Abstract
We studied the impact of baseline systolic blood pressure (SBP) on outcomes in mild to moderate chronic systolic and diastolic heart failure (HF) patients in the Digitalis Investigation Group trial using propensity-matched design. Of the 7788 patients, 7785 had baseline SBP data and 3538 had SBP ≤120 mm Hg. Propensity scores for SBP ≤120 mm Hg, calculated for each of the 7785 patients, were used to assemble a matched cohort of 3738 patients with SBP ≤120 and >120 mm Hg who were well-balanced on 32 baseline characteristics. All-cause mortality occurred in 35% and 32% of matched patients with SBP ≤120 and >120 mm Hg respectively during 5 years of follow-up (hazard ratio {HR} when SBP ≤120 was compared with >120 mm Hg, 1.10; 95% confidence interval {CI}, 0.99–1.23; p=0.088). HRs (95% CIs) for cardiovascular and HF mortality associated with SBP ≤120 mm Hg were 1.15 (1.01–1.30; p=0.031) and 1.30 (1.08–1.57; p=0.006). Cardiovascular hospitalization occurred in 53% and 49% of matched patients with SBP ≤120 and >120 mm Hg respectively (HR for SBP ≤120 was compared with >120 mm Hg, 1.13; 95% CI, 1.03–1.24; P=0.008). HRs (95% CIs) for all-cause and HF hospitalization associated with SBP ≤120 mm Hg were 1.10 (1.02–1.194; p=0.017) and 1.21 (1.07–1.36; p=0.002). In conclusion, in patients with mild to moderate chronic systolic and diastolic HF, baseline SBP ≤120 mm Hg was associated with increased cardiovascular and HF mortality and all-cause, cardiovascular and HF hospitalization that was independent of other baseline characteristics.
Keywords: heart failure, systolic blood pressure, mortality, hospitalization
Hypertension is a known risk factor for incident heart failure (HF).1, 2 However, several studies have demonstrated that in patients with acute decompensated HF, a low systolic blood pressure (SBP) is associated with poor outcomes.3–8 We have recently demonstrated similar associations between low SBP and poor outcomes in a propensity-matched cohort of chronic advanced systolic HF patients.9 However, the role of baseline SBP on outcomes in patients with chronic mild to moderate systolic and diastolic HF is relatively less known and has not been investigated using propensity-matched design. The purpose of the current study was to examine the association between baseline SBP and outcomes in a propensity-matched cohort of mild to moderate chronic systolic and diastolic HF patients.
Methods
A public-use copy of the Digitalis Investigation Group (DIG) data set was used for the current analysis. DIG was a multicenter randomized placebo-controlled clinical trial of digoxin in patients with HF.10 Briefly, 7788 patients with advanced chronic systolic HF were enrolled from 302 different sites across the United States and Canada between February 1991 and August 1993. At baseline, patients had a mean duration of 17 months of HF and had a mean left ventricular ejection fraction (LVEF) of 29%. Most patients had New York Heart Association (NYHA) class I-III symptoms and over 80% of all patients were receiving angiotensin-converting enzyme (ACE) inhibitors and diuretics.
Data on baseline SBP was available from 7785 patients and were documented by study investigators. Of these, 3538 (45%) had SBP ≤120 mm Hg (median, 110 mm Hg, interquartile range, 8 mm Hg), and 4247 (54%) had SBP >120 mm Hg (median, 140 mm Hg, interquartile range, 20 mm Hg). We chose SBP of 120 mm Hg as our cutoff as it is often considered the upper limit of normal range. Taking into account the significant imbalances in baseline characteristics between the two groups (Table 1), we used propensity scores to assemble a matched cohort of 1869 pairs of patients who were well-balanced on 32 baseline characteristics.11, 12 We began by estimating propensity scores for SBP ≤120 mmHg for each of the 7785 patients using a non-parsimonious multivariable logistic regression model and then assembled a cohort of 1869 pairs (n=3838) of propensity-matched patients with SBP ≤120 and >120 mm Hg who were well-balanced on 32 baseline characteristics.13–21 Absolute standardized differences were estimated to evaluate the pre-match imbalance and post-match balance, and presented as a Love plot.13–21 An absolute standardized difference of 0% indicates no residual bias and differences <10% are considered inconsequential.
Table 1.
Baseline characteristics of heart failure patient, by systolic blood pressure (BP), pre and post–propensity score matching
| Pre-match |
Post-match |
|||||
|---|---|---|---|---|---|---|
| Systolic BP (mm Hg) |
Systolic BP (mm Hg) |
|||||
| >120 (n = 4247) | ≤120 (n = 3538) | P Value | >120 (n = 1869) | ≤120 (n = 1869) | P Value | |
| Age (years) | 65 ± 10 | 62 ± 11 | <0.001 | 64 ± 10 | 64 ± 11 | 0.511 |
| Female | 1163 (27%) | 762 (22%) | <0.001 | 438 (23%) | 444 (24%) | 0.847 |
| Non-white | 657 (16%) | 470 (13%) | 0.006 | 254 (14%) | 242 (13%) | 0.595 |
| Body mass index (kg/m2) | 28 ± 6 | 27 ± 5 | <0.001 | 27 ±5 | 27 ±6 | 0.410 |
| Duration of heart failure (months) | 30 ±37 | 29 ±35 | 0.434 | 30 ±37 | 30 ±36 | 0.565 |
| Primary cause of heart failure | ||||||
| Ischemic | 2811 (66%) | 2548 (72%) | <0.001 | 1330 (71%) | 1330 (71%) | 0.223 |
| Hypertensive | 622 (15%) | 182 (5%) | 150 (8%) | 133 (7%) | ||
| Idiopathic | 545 (13%) | 565 (16%) | 261 (14%) | 293 (15%) | ||
| Others | 269 (6%) | 243 (7%) | 128 (7%) | 113 (6%) | ||
| Prior myocardial infarction | 2546 (60%) | 2361 (67%) | <0.001 | 1237 (66%) | 1217 (65%) | 0.507 |
| Current angina pectoris | 1170 (28%) | 944 (27%) | 0.392 | 520 (28%) | 524 (28%) | 0.913 |
| Hypertension | 1159 (59%) | 2514 (33%) | <0.001 | 792 (42%) | 794 (43%) | 0.972 |
| Diabetes mellitus | 1366 (32%) | 852 (24%) | <0.001 | 519 (28%) | 524 (28%) | 0.883 |
| Chronic kidney disease | 2017 (48%) | 1508 (43%) | <0.001 | 854 (46%) | 840 (45%) | 0.646 |
| Medications | ||||||
| Pre–trial digoxin use | 1780 (42%) | 1584 (45%) | 0.011 | 811 (43%) | 831 (45%) | 0.529 |
| Trial use of digoxin | 2131 (50%) | 1757 (50%) | 0.650 | 938 (50%) | 919 (50%) | 0.554 |
| ACE inhibitors | 3929 (93%) | 3343 (95%) | <0.001 | 1740 (93%) | 1745 (93%) | 0.793 |
| Nitroglycerine and hydralazine | 80 (2%) | 31 (1%) | <0.001 | 20 (1%) | 23 (1%) | 0.761 |
| Diuretics | 3311 (78%) | 2762 (78%) | 0.911 | 1464 (78%) | 1438 (77%) | 0.338 |
| Potassium-sparing diuretics | 284 (7%) | 312 (9%) | <0.001 | 146 (8%) | 156 (8%) | 0.581 |
| Potassium supplement | 1185 (28%) | 1013 (29%) | 0.476 | 545 (29%) | 529 (28%) | 0.595 |
| Symptoms and signs of heart failure | ||||||
| Dyspnea at rest | 922 (22%) | 782 (22%) | 0.676 | 395 (21%) | 381 (20%) | 0.602 |
| Dyspnea on exertion | 3155 (74%) | 2705 (77%) | 0.027 | 1422 (76%) | 1410 (75%) | 0.673 |
| Jugular venous distension | 519 (12%) | 501 (14%) | 0.012 | 251 (13%) | 227 (12%) | 0.249 |
| Third heart sound | 864 (20%) | 981 (28%) | <0.001 | 455 (24%) | 435 (23%) | 0.471 |
| Pulmonary rales | 657 (16%) | 644 (18%) | <0.001 | 314 (17%) | 289 (16%) | 0.272 |
| Lower extremity edema | 961 (23%) | 672 (19%) | <0.001 | 393 (21%) | 375 (20%) | 0.489 |
| Number of symptom/signs | 5.4 ±2.0 | 5.5 ±2.0 | 0.021 | 5.5 ±2.0 | 5.5 ±2.0 | 0.251 |
| New York Heart Association | ||||||
| Class I | 650 (15%) | 453 (13%) | 254 (14%) | 267 (14%) | ||
| Class II | 2404 (57%) | 1838 (52%) | 1026 (55%) | 1038 (56%) | ||
| Class III | 1127 (27%) | 1159 (33%) | 557 (30%) | 538 (29%) | ||
| Class IV | 66 (2%) | 88 (3%) | 32 (2%) | 26 (1%) | ||
| Heart rate (beats /minute) | 78 ±12 | 79 ±13 | 0.393 | 78 ±12 | 78 ±13 | 0.819 |
| Diastolic blood pressure (mm Hg) | 80 ±11 | 69 ±9 | <0.001 | 74 ±9 | 74 ±8 | 0.578 |
| Chest radiograph findings | ||||||
| Pulmonary congestion | 550 (13%) | 559 (16%) | <0.001 | 276 (15%) | 256 (14%) | 0.365 |
| Cardiothoracic ratio >0.5 | 2541 (60%) | 2148 (61%) | 0.429 | 1115 (60%) | 1086 (58%) | 0.349 |
| Serum creatinine (mg/dL) | 1.29 ±0.38 | 1.27±0.36 | 0.004 | 1.29±0.38 | 1.27 ±0.36 | 0.208 |
| Serum potassium (mEq/L) | 4.33 ±0.44 | 4.34 ±0.44 | 0.078 | 4.35 ±0.44 | 4.35 ±0.45 | 0.923 |
| LV ejection fraction (%) | 35 ±13 | 29 ±11 | <0.001 | 31 ±11 | 31 ±12 | 0.834 |
| LV ejection fraction >45% | 725 (17%) | 263 (7%) | <0.001 | 177 (10%) | 211 (11%) | 0.068 |
ACE =angiotensin-converting enzyme; HF=; LV=left ventricular
The primary outcome for the current analysis was all-cause mortality during 5 years of follow-up. The secondary outcomes included various cause-specific mortalities and hospitalizations. Kaplan-Meier and Cox regression analyses were used to determine associations between SBP ≤120 mm Hg and outcomes during 5 years of follow-up. Subgroup analyses were conducted to determine the homogeneity of association between SBP≤120 mm Hg and all-cause mortality. Formal sensitivity analyses were conducted to determine the impact of an unmeasured confounder. All statistical tests were two-tailed with a p-value <0.05 considered significant. All data analyses were performed using SPSS for Windows version 18 (SPSS Inc., Chicago, IL).
Results
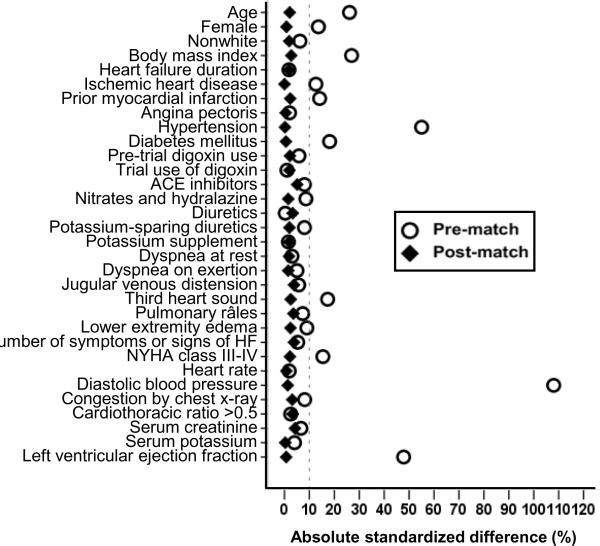
Matched patients had a mean age of 64 (±10) years with 23% women and 14% non-whites. Matched patients with SBP ≤120 mm Hg had a median SBP of 114 mm Hg (interquartile range, 10 mm Hg) and those with SBP >120 mm Hg had a median SBP of 134 mm Hg (interquartile range, 10 mm Hg). Over 90% of the matched patients with SBP ≤120 mm Hg had their SBP between 110 and 120 mm Hg. Before matching, patients with SBP ≤120 mm Hg were younger (by a mean age of 3 years) and had a lower prevalence of hypertension, diabetes, chronic kidney disease (Table 1). They were also more likely to be male, have higher prevalence of ischemic cardiomyopathy, higher symptom burden and a lower mean LVEF. These and other pre-match imbalances in baseline covariates were balanced after matching (Table 1). Post-match standardized differences for all measured covariates were <10% (most were <5%), suggesting substantial covariate balance across the groups (Figure 1).
Figure 1.
Love plot for pre-and post-match absolute standardized differences for baseline covariates for patients with systolic blood pressure ≤120 and >120 mm Hg (ACE=angiotensin converting enzyme; HF=heart failure; NYHA=New York Heart Association)
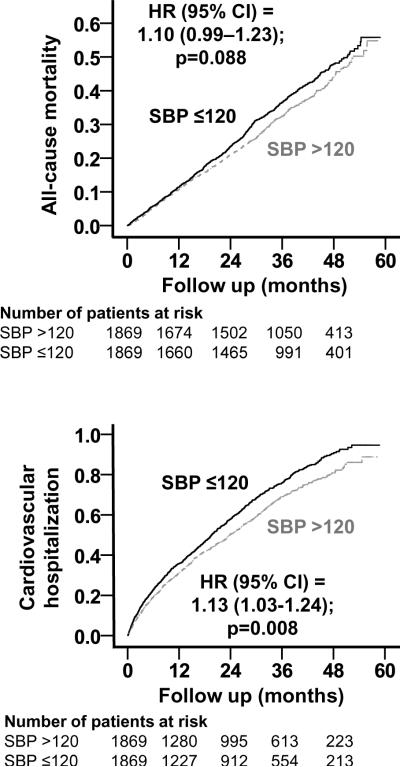
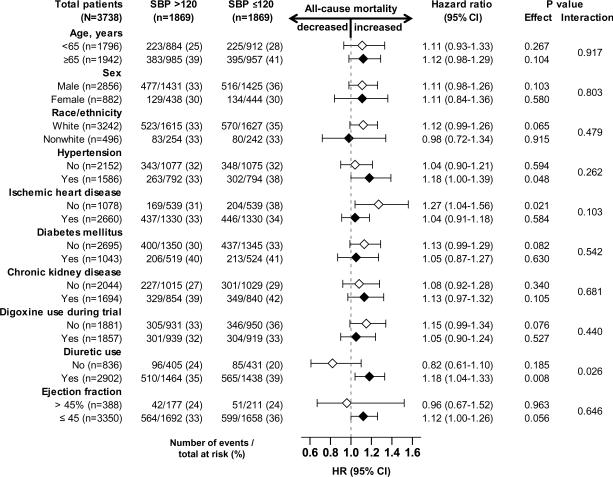
All-cause mortality occurred in 35% and 32% of matched patients with SBP ≤120 and >120 mm Hg respectively during 5 years of follow-up (hazard ratio {HR} when SBP ≤120 was compared with SBP >120 mm Hg, 1.10; 95% confidence interval {CI}, 0.99–1.23; p=0.088; Table 2 and Figure 2). This association was homogeneous across various subgroups of patients except that it was only observed among those receiving diuretics (Figure 3). In the absence of a hidden bias, a sign-score test for matched data with censoring provides evidence (p=0.0147) that patients with SBP ≤120 mm Hg clearly had higher mortality than those with SBP >120 mm Hg. A hidden covariate that is a near-perfect predictor of mortality could explain away this association if it also increased the odds of having SBP≤120 by only 3.13%. Associations of SBP ≤120 mm Hg with other cause-specific mortalities before and after matching are displayed in Table 2.
Table 2.
Baseline systolic blood pressure (SBP) and mortality
| Events, n (%) | Systolic blood pressure (mm Hg) |
Absolute risk increase* | Hazard ratio† (95% CI) | p value | |
|---|---|---|---|---|---|
| >120 | ≤120 | ||||
| Pre-match | (n=4247) | (n=3538) | |||
| All-cause | 1289 (30%) | 1316 (37%) | 7% | 1.31 (1.21–1.41) | <0.001 |
| Cardiovascular | 987 (23%) | 1064 (30%) | 7% | 1.38 (1.27–1.51) | <0.001 |
| Heart failure | 396 (9%) | 511 (14%) | 5% | 1.66 (1.45–1.89) | <0.001 |
| Other cardiovascular | 591 (14%) | 553 (16%) | 2% | 1.20 (1.06–1.34) | 0.003 |
| Non-cardiovascular | 232 (6%) | 178 (5%) | −1% | 0.99 (0.81–1.20) | 0.894 |
| Unknown | 70 (2%) | 74 (2%) | 0% | 1.36 (0.98–1.88) | 0.068 |
| Post-match | (n=1869) | (n=1869) | |||
| All-cause | 606 (32%) | 650 (35%) | 3% | 1.10 (0.99–1.23) | 0.088 |
| Cardiovascular | 459 (25%) | 514 (28%) | 3% | 1.15 (1.01–1.30) | 0.031 |
| Heart failure | 194 (10%) | 246 (13%) | 3% | 1.30 (1.08–1.57) | 0.006 |
| Other cardiovascular | 265 (14%) | 268 (14%) | 0% | 1.04 (0.87–1.23) | 0.682 |
| Non-cardiovascular | 110 (6%) | 99 (5%) | −1% | 0.93 (0.71–1.22) | 0.581 |
| Unknown | 37 (2%) | 37 (2%) | 0% | 1.03 (0.65–1.62) | 0.907 |
Absolute risk increase was calculated by subtracting events in the ≤120 SBP group from those in the >120 SBP group.
Hazard ratios and confidence intervals (CI) when SBP≤120 was compared with SBP>120 mm Hg
Figure 2.
Kaplan-Meier plots for all-cause mortality (top panel) and cardiovascular hospitalization (bottom panel) by systolic blood pressure (SBP) (CI=confidence interval; HR=hazard ratio)
Figure 3.
Hazard ratio and 95% confidence interval (CI) for all-cause mortality associated with systolic blood pressure (SBP) ≤ 120 mm Hg in subgroups of matched patients with heart failure
Cardiovascular hospitalization occurred in 53% and 49% of matched patients with SBP ≤120 and >120 mm Hg respectively (HR for SBP ≤120, 1.13; 95% CI, 1.03–1.24; P=0.008; Table 3 and Figure 2). Associations of SBP ≤120 mm Hg with all-cause and other cause-specific hospitalizations before and after matching are displayed in Table 3.
Table 3.
Baseline systolic blood pressure (SBP) and hospitalization
| Events, n (%) | Systolic blood pressure (mm Hg) |
Absolute risk increase* | Hazard ratio† (95% CI) | p value | |
|---|---|---|---|---|---|
| >120 | ≤120 | ||||
| Pre-match | (n=4247) | (n=3538) | |||
| All-cause | 2779 (65%) | 2347 (66%) | 1% | 1.08 (1.03–1.15) | 0.004 |
| Cardiovascular | 2129 (50%) | 1880 (53%) | 3% | 1.15 (1.08–1.22) | <0.001 |
| Heart failure | 1136 (27%) | 1150 (33%) | 6% | 1.33 (1.22–1.44) | <0.001 |
| Myocardial infarction | 266 (6%) | 184 (5%) | 1% | 0.88 (0.73–1.06) | 0.177 |
| Unstable angina pectoris | 504 (12%) | 437 (12%) | 0% | 1.11 (0.98–1.26) | 0.112 |
| Post-match | (n=1869) | (n=1869) | |||
| All-cause | 1203 (64%) | 1255 (67%) | 3% | 1.10 (1.02–1.19) | 0.017 |
| Cardiovascular | 918 (49%) | 985 (53%) | 4% | 1.13 (1.03–1.24) | 0.008 |
| Heart failure | 496 (27%) | 576 (31%) | 4% | 1.21 (1.07–1.36) | 0.002 |
| Myocardial infarction | 117 (6%) | 106 (6%) | 0% | 0.92 (0.71–1.20) | 0.549 |
| Unstable angina pectors | 216 (12%) | 255 (14%) | 2% | 1.23 (1.02–1.47) | 0.028 |
Absolute risk increase was calculated by subtracting events in the ≤120 SBP group from those in the >120 SBP group.
Hazard ratios and confidence intervals (CI) when SBP ≤120 was compared with SBP>120 mm Hg
Discussion
The result of the current analysis demonstrate that in patients with mild to moderate HF, baseline SBP≤120 was associated with increased long-term mortality and hospitalization, which remained significant for all outcome except all-cause mortality in a well-balanced propensity-matched cohort. These findings suggest that baseline SBP ≤120 mm Hg is a powerful predictor of poor outcomes even among ambulatory patients with mild to moderate chronic HF and that these associations were at least part intrinsic in nature. These findings are important as over 90% of the patients in the group with SBP ≤120 mm Hg had their SBP between 110 and 120 mm Hg, a range often considered optimal. And, yet these patients consistently had poor outcomes from all-cause, cardiovascular and HF mortalities and hospitalizations.
Significant unadjusted associations are often in part confounded by covariates that maybe imbalanced at baseline. However, potential confounding covariates were equally distributed between patients with SBP ≤120 and >120 mm Hg. While patients with SBP ≤120 mm Hg were younger with a lower prevalence of hypertension, diabetes and chronic kidney disease, they were also more likely to be male with a higher prevalence of ischemic cardiomyopathy, a higher symptom burden and a lower mean LVEF. It appears that imbalances in these latter characteristics may have at least in part confounded the unadjusted associations between SBP ≤120 mm Hg and poor outcomes. This is also supported by the substantial attenuation of the magnitude of these associations after propensity matching. However, the persistence of significant associations of SBP ≤120 mm Hg with poor outcomes among matched cohort suggest that these associations were also independent of those measured baseline covariates.
The intrinsic association between SBP ≤120 mm Hg and poor outcomes is unlikely to be solely or primarily due to hypoperfusion as the vast majority of these patients had their SBP between 110 and 120 mm Hg. It is therefore possible that SBP ≤120 mm Hg was rather a marker than cause of poor outcomes. HF patients SBP ≤120 mm Hg were more likely to have ischemic cardiomyopathy at baseline. Although the prevalence of ischemic heart disease between the two SBP groups were balanced after matching, it is possible that HF patients with SBP ≤120 mm Hg had more severe ischemia, the continuation of which during follow-up may have resulted in further lowering of SBP and hypoperfusion. This notion is also supported by our findings of an association between SBP ≤120 mm Hg and increased risk of hospitalizations due to unstable angina. However, the interaction between ischemic heart disease, SBP ≤120 mm Hg and all-cause mortality in mild to moderate HF may be more complex. While it is possible that myocardial ischemia may contribute to low SBP, and yet findings from our subgroup analysis suggest that in these patients, baseline SBP ≤120 mm Hg had no association with all-cause mortality. This is important as over 70% of matched patients in our study had ischemic cardiomyopathy at baseline and findings from our study suggest that baseline SBP had no association with outcomes in these patients.
As the cardiac performance deteriorates with advancing HF, a drop in SBP may be part of the natural history of HF. However, therapy with neurohormonal blockade and diuretics may also contribute to that process. Interestingly, even before matching, patients in both SBP groups in our study had similar duration of HF and over 90% were receiving angiotensin-converting enzyme inhibitors. Although little is known about the benefits of treating hypertension in patients with HF, the American College of Cardiology/American Heart Association guideline for management of chronic HF recommends that it is prudent to manage hypertension in patients with HF as if they did not have HF.22 HF patients with a history of hypertension may be less likely to have low SBP and yet findings from our subgroup analysis suggest that in those with hypertension, baseline SBP ≤120 mm Hg was associated with increased mortality. This is in contrast to patients with hypertension but without HF in whom a lower SBP has been shown to be associated with improved outcomes.23 However, intensive lowering of SBP has not been shown to be beneficial in patients with hypertension and other morbidities such as diabetes and coronary artery disease.24–26
Several studies have examined the association between SBP and outcomes in chronic HF.5, 7–9, 27, 28 One of these studies has examined the association of SBP and mortality among DIG participants with low LVEF and NYHA class I–III.28 Our study is distinguished by the inclusion of DIG participants with normal LVEF and other outcomes, the use of SBP 120 as a cutoff, a often-used goal for SBP in HF, and insightful subgroup analyses. Further, our study is distinguished by the use of propensity-matched design which allowed us to assemble a balanced cohort. Although traditional regression-based multivariable models are useful for risk adjustment, they may be limited by strong untenable model assumptions, and concerns for residual bias and procedural transparency.12, 29
Several limitations of the current study must be acknowledged. Although propensity score technique accounts for imbalances in all of the measured covariates, it may or may not balance unmeasured covariates. Despite a relatively modest sensitivity of our findings to an unmeasured covariate, for such a covariate to become a confounder, in addition to be a near-perfect predictor of HF hospitalization plus all-cause mortality it must also be associated with SBP and should not be strongly correlated with any of the 32 baseline characteristics. Considering the strong correlation between various clinical covariates, it seems unlikely. Finally, the findings of this study need to be replicated in more contemporary HF patients. In conclusion, in patients with mild to moderate chronic systolic and diastolic HF, baseline SBP ≤120 mm Hg is associated with poor clinical outcomes.
Acknowledgement
“The Digitalis Investigation Group (DIG) study was conducted and supported by the NHLBI in collaboration with the DIG Investigators. This manuscript was prepared using a limited access dataset obtained by the NHLBI and does not necessarily reflect the opinions or views of the DIG Study or the NHLBI.”
Funding/Support: Dr. Ahmed is supported by the National Institutes of Health through grants (R01-HL085561 and R01-HL097047) from the National Heart, Lung, and Blood Institute and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None
References
- 1.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. Jama. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 2.Britton KA, Gaziano JM, Djousse L. Normal systolic blood pressure and risk of heart failure in US male physicians. Eur J Heart Fail. 2009;11:1129–1134. doi: 10.1093/eurjhf/hfp141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O'Connor CM, She L, Stough WG, Yancy CW, Young JB, Fonarow GC. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. Jama. 2006;296:2217–2226. doi: 10.1001/jama.296.18.2217. [DOI] [PubMed] [Google Scholar]
- 4.Fonarow GC, Adams KF, Jr., Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. Jama. 2005;293:572–580. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 5.Grigorian-Shamagian L, Gonzalez-JuAnatey JR, Vazquez R, Cinca J, Bayes-Genis A, Pascual D, Fernandez-Palomeque C, Bardaji A, Almendral J, Nieto V, Macaya C, Jimenez RP, de Luna AB. Association of blood pressure and its evolving changes with the survival of patients with heart failure. J Card Fail. 2008;14:561–568. doi: 10.1016/j.cardfail.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Rihal CS, Nishimura RA, Hatle LK, Bailey KR, Tajik AJ. Systolic and diastolic dysfunction in patients with clinical diagnosis of dilated cardiomyopathy. Relation to symptoms and prognosis. Circulation. 1994;90:2772–2779. doi: 10.1161/01.cir.90.6.2772. [DOI] [PubMed] [Google Scholar]
- 7.Raphael CE, Whinnett ZI, Davies JE, Fontana M, Ferenczi EA, Manisty CH, Mayet J, Francis DP. Quantifying the paradoxical effect of higher systolic blood pressure on mortality in chronic heart failure. Heart. 2009;95:56–62. doi: 10.1136/hrt.2007.134973. [DOI] [PubMed] [Google Scholar]
- 8.Guder G, Frantz S, Bauersachs J, Allolio B, Wanner C, Koller MT, Ertl G, Angermann CE, Stork S. Reverse epidemiology in systolic and nonsystolic heart failure: cumulative prognostic benefit of classical cardiovascular risk factors. Circ Heart Fail. 2009;2:563–571. doi: 10.1161/CIRCHEARTFAILURE.108.825059. [DOI] [PubMed] [Google Scholar]
- 9.Desai RV, Banach M, Ahmed MI, Mujib M, Aban I, Love TE, White M, Fonarow G, Deedwania P, Aronow WS, Ahmed A. Impact of baseline systolic blood pressure on long-term outcomes in patients with advanced chronic systolic heart failure (insights from the BEST trial) Am J Cardiol. 2010;106:221–227. doi: 10.1016/j.amjcard.2010.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Digitalis Investigation Group The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 12.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 13.Desai RV, Ahmed MI, Fonarow GC, Filippatos GS, White M, Aban IB, Aronow WS, Ahmed A. Effect of serum insulin on the association between hyperuricemia and incident heart failure. Am J Cardiol. 2010;106:1134–1138. doi: 10.1016/j.amjcard.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer P, Ekundayo OJ, Adamopoulos C, Mujib M, Aban I, White M, Aronow WS, Ahmed A. A propensity-matched study of elevated jugular venous pressure and outcomes in chronic heart failure. Am J Cardiol. 2009;103:839–844. doi: 10.1016/j.amjcard.2008.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekundayo OJ, Muchimba M, Aban IB, Ritchie C, Campbell RC, Ahmed A. Multimorbidity due to diabetes mellitus and chronic kidney disease and outcomes in chronic heart failure. Am J Cardiol. 2009;103:88–92. doi: 10.1016/j.amjcard.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aronow WS, Ahmed MI, Ekundayo OJ, Allman RM, Ahmed A. A propensity-matched study of the association of peripheral arterial disease with cardiovascular outcomes in community-dwelling older adults. Am J Cardiol. 2009;103:130–135. doi: 10.1016/j.amjcard.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed A, Pitt B. A history of systemic hypertension and incident heart failure hospitalization in patients with acute myocardial infarction and left ventricular systolic dysfunction. Am J Cardiol. 2009;103:1374–1380. doi: 10.1016/j.amjcard.2009.01.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giamouzis G, Sui X, Love TE, Butler J, Young JB, Ahmed A. A propensity-matched study of the association of cardiothoracic ratio with morbidity and mortality in chronic heart failure. Am J Cardiol. 2008;101:343–347. doi: 10.1016/j.amjcard.2007.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filippatos GS, Adamopoulos C, Sui X, Love TE, Pullicino PM, Lubsen J, Bakris G, Anker SD, Howard G, Kremastinos DT, Ahmed A. A propensity-matched study of hypertension and increased stroke-related hospitalization in chronic heart failure. Am J Cardiol. 2008;101:1772–1776. doi: 10.1016/j.amjcard.2008.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, Zile MR, Love TE, Aban IB, Shlipak MG. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99:393–398. doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed A. A propensity matched study of New York Heart Association class and natural history end points in heart failure. Am J Cardiol. 2007;99:549–553. doi: 10.1016/j.amjcard.2006.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr., Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B, American College of C. American Heart Association Task Force on Practice G. American College of Chest P. International Society for H, Lung T, Heart Rhythm S ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 23.Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, Levy D. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 24.Cushman WC, Evans GW, Byington RP, Goff DC, Jr., Grimm RH, Jr., Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F, Group ftAS Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper-DeHoff RM, Gong Y, Handberg EM, Bavry AA, Denardo SJ, Bakris GL, Pepine CJ. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010;304:61–68. doi: 10.1001/jama.2010.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, Hua T, Laragh J, McInnes GT, Mitchell L, Plat F, Schork A, Smith B, Zanchetti A, group Vt Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 27.Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. 2004;43:1439–1444. doi: 10.1016/j.jacc.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 28.Lee TT, Chen J, Cohen DJ, Tsao L. The association between blood pressure and mortality in patients with heart failure. Am Heart J. 2006;151:76–83. doi: 10.1016/j.ahj.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Fitzmaurice G. Confounding: regression adjustment. Nutrition. 2006;22:581–583. doi: 10.1016/j.nut.2006.02.004. [DOI] [PubMed] [Google Scholar]