Abstract
Background. Schistosomes infect 200 million individuals annually and cause significant hepatic fibrosis in up to 20%. Little is known regarding the mechanisms of schistosome-associated hepatic fibrosis in humans, and few biomarkers for risk of fibrosis have been identified.
Methods. We treated 611 Schistosoma japonicum–infected Filipinos with praziquantel (PZQ) and performed ultrasound to quantify hepatic fibrosis at baseline and 12 months after PZQ treatment. We developed a multiplexed assay (FibroPlex) that quantifies predictors and effect modifiers of fibrosis. We measured FibroPlex analytes produced by peripheral blood mononuclear cells stimulated with schistosome egg antigen 4 weeks after PZQ treatment and related these levels to risk of fibrosis 1 year after PZQ treatment.
Results. After adjusting for potential confounders, including baseline grade of fibrosis, individuals with detectable tissue inhibitor of matrix-metalloprotease–1 (TIMP-1) had a 3.5-fold greater risk of fibrosis 1 year after PZQ treatment, compared with individuals with undetectable levels (odds ratio, 3.48; 95% confidence interval, 1.41–8.43; P = .007).
Discussion Because TIMP-1 inhibits most matrix metalloproteases, which are responsible for collagen degradation, these data suggest that schistosome-associated hepatic fibrosis results, in part, from excessive inhibition of collagen remodeling. These data further suggest that TIMP-1 is a promising biomarker for assessing risk of hepatic fibrosis in schistosomiasis and, potentially, other infectious and noninfectious causes of liver disease.
Schistosomiasis currently affects >250 million people per year worldwide [1] and results in 1.53 million disability-adjusted life years lost per annum [2]. A recent reassessment of the global burden of schistosomiasis suggests that the actual health burden is 4–30 times greater than the previous World Health Organization (WHO) estimate [3, 4]. Despite the availability of chemotherapy with praziquantel, hepatic fibrosis remains among the most serious sequelae of chronic schistosome infection, occurring in up to 20% of infected individuals [5].
Hepatic fibrosis is considered to be a wound healing response characterized by persistent tissue damage, activation of multiple cell types that produce extracellular matrix (ECM), culminating in tissue scarring, loss of normal parenchyma, and eventually, organ failure [6]. Animal and human studies have attributed schistosome-associated fibrosis to the T-helper 2 (Th2) immune response induced by egg antigens trapped in sinusoidal spaces [7–9]. Activated lymphocytes and macrophages produce profibrotic cytokines (IL-13, TGFβ-1), chemokines, growth factors (TGFβ-1, EGF), and molecules that regulate ECM turnover, such as matrix metalloproteases (MMPs) and their inhibitors, the tissue inhibitors of matrix-metalloproteases (TIMPs) [10, 11]. Whether fibrosis results from increased synthesis of ECM, decreased degradation of ECM, or a combination of both has not been clearly established, although the TIMP:MMP ratio may determine the net balance of ECM turnover [12, 13].
Many studies have investigated the pathophysiology of schistosomiasis-associated hepatic fibrosis; however, the immunological mechanisms involved in human fibrosis remains incomplete. In both murine and human studies, Th2 cytokines (interleukin [IL]-4, IL-5, and IL-13) have been associated with an increased risk of fibrosis [7, 14–16], and IL-10 is thought to play a critical immunoregulatory role [14, 17]. In contrast to animal models, human studies have shown that tumor necrosis factor (TNF)–α and interferon (IFN)–γ are associated with increased and decreased risk of fibrosis, respectively [15, 17–20]. The roles of the MMP:TIMP axis in human schistosome infection remains unclear [21].
In previous studies, we demonstrated that high levels of IL-5 and IL-13 produced by peripheral blood mononuclear cells (PBMCs) stimulated with schistosome egg antigens (SEAs) were associated with persistent fibrosis in a cohort of 611 Schistosoma japonicum–infected individuals [7]. To better understand the pathogenesis of S. japonicum–associated hepatic fibrosis, we measured multiple predictors and modifiers of fibrosis in this same cohort of individuals and analyzed their relationship to risk of fibrosis 1 year after treatment with praziquantel (PZQ).
METHODS
Study Site and Population
Study subjects participated in a longitudinal treatment-reinfection study conducted in 3 rice-farming villages in Leyte, the Philippines, where S. japonicum is endemic and malaria is not endemic. These villages participate in the annual mass chemotherapy program of the Philippine Department of Health; however, multiple factors have resulted in suboptimal participation in this program. In total, 1262(74.3%) of 1699 individuals aged 7–30 years who resided in the study villages were screened for the presence of S. japonicum infection by duplicate examination of 3 stool samples before enrollment. The prevalence of infection with S. japonicum among persons in this age range was 60.0%. Subjects were eligible if they were infected with S. japonicum, lived primarily in a study village, were aged 7–30 years, were not pregnant or lactating, had no severe fibrosis (grade II or III) assessed by ultrasound examination, did not have severe wasting (defined as body mass index less than the third percentile of a reference population of same age and sex derived from the US National Health & Anthropometric Examination Survey) or severe anemia (hemoglobin level <7 g/dL), and provided both child assent and parental consent or adult consent if >18 years of age. Individuals with severe fibrosis were ineligible because of the lengthy follow-up period and were referred for intensive antihelminthic treatment. Six hundred eleven enrolled participants were treated with PZQ (60 mg/kg in a split dose) at baseline. Socioeconomic status was measured by questionnaire, and water contact was measured by direct observation, as described elsewhere [22, 23]. The study was approved by the Institutional Review Boards of Brown University and The Philippines Research Institute of Tropical Medicine. All S. japonicum–reinfected subjects were treated at 18 months follow-up, after completion of the longitudinal study.
Stool Examination
At baseline and 3, 6, 9, and 12 months after treatment, egg counts were determined by duplicate examination of 3 consecutive stool specimens obtained from each study participant by the Kato Katz method. Stool samples negative for S. japonicum eggs after documented reinfection were considered to be misclassified and were imputed using the last value carried forward method [24]. Intermittent missing stool samples were imputed using the same method [24]. Cumulative egg count after treatment was defined as the sum of egg counts at 3, 6, 9, and 12 months follow-up. Intensity of infection was determined using WHO criteria; low, moderate, and high intensity infections were defined as 1–99, 100–399, and ≥400 epg, respectively [25].
Ultrasound Evaluation
At baseline and 12 months follow-up, study subjects were evaluated by ultrasound by 2 observers (JDK and RMO) using a EUB-200 device with a 3.5-Mhz probe (Hitachi). Both observers were blinded to the FibroPlex results of participants. Liver span was measured as size of the left liver lobe in centimeters in the right parasternal line. Reference measurements for liver and spleen size among healthy Filipinos are not available; therefore, height-specific normal values from a healthy Chinese population were used [26]. Hepatomegaly was defined as >2 standard deviations (SDs) above the mean. Grading of hepatic fibrosis was based on a modification of the grading system described by Doehring-Schwerdtfeger et al [27]. Severe fibrosis (grade II or III) was an exclusion criterion for participation in the study; thus, only subjects with no or grade I fibrosis were included at baseline. Persistent fibrosis was defined as presence of fibrosis both at baseline and at follow-up. Reversible fibrosis was defined as presence of fibrosis at baseline, but not at follow-up.
PBMC Collection and S. japonicum Antigens
Four weeks after treatment, venipuncture was performed and blood samples were collected in Vacutainer tubes (Becton Dickinson) containing heparin as anticoagulant. PBMCs were isolated and placed in culture within 4 h after collection, as described elsewhere [23]. SEA was prepared as described elsewhere [28, 29]. PBMCs were stimulated with SEA, phytohemagglutinin (PHA), and control media, as described elsewhere [23]. All samples produced detectible IFN-γ in response to PHA.
Multiplexed Fibrosis Assays
Our multiplexed fibrosis assay (FibroPlex) was performed on culture supernatants by means of a multiplexed bead-based platform and custom assay kits. The FibroPlex is composed of a 7-plex sandwich format and a 1-plex (TGF-β1) sandwich assay. The 1 plex is not multiplexed, because the samples for TGF-β1 must be acid-activated before analysis.
For the 7-plex and 1-plex sandwich components, 500 μg of detection antibody (TGF-β1, TIMP-1, MIP-1α, IL-13Rα2, BMP-7 [R&D]; CTGF [PetroTech]; MMP-1 [Abcam]; and IL-13 [BD Pharmingen]) was coupled to 6.25 × 107 microspheres from unique bead regions, according to the manufacturer's (Luminex) instructions. Beads were pooled as a single lot, lyophilized in single-use aliquots, and stored at -80°C. Standards were pooled as a single lot at appropriate concentrations, were aliquoted into single-use tubes, were lyophilized, and were stored at −80°C. Biotinylated detection antibodies were pooled as a single lot into single-use aliquots, were lyophilized, and were stored at −80°C. Custom (all analytes) controls were pooled into single-use aliquots, were lyophilized, and were stored at −80°C.
The FibroPlex kit demonstrates <10% median interanalyte interference, and the median intraassay coefficient of variation, as assessed by 28 replicate serum controls, was 18%. The mean (standard error of the mean) for the 28 replicate serum controls was 4975 (131.0) pg/mL for TIMP-1, 493 (10.3) pg/mL for MIP-1α, 563 (38.9) pg/mL for IL-13Rα2, 1062 (42.3) pg/mL for BMP-7, 458 (15.5) pg/mL for CTGF, 765 (24.5) pg/mL for MMP-1, and 229 (8.0) pg/mL for IL-13; TGF-β1 was not tested. The lower limit of detection was 2.6 pg/mL for TIMP-1, 1.97 pg/mL was MIP-1α, 4.82 pg/mL for IL-13Rα2, 30.6 pg/mL for BMP-7, 1.4 pg/mL for CTGF, 1.75 pg/mL for MMP-1, 2.4 pg/mL for IL-13, and 1.86 pg/mL for TGF-β1.
All specimen identification and pipetting was performed by a bar-code-enabled, high-speed pipetting robot (Tecan). For each sample, values for PBMCs stimulated with media alone were subtracted from the values for PBMCs stimulated with SEA.
Hepatitis B
The prevalence of detectible hepatitis B surface antigen was measured in plasma with use of an enzyme-linked immunosorbent assay–based method (Shangai Kehua Biotech) according to manufacturer's instructions. Results were read as negative or positive. Vaccination with hepatitis B vaccine is not available in the study area; therefore, a positive test result indicates infection with hepatitis B virus [30].
Statistical Analyses
S. japonicum egg counts and FibroPlex results were loge transformed to produce more normal distributions. FibroPlex results were analyzed as both continuous and categorical variables. FibroPlex results were analyzed dichotomously (detectible vs not detectible) if <25% of the subjects had responses greater than the detection threshold (TIMP-1) or as tertiles of their distribution (all other analytes). Multivariate linear and logistic regression models were used to evaluate the relationship between FibroPlex analytes and liver span and grade of fibrosis 1 year after PZQ treatment. Potential confounders were selected on the basis of known determinants of fibrosis and results of bivariate analyses.
Models evaluating liver span 1 year after PZQ treatment were adjusted for baseline liver span, sex, socioeconomic status, cumulative water contact, egg counts at baseline and 1 year after treatment, height, and hepatitis B antigenemia. Models evaluating fibrosis 1 year after PZQ treatment were adjusted for baseline grade of liver fibrosis, sex, age, socioeconomic status, cumulative water contact, egg count at baseline and 1 year after treatment, and hepatitis B antigenemia. All analyses were performed in JMP, version 8 (SAS Institute)
RESULTS
Descriptive Characteristics
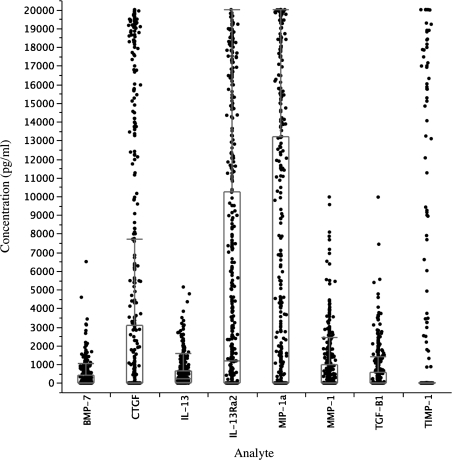
Table 1 presents the characteristics of all the participants at the beginning of the study (n = 611) and of those who were available for ultrasound at 12 months of follow-up. At baseline, sex, age, baseline liver span and fibrosis, and mean egg count were not significantly different between those who completed follow-up at 12 months (438 [71.5%]) and those who were lost to follow-up (173 [28.5%]; all P values were not statistically significant). Because of the eligibility criteria, at baseline, the prevalence of infection was 100% and all cases of fibrosis (7%) were classified as mild. At follow-up, the prevalence of fibrosis had increased to 20%, with 10.1% graded as moderate and 1.1% graded as severe fibrosis. The prevalence of reinfection at 12 months after treatment was 82.6%. Figure 1 presents the SEA-specific levels of FibroPlex analytes in PBMC culture supernatants.
Table 1.
Descriptive characteristics of cohort at baseline and at 12 months after praziquantel treatment
| Variable | Baseline | 12 months |
| Sample size | 611 | 438 |
| Age, years, mean (95% CI) | 15 (14.5–15.4) | 16 |
| Female sex, % | 36 | 36 |
| Prevalence of Schistosoma Japonicum | 100 | 82.6 |
| S. japonicum intensity, % | ||
| Low | 70.5 | 66.1 |
| Moderate | 23.6 | 12.6 |
| High | 5.9 | 3.9 |
| Prevalence of fibrosis, % (no) | 7 (41) | 20 (89) |
| Mild | 100 (41) | 88.8 (79) |
| Moderate | 10.1 (9) | |
| Severe | 1.1 (1) | |
| Liver span, cm,a mean (95% CI) | 7.14 (7.05–7.22) | 6.94 (6.83–7.05) |
| Prevalence of HBsAg, % (no) | 11.9 |
NOTE. Abbreviations: CI, confidence interval; HsAg, Hepatitis B Surface antigen.
Liver size measured by ultrasound as size of the left liver lobe in cm in the right parasternal line.
Figure 1.
Concentration of FibroPlex analytes in culture supernatants from peripheral blood mononuclear cells (PBMCs; 542–556) stimulated with schistosome egg antigens (SEAs). Analyte concentrations of SEA-stimulated wells after subtraction of value in wells stimulated with media alone are shown. Box plots indicate medians (center lines), 75th percentiles (boxes), and 90th percentiles (bars).
IL-13 Predicts Decreased Liver Span 1 Year after PZQ Treatment
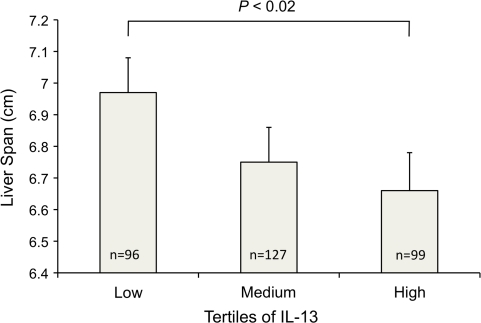
In previous work in this same cohort, we demonstrated that high levels of IL-5 and IL-13 produced by PBMCs stimulated with SEA were associated with persistent fibrosis in individuals with fibrosis at baseline, independent of intensity of infection and reinfection [7]. Because scarring is accompanied by wound contracture, hepatic fibrosis is often associated with loss of hepatic volume [31]. We therefore evaluated whether profibrotic, Th2 cytokines produced by PBMCs would predict liver span measured by ultrasound at 12 months of follow-up. SEA-specific IL-13 level was a significant predictor (β = −.064; P = .003) of decreased liver span 12 months after PZQ treatment, even after adjusting for important predictors of liver span, including baseline liver span, sex, socioeconomic status, cumulative water contact, egg count at baseline and 1 year after treatment, height, and hepatitis B antigenemia. When analyzed as tertiles, individuals with high IL-13 levels had liver spans that were .31-cm smaller 12 months after PZQ treatment than did individuals with low IL-13 levels, after adjusting for the same confounders (P = .02) (Figure 2).
Figure 2.
IL-13 responses to Schistosoma japonicum egg antigen predicts decreased liver span 1 year after praziquantel (PZQ) treatment. Peripheral blood mononuclear cells (PBMCs) from S. japonicum–infected individuals aged 7–30 years were collected 1 month after PZQ treatment, stimulated with egg antigen or media; FibroPlex analytes were measured in supernatants with a multiplexed assay. Antigen-specific cytokine levels were evaluated for their relationship with liver span measured by ultrasound in right parasternal line. Potential confounders included baseline liver span, sex, socioeconomic status, cumulative water contact, egg counts at baseline and 1 year after treatment, height, and hepatitis B antigenemia. Error bars represent standard error of the mean.
TIMP-1 Predicts Increased Hepatic Fibrosis 1 Year after PZQ Treatment
We evaluated the relationships between multiple measures of collagen metabolism and risk of future hepatic fibrosis with use of multiple linear regression models to adjust for known determinants of hepatic fibrosis. We measured TIMP-1, MMP-1, BMP-7, TGFβ-1, IL-13Rα2, and CTGF levels in culture supernatants from PBMCs obtained 4 weeks after PZQ treatment that stimulated with SEA or media alone. No significant correlation was found between SEA-specific levels of TGFβ-1, CTGF, BMP-7, or MMP-1 in PBMC culture supernatants and prevalence of fibrosis at 12 months after PZQ treatment.
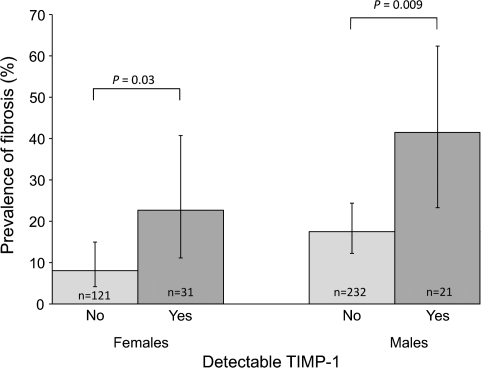
However, SEA-specific TIMP-1 level was a significant predictor of the presence of hepatic fibrosis 12 months after PZQ treatment, even after adjusting for important predictors of hepatic fibrosis, including baseline grade of hepatic fibrosis, sex, age, socioeconomic status, cumulative water contact, egg count at baseline and 1 year after treatment, and hepatitis B antigenemia (P = .004). Individuals with detectible TIMP-1 in culture supernatants from SEA-stimulated PBMCs had a 3.5-fold higher risk of fibrosis at 12 months of follow-up, compared with individuals with undetectable TIMP-1 levels after adjusting for the same confounders (odds ratio, 3.48; 95% confidence interval, 1.41–8.43; P = .007) (Figure 3).
Figure 3.
TIMP-1 produced in response to Schistosoma japonicum egg antigen predicts fibrosis 1 year after praziquantel (PZQ) treatment. Peripheral blood mononuclear cells (PBMCs) from S. japonicum–infected individuals aged 7–30 years, collected at 1 month after PZQ treatment, were stimulated with egg antigen, and FibroPlex analytes were measured in supernatants with a multiplexed assay. Antigen-specific TIMP-1 levels (evaluated as detectable or not detectable) were analyzed for their relationship with hepatic fibrosis measured by ultrasound 1 year after PZQ treatment. Potential confounders included baseline grade of liver fibrosis, age, socioeconomic status, cumulative water contact, egg count at baseline and 1 year after treatment, and hepatitis B antigenemia. Results presented are stratified by sex. Error bars represent 95% confidence intervals.
DISCUSSION
In the present study, we evaluated the relationship between modulators of collagen metabolism produced in response to S. japonicum egg antigens and risk of hepatomegaly and hepatic fibrosis measured 1 year after PZQ treatment in children, adolescents, and young adults. The goals of this work were to identify the mechanisms of human fibrosis in S. japonicum and potential noninvasive biomarkers for assessing fibrosis risk.
Schistosome-associated fibrosis in humans is associated with Th2 cytokine responses to egg antigens measured in PBMCs [7, 16]. Because fibrosis may result from imbalance in collagen secretion and degradation [13], in the current study, we explored the relationship between S. japonicum–induced modulators of collagen metabolism and risk of hepatomegaly and fibrosis 1 year after PZQ treatment. To evaluate multiple measures of collagen metabolism, we developed a multiplexed, sandwich-based immunoassay (Fibroplex) that measured TGF-β1, IL-13Rα2, IL-13, MIP-1 α, CTGF, BMP-7, TIMP-1, and MMP-1.
TGF-β1 is a potent profibrotic cytokine that promotes hepatic stellate cell activation, upregulates TIMPs, and down-modulates MMPs [11, 13, 32]. IL-13Rα2 is a soluble IL-13 receptor initially described as an inhibitor of IL-13 (decoy receptor) [11]; however, in the presence of TNF-α, IL-13Rα2 may promote fibrosis [33]. IL-13 is a Th2 cytokine that promotes alternative activation of macrophages, induces TGF-β1 production [11, 34, 35], and is associated with fibrosis in schistosome-infected individuals [7, 16]. MIP-1α is chemotactic for monocytes and is associated with pulmonary fibrosis [11]. BMP-7 is a natural TGF-β1 antagonist [34], and CTGF enhances TGF-β1 signaling by inhibiting BMP-7 [34]. MMP-1 is a protease that degrades collagen I and III and is inhibited by TIMP-1.
Fibrotic livers are often smaller than normal livers because of volume loss that accompanies replacement of normal parenchyma by scar tissue with subsequent wound contraction [31]. In the same cohort as that used in the current study, we previously demonstrated that high IL-13 production in responses to SEA predicted persistent fibrosis 1 year after PZQ treatment in S. japonicum–infected individuals with fibrosis at baseline [7]. Here, we show that high levels of IL-13 are associated with decreased liver span 1 year after treatment after adjusting for important confounders of liver span including age, height, sex, socioeconomic status, baseline liver span, baseline S. japonicum intensity, S. japonicum intensity 1 year after PZQ treatment, prevalence of Hepatitis B Surface antigen (HBsAg), and cumulative water contact measured during the follow-up period. These data indicate that liver span is influenced by heterogeneity in an individual's cytokine response to egg antigens, not just the presence and intensity of eggs per se. Although the absolute liver span differed by only .31 cm between the high and low IL-13 groups, we note that this represents a change from baseline over a relatively short interval and assessment of its clinical significance requires longer follow-up. We did not detect any correlation between TGF-β1 levels and liver span, which may reflect difficulties in measuring active TGF-β1 versus inactive TGF-β1 [36], or the possibility that IL-13 acts independently of TGF-β1 in inducing liver fibrosis as reported in murine S. mansoni infection [36].
Advanced hepatic fibrosis is relatively hypocellular, supporting the hypothesis that extensive scarring results from low levels of MMPs [37]. However, after adjusting for baseline fibrosis and other confounders, we did not detect a relationship between MMP-1 production by SEA-stimulated PBMCs and risk of fibrosis 1 year after PZQ treatment. In contrast, we detected a strong relationship between TIMP-1 levels and risk of fibrosis. Individuals whose PBMCs made detectible TIMP-1 in response to SEA stimulation had a 3.5 fold (P < .007) higher risk of fibrosis 1 year after PZQ treatment, compared with individuals with undetectable TIMP-1 levels. These data suggest that schistosome-associated fibrosis results from excessive inhibition of collagen remodeling through TIMP-1 and may reflect defects in trafficking of MMP producing PBMCs to the liver. In addition to inhibiting MMPs, TIMP-1 has anti-apoptotic and proliferative effects on fibroblasts, which may account, in part, for their profibrotic role in schistosome infection [38]. We are currently designing assays to measure the soluble products of collagen deposition and subsequent degradation to further parse the roles of MMPs and TIMPs in our cohort.
TIMP-1 gene expression peaks at the fibrotic stage of S. mansoni infection in mice [39], and fibrosis regression in response to PZQ therapy is associated with a dramatic decrease in the levels of TIMP-1 [12]. The relationship between increased levels of TIMP-1 and fibrosis has been demonstrated in several animal models and in cirrhotic human liver [40, 41]. In contrast, S. mansoni–infected TIMP-1 and -2 knockout mice did not differ in granuloma volume or hydroxyproline content, compared with wild-type mice [42], and reconciling these disparate results remains a priority. In contrast to our IL-13 results, we did not detect a significant relationship between TIMP-1 levels and liver span. This discordance may reflect differences in the kinetics of loss of hepatic parenchyma (a late finding) with collagen accumulation (an early finding).
The reinfection rate in our cohort was high, with >80% reinfected by 12 months after PZQ treatment. At baseline, 7% of our enrolled cohort had hepatic fibrosis, which increased to 20% 1 year after PZQ treatment. Seventy-three (17.6%) of 414 individuals without fibrosis at baseline and 16 (53.3%) of 30 individuals with grade 1 fibrosis at baseline had detectible hepatic fibrosis 1 year after PZQ treatment. These results are concordant with the rapid development of hepatomegaly in newly infected individuals [43]. This alarming increase in the prevalence of fibrosis despite effective annual treatment requires further study to determine the optimal interval for PZQ treatment in areas of high transmission and supports efforts to develop biomarkers of fibrosis risk to target individuals for more frequent anti-schistosomal treatment.
In our study, TIMP-1 predicted risk of liver fibrosis after controlling for important confounders, highlighting the impact of heterogeneity of the host's immune response on fibrosis. Specifically, by adjusting for intensity of reinfection and water contact exposure, our analyses highlight the importance of host-specific immune responses that are independent of infection intensity. Others have previously documented the effect of host genome on fibrosis with polymorphisms in CTGF, IFN-γ, IFN-γ receptor, IL-13, and specific MHC II alleles being associated with increased risk of fibrosis [44-47]. We are currently examining the role of polymorphisms in the promoter regions of TIMP and MMP genes in predicting risk of fibrosis [48].
Several study limitations should be addressed. First, we only measured FibroPlex analytes at a single time point; therefore, we cannot make inferences regarding the impact of PZQ treatment on these factors. Second, we did not measure other potential causes of hepatic fibrosis in our population, such as prevalence of hepatitis C virus infection or detailed measures of alcohol ingestion. The prevalence of hepatitis C virus infection in the general Filipino population has been reported as <1% [49] and, therefore, was not investigated in our cohort. The reported prevalence of hepatitis B virus infection is higher in the Philippines (>4% [49]), with a HBsAg prevalence of 11.9% in our cohort. Of importance, HBsAg was not related to liver span or liver fibrosis in any of our analyses. A recent WHO survey reported a prevalence of heavy drinking of 1.1% in Filipinos aged ≥18 years [50]. Because the mean age in our study sample was 15 years, alcohol remains an unlikely contributor to fibrosis among our participants.
A significant limitation to understanding fibrosis in human schistosome infection remains the relative inaccessibility of human liver tissue for study. We sampled and analyzed PBMCs based on the observation that recruitment of monocytes to the liver with their subsequent differentiation in macrophages secreting pro-fibrotic mediators and in fibroblasts is central to the process of hepatic fibrosis [32]. This sampling limitation fails to measure the contribution of hepatic stellate cells, a major source of myofibroblasts in the liver. Despite this limitation, we detected a significant role for TIMP-1 production by SEA-stimulated PBMCs in predicting risk of hepatic fibrosis.
Dissecting the immunologic network promoting schistosome-associated hepatic fibrosis remains daunting because of the complexity of the populations studied, the limited opportunity to perform multiple experiments, and the relative inaccessibility of relevant tissue samples. Despite these difficulties, the goal of identifying biomarkers of fibrosis risk remains critical because of the high prevalence of fibrosis despite effective treatment. We are currently evaluating the performance characteristic of serum measures of the TIMP-MMP axis as a step toward identifying patients at greatest risk of hepatic fibrosis.
Funding
National Institutes of Health (grants R01AI48123 and K23AI52125).
Supplementary Material
Acknowledgments
We thank our field staff, including Archie Pablo, Raquel Pacheco, Patrick Sebial, Mary Paz Urbina, and Jemaima Yu, for their diligence and energy, and the study participants from Macanip, Buri, and Pitogo in Leyte.
References
- 1.Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Trop. 2000;77:41–51. doi: 10.1016/s0001-706x(00)00122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJL, Lopez AD. The Global Burden of Disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Cambridge, MA: Harvard School of Public Health; 1996. [Google Scholar]
- 3.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–18. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 4.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–9. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- 5.Arnaud V, Li J, Wang Y, et al. Regulatory role of interleukin-10 and interferon-gamma in severe hepatic central and peripheral fibrosis in humans infected with Schistosoma japonicum. J Infect Dis. 2008;198:418–26. doi: 10.1086/588826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–9. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coutinho HM, Acosta LP, Wu HW, et al. Th2 cytokines are associated with persistent hepatic fibrosis in human Schistosoma japonicum infection. J Infect Dis. 2007;195:288–95. doi: 10.1086/510313. [DOI] [PubMed] [Google Scholar]
- 8.Cheever AW, Williams ME, Wynn TA, et al. Anti-IL-4 treatment of Schistosoma mansoni-infected mice inhibits development of T cells and non-B, non-T cells expressing Th2 cytokines while decreasing egg-induced hepatic fibrosis. J Immunol. 1994;153:753–9. [PubMed] [Google Scholar]
- 9.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest. 1999;104:777–85. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaudin P, Trocme C, Berthier S, et al. TIMP-1/MMP-9 imbalance in an EBV-immortalized B lymphocyte cellular model: evidence for TIMP-1 multifunctional properties. Biochim Biophys Acta. 2000;1499:19–33. doi: 10.1016/s0167-4889(00)00084-7. [DOI] [PubMed] [Google Scholar]
- 11.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh KP, Gerard HC, Hudson AP, Boros DL. Expression of matrix metalloproteinases and their inhibitors during the resorption of schistosome egg-induced fibrosis in praziquantel-treated mice. Immunology. 2004;111:343–52. doi: 10.1111/j.0019-2805.2004.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007;117:539–48. doi: 10.1172/JCI30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann KF, Wynn TA, Dunne DW. Cytokine-mediated host responses during schistosome infections; walking the fine line between immunological control and immunopathology. Adv Parasitol. 2002;52:265–307. doi: 10.1016/s0065-308x(02)52014-5. [DOI] [PubMed] [Google Scholar]
- 15.Silva-Teixeira DN, Contigli C, Lambertucci JR, Serufo JC, Rodrigues V., Jr Gender-related cytokine patterns in sera of schistosomiasis patients with Symmers' fibrosis. Clin Diagn Lab Immunol. 2004;11:627–30. doi: 10.1128/CDLI.11.3.627-630.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alves Oliveira LF, Moreno EC, Gazzinelli G, et al. Cytokine production associated with periportal fibrosis during chronic schistosomiasis mansoni in humans. Infect Immun. 2006;74:1215–21. doi: 10.1128/IAI.74.2.1215-1221.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Booth M, Mwatha JK, Joseph S, et al. Periportal fibrosis in human Schistosoma mansoni infection is associated with low IL-10, low IFN-gamma, high TNF-alpha, or low RANTES, depending on age and gender. J Immunol. 2004;172:1295–303. doi: 10.4049/jimmunol.172.2.1295. [DOI] [PubMed] [Google Scholar]
- 18.Henri S, Chevillard C, Mergani A, et al. Cytokine regulation of periportal fibrosis in humans infected with Schistosoma mansoni: IFN-gamma is associated with protection against fibrosis and TNF-alpha with aggravation of disease. J Immunol. 2002;169:929–36. doi: 10.4049/jimmunol.169.2.929. [DOI] [PubMed] [Google Scholar]
- 19.Dessein AJ, Hillaire D, Elwali NE, et al. Severe hepatic fibrosis in Schistosoma mansoni infection is controlled by a major locus that is closely linked to the interferon-gamma receptor gene. Am J Hum Genet. 1999;65:709–21. doi: 10.1086/302526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coutinho HM, McGarvey ST, Acosta LP, et al. Nutritional status and serum cytokine profiles in children, adolescents, and young adults with Schistosoma japonicum-associated hepatic fibrosis, in Leyte, Philippines. J Infect Dis. 2005;192:528–36. doi: 10.1086/430929. [DOI] [PubMed] [Google Scholar]
- 21.Gomez DE, De Lorenzo MS, Alonso DF, Andrade ZA. Expression of metalloproteinases (MMP-1, MMP-2, and MMP-9) and their inhibitors (TIMP-1 and TIMP-2) in schistosomal portal fibrosis. Am J Trop Med Hyg. 1999;61:9–13. doi: 10.4269/ajtmh.1999.61.9. [DOI] [PubMed] [Google Scholar]
- 22.Friedman JF, Kanzaria HK, Acosta LP, et al. Relationship between Schistosoma japonicum and nutritional status among children and young adults in leyte, the Philippines. Am J Trop Med Hyg. 2005;72:527–33. [PubMed] [Google Scholar]
- 23.Leenstra T, Acosta LP, Wu HW, et al. T-Helper-2 cytokine responses to Sj97 predict resistance to reinfection with Schistosoma japonicum. Infect Immun. 2006;74:370–81. doi: 10.1128/IAI.74.1.370-381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Twisk J, de Vente W. Attrition in longitudinal studies: how to deal with missing data. J Clin Epidemiol. 2002;55:329–37. doi: 10.1016/s0895-4356(01)00476-0. [DOI] [PubMed] [Google Scholar]
- 25.WHO. Prevention and control of intestinal parasitic infections. WHO Technical Report Series 749. Geneva: World Health Organization; 1987. pp. 8–28. [PubMed] [Google Scholar]
- 26.Li YS, Kardorff R, Richter J, et al. Ultrasound organometry: the importance of body height adjusted normal ranges in assessing liver and spleen parameters among Chinese subjects with Schistosoma japonicum infection. Acta Trop. 2004;92:133–8. doi: 10.1016/j.actatropica.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Doehring-Schwerdtfeger E, Mohamed-Ali G, Abdel-Rahim IM, et al. Sonomorphological abnormalities in Sudanese children with Schistosoma mansoni infection: a proposed staging-system for field diagnosis of periportal fibrosis. Am J Trop Med Hyg. 1989;41:63–9. [PubMed] [Google Scholar]
- 28.Boros DL, Warren KS. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970;132:488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acosta LP, Waine G, Aligui GD, Tiu WU, Olveda RM, McManus DP. Immune correlate study on human Schistosoma japonicum in a well-defined population in Leyte, Philippines. II. Cellular immune responses to S. japonicum recombinant and native antigens. Acta Trop. 2002;84:137–49. doi: 10.1016/s0001-706x(02)00177-8. [DOI] [PubMed] [Google Scholar]
- 30.Lunn ER, Hoggarth BJ, Cook WJ. Prolonged hepatitis B surface antigenemia after vaccination. Pediatrics. 2000;105:E81. doi: 10.1542/peds.105.6.e81. [DOI] [PubMed] [Google Scholar]
- 31.Heidelbaugh JJ, Bruderly M. Cirrhosis and chronic liver failure: part I. Diagnosis and evaluation. Am Fam Physician. 2006;74:756–62. [PubMed] [Google Scholar]
- 32.Gressner OA, Weiskirchen R, Gressner AM. Biomarkers of hepatic fibrosis, fibrogenesis and genetic pre-disposition pending between fiction and reality. J Cell Mol Med. 2007;11:1031–51. doi: 10.1111/j.1582-4934.2007.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 34.Gressner OA, Gressner AM. Connective tissue growth factor: a fibrogenic master switch in fibrotic liver diseases. Liver Int. 2008;28:1065–79. doi: 10.1111/j.1478-3231.2008.01826.x. [DOI] [PubMed] [Google Scholar]
- 35.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 36.Kaviratne M, Hesse M, Leusink M, et al. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-beta independent. J Immunol. 2004;173:4020–9. doi: 10.4049/jimmunol.173.6.4020. [DOI] [PubMed] [Google Scholar]
- 37.Issa R, Zhou X, Constandinou CM, et al. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology. 2004;126:1795–808. doi: 10.1053/j.gastro.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal. 2008;1:re6. doi: 10.1126/scisignal.127re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh KP, Gerard HC, Hudson AP, Boros DL. Dynamics of collagen, MMP and TIMP gene expression during the granulomatous, fibrotic process induced by Schistosoma mansoni eggs. Ann Trop Med Parasitol. 2004;98:581–93. doi: 10.1179/000349804225021316. [DOI] [PubMed] [Google Scholar]
- 40.Iredale JP, Benyon RC, Arthur MJ, et al. Tissue inhibitor of metalloproteinase-1 messenger RNA expression is enhanced relative to interstitial collagenase messenger RNA in experimental liver injury and fibrosis. Hepatology. 1996;24:176–84. doi: 10.1002/hep.510240129. [DOI] [PubMed] [Google Scholar]
- 41.Lichtinghagen R, Breitenstein K, Arndt B, Kuhbacher T, Boker KH. Comparison of matrix metalloproteinase expression in normal and cirrhotic human liver. Virchows Arch. 1998;432:153–8. doi: 10.1007/s004280050149. [DOI] [PubMed] [Google Scholar]
- 42.Vaillant B, Chiaramonte MG, Cheever AW, Soloway PD, Wynn TA. Regulation of hepatic fibrosis and extracellular matrix genes by the th response: new insight into the role of tissue inhibitors of matrix metalloproteinases. J Immunol. 2001;167:7017–26. doi: 10.4049/jimmunol.167.12.7017. [DOI] [PubMed] [Google Scholar]
- 43.Olveda RM, Daniel BL, Ramirez BD, et al. Schistosomiasis japonica in the Philippines: the long-term impact of population-based chemotherapy on infection, transmission, and morbidity. J Infect Dis. 1996;174:163–72. doi: 10.1093/infdis/174.1.163. [DOI] [PubMed] [Google Scholar]
- 44.Marquet S, Abel L, Hillaire D, et al. Genetic localization of a locus controlling the intensity of infection by Schistosoma mansoni on chromosome 5q31-q33. Nat Genet. 1996;14:181–4. doi: 10.1038/ng1096-181. [DOI] [PubMed] [Google Scholar]
- 45.Hirayama K, Chen H, Kikuchi M, et al. Glycine-valine dimorphism at the 86th amino acid of HLA-DRB1 influenced the prognosis of postschistosomal hepatic fibrosis. J Infect Dis. 1998;177:1682–6. doi: 10.1086/515299. [DOI] [PubMed] [Google Scholar]
- 46.Hirayama K, Matsushita S, Kikuchi I, Iuchi M, Ohta N, Sasazuki T. HLA-DQ is epistatic to HLA-DR in controlling the immune response to schistosomal antigen in humans. Nature. 1987;327:426–30. doi: 10.1038/327426a0. [DOI] [PubMed] [Google Scholar]
- 47.Dessein A, Chevillard C, Arnaud V, et al. Variants of CTGF are associated with hepatic fibrosis in Chinese, Sudanese, and Brazilians infected with schistosomes. J Exp Med. 2009;206:2321–8. doi: 10.1084/jem.20090383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lose F, Thompson PJ, Duffy D, Stewart GA, Kedda MA. A novel tissue inhibitor of metalloproteinase-1 (TIMP-1) polymorphism associated with asthma in Australian women. Thorax. 2005;60:623–8. doi: 10.1136/thx.2004.026930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yanase Y, Ohida T, Kaneita Y, Agdamag DM, Leano PS, Gill CJ. The prevalence of HIV, HBV and HCV among Filipino blood donors and overseas work visa applicants. Bull World Health Organ. 2007;85:131–7. doi: 10.2471/BLT.06.033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ustun T, Chatterji S, Meghbal A, Murray CJL. The world health surveys. In: Murry CJL, Evans DE, editors. Health systems performance assessment: debates, methods and empiricism. Geneva: WHO; 2003. pp. 797–808. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.