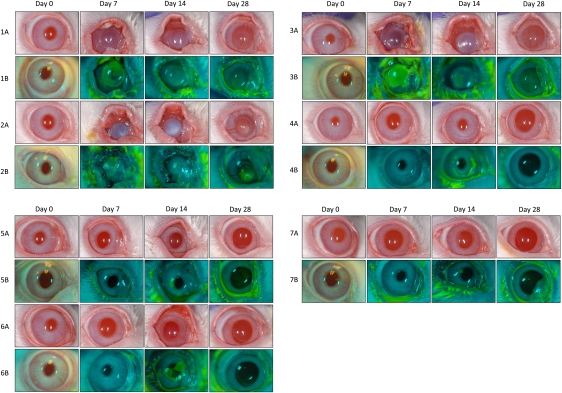
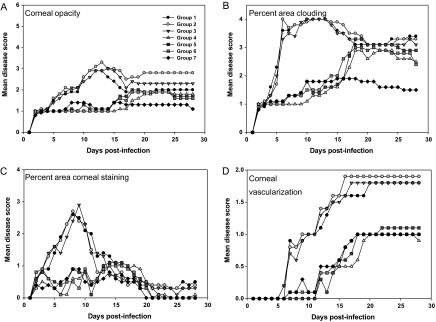
The progression of the disease over the course of the study for representative rabbits is shown in the photographs in Figure 1 and the ranges for each parameter scored are shown in Supplementary Table 1. For corneal disease (Figure 2), results were similar for the albumin- and VIG-only groups (groups 1–3). Opacity scores increased between days 5 and 12 and then began to decline. For corneal vascularization, scores remained at the peak through day 28. The albumin- or VIG-only groups (groups 1–3) did not differ significantly from each other (P > .05; Kruskal-Wallis). In all groups receiving prednisolone (groups 4–6), disease scores rose initially, plateaued, and then began to increase again at about day 15. From days 5–14 after infection, the disease scores for groups 4–6 (prednisolone with or without VIG or TFT) were significantly different from those for groups 1–3 (albumin and VIG only) (P < .05) but not from each other (P > .05). Except for vascularization, the TFT-only group (group 7) showed the best therapeutic effect, because the rebound disease phenomenon observed in prednisolone-treated groups was not seen. From day 15 after infection, corneal opacity and area of clouding scores in group 7 (TFT only) were significantly different from those in all other groups (P < .05).
Figure 1.
Photographic time course of vaccinia keratitis recorded with digital photography. Light image panels (denoted A) were obtained with a Nikon D100 single-lens reflex digital camera and Nikon AF Micro-NIKKOR 105mm 1:2.8D lens fitted with a Nikon Macro Speedlight SB-29s ring flash and Promaster Skylight 1A Filter 52mm. Fluorescein staining panels (denoted B) were obtained with a Nikon D200 single-lens reflex digital camera and Nikon AF-S Micro-NIKKOR 105mm 1:2.8G ED lens fitted with a Nikon R1 Flash System. Images shown are time courses for 1 representative rabbit per group during the course of the study, including groups 1 (albumin), 2 (single-dose vaccinia immunoglobulin [VIG]), 3 (2 doses of VIG), 4 (single-dose VIG, trifluorothymidine [TFT], prednisolone), 5 (2 doses of VIG, TFT, prednisolone), 6 (TFT and prednisolone), and 7 (TFT only).
Figure 2.
Corneal disease. Infected eyes were examined daily by a board-certified ophthalmologist using a slit-lamp biomicroscope and scored according to a modified MacDonald-Shadduck scoring system, as described elsewhere [19]. A, Severity of corneal opacity. B, Percentage of corneal surface with stromal clouding. C, Percentage of corneal surface staining positive for epithelial disruption with fluorescein. D, Extent of corneal neovascularization. Data points represent the mean of 8 animals per group. Symbol key for all groups is provided in the inset in A.
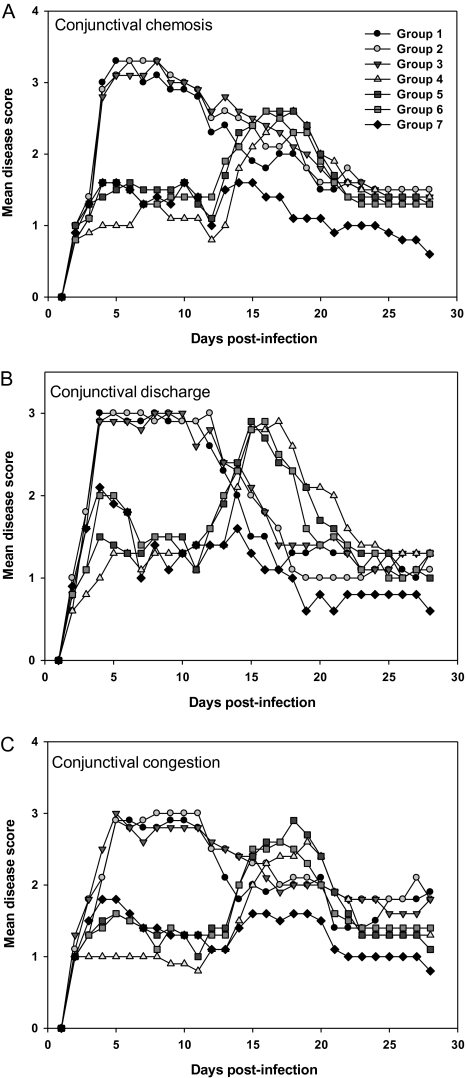
Conjunctival disease scores clustered in a manner similar to that seen for corneal disease (Figure 3). The TFT-only group (group 7) had the best therapeutic effect. The VIG-only groups were not different from the albumin-only group (P > .05). In all groups treated with topical prednisolone (groups 4–6), the severity of conjunctival disease was lower than in the albumin–or VIG-only groups, plateaued until day 15, and then increased, suggesting a rebound effect once treatment was halted. These results indicate that TFT alone had the best therapeutic effect and that VIG treatment neither enhanced nor reduced ocular disease.
Figure 3.
Conjunctival disease in infected eyes was scored daily. Data points represent means of 8 animals per group. A, Conjunctival chemosis. B, Conjunctival discharge. C, Conjunctival congestion.
Corneal Thickness
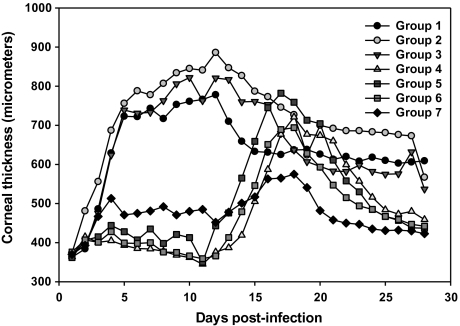
Increased corneal thickness, indicating edema and epithelial cell hyperplasia, was evident within 48 h after infection in all groups (Figure 4). For groups 1–3 (albumin and VIG only), corneal thickness increased rapidly through day 5 (∼750 μmol/L); continued to increase at a slower rate, peaking at approximately day 12 (750–900 μmol/L); and then gradually declined (550–620 μmol/L). Corneal thickness in groups 4–6 (prednisolone with or without VIG or TFT) remained low (∼400 μmol/L) through day 14. Corneal thickness in these groups then increased, peaking at 700–800 μmol/L on day 16 or 17, before declining to ∼450 μmol/L on day 28. Corneal thickness in group 7 (TFT only) remained at 500 to –∼600 μmol/L through day 17 and then gradually declined to ∼420 μmol/L (Figure 4). Corneas in groups 1–3 (albumin or VIG only) were significantly thicker than in groups 4–6 (prednisolone with or without VIG or TFT) between days 3 and 14 (Figure 4) (P < .05). Corneas in group 7 (TFT only) were significantly thinner from those in groups 1–3 (albumin or VIG only) between days 3 and 14 (P < .05). There were no significant differences between groups after day 14.
Figure 4.
Corneal thickness was measured daily using an Accutome AccuPach V pachymeter. Points represent means for 8 animals per group.
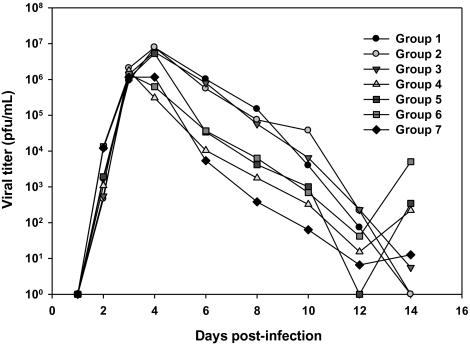
Viral Titers
Corneal swab samples were collected on days 2, 3, 4, 6, 8, 10, 12, and 14 and titered by plaque assay (Figure 5). Titers rose rapidly in all groups, peaking on day 3 at 1 × 106 PFUs/mL for groups 4, 6, and 7. Viral titers peaked at 1 × 107 in groups 1–3 on day 4. On days 4, 6, 8, and 10 the titers in groups 4–6 were 1–2 logs lower than those in groups 1–3. The titers in group 7 (TFT only) were 2–3 logs lower than in groups 1–3 (albumin or VIG only) on days 4–6. By day 14, viral titers were low (∼1 × 101) or undetectable for groups 1–3 and 7. However, viral titers in the 3 groups that were treated with prednisolone rebounded on day 14 with titers ranging from 5 × 102 to 1 × 104. This rebound in viral replication in all groups treated with prednisolone is significant in light of the increased disease scores seen for these 3 groups after day 15 (Figures 2 and 3).
Figure 5.
Viral titers. Corneal swab samples from infected eyes were obtained on the indicated days and stored at −80°C. Samples were titered by plaque assay on HeLa cells. Points represent means for 8 animals per group, titered in duplicate.
The titers in group 7 were significantly different from those in groups 1–3 on days 4–12 (P < .05). The mean peak viral titers in groups 1 and 3 (7.2 × 106 and 6.53 × 106 PFUs/mL, respectively) were significantly higher than the mean peak titer for group 4 (1.9 × 106 PFUs/mL) (P < .05). The time to reach peak titer did not differ significantly between groups.
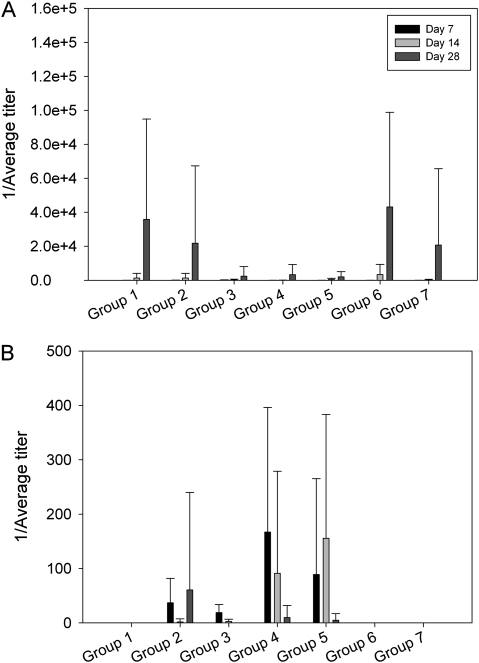
Serology
Samples for serology were obtained on days 7, 14, and 28, and the concentration of rabbit anti-VACV antibodies and VIG were determined by enzyme-linked immunosorbent assay (Figure 6). The day 7 samples were taken after the administration of VIG. The anti-VACV antibody response is shown in Figure 6A. On day 7, anti-VACV antibodies were detected in groups 1–4 and 7 but were low (median score, ∼1:8–1:32; Figure 6A). Anti-VACV antibodies were detected in all groups on day 14 and were higher than on day 7 (median score, ∼1:16–1:512). There were no significant differences in antibody titers between groups. These results indicated that the treatments did not interfere with the development of an antibody response to the virus. To confirm the administration of VIG and to determine how long VIG persisted, samples were also tested for antibodies to human immunoglobulin G (Figure 6B). On day 7, VIG was detected in all groups given intravenous VIG (groups 2–5), as expected. On day 14, VIG was present only in groups 4 and 5, and by day 28 VIG titers were either very low or below the baseline of the assay.
Figure 6.
Antibody titers. Bar graphs show the mean titers (± standard deviations) for vaccinia virus (VACV)-specific rabbit antibodies (A) and antibodies to human immunoglobulin G (vaccinia immunoglobulin [VIG]) (B). Blood was collected once before infection (day 0) and on days 7, 14, and 28. Samples were considered positive if absorbance values were >3 standard deviations greater than baseline (day 0) values.
Animal Weight
In general, the animals lost weight during the first 8 days of the study before recovering (data not shown). There was no significant difference in animal weights between groups on any day.
Histology
Paraformaldehyde-fixed sections were scored for evidence of inflammation in the eyelids, the palpebral conjunctiva, the bulbar conjunctiva, the meibomian glands, and the cornea. The cornea showed the most severe inflammation of any tissues in all groups. Groups 4–6 (prednisolone with VIG and/or TFT) and 7 (TFT only) displayed less severe disruption of the corneal epithelium, less severe inflammation in the superficial cornea near the epithelium, and reduced corneal stromal disease compared to groups 1–3 (albumin or VIG only), although the differences were not statistically significant. Groups 4–6, treated with prednisolone acetate, had slightly less severe inflammation than group 7. Overall, the differences in disease severity in terms of inflammation were not significant between groups (data not shown).
Mortality
On day 10, 1 rabbit from group 5 died shortly after the administration of the second dose of intravenous VIG. Findings of pathological examination after necropsy suggested that the death was due to acute pulmonary reaction associated with VIG administration.
DISCUSSION
Vaccination against smallpox is associated with a high risk of adverse reactions, ranging from mild fever and muscle pain to systemic infection, encephalitis, myocarditis, and death [4, 20–23]. Accidental transfer of virus to another part of the body is not unusual, with the most common sites being the face, genitalia, and anus [24]. Accidental transfer of the virus to the eye occurred in ∼1–4 recipients per 40,000 primary vaccinations during the smallpox eradication effort [1, 4]. The estimated rates of corneal involvement (keratitis) ranged from 6% to 30% of ocular vaccinia cases, depending on reporting conditions [2, 3]. Currently, the CDC recommends the use of VIG as a first-line treatment for adverse reactions against smallpox vaccination unless keratitis is present [24]. Off-label use of TFT is currently recommended to treat VACVK. The use of topical corticosteroids to treat VACVK is not recommended without concurrent treatment with a topical antiviral [24]. To date, a rigorous comparison of all of the potential treatments for VACVK in an animal model has not been done [24].
There were 2 goals for this investigation: first, to determine whether VIG administered intravenously exacerbated corneal scarring and, second, to compare the efficacy of different therapeutic strategies. Our study is not directly comparable to the study by Fulginiti et al [6], because we administered the VIG intravenously a maximum of 2 times during the course of 7 days, whereas Fulginiti et al administered VIG intramuscularly up to 5 times during the course of 5 days. Our results showed that intravenous administration of VIG, either once or twice at weekly intervals, neither increased the severity of the disease nor had a significant therapeutic effect. We therefore conclude that passive immunization should not be contraindicated in patients with VACVK.
Corticosteroids have been suggested for use in VACVK. It was therefore of interest to also test prednisolone acetate [25], alone or in combination with VIG and trifluridine, for efficacy in treating VACVK. All groups that received TFT (groups 4–7) had significantly lower peak disease scores for most disease parameters compared with groups 1–3 (albumin or VIG only). During the period during which the animals were actively treated (through 10 days after the onset of VACVK), groups 4–7 had significantly less severe disease than did the mock-treated (albumin) and VIG-only groups. However, after the cessation of treatment, groups 4–6 (prednisolone with VIG or TFT) experienced worsening corneal opacity, corneal clouding, conjunctival disease and corneal thickness during the same period in which groups 1–3 and 7 had begun to heal (Figures 1, 2, and 3). Virus titers in the groups that received prednisolone acetate or TFT were lower than in the other groups from days 4–12; however, in all of the groups receiving the anti-inflammatory steroid, viral titers increased between day 12 and 14, indicating that virus replication resumed after treatment ended (Figure 4). A similar relapse in disease severity and viral titer was not seen in group 7 (TFT only). These results indicate that prednisolone delays the clearance of infectious virus even in the presence of TFT and/or VIG and suggest that corticosteroids should not be used to treat VACVK.
Trifluridine has been shown to inhibit VACV replication in vitro [25] and to reduce the severity of VACVK in rabbits [15]. We found that TFT treatment alone reduced peak viral titers by 80% relative to the albumin-treated group. Corneal and conjunctival disease scores in group 7 animals were comparable to those in the groups treated with a combination of TFT and prednisolone acetate (group 6) or TFT, prednisolone acetate, and VIG (groups 4 and 5) during the treatment phase of the study. These data suggest that reducing viral replication alone was enough to reduce the severity of keratitis even after treatment ended and that corticosteroid use did not improve the efficacy of TFT. Additionally, all groups that received TFT experienced a delay in the onset of vascularization relative to the mock-treated and VIG-only groups, suggesting that viral replication may play a role in angiogenesis.
In summary, we have shown that treatment with VIG, either alone or in conjunction with antivirals and anti-inflammatory steroids, neither exacerbates corneal scarring nor reduces the severity of VACVK. The lack of detrimental effect of VIG indicates that use of VIG should be considered clinically to treat adverse reactions to smallpox vaccination, even in isolated cases of keratitis. Our results also clearly show that the inclusion of prednisolone acetate interfered with viral clearance. We therefore conclude that topical corticosteroids should not be used for treating VACVK. Finally, the data show that the most effective treatment for VACVK was 9 topical applications of a 1% TFT solution a day for 10 days. Based on these findings we recommend that 1% TFT, with or without intravenous VIG, is the preferred therapeutic regimen for treating VACVK.
Funding
This work was supported by the Centers for Disease Control via Cangene Corporation (grant number AGR DTD 05/09/07) and the National Eye Institute (grant number P30 EY016665).
Supplementary Material
Acknowledgments
The authors would like to thank Elizabeth Froelich and Inna Larsen for administrative support.
References
- 1.Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968. N Engl J Med. 1969;281:1201–8. doi: 10.1056/NEJM196911272812201. [DOI] [PubMed] [Google Scholar]
- 2.Ruben FL, Lane JM. Ocular vaccinia: an epidemiologic analysis of 348 cases. Arch Ophthalmol. 1970;84:45–8. doi: 10.1001/archopht.1970.00990040047012. [DOI] [PubMed] [Google Scholar]
- 3.Duke-Elder S. Diseases of the outer eye. In: Duke-Elder S, editor. System of ophthalmology. Vol. 8. St Louis: CV Mosby; 1965. [Google Scholar]
- 4.Pepose JS, Margolis TP, LaRussa P, Pavan-Langston D. Ocular complications of smallpox vaccination. Am J Ophthalmol. 2003;136:343–52. doi: 10.1016/s0002-9394(03)00293-9. [DOI] [PubMed] [Google Scholar]
- 5.Rotz LD, Dotson DA, Damon IK, Becher JA. Vaccinia (smallpox) vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2001. MMWR Recomm Rep. 2001;50:1–25. [PubMed] [Google Scholar]
- 6.Fulginiti VA, Winograd LA, Jackson M, Ellis P. Therapy of experimental vaccinal keratitis: effect of idoxuridine and VIG. Arch Ophthalmol. 1965;74:539–44. doi: 10.1001/archopht.1965.00970040541019. [DOI] [PubMed] [Google Scholar]
- 7.Jones BR, Al-Hussaini MK. Therapeutic considerations in ocular vaccinia. Trans Ophthalmol Soc UK. 1963;83:613–31. [PubMed] [Google Scholar]
- 8.Rennie AG, Cant JS, Foulds WS, Pennington TH, Timbury MC. Ocular vaccinia. Lancet. 1974;2:273–5. doi: 10.1016/s0140-6736(74)91427-5. [DOI] [PubMed] [Google Scholar]
- 9.Rydberg M, Pandolfi M. Treatment of vaccinia keratitis with post-vaccinial gamma globulin. Acta Ophthalmol (Copenh) 1963;41:713–8. doi: 10.1111/j.1755-3768.1963.tb03592.x. [DOI] [PubMed] [Google Scholar]
- 10.Jones BR, Galbraith JE, Al-Hussaini MK. Vaccinial keratitis treated with interferon. Lancet. 1962;1:875–9. doi: 10.1016/s0140-6736(62)91908-6. [DOI] [PubMed] [Google Scholar]
- 11.Jack MK, Sorenson RW. Vaccinial keratitis treated with IDU. Arch Ophthalmol. 1963;69:730–2. doi: 10.1001/archopht.1963.00960040736009. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman HL, Nesburn AB, Maloney ED. Cure of vaccinia infection by 5-iodo-2'-deoxyuridine. Virology. 1962;18:567–9. doi: 10.1016/0042-6822(62)90058-2. [DOI] [PubMed] [Google Scholar]
- 13.Kimura SJ, Thygeson P, Geller HO. Observations on the effect of adrenal steroids on vaccinia virus. I. The effect of cortisone in experimental vaccinia-virus keratoconjunctivitis of the rabbit. Am J Ophthalmol. 1953;36:116–20. doi: 10.1016/0002-9394(53)90160-3. [DOI] [PubMed] [Google Scholar]
- 14.Sudarsky RD. Postvaccinial disciform keratitis; recurrent episodes treated with steroids. Am J Ophthalmol. 1957;44:810–2. [PubMed] [Google Scholar]
- 15.Hyndiuk RA, Seideman S, Leibsohn JM. Treatment of vaccinial keratitis with trifluorothymidine. Arch Ophthalmol. 1976;94:1785–6. doi: 10.1001/archopht.1976.03910040559015. [DOI] [PubMed] [Google Scholar]
- 16.Parkhurst JR. Danenberg PV, Heidelberger C. Growth inhibition of cells in cultures and of vaccinia virus infected HeLa cells by derivatives of trifluorothymidine. Chemotherapy. 1976;22:221–31. doi: 10.1159/000221929. [DOI] [PubMed] [Google Scholar]
- 17.Fillmore GL, Ward TP, Bower KS, et al. Ocular complications in the Department of Defense Smallpox Vaccination Program. Ophthalmology. 2004;111:2086–93. doi: 10.1016/j.ophtha.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 18.Altmann SE, Jones JC, Schultz-Cherry S, Brandt CR. Inhibition of vaccinia virus entry by a broad spectrum antiviral peptide. Virology. 2009;388:248–59. doi: 10.1016/j.virol.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altmann S, Emanuel A, Toomey M, et al. A quantitative rabbit model of vaccinia keratitis. Invest Ophthalmol Vis Sci. 2010;51:4531–40. doi: 10.1167/iovs.09-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CDC Adverse reaction to smallpox vaccine. Commun Dis Rep CDR Wkly. 1994;4:157. [PubMed] [Google Scholar]
- 21.Baggs J, Chen RT, Damon IK, et al. Safety profile of smallpox vaccine: insights from the laboratory worker smallpox vaccination program. Clin Infect Dis. 2005;40:1133–40. doi: 10.1086/428731. [DOI] [PubMed] [Google Scholar]
- 22.Casey CG, Iskander JK, Roper MH, et al. Adverse events associated with smallpox vaccination in the United States, January–October 2003. JAMA. 2005;294:2734–43. doi: 10.1001/jama.294.21.2734. [DOI] [PubMed] [Google Scholar]
- 23.Fulginiti VA, Papier A, Lane JM, Neff JM, Henderson DA. Smallpox vaccination: a review, part II. Adverse events. Clin Infect Dis. 2003;37:251–71. doi: 10.1086/375825. [DOI] [PubMed] [Google Scholar]
- 24.Cono J, Casey CG, Bell DM. Smallpox vaccination and adverse reactions: guidance for clinicians. MMWR Recomm Rep. 2003;52:1–28. [PubMed] [Google Scholar]
- 25.Heidelberger C. On the molecular mechanism of the antiviral activity of trifluorothymidine. Ann N Y Acad Sci. 1975;255:317–25. doi: 10.1111/j.1749-6632.1975.tb29239.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.