Abstract
In patients with falciparum malaria, plasma concentrations of cell-derived microparticles correlate with disease severity. Using flow cytometry, we quantified red blood cell–derived microparticles (RMPs) in patients with malaria and identified the source and the factors associated with production. RMP concentrations were increased in patients with Plasmodium falciparum (n = 29; median, 457 RMPs/μL [range, 13–4,342 RMPs/μL]), Plasmodium vivax (n = 5; median, 409 RMPs/μL [range, 281–503/μL]), and Plasmodium malariae (n = 2; median, 163 RMPs/μL [range, 127–200 RMPs/μL]) compared with those in healthy subjects (n = 11; median, 8 RMPs/μL [range, 3–166 RMPs/μL]; P = .01). RMP concentrations were highest in patients with severe falciparum malaria (P = .01). Parasitized red cells produced >10 times more RMPs than did unparasitized cells, but the overall majority of RMPs still derived from uninfected red blood cells (URBCs). In cultures, RMP production increased as the parasites matured. Hemin and parasite products induced RMP production in URBCs, which was inhibited by N-acetylcysteine, suggesting heme-mediated oxidative stress as a pathway for the generation of RMPs.
In recent years, circulating cell-derived microparticles (MPs), which expose the phospholipid phosphatidyl serine (PS), have been identified increasingly in a broad range of diseases. Membrane PS is usually localized in the inner leaflet of the lipid bilayer of resting cells, but upon activation or apoptosis, PS becomes exposed on the external surface of the cell membrane [1, 2]. The presence of PS in the outer leaflet facilitates membrane blebbing and release of MPs with a diameter of <1 μm [3]. MPs display the cell surface proteins of the parent cell, allowing identification of their origin [4]. An increase in MP production has been found in a variety of conditions, including cardiovascular disease [5], idiopathic thrombocytopenic purpura [6], and thalassemia [7, 8]. MPs play an important role in inflammation, coagulation, and vascular homeostasis [4, 9]. Malaria is associated with an increase in the plasma concentrations of endothelial MPs (EMPs) in proportion to disease severity [10, 11]. This may result from endothelial activation or be a direct mechanical result of cytoadherence of parasitized red cells to the endothelium. The origin and role of red blood cell–derived MPs (RMPs) in malaria, which comprise numerically the most important fraction of plasma MPs, have not been established. We quantified circulating RMPs in patients with malaria on admission and then after antimalarial treatment by use of flow cytometry and determined whether uninfected red blood cells (URBCs) or infected red blood cells (IRBCs) were the main origin of these RMPs. We investigated a candidate precipitant of RMP production, hemin, an oxidative Plasmodium falciparum heme product released at schizont rupture. We also evaluated the parasite stage-specific production of IRBC-derived RMPs in an in vitro culture system.
METHODS
Detection of RMPs in Plasma Samples From Patients With Malaria
Blood Samples.
This study was performed at the Hospital for Tropical Diseases, Bangkok, Thailand. Blood samples were collected from 11 healthy subjects, 19 patients with severe falciparum malaria, 10 patients with uncomplicated falciparum malaria, 5 patients with vivax malaria, and 2 patients with Plasmodium malariae infections. Malaria diagnosis was made by light microscopic analysis of a peripheral blood sample slide. Severe malaria was defined according to standard criteria [12]. Patients were treated with standard courses of artemisinin derivatives. Blood samples were collected into plastic tubes containing trisodium citrate (1:9 vol/vol) on admission and on days 1, 2, 3, 5, 7, and 14 after the start of antimalarial drug treatment. Plasma for RMP assessment was centrifuged at 1,500 g for 15 min followed by additional centrifugation of the supernatant at 13,000 g for 2 min [11]. Routine blood samples collected from patients hospitalized with trauma (n = 5) and sepsis (n = 6) were assessed as nonmalaria severely ill controls. This study was conducted as part of a clinical trial conducted at the Faculty of Tropical Medicine, Mahidol University, and approved by the ethics committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand.
Quantitation of MPs Using Flow Cytometry.
A specific marker for phospholipid PS (fluorescein isothiocyanate [FITC]–conjugated annexin V) and phycoerythrin (PE) –conjugated anti-glycophorin A were used for identification of RMPs. Plasma (30 μL) was mixed with 2 μL of FITC-conjugated annexin V (Becton Dickinson Biosciences) and 2 μL of PE-conjugated anti-glycophorin A (Becton Dickinson Biosciences). These mixtures were incubated with 16 μL of binding buffer at room temperature and protected from light for 15 min, after which they were diluted with 1000 μL of binding buffer and quantified by flow cytometry (FACsCalibur; Becton Dickinson Biosciences) within 1 h by use of a modification of a flow-rate-based assay reported elsewhere [13–15]. RMPs were localized within region R1 and were distinguished from debris by annexin V– and glycophorin A–associated responses in the upper right quadrant (Figures 1A and 1B). The absolute number of RMPs was calculated using the following formula:
where # = (number of prebead RMPs + number of postbead RMPs)/2 and K = (dilution factor × calibration factor)/diluent volume = 2.9 × 10−5 [15].
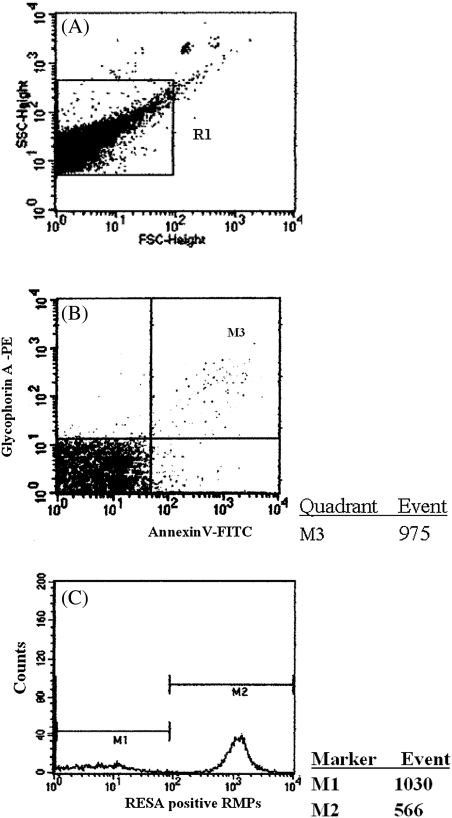
Figure 1.
Flow cytometric quantitation of plasma red blood cell–derived microparticles (RMPs) in blood samples from patients with malaria. A, RMPs gated by size on a forward scatter and side scatter plot. B, Only events included within gate M3 were further analyzed for fluorescence associated with annexin V– and glycophorin A–positive labeling. C, Analysis of the origin of RMPs with positive staining by anti–ring-infected erythrocyte surface antigen (RESA) antibodies denoting a parasitized red cell origin. RMPs were identified as either RESA positive (M2) or RESA negative (M1).
RMP Production in an In Vitro Culture of P. falciparum
Parasite Culture.
The Thai laboratory strain of P. falciparum (TM267) was cultured in vitro at 3% hematocrit in Roswell Park Memorial Institute 1640 medium (RPMI1640; ICN Biomedical) containing 10% human AB serum in a 5% carbon dioxide environment at 37°C, as described elsewhere [16]. To ensure synchronous parasite cultures, the samples were treated with 5% sorbitol [17]. Culture supernatant was sampled every 6 h for detection of RMPs, and slides for microscopy were prepared at each time point to assess parasitemia and parasite development. Morphological criteria for the light microscopic assessment of developmental stages of P. falciparum have been described elsewhere [18].
Assessment of Parasitized Versus Nonparasitized Red Blood Cells as the Source of RMPs.
In contrast to unparasitized red blood cells (RBCs), parasitized RBCs contain membrane-associated ring-infected erythrocyte surface antigen (RESA) and possibly other parasite-derived antigens, which can be identified by immunofluorescence by use of plasma obtained from immune P. falciparum–infected patients. Thus, MPs derived from parasitized red cells, and also from cells that have been once parasitized and then pitted, are RESA positive, and MPs derived from erythrocytes that have never been parasitized are RESA negative. RMPs in 5 mL of culture medium were prepared as described above by 2-step centrifugation [10, 11]. Annexin V-PE (Becton Dickinson Biosciences; catalogue no. 5165875X) and Cy5-conjugated anti-glycophorin A (Becton Dickinson Biosciences; catalogue no. 559944) was used to quantify the combined fractions of RMPs as described above. Samples were fixed with 100 μL of .05% glutaraldehyde for 30 min and then incubated with 100 μL of plasma containing anti-RESA-positive antibody for 30 min. Five microliters of cell suspension was then labeled with 5 μL of FITC-conjugated human immunoglobulin G (IgG; Dako; catalogue no. F0202), 5 μL of PE-conjugated annexin V, and 5 μL of Cy5-conjugated anti-glycophorin A. This mixture was subsequently incubated for 30 min at room temperature and protected from light. After incubation, 1 mL of diluted binding buffer solution (1:10 vol/vol in distilled water) was added and the sample was analyzed by flow cytometry using flow-rate-based calibration [15]. The origin of the RMPs was identified by analysis of the forward scatter and side scatter patterns, which denote the RESA-positive IRBC-derived RMPs as a fraction of the total number of RMPs (Figure 1C). The fraction of RESA-positive RMPs was calculated using CellQuest software version 3.3 (Becton Dickinson Biosciences). The contribution of circulating parasitized erythrocytes to the total RMP concentration was derived as follows: ratio of RMPs produced by parasitized cells to RMPs produced by unparasitized cells = (RESA-positive RMPs/RESA-negative RMPs) × (100 − parasitemia/parasitemia), where parasitemia is a percentage. This estimate assumes RMPs are derived from the circulating parasitized and unparasitized erythrocytes, and that there is no significant contribution from previous generations of parasitized cells.
Effects of Hemin on RMP Production
A stock hemin solution was freshly prepared at the beginning of each experiment by dissolving .1 g of hemin chloride (Sigma) in 1 mL of .1 mol/L sodium hydroxide and 9 mL of phosphate-buffered saline. The hemin solution was then diluted to 12.5, 25, 50, or 100 μg/mL with RPMI 1640 and used immediately. Blood samples were obtained from healthy human volunteers by venipuncture into heparinized tubes and centrifuged at 1,100 g for 10 min at 4°C. Plasma was removed and packed RBCs were washed with RPMI 1640. After removal of the buffy coat, washed RBCs were resuspended in hemin in concentrations ranging from 12.5 to 100 μg/mL with a final hematocrit of 5% in the presence or absence of 1 mg/mL N-acetylcysteine (Parvolex injection; 200 mg/mL). RMPs were separated from RBC remnants by centrifugation at 1,500 g for 15 min, and then the supernatant was further centrifuged at 13,000 g for 2 min. RMPs were quantitated by flow cytometry using the specific RBC monoclonal antibody (PE-CD235; Beckman Coulter Immunotech) doubled with FITC-conjugated annexin V. Results are expressed as the median (range) number of RMPs per microliter.
Statistical Analysis
Statistical analyses were performed using the SPSS statistical program (version 11.0; SPSS). Non-normally distributed parameters were compared using the Kruskal-Wallis and Mann-Whitney U tests. Correlations were assessed by the method of Spearman for non-normally distributed variables. A P value of <.05 was considered to be statistically significant.
RESULTS
Quantification of RMPs in Patients with Malaria
A total of 36 patients with acute P. falciparum, Plasmodium vivax, or P. malariae infection were studied. Baseline clinical and laboratory characteristics are summarized in Table 1. Patients with severe malaria had higher peripheral blood parasitemia (P = .01), higher serum aspartate aminotransferase (AST) levels (P = .01), and lower plasma glucose levels (P = .01) compared with those of patients with uncomplicated malaria (Table 2).
Table 1.
Baseline Characteristics of Enrolled Patients with Plasmodium falciparum, Plasmodium vivax, and Plasmodium malariae Infection
| Characteristic | Patients with PF (n = 28) | Patients with PV (n = 5) | Patients with PM (n = 2) |
| Parasitemia, parasites/μL | 37,077 (400–1,180,690) | 10,500 (4,635–28,109) | 6,029 (3,818–8,239) |
| Hematocrit, % | 37.7 (14.5–44.7) | 37.3 (24.10–41.80) | 31.6 (30.4–32.8) |
| Hb level, g/dL | 12.4 (4.8–15.4) | 12.8 (7.6–14) | 11 (10.5–11.5) |
| AST level, IU/L | 44 (13–184) | 35 (25–50) | 36 (19–53) |
| ALT level, IU/L | 36 (17–126) | 26 (16–37) | 35 (32–38) |
| Serum bilirubin level, mg/dL | 1.17 (.11–18.37) | .46 (.2–.78) | .5 (.21–.79) |
| Total bilirubin level, mg/dL | 2.52 (.63–23.86) | 1.26 (.84–2.22) | 1.39 (.74–2.04) |
| BUN level, mmol/L | 21 (8–85) | 16 (10–138) | 12.5 (9–16) |
| Creatinine level, μmol/L | 1.1 (.6–3.98) | .99 (.62–.99) | .94 (.92–.95) |
| Glucose level, mmol/L | 6.6 (4.3–13.1) | 7.1 (5.2–8.7) | 6.2 (5.9–6.4) |
| Plasma lactate level, mmol/L | 4.39 (2.45–12.70) | NA | NA |
NOTE. Data are median (range) values. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Hb, hemoglobin; NA, not available; PF, P. falciparum infection; PM, P. malariae infection; PV, P. vivax infection.
Table 2.
Baseline Characteristics of Enrolled Patients With Uncomplicated Falciparum Malaria and Severe Falciparum Malaria
| Characteristic | Patiens with UM (n = 10) | Patients with SM (n = 18) |
| Parasitemia, parasites/μL | 14,821 (1,160–92,115) | 314,654 (139,332–489,975)a |
| Hematocrit, % | 37.8 (28.6–42.8) | 34 (30–39) |
| Hb level, g/dL | 12.4 (8.9–14.8) | 11.63 (9.98–13.27) |
| AST level, IU/L | 23 (13–46) | 75 (51–98)a |
| ALT level, IU/L | 28 (17–41) | 52 (36–69) |
| Serum bilirubin level, mg/dL | .35 (.11–.89) | 2.47 (.73–18.37)a |
| Total bilirubin level, mg/dL | 1.2 (.63–2.24) | 5.95 (1.65–23.86)a |
| BUN level, mmol/L | 17 (8–24) | 32 (21–43)a |
| Creatinine level, μmol/L | .86 (.65–1.27) | 1.17 (.8–1.55) |
| Glucose level, mmol/L | 6.4 (4.3–13.1) | 6.8 (5.9–7.8) |
| Plasma lactate level, mmol/L | 3.78 (3.03–6.18) | 6.58 (3.15–12.7)a |
NOTE. Data are median (range) values. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Hb, hemoglobin; SM, severe falciparum malaria; UM, uncomplicated falciparum malaria.
aP < .01 compared with patients with UM.
On admission, concentrations of RMPs were increased in all malaria patients, with a median concentration in P. falciparum malaria (n = 29) of 457 RMPs/μL (range, 13–4,342 RMPs/μL), in P. vivax malaria (n = 5) of 409 RMPs/μL (range, 281–503 RMPs/μL), and in P. malariae malaria (n = 2) of 163 RMPs/μL (range, 127–200 RMPs/μL) compared with 8 RMPs/μL (range, 3–166 RMPs/μL) in healthy controls (n = 11; P < .001) (Table 3). The median RMP concentration in patients with trauma was 208 RMPs/μL (range, 85–463 RMPs/μL; n = 6), and that in patients with sepsis was 175 RMPs/μL (range, 125–205 RMPs/μL; n = 6).
Table 3.
Total Red Cell–derived Microparticles in Patients With Severe Falciparum Malaria, Nonsevere Falciparum Malaria, Vivax Malaria, or Malariae Malaria and Healthy Subjects
| No. of red cell–derived microparticles, median (range) | |||||
| Day | Patients with SM | Patients with UM | Patients with PV | Patients with PM | Healthy subjects |
| 0 | 535 (13–4,342)a | 276 (15–2,150)a | 409 (281–503)a | 163 (127–200)a | 8 (3–166) |
| 1 | 269 (41–1,979)a | 150 (33–360)a | 47 (14–266)ab | 122 (114–130) | NA |
| 2 | 159 (16–524)a | 96 (33–478)a | 162 (24–369)ab | 268 (NA) | NA |
| 3 | 146 (28–963)ab | 84 (13–759)a | 52 (38–316)ab | 155 (134–175) | NA |
| 5 | 115 (35–476)ab | 156 (7–1,387)a | 121 (11–774)ab | 102 (100–105) | NA |
| 7 | 112 (35–1,830)ab | 38 (9–317)ab | 49 (13–808)ab | 125 (46–205) | NA |
| 14 | 156 (9–921)ab | 45 (10–410)ab | 18 (6–64)b | 48 (NA) | NA |
NOTE. NA, not available; PM, malariae malaria; PV, vivax malaria; SM, severe falciparum malaria; UM, nonsevere falciparum malaria.
P < .05 compared with healthy subjects.
P < .01 compared with value on admission day (day 0).
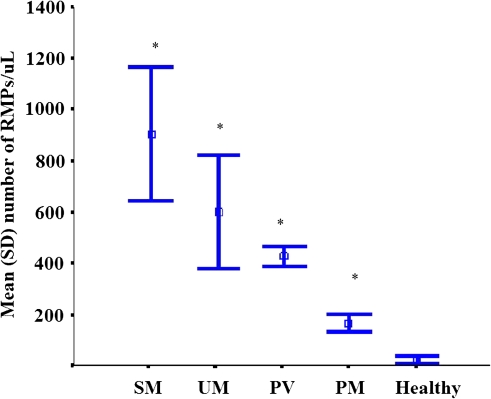
RMP concentrations in patients with severe falciparum malaria (n = 19) were higher than in patients with uncomplicated falciparum malaria (n =10), with a median concentration of 535 RMPs/μL (range, 13–4,342 RMPs/μL) versus 276 RMPs/μL (range, 15–2,150 RMPs/μL), respectively (P = .01) (Figure 2). In patients with falciparum malaria, the concentration of RMPs was correlated positively with the peripheral blood parasitemia (rs = .73; P = .01).
Figure 2.
Plasma red blood cell–derived microparticles in blood samples collected at admission from healthy subjects, patients with uncomplicated Plasmodium falciparum infection (UM), patients with severe P. falciparum infection (SM), patients with Plasmodium vivax infection (PV), and patients with Plasmodium malariae infection (PM). *P < .001 compared with healthy subjects. Data are represented as the mean (± SD).
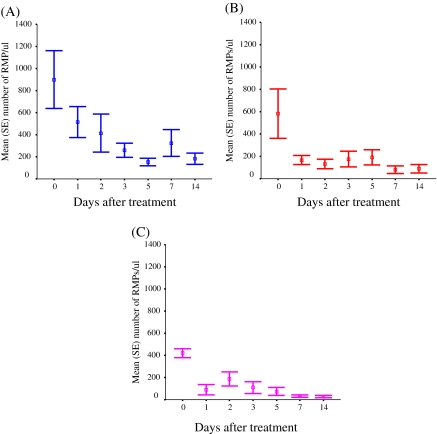
After antimalarial treatment, the level of RMPs decreased rapidly to <400 RMPs/μL after 24 h and continued to decrease further between days 3 and 14. Nevertheless, the median RMP concentrations still remained above reference levels at 14 d after the start of antimalarial treatment (median, 96 RMPs/μL [range, 9–921 RMPs/μL] for all groups) (Figure 3). The origin of the RMPs in the plasma of severe malaria patients (N = 5) was investigated. If the contribution of previous generations of parasitized cells and schizont rupture is ignored, and if RESA-positive RMPs are assumed to come from the circulating parasitized erythrocytes, then each parasitized erythrocyte contributed a median of 13 (range, 4–68) times more MPs than each unparasitized cell.
Figure 3.
Plasma red blood cell–derived microparitcle (RMP) concentrations in malaria patients with severe Plasmodium falciparum infection (A), uncomplicated P. falciparum infection (B), and Plasmodium vivax infection (C) following antimalarial drug treatment. Data are represented as the mean (± SE).
Release of RMPs During Parasite Development
RMP concentrations were assessed every 6 h in the supernatant of a synchronized P. falciparum in-vitro culture (N = 5) and analyzed in relation to parasite stage of development. RMP production in vitro increased as the parasites matured, with lower-level production in ring-stage-infected RBCs (median resulting concentration, 200 RMPs/μL [range, 168–220 RMPs/μL] increasing to 390 RMPs/uL [range, 369-498 RMPs/uL]; P = .01 for trend). The fraction of RMPs derived from infected red blood cells in vitro, defined as RMPs positively staining with both annexin V and anti-RESA antibody plus FITC-conjugated IgG, was overall 39% of the total RMPs. In synchronized culture (5% parasitemia and 5% hematocrit), the mean proportion of the total RMPs positively staining with anti-RESA was 39% (SD, 2%) for ring-stage parasites, 31% (SD, 8%) for trophozoite-stage parasites, and 39% (SD, 5%) for schizont-stage parasites. After schizont rupture, the mean proportion of RMPs positively staining with anti-RESA was 47% (SD, 4%). The mean proportion of RMPs released by infected cells compared with that released by uninfected cells was estimated to be 12 (SD, 1) for the ring stage, 9 (SD, 3) for the trophozoite state, 12 (SD, 3) for the schizont stage, and 17 (SD, 1) after schizont rupture.
Release of RMPs After Hemin Treatment
Uninfected red blood cells were incubated with hemin (12.5–100 μg/mL) for 2–6 h in the presence or absence of the antioxidant N-acetylcysteine in a concentration of 1 mg/mL. RMP production increased with increasing hemin concentrations and was maximal after incubation with 100 μg/mL of hemin exposure in the supernatant for 6 h with a median concentration of 700 RMPs/μL (range, 638882 RMPs/μL). This effect was inhibited when the red blood cells were incubated concomitantly with N-acetylcysteine (1 mg/mL) and hemin (P = .01).
DISCUSSION
This study quantifies circulating RMPs during malaria infection. Plasma RMP concentrations were increased in patients with falciparum malaria in proportion to disease severity, and were also increased in patients with P. vivax and P. malariae infections, although to a lesser extent. RMP concentrations were also higher in patients with severe malaria than in patients with trauma or severely ill in sepsis. Concentrations of RMPs decreased 24 h after initiation of antimalarial drug treatment, although in patients with P. falciparum malaria, the circulating RMP levels remained increased for 2 weeks after the start of antimalarial treatment. In contrast, in patients with P. vivax and P. malariae infections, MPs concentrations were lower and decreased to baseline levels within 2 weeks after the start of treatment. Removal by the spleen is likely to be the most important contributor to the clearance of RMPs from the circulation, since it has been shown that splenectomized malaria patients have increased levels and prolonged circulation of MPs [7]. During malaria infection the spleen enlarges and is activated, increasing its clearance capacity [19]. Cells and particles that express PS on their surface are removed by the splenic reticuloendothelial system. The liver and the lungs can also contribute to the clearance of RMPs as has been shown in a murine animal model [20]. The production of RMPs in a P. falciparum in vitro culture system was increased during the latter stages of the parasite asexual life cycle. Parasitized cells produced considerably more RMPs per cell at all stages of development than did unparasitized cells both in vivo and in vitro. It was estimated that parasitized red cells contributed ∼13 times more RMPs than do uninfected red cells in severe malaria. This is consistent with other observed changes in URBCs during severe malaria infections, which include a marked reduction in their deformability [21]. RMP production by URBCs could be mediated by the release of malaria heme products at the schizont rupture, which is also one of the proposed mechanisms for the reduction in uninfected red cell deformability. We showed that hemin induces the production of RMPs in a concentration- and time-dependent manner. This is probably mediated through an oxidative mechanism, since the antioxidant N-acetylcysteine almost completely blocked RMP production. Oxidative stress is increased during malaria infection and is thought to be generated by both malaria heme products and the host immune response [22, 23].
It has been shown in previous studies that PS is expressed on the outer membrane leaflet in IRBCs and that PS expression is related to parasite maturation [24, 25], which suggests that the vesiculation process leading to MP formation is related to active red cell membrane changes induced by the growing parasite. Studies by Lang and colleagues [26] showed that infection of RBCs by P. falciparum leads to activation of several distinct anion channels and a nonselective, Ca2+-permeable cation channel. These channels could be activated by oxidative stress generated by the parasite. Similar or identical channels are activated by oxidation of noninfected erythrocytes [22]. Activation of the nonselective cation channel allows entry of Ca2+ and Na+, both of which are required for intracellular growth of the parasite. Entry of Ca2+ is known to stimulate a phospholipid scramblase, which is a protein responsible for the bidirectional phospholipid migration across the lipid bilayer, resulting in breakdown of the PS asymmetry of the cell membrane. The exposure of PS at the outer surface of the cell membrane could be followed by binding to PS receptors on macrophages and subsequent phagocytosis of the affected RBCs [1, 5]. Maintenance of the normal asymmetry by vesiculation may represent an important parasite strategy to avoid recognition and destruction by the host reticuloendothelial system [27, 28]. This study identifies different sources of plasma RMPs in patients with malaria and reconfirms the relationship of plasma RMP concentrations with disease severity. Parasite maturation evokes the release of RMPs from infected erythrocytes, whereas the quantatively more important production of RMPs from uninfected erythrocytes might be triggered by an oxidative-stress-related mechanism through heme exposure.
Funding
This work was supported by the Wellcome Trust Mahidol University–Oxford Tropical Medicine Research program funded by the Wellcome trust of Great Britain; and the Royal Golden Jubilee PhD program of the Thailand Research Fund (R.G.J. and T.R.F. [Senior Research Scholar]).
Acknowledgments
We thank the staff of the Hospital for Tropical Diseases, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, for their help and technical support.
References
- 1.Schroit AJ, Madsen JW, Tanaka Y. In vivo recognition and clearance of red blood cells containing phosphatidylserine in their plasma membranes. J Biol Chem. 1985;260:5131–8. [PubMed] [Google Scholar]
- 2.Utsugi T, Schroit AJ, Connor J, Bucana CD, Fidler IJ. Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res. 1991;51:3062–6. [PubMed] [Google Scholar]
- 3.Zwaal RF, Schroit AJ. Pathophysiology implication of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–31. [PubMed] [Google Scholar]
- 4.Distler JH, Huber LC, Hueber AJ, et al. The release of microparticles by apoptotic cells and their effects on macrophages. Apoptosis. 2005;10:731–41. doi: 10.1007/s10495-005-2941-5. [DOI] [PubMed] [Google Scholar]
- 5.VanWijk MJ, VanBavel E, Sturk A, Nieuwland R. Microparticles in cardiovascular diseases. Cardiovasc Res. 2003;59:277–87. doi: 10.1016/s0008-6363(03)00367-5. [DOI] [PubMed] [Google Scholar]
- 6.Jy W, Horstman LL, Arce M, Ahn YS. Clinical significance of platelet microparticles in autoimmune thrombocytopenias. J Lab Clin Med. 1992;119:334–45. [PubMed] [Google Scholar]
- 7.Pattanapanyasat K, Gonwong S, Chaichompoo P, et al. Activated platelet-derived microparticles in thalassaemia. Br J Haematol. 2007;136:462–71. doi: 10.1111/j.1365-2141.2006.06449.x. [DOI] [PubMed] [Google Scholar]
- 8.Pattanapanyasat K, Noulsri E, Fucharoen S, et al. Flow cytometric quantitation of red blood cell vesicles in thalassemia. Cytometry B Clin Cytom. 2004;57:23–31. doi: 10.1002/cyto.b.10064. [DOI] [PubMed] [Google Scholar]
- 9.Coltel N, Combes V, Wassmer SC, Chimini G, Grau GE. Cell vesiculation and immunopathology: implications in cerebral malaria. Microbes Infect. 2006;8:2305–16. doi: 10.1016/j.micinf.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Combes V, Simon AC, Grau GE, et al. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest. 1999;104:93–102. doi: 10.1172/JCI4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Combes V, Taylor TE, Juhan-Vague I, et al. Circulating endothelial microparticles in Malawian children with severe falciparum malaria complicated with coma. JAMA. 2004;291:2542–4. doi: 10.1001/jama.291.21.2542-b. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization, Communicable Diseases Cluster. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94(suppl 1):1–90. [PubMed] [Google Scholar]
- 13.Storie I, Sawle A, Whitby L, et al. Flow rate calibration II: a clinical evaluation study using Pan LeucoGating as a single-platform protocol. Cytometry B Clin Cytom. 2003;55:8–13. doi: 10.1002/cyto.b.10050. [DOI] [PubMed] [Google Scholar]
- 14.Storie I, Sawle A, Goodfellow K, et al. Flow rate calibration I: a novel approach for performing absolute cell counts. Cytometry B Clin Cytom. 2003;55:1–7. doi: 10.1002/cyto.b.10051. [DOI] [PubMed] [Google Scholar]
- 15.Nantakomol D, Chimma P, Day N, et al. Quantitation of cell-derived microparticles in plasma using flow-rate based calibration. Southeast Asian J Trop Med Public Health. 2008;39:146–53. [PubMed] [Google Scholar]
- 16.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–5. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 17.Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–20. [PubMed] [Google Scholar]
- 18.Silamut K, White NJ. Relation of the stage of parasite development in the peripheral blood to prognosis in severe falciparum malaria. Trans R Soc Trop Med Hyg. 1993;87:436–43. doi: 10.1016/0035-9203(93)90028-o. [DOI] [PubMed] [Google Scholar]
- 19.Looareesuwan S, Ho M, Wattanagoon Y, et al. Dynamic alteration in splenic function during acute falciparum malaria. N Engl J Med. 1987;317:675–9. doi: 10.1056/NEJM198709103171105. [DOI] [PubMed] [Google Scholar]
- 20.Bocci V, Pessina GP, Paulesu L. Studies of factors regulating the aging of human erythrocytes–III. Metabolism and fate of erythrocytic vesicles. Int J Biochem. 1980;11:139–42. doi: 10.1016/0020-711x(80)90246-3. [DOI] [PubMed] [Google Scholar]
- 21.Dondorp AM, Pongponratn E, White NJ. Reduced microcirculatory flow in severe falciparum malaria: pathophysiology and electron-microscopic pathology. Acta Trop. 2004;89:309–17. doi: 10.1016/j.actatropica.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Omodeo-Sale F, Monti D, Olliaro P, Taramelli D. Prooxidant activity of beta-hematin (synthetic malaria pigment) in arachidonic acid micelles and phospholipid large unilamellar vesicles. Biochem Pharmacol. 2001;61:999–1009. doi: 10.1016/s0006-2952(01)00558-5. [DOI] [PubMed] [Google Scholar]
- 23.Pichyangkul S, Saengkrai P, Webster HK. Plasmodium falciparum pigment induces monocytes to release high levels of tumor necrosis factor-alpha and interleukin-1 beta. Am J Trop Med Hyg. 1994;51:430–5. [PubMed] [Google Scholar]
- 24.Sherman IW, Prudhomme J. Phosphatidylserine expression on the surface of malaria-parasitized erythrocytes. Parasitol Today. 1996;12:122. doi: 10.1016/0169-4758(96)80673-9. [DOI] [PubMed] [Google Scholar]
- 25.Sherman IW, Eda S, Winograd E. Erythrocyte aging and malaria. Cell Mol Biol. 2004;50:159–69. [PubMed] [Google Scholar]
- 26.Eda S, Sherman IW. Cytoadherence of malaria-infected red blood cells involves exposure of phosphatidylserine. Cell Physiol Biochem. 2002;12:373–84. doi: 10.1159/000067908. [DOI] [PubMed] [Google Scholar]
- 27.Lang F, Lang PA, Lang KS, et al. Channel-induced apoptosis of infected host cells-the case of malaria. Pflugers Arch. 2004;448:319–24. doi: 10.1007/s00424-004-1254-9. [DOI] [PubMed] [Google Scholar]
- 28.Willekens FL, Roerdinkholder-Stoelwinder B, Groenen-Dopp YA, et al. Hemoglobin loss from erythrocytes in vivo results from spleen-facilitated vesiculation. Blood. 2003;101:747–51. doi: 10.1182/blood-2002-02-0500. [DOI] [PubMed] [Google Scholar]