Abstract
(See the editorial commentary by Grebely and Dore, on pages 571–4.)
Background. Population-level hepatitis C virus (HCV) infection incidence is a surrogate for community drug-related risk.
Methods. We characterized trends in human immunodeficiency virus (HIV) and HCV infection incidence and HCV infection prevalence among injection drug users (IDUs) recruited over 4 periods: 1988–1989, 1994–1995, 1998, and 2005–2008. We calculated HIV and HCV infection incidence within the first year of follow-up among IDUs whose test results were negative for these viruses at baseline (n = 2061 and n = 373, respectively). We used Poisson regression to compare trends across groups.
Results. HIV infection incidence declined significantly from 5.5 cases/100 person-years (py) in the 1988–1989 group to 2.0 cases/100 py in the 1994–1995 group to 0 cases/100 py in the 1998 and 2005–2008 groups. Concurrently, HCV infection incidence declined but remained robust (22.0 cases/100 py in the 1988–1989 cohort to 17.2 cases/100 py in the 1994–1995 cohort, 17.9 cases/100 py in the 1998 cohort, and 7.8 cases/100 py in the 2005–2008 cohort; P = .07). Likewise, HCV infection prevalence declined, but chiefly in younger IDUs. For persons aged <39 years, relative to the 1988–1989 cohort, all groups exhibited significant declines (adjusted prevalence ratio [PR] for the 2005–08 cohort, .73; 95% confidence interval [CI], .65–.81). However, for persons aged ≥39 years, only the 2005–2008 cohort exhibited declining prevalence compared with the 1988–1989 cohort (adjusted PR, .87; 95% CI, .77–.99).
Conclusions. Although efforts to reduce blood-borne infection incidence have had impact, this work will need to be intensified for the most transmissible viruses, such as HCV.
Nearly 30 years into the HIV epidemic, injection drug users (IDUs) remain at high risk for HIV infection. Data from surveillance systems and cohort studies have collectively suggested that HIV infection incidence among IDUs has declined [1–11], a trend attributed at least in part to harm-reduction measures including needle-exchange programs (NEPs) and substance-abuse treatment. Hepatitis C virus (HCV) is nearly 10 times more transmissible by needlestick than is HIV [12]. Prevalence estimates of HCV infection among IDUs have been reported to exceed 50% in most IDU populations, ranging as high as 95% [13]. Sharing a needle even once is enough to transmit or acquire HCV [14]. Harm-reduction measures that have led to declines in HIV infection incidence have not been as successful for HCV infection [15, 16].
HCV transmission is almost exclusively parenteral, in contrast to the multiple routes of transmission for HIV. Furthermore, drug use is much more likely to result in exposure to HCV than to HIV, and each exposure is more likely to result in transmission, making population-level HCV infection incidence a surrogate for drug-related risk behavior in the community [17, 18]. There are few recent reports on HCV infection incidence among community-based IDUs, but some data suggest that high-risk drug behaviors persist among IDUs. In 2009, the US Centers for Disease Control and Prevention published a report on HIV-associated risk behaviors among ∼10,000 IDUs sampled from 23 US cities [19]. Overall, one-third of IDUs reported sharing injection equipment in the preceding year and fewer than one-third had participated in an HIV behavioral intervention. Current estimates of HCV infection incidence would provide further context for these reports.
In a community-based cohort of IDUs in Baltimore, Maryland, we previously demonstrated declining HIV infection incidence from 1988 through 2007 [10, 20]. We have also reported on high HCV infection prevalence and incidence among this study's initial cohort, recruited in 1988–1989 [21]. We have recruited additional cohorts of IDUs in more recent periods. The objective of this analysis was to characterize trends in HIV and HCV infection incidence as well as HCV infection prevalence among IDUs over 4 recruitment periods spanning 20 years.
METHODS
Ethics Statement
All participants provided written informed consent, and the study was approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Study Population
The AIDS Linked to the Intravenous Experience (ALIVE) study follows a community-based cohort of IDUs in Baltimore that has been described elsewhere [22]. In 1988–1989, 2946 IDUs were enrolled through community outreach and were followed up at 6-month intervals. All participants acknowledged nonmedical injection-drug use within the preceding 11 years, were ≥18 years of age, and were free of AIDS at entry into the study. In a similar manner, and from the same community, additional persons were recruited into this cohort in 1994–1995 (n = 391), 1998 (n = 244), and 2005–2008 (n = 875). Some recruitment criteria changed over time. In the fourth period, persons were no longer required to be AIDS-free at entry. To replenish with active injectors, in 1994–1995, persons had to have injected in the preceding 3 years, and in 1998 and 2005–2008, in the preceding year.
We calculated HIV infection incidence for each recruitment cohort among persons who were HIV antibody negative at the baseline visit and who had a follow-up visit within 1 year of baseline (n = 2061). Individuals who were lost-to-follow-up or did not have at least one follow-up visit within 1 year of baseline were excluded. Incidence was calculated to reflect only time until the first follow-up visit that occurred within 1 year of baseline. Incidence was calculated to reflect only time until the first semi-annual visit that occurred among this group. HCV infection incidence analysis was performed similarly among those individuals who were HCV antibody negative at the baseline visit and had a follow-up visit within 1 year of baseline (n = 373).
Individuals were eligible for the HCV infection prevalence analysis only if a serum sample was available for us to test for HCV antibodies at baseline. From the original cohort, we randomly selected 250 persons, 4 of whom had no sample. The random sample was comparable to the full population with respect to demographics and risk behaviors (data not shown). Serum samples were not available for 12 participants in the 1994–1995 cohort (3.1%), 12 (4.9%) in the 1998 cohort, and 1 person (.1%) of the 2005–2008 cohort. Final sample sizes for the HCV infection prevalence analysis were 246 from the 1988–1989 recruitment group, 379 from 1994–1995, 232 from 1998, and 874 from 2005–2008.
Measurements
At all study visits, each participant underwent a blood draw and answered a questionnaire that included a combination of interviewer-administered and audio computer-assisted self-interview (ACASI) questions. Blood samples were stored frozen at −70°C in a repository for future testing. We performed HCV antibody testing on frozen specimens using a second-generation or later enzyme immunoassay (Ortho Diagnostics).
At the baseline visit, we elicited information on demographics, lifetime medical history, and lifetime drug-use and sexual history. Participants were asked about when they started using and injecting drugs; lifetime experience with needle sharing, attending shooting galleries (venues where IDUs can rent, borrow, or purchase injection equipment and where injection equipment is typically used repeatedly), and being in drug treatment of any form, including methadone maintenance and detoxification; and number of sexual partners in the preceding 10 years. At baseline and follow-up visits, we collected data on behaviors in the preceding 6 months, including frequency (categorized as less than daily and at least daily) and types (heroin, cocaine, and speedball) of drugs injected; number of needle-sharing partners (eg, the number of persons with whom needles were shared or number of persons from whom the participant borrowed or rented a needle that had been previously used); shooting-gallery attendance; any drug treatment, specifically methadone maintenance; and number and types of sexual partners. Data on NEP attendance was available only after 1998, so it was not included. Data on sensitive information (eg, drug use) was collected using ACASI after 1998.
Statistical Analysis
We compared characteristics of participants across the 4 recruitment periods using χ2 tests for categorical variables and the Kruskal-Wallis test for continuous variables. We calculated incidence as the number of new HIV or HCV infections divided by person-years (py) of follow-up between baseline and the first semi-annual follow-up visit (as long as the visit was within 1 year of baseline) for persons initially HIV or HCV antibody negative. We did not perform multivariate analysis for HIV because we observed no infections in either the 1998 or 2005–2008 cohorts. We used Poisson regression to calculate incidence-rate ratios of HCV infection by recruitment cohort after adjustment. We built models sequentially to assess the impact of different factors. Initially, we included differences across recruitment cohorts to ensure that the differences observed were not explained by changes in the populations over time. First, we included only age and time since first injection followed by demographic variables (sex, race, and educational attainment) and HIV serostatus. We constructed subsequent models to determine whether any of the changes observed were explained by changes in recent drug-use or sexual risk behaviors over time. Drug-related risk behaviors included frequency of injection, needle sharing, shooting gallery attendance, and drug treatment in the preceding 6 months; sexual risk behaviors included number of sexual partners and self-reported sexually transmitted infections in the preceding 6 months. We also constructed models varying the order in which variables were included (eg, drug behaviors before demographics). We compared HCV infection prevalence across the 4 cohorts using the χ2 test for trend. We used Poisson regression with robust variance estimation to calculate prevalence ratios of HCV infection by recruitment cohort after adjusting for differences in confounders across recruitment cohorts and the impact of changes in risk behavior. The model-building strategy was similar to that described for HCV infection incidence. The primary difference was that in the prevalence models, we included lifetime risk behaviors rather than recent risk behaviors. We assessed effect modification by age and years since first reported injection on the association between recruitment cohort and HCV infection prevalence by including interaction terms in regression models. All statistical analyses were performed using Stata software (version 10.1; StataCorp).
RESULTS
Table 1 illustrates characteristics of the 4 recruitment cohorts at enrollment. Over time, the cohorts were older and the median duration of injection drug use was longer. Compared with the 1988–1989 cohort, subsequent recruitments included more women. The first 3 cohorts had a higher proportion of African Americans compared with the most recent recruitment (P < .001 for all). There were no statistically significant differences in marital status or educational attainment over time. The 2 later cohorts had a higher proportion of individuals with no formal income. HIV infection prevalence fluctuated from 23% in 1988–89 to 11 % in 1994–95 to 31% in 1998 and 23% in 2005–08. There were some variations in injection practices over time, some of which reflect recruitment differences. The proportion of participants who reported ever sharing needles over time decreased slightly; however, the proportion who reported ever attending a shooting gallery increased, as did the proportion who reported ever being in drug treatment (P < .001). The number of sexual partners in the preceding 10 years decreased over time. The proportion not injecting within 6 months of baseline was highest in the original and 2005–2008 recruitments. The proportion of participants injecting only heroin was higher in the 1994–1995 and 1998 cohorts than in those from other periods.
Table 1.
Description of study population by calendar period of recruitment*
| 1988-89 (n=246) | 1994-95 (n=379) | 1998 (n=232) | 2005-08 (n=874) | P value | |
| Median age (interquartile range) | 34 (30 – 38) | 37 (32 – 42) | 40 (31 – 40) | 43 (36 – 48) | <0.0001 |
| Duration of injection drug use | 12.9 (6.9 – 18.8) | 15.4 (8.0 – 23.0) | 17.9 (10.0 – 25.5) | 18.5 (10.2 – 27.3) | <0.0001 |
| Male gender | 201 (81.7) | 253 (66.8) | 150 (64.7) | 562 (64.3) | <0.0001 |
| African-American | 216 (87.8) | 360 (95.0) | 219 (94.8) | 573 (65.6) | <0.0001 |
| Never married | 154 (62.6) | 255 (67.3) | 151 (66.2) | 549 (62.9) | 0.4068 |
| ≥High school education | 114 (46.5) | 177 (47.0) | 95 (41.7) | 367 (42.0) | 0.2796 |
| Income/6 months | <0.0001 | ||||
| None 1–5000 5001 – 10,000 >10,000 |
10 (4.2) 169 (71.0) 30 (12.6) 29 (12.2) |
35 (9.3) 277 (73.7) 41 (10.9) 23 (6.1) |
67 (31.2) 95 (44.2) 31 (14.4) 22 (10.2) |
206 (23.7) 392 (45.2) 169 (19.5) 101 (11.6) |
|
| HIV positive | 56 (22.8) | 42 (11.1) | 71 (30.6) | 201 (23.0) | <0.0001 |
| Lifetime risk behaviors | |||||
| Ever shared needles | 236 (96.0) | 312 (82.5) | 165 (71.7) | 755 (86.5) | <0.0001 |
| Ever attend shooting gallery | 114 (46.3) | 222 (58.7) | 140 (60.6) | 759 (86.8) | <0.0001 |
| Ever been in drug treatment | 125 (50.8) | 232 (61.4) | 143 (61.9) | 770 (88.2) | <0.0001 |
| Sexual partners in prior 10 years | |||||
|
0–2 3–5 6–10 ≥11 |
27 (11.0) 64 (26.0) 45 (18.3) 110 (44.7) |
64 (16.9) 99 (26.2) 66 (17.5) 149 (39.4) |
83 (35.9) 67 (29.0) 31 (13.4) 50 (21.7) |
196 (22.6) 221 (25.5) 159 (18.3) 292 (33.6) |
<0.0001 |
| Recent risk behaviors | |||||
| Injection drug use in prior year | 225 (91.5) | 374 (98.9) | 232 (100) | 874 (100) | <0.0001 |
| Drug injected in prior six months* | |||||
| None Heroin Cocaine Both Other |
27 (11.0) 19 (7.7) 37 (15.0) 161 (65.5) 2 (0.81) |
11 (3.3) 38 (11.5) 20 (6.0) 250 (75.3) 13 (3.9) |
0 (0.0) 37 (16.2) 4 (1.8) 187 (82.0) 0 (0.0) |
84 (9.8) 158 (18.4) 20 (2.3) 581 (67.8) 14 (1.6) |
<0.0001 |
| Shared needles in prior six months | 149 (60.6) | 130 (39.3) | 89 (39.4) | 562 (64.5) | <0.0001 |
| Attended shooting gallery in prior six months | 70 (28.5) | 42 (12.7) | 33 (14.5) | 213 (24.4) | <0.0001 |
NOTE.* Numbers do not add up due to missing values; p <0.05 for chi-squared test for trend for all but male gender, African-American race, marital status and HIV serostatus.
Trends in HIV and Hepatitis C Virus Infection Incidence
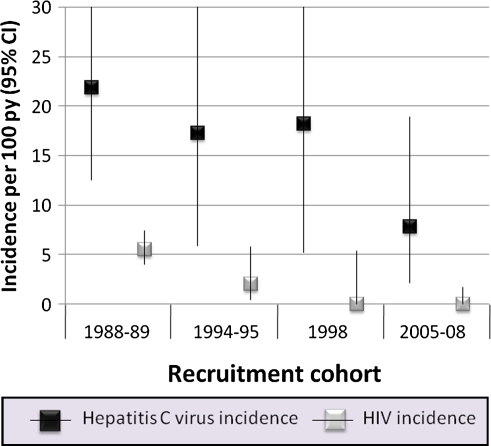
HIV infection incidence declined significantly over time from 5.5 cases/100 py in the 1988–1989 group to 2.0 cases/100 py in the 1994–1995 group to 0 cases/100 py in the 1998 and 2005–2008 groups (Figure 1). HCV infection incidence decreased over time from 22.0 cases/100 py in the 1988–1989 group to 17.2 cases/100 py in the 1994–1995 group, 17.8 cases/100 py in the 1998 group, and 7.8 cases/100 py in the 2005–2008 group (P = .07, χ2 test for trend) (Figure 1). The decline between the 1988–1989 and 1994 recruitment groups was not statistically significant (incidence rate ratio [IRR], .79; 95% confidence interval [CI], .28–2.19; Table 2). Relative to 1988–89, there were also statistically nonsignificant declines in HCV infection incidence in the 1998 and 2005–2008 groups. There was no decline between the 1994–1995 and 1998 groups (IRR, 1.04; 95% CI, .28–3.87) and a statistically nonsignificant decline between the 1998 and 2005–2008 groups (IRR, .43; 95% CI, .11–1.75). These differences strengthened in magnitude after adjustment for demographics, duration of injection, and HIV serostatus; however, none were statistically significant (Table 2, models 2 and 3). Neither drug-related nor sexual-related risk behavior explained a large proportion of the change in HCV infection incidence over time (Table 2, models 4 and 5). For example, the IRR before adjusting for drug-related risk behavior was .38 (95% CI, .09–1.62), whereas after adjusting for drug-related risk behavior the IRR was .43 (95% CI, .09–2.18).
Figure 1.
Incidence per 100 person-years of human immunodeficiency virus and hepatitis C virus infection by recruitment cohort in the AIDS Linked to the Intravenous Experience (ALIVE) cohort, 1988–2009.
Table 2.
Incidence rate ratios of HCV infection by calendar period of enrollment*
| Incidence rate ratio (95% CI) | ||||
| 1988–89 | 1994–95 | 1998 | 2005–08 | |
| Model 1: unadjusted | REF | 0.79 (0.28 – 2.19) | 0.82 (0.27 – 2.47) | 0.36 (0.12 – 1.09) |
| Model 2: adjusted for age, time since 1st injection | REF | 0.80 (0.27 – 2.33) | 0.98 (0.31 – 3.06) | 0.55 (0.14 – 2.14) |
| Model 3: adjusted age, time since 1st injection, gender, race, HIV, education, income | REF | 0.70 (0.21 – 2.38) | 0.95 (0.26 – 3.45) | 0.38 (0.09 – 1.62) |
| Model 4: adjusted age, time since 1st injection, gender, race, HIV, education, income, drug-related risk behaviors** | REF | 0.98 (0.28 – 3.37) | 0.99 (0.27 – 3.63) | 0.43 (0.09 – 2.18) |
| Model 5: adjusted age, time since 1st injection, gender, race, HIV, education, income, drug and sex-related risk behaviors*** | REF | 1.00 (0.29 – 3.42) | 0.90 (0.26 – 3.20) | 0.36 (0.07 – 1.87) |
NOTE. *Results from Poisson regression; age was included in all models as a continuous variable.
Summary drug-related risk behavior including recent (past six months) frequency of drug injection, needle sharing, shooting gallery attendance and drug treatment
Summary sex-related risk behavior includes recent number of partners in the last 6 months categorized as 0, 1, and >2 and any sexually transmitted infection.
Trends in Hepatitis C Virus Infection Prevalence
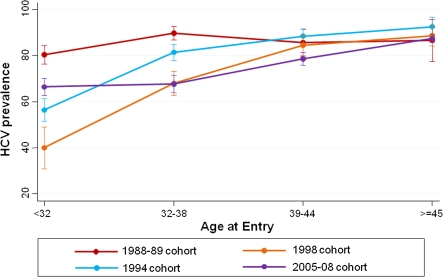
We observed similar trends in the association between recruitment cohort and HCV infection prevalence across strata of age and years since first injection. A statistically significant decline in HCV infection prevalence was observed among persons who were younger (<39 years of age) and had shorter injection history (<15 years) but not among those who were older and injecting for longer. Among those who had been injecting for <5 years at entry, the HCV infection prevalence was 70% in the 1988–1989 group, 65% in the 1994–1995 group, 50% in the 1998 group, and 52% in the 2005–2008 group (P = .02). Furthermore, >80% prevalence was reached by 5–9 years of injecting in the 1988–1989 group versus 15–19 years of injecting in the 2005–2008 group. Figure 2 shows HCV infection prevalence by age. Because differences across cohorts over time were heterogeneous across strata of age, all analyses included an interaction between age (stratified at the median of 39 years) and recruitment cohort.
Figure 2.
Hepatitis C virus infection prevalence by age at entry and recruitment cohort in the AIDS Linked to the Intravenous Experience (ALIVE) cohort, 1988–2008 (n = 1731).
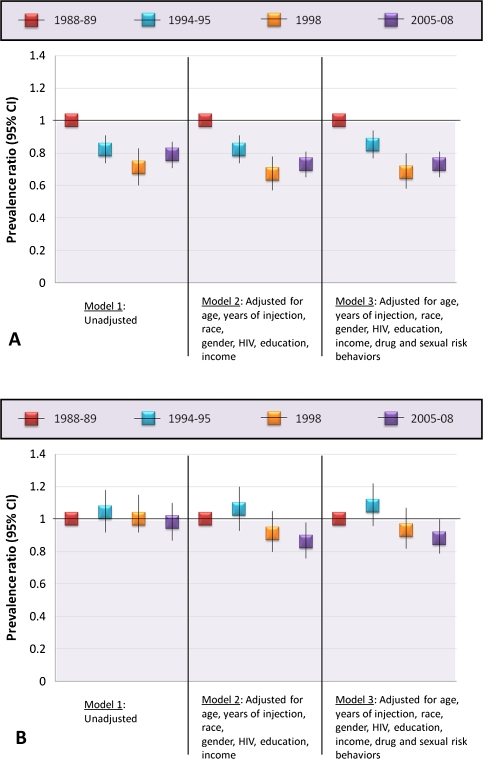
The pattern of decline in HCV infection prevalence over time among individuals aged <39 years persisted after adjustment for demographic factors and injection duration differences across recruitment cohorts (Figure 3A). All cohorts exhibited statistically significant differences in HCV infection prevalence relative to the 1988–1989 cohort (model 2); there was also a decline between the 1998 and 1994–1995 cohorts (PR, .82; 95% CI, .69–.97; P = .02) and a statistically nonsignificant increase between the 1998 and 2005–2008 cohorts (PR, 1.08; 95% CI, .91–1.29; P = .38). These changes were not largely explained by differences in lifetime drug-related or sexual-related risk behavior across the cohorts, as adjustment for these behaviors did not substantially attenuate the PRs (model 3).
Figure 3.
Prevalence ratios of hepatitis C virus infection by recruitment cohort for persons aged <39 years and persons aged ≥39 years. Results are from Poisson regression with robust variance estimates are stratified by age because there was a statistically significant interaction between age and recruitment cohort (P < .01). Panel A reflects ages of <39 years and panel B reflects ages of ≥39 years. The reference group for all models is the 1988–1989 cohort. P values for all prevalence ratios in panel A are statistically significant. Only the P value for the 2005–2008 cohort in model 2 of panel A is statistically significant. Age was included as a continuous variable in both models to account for residual confounding. Drug-related risk behaviors include lifetime history of needle sharing, shooting gallery attendance, and drug treatment; sexual risk behavior includes number of sexual partners in the preceding 10 years.
Among individuals aged ≥39 years, after adjustment for demographic and time since injection differences, there were no statistically significant differences between the 1994–1995 or the 1988 cohort and the 1988–1989 cohort (Figure 3B), but the 2005–2008 cohort had significantly lower HCV infection prevalence compared with the 1988–1989 cohort (PR, .86; 95% CI, .76–.98). Only a small proportion of this decline was explained by changes in drug-related risk behavior over time (model 3).
DISCUSSION
In this cohort of IDUs, we observed a dramatic decline in HIV infection incidence over 2 decades; no new infections occurred within the first year of follow-up in cohorts recruited in 1998 forward. Reductions in HCV infection incidence and prevalence were also observed over the same period, most notably among persons who had started injection recently and were younger. However, similar prevalences of HCV infection over time among those who were injecting longer and were older suggest that these improvements delay but do not prevent HCV infection at the population level. Collectively, these data support intensifying the harm-reduction strategies that have markedly reduced HIV transmission to reduce further the risk of HCV infection.
Large-scale expansion of NEPs and opiate substitution treatment programs appear to have reduced HIV transmission among IDUs [6, 7, 23]. Accordingly, in this Baltimore IDU cohort, we have seen marked reductions in HIV infection incidence over 2 decades [10, 20]. In this analysis, we also detected a decline in HCV infection incidence as well as HCV infection prevalence among those who were younger or had recently started injection. Importantly, we observed that HCV acquisition may be delayed by up to 10 years among IDUs compared with that in the late 1980s when the epidemic was at peak. These results are consistent with a recent meta-analysis by Hagan and colleagues [24] that suggested that time to HCV infection has lengthened in developed countries as well as a number of reports suggesting declines among younger injectors and new initiates [25–30]. Although we hypothesize that these declines are due to expanded harm-reduction efforts and reduction in drug-related risk behavior, our data did not demonstrate that changes in self-reported injection behavior had substantial impact. Of note, we were not able to account for changes in NEP attendance.
Despite reductions in HCV infection prevalence among younger individuals, we did not observe declines in HCV burden in those who were older and had longer injection histories, with the exception of a slight reduction among the most recent cohort compared with the earliest. It is possible that we failed to observe a difference over time among older IDUs with longer history of injection because it will simply take longer for reductions in HCV infection incidence to impact prevalence among older individuals, given that many of the individuals in this older age group were likely infected 20–25 years ago, prior to expanded harm-reduction strategies. This hypothesis is supported by the significantly lower prevalence among older IDUs in the most recent recruitment cohort.
However, it is important to consider other reasons why analogous reductions in HCV infection among older persons with a history of drug injection have not been detected. HCV is an order of magnitude more transmissible than HIV by a single needlestick [12]. Thus, measures that reduce the likelihood of viral exposure or that reduce the number of viruses in each exposure might be sufficient to affect HIV infection incidence without having a commensurate effect on HCV infection. Interventions such as NEPs and opiate substitution may reach IDUs too late in their injecting careers to have a significant impact on HCV infection incidence. Furthermore, although these measures may reduce the frequency of needle sharing that may be sufficient to impact HIV transmission, they may not completely eliminate risk behavior, making them insufficient to effectively prevent HCV transmission throughout an IDU's injection career.
Data from this cohort and many others indicate the risk of HCV-related liver disease sharply rises in persons aged >40 years [31–33]. It is difficult to disentangle the effects of age and duration of HCV infection on disease progression, and despite some public health benefits of delaying HCV infection, it will likely not be sufficient to prevent the long-term complications of disease in a large proportion of IDUs. Even with some reduction in transmission, of the 2005–2008 cohort, >80% of individuals ≥39 years old are infected with HCV and at risk for developing any of the complications associated with chronic liver disease. Furthermore, this level of prevalence points to a large reservoir of HCV infection among this population, which significantly hampers prevention efforts.
Efforts need to be intensified on both the prevention and treatment fronts to reduce the reservoir of HCV-infected IDUs. For many persons, the interval between initiation and injection simply remains too brief for prevention strategies to be successful. Targeting very young IDUs or drug users who have not yet transitioned to injection is critical. However, because transmission continues to occur even among older IDUs, prevention strategies must target IDUs at all stages and ages, and it may be that different strategies are needed for different subpopulations. The other method for reducing the size of the reservoir is HCV infection treatment. Current treatment regimens have limited efficacy among certain subpopulations. In particular, our population in Baltimore is predominantly infected with genotype 1 and African American, with a high prevalence of HIV co-infection, all of which are factors associated with reduced treatment success [34–36]. The impact of treatment in reducing HCV burden is likely to have been minimal, because we and others have demonstrated that few IDUs are engaged in care for HCV infection and even fewer successfully clear their HCV infections [40]. With new, more efficacious therapeutics on the horizon [41], it is critical that strategies to improve uptake and completion of HCV infection treatment of IDUs be implemented, because treatment is the only option for the large numbers of IDUs already infected with HCV.
We were limited in this analysis by not having HCV testing on the full baseline recruitment cohort; however, the random sample did appear to be similar to the full baseline cohort with respect to key covariates that would be associated with HCV infection prevalence. Our analysis was further limited by the small number of persons who were HCV antibody negative at baseline; this is a common problem in epidemiologic studies among IDUs. All of our behavioral data were collected via self-report, a method that has inherent limitations, some of which may have impaired our ability to assess how much impact behavior change had, particularly in the analysis of HCV infection prevalence. We cannot rule out the possibility of variability in unmeasured individual factors or secular changes across the cohorts that could have impacted prevalence and incidence. Eligibility criteria slightly changed over time, and we observed that a number of factors, including HIV serostatus, differed across recruitment cohorts. Although we adjusted for all measured confounders, we cannot rule out the possibility of residual confounding. Finally, prevalence estimates are impacted by both incidence and mortality, and it is possible that some of the early changes in prevalence observed actually reflect the high levels of mortality due to drug overdose and AIDS in the era prior to the use of highly active antiretroviral therapy (HAART). However, continued declines even after 1996 suggest that some of this difference may be because of reduced incidence as well. Finally, behavioral data were collected by interviewer-administered questionnaire prior to 1998 and via ACASI after 1998, which may have further impacted differences.
These limitations notwithstanding, the data collectively suggest that harm-reduction strategies that have been successful for HIV infection may also be contributing to declines in HCV acquisition. However, additional, more intensive strategies, particularly those that target new initiates into drug injection, are needed to significantly impact community-level drug-related risk. Furthermore, HCV infection prevalence and incidence remain up to 10-fold higher than those of HIV infection in this population, reinforcing not only the continued need for preventive measures but also the need to expand care and treatment to those already infected.
Funding
This study was supported by Public Health Service Grants from the National Institute on Drug Abuse (DA04334, DA12568, DA16078 and DA013868). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
We thank the ALIVE study staff and participants, without whom this would not have been possible.
References
- 1.Fleming PL, Ward JW, Karon JM, Hanson DL, De Cock KM. Declines in AIDS incidence and deaths in the USA: A signal change in the epidemic. AIDS. 1998;12:S55–61. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. HIV and AIDS–United States, 1981–2000. MMWR Morb Mortal Wkly Rep. 2001;50:430–4. [PubMed] [Google Scholar]
- 3.Longshore D, Anglin MD. Seroprevalence stable and seroconversions rare among injection drug users in Los Angeles. J Acquir Immune Defic Syndr. 1992;5:212–3. [PubMed] [Google Scholar]
- 4.Watters JK. Trends in risk behavior and HIV seroprevalence in heterosexual injection drug users in San Francisco, 1986–1992. J Acquir Immune Defic Syndr. 1994;7:1276–81. [PubMed] [Google Scholar]
- 5.Des Jarlais C, Perlis T, Friedman SR, et al. Behavioral risk reduction in a declining HIV epidemic: Injection drug users in New York City, 1990–1997. Am J Public Health. 2000;90:1112–6. doi: 10.2105/ajph.90.7.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Des Jarlais DC, Marmor M, Friedmann P, et al. HIV incidence among injection drug users in New York City, 1992–1997: Evidence for a declining epidemic. Am J Public Health. 2000;90:352–9. doi: 10.2105/ajph.90.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kral AH, Lorvick J, Gee L, et al. Trends in human immunodeficiency virus seroincidence among street-recruited injection drug users in San Francisco, 1987–1998. Am J Epidemiol. 2003;157:915–22. doi: 10.1093/aje/kwg070. [DOI] [PubMed] [Google Scholar]
- 8.Moss AR, Vranizan K, Gorter R, Bacchetti P, Watters J, Osmond D. HIV seroconversion in intravenous drug users in San Francisco, 1985–1990. AIDS. 1994;8:223–31. doi: 10.1097/00002030-199402000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Wiebel WW, Jimenez A, Johnson W, et al. Risk behavior and HIV seroincidence among out-of-treatment injection drug users: A four-year prospective study. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12:282–9. doi: 10.1097/00042560-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Nelson KE, Galai N, Safaeian M, Strathdee SA, Celentano DD, Vlahov D. Temporal trends in the incidence of human immunodeficiency virus infection and risk behavior among injection drug users in Baltimore, Maryland, 1988–1998. Am J Epidemiol. 2002;156:641–53. doi: 10.1093/aje/kwf086. [DOI] [PubMed] [Google Scholar]
- 11.Van Den Berg C, Smit C, Van Brussel G, Coutinho R, Prins M. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: Evidence from the Amsterdam Cohort Studies among drug users. Addiction. 2007;102:1454–62. doi: 10.1111/j.1360-0443.2007.01912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sulkowski MS, Ray SC, Thomas DL. Needlestick transmission of hepatitis C. JAMA. 2002;287:2406–13. doi: 10.1001/jama.287.18.2406. [DOI] [PubMed] [Google Scholar]
- 13.Aceijas C, Rhodes T. Global estimates of prevalence of HCV infection among injecting drug users. Int J Drug Policy. 2007;18:352–8. doi: 10.1016/j.drugpo.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: The prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J Public Health. 1996;86:655–61. doi: 10.2105/ajph.86.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright NM, Tompkins CN. A review of the evidence for the effectiveness of primary prevention interventions for hepatitis C among injecting drug users. Harm Reduct J. 2006;3:27. doi: 10.1186/1477-7517-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmateer N, Kimber J, Hickman M, Hutchinson S, Rhodes T, Goldberg D. Evidence for the effectiveness of sterile injecting equipment provision in preventing hepatitis C and human immunodeficiency virus transmission among injecting drug users: A review of reviews. Addiction. 2010;105:860–1. doi: 10.1111/j.1360-0443.2009.02888.x. [DOI] [PubMed] [Google Scholar]
- 17.Vickerman P, Hickman M, May M, Kretzschmar M, Wiessing L. Can hepatitis C virus prevalence be used as a measure of injection-related human immunodeficiency virus risk in populations of injecting drug users? An ecological analysis. Addiction. 2010;105:311–8. doi: 10.1111/j.1360-0443.2009.02759.x. [DOI] [PubMed] [Google Scholar]
- 18.Des Jarlais DC, Arasteh K, McKnight C, Hagan H, Perlman D, Friedman SR. Using hepatitis C virus and herpes simplex virus-2 to track HIV among injecting drug users in New York City. Drug Alcohol Depend. 2009;101:88–91. doi: 10.1016/j.drugalcdep.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. HIV-associated behaviors among injecting-drug users–23 cities, United States, May 2005–February 2006. MMWR Morb Mortal Wkly Rep. 2009;58:329–32. [PubMed] [Google Scholar]
- 20.Mehta SH, Galai N, Astemborski J, et al. HIV incidence among injection drug users in Baltimore, Maryland (1988–2004) J Acquir Immune Defic Syndr. 2006;43:368–72. doi: 10.1097/01.qai.0000243050.27580.1a. [DOI] [PubMed] [Google Scholar]
- 21.Villano SA, Vlahov D, Nelson KE, Lyles CM, Cohn S, Thomas DL. Incidence and risk factors for hepatitis C among injection drug users in Baltimore, Maryland. J Clin Microbiol. 1997;35:3274–7. doi: 10.1128/jcm.35.12.3274-3277.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vlahov D, Anthony JC, Munoz A, et al. The ALIVE study, a longitudinal study of HIV-1 infection in intravenous drug users: Description of methods and characteristics of participants. NIDA Res Monogr. 1991;109:75–100. [PubMed] [Google Scholar]
- 23.Des Jarlais DC, Perlis T, Arasteh K, et al. HIV incidence among injection drug users in New York City, 1990 to 2002: Use of serologic test algorithm to assess expansion of HIV prevention services. Am J Public Health. 2005;95:1439–44. doi: 10.2105/AJPH.2003.036517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hagan H, Pouget ER, Des Jarlais DC, Lelutiu-Weinberger C. Meta-regression of hepatitis C virus infection in relation to time since onset of illicit drug injection: The influence of time and place. Am J Epidemiol. 2008;168:1099–109. doi: 10.1093/aje/kwn237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burt RD, Hagan H, Garfein RS, Sabin K, Weinbaum C, Thiede H. Trends in hepatitis B virus, hepatitis C virus, and human immunodeficiency virus prevalence, risk behaviors, and preventive measures among Seattle injection drug users aged 18–30 years, 1994–2004. J Urban Health. 2007;84:436–54. doi: 10.1007/s11524-007-9178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Des Jarlais DC, Perlis T, Arasteh K, et al. Reductions in hepatitis C virus and HIV infections among injecting drug users in New York City, 1990–2001. AIDS. 2005;19:S20–5. doi: 10.1097/01.aids.0000192066.86410.8c. [DOI] [PubMed] [Google Scholar]
- 27.Tseng FC, O'Brien TR, Zhang M, et al. Seroprevalence of hepatitis C virus and hepatitis B virus among San Francisco injection drug users, 1998 to 2000. Hepatology. 2007;46:666–71. doi: 10.1002/hep.21765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldberg D, Burns S, Taylor A, Cameron S, Hargreaves D, Hutchinson S. Trends in HCV prevalence among injecting drug users in Glasgow and Edinburgh during the era of needle/syringe exchange. Scand J Infect Dis. 2001;33:457–61. doi: 10.1080/00365540152029936. [DOI] [PubMed] [Google Scholar]
- 29.Crofts N, Aitken CK. Incidence of bloodborne virus infection and risk behaviours in a cohort of injecting drug users in Victoria, 1990–1995. Med J Aust. 1997;167:17–20. doi: 10.5694/j.1326-5377.1997.tb138757.x. [DOI] [PubMed] [Google Scholar]
- 30.van den Berg CH, Smit C, Bakker M, et al. Major decline of hepatitis C virus incidence rate over two decades in a cohort of drug users. Eur J Epidemiol. 2007;22:183–93. doi: 10.1007/s10654-006-9089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: Host, viral, and environmental factors. JAMA. 2000;284:450–6. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 32.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–32. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 33.Grebely J, Raffa JD, Lai C. J Viral Hepat. 2011. Impact of hepatitis C virus infection on all-cause and liver-related mortality in a large community-based cohort of inner city residents [published online ahead of print 25 February, 2010] pp. 32–41. doi: 10.1111/j.1365-2893.2010.01279.x. [DOI] [PubMed] [Google Scholar]
- 34.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet. 2001;358:958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 35.Chung RT, Andersen J, Volberding P, et al. Peginterferon alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–9. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeffers LJ, Cassidy W, Howell CD, Hu S, Reddy KR. Peginterferon alfa-2a (40 kd) and ribavirin for black American patients with chronic HCV genotype 1. Hepatology. 2004;39:1702–8. doi: 10.1002/hep.20212. [DOI] [PubMed] [Google Scholar]
- 37.Mehta SH, Lucas GM, Mirel LB, et al. Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV clinic. AIDS. 2006;20:2361–9. doi: 10.1097/QAD.0b013e32801086da. [DOI] [PubMed] [Google Scholar]
- 38.Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33:126–33. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grebely J, Raffa JD, Lai C, et al. Low uptake of treatment for hepatitis C virus infection in a large community-based study of inner city residents. J Viral Hepat. 2009;16:352–8. doi: 10.1111/j.1365-2893.2009.01080.x. [DOI] [PubMed] [Google Scholar]
- 40.Hagan H, Latka MH, Campbell JV, et al. Eligibility for treatment of hepatitis C virus infection among young injection drug users in 3 US cities. Clin Infect Dis. 2006;42:669–72. doi: 10.1086/499951. [DOI] [PubMed] [Google Scholar]
- 41.Foster GR, Hezode C, Bronowicki JP, et al. In: Scientific Programme of the 44th Annual Meeting of the European Association for the Study of the Liver (Copenhagen, Denmark). Geneva Switzerland: European Association for the Study of the Liver; 2009. Activity of telaprevir alone or in combination with peginterferon alfa-2A and ribavirin in treatment-naïve genotype 2 and 3 hepatitis-C infected patients: Interim results of study C209. [Google Scholar]