Abstract
Background
Outcomes among inotrope-treated heart failure (HF) patients receiving cardiac resynchronization (CRT) have not been well characterized, particularly in those requiring intravenous inotropes at the time of implant.
Methods
We analyzed 759 consecutive CRT-defibrillator recipients who were categorized as never on inotropes (NI; n=585), weaned from inotropes before implant (PI; n=124), or on inotropes at implant (II; n=50). Survival free from heart transplant or ventricular assist device (VAD) and overall survival were compared using the Social Security Death Index. A patient cohort who underwent unsuccessful CRT implantation and received a standard defibrillator (SD; n=94) comprised a comparison group. Propensity score analysis was used to control for inter-group baseline differences.
Results
Compared to the other cohorts, II patients had more comorbidities. Both survival endpoints differed significantly (p<0.001) among the 4 cohorts; II patients demonstrated shorter survival than NI patients, with the PI and SD groups having intermediate survival. After adjusting for propensity scores, overall differences and patterns in survival endpoints persisted (p<0.01), but the only statistically significant pairwise difference was overall survival between the NI and II groups at 12 months (HR 2.95, 95% CI 1.05-8.35). CRT recipients ever on inotropes (PI and II) and SD patients ever requiring inotropes (n=17) experienced similar survival endpoints. Among II patients, predictors of hospital discharge free from inotropes after CRT included male gender, older age, and ability to tolerate β-blockade.
Conclusions
Inotrope-dependent HF patients show significantly worse survival despite CRT than inotrope-naïve patients, in part because of more comorbid conditions at baseline. CRT may not provide a survival advantage over a standard defibrillator among patients who have received inotropes prior to CRT. Weaning inotropes and initiating neurohormonal antagonists prior to CRT should be an important goal among inotrope-dependent HF patients.
Keywords: Cardiac resynchronization, inotropes, heart failure, defibrillators
Introduction
The prognosis of New York Heart Association (NYHA) class 4 heart failure (HF) patients requiring continuous inotrope infusion is poor; one-half of such patients die within 6 months. (1, 2) Accordingly, these patients have been excluded from multicenter clinical trials evaluating cardiac resynchronization therapy (CRT), (3-7) and no controlled studies have compared survival between CRT recipients who are inotrope-dependent and those never requiring inotropes. Small, retrospective analyses have provided limited insight into the morbidity and mortality benefits of CRT in inotrope-treated HF patients. (8, 9)
We examined survival outcomes in patients requiring inotrope infusion at the time of CRT implantation, comparing these outcomes to CRT recipients successfully weaned from inotropes prior to CRT implant and inotrope-naïve patients. A cohort of patients with unsuccessful CRT implant procedures but in whom a standard defibrillator was implanted provided perspective in these comparisons.
Methods
Patient Selection
We conducted a systemic review of the medical records of all patients (n=759) receiving CRT–defibrillators (CRT-D) for currently accepted indications (10, 11) from 2002-2008 at Presbyterian University Hospital. As such, all study patients had LVEF ≤35%, QRS duration ≥120 ms, and NYHA class 3-4 HF despite optimally tolerated medical therapy. Patients who had ever been treated with intravenous milrinone or dobutamine were identified and divided into those successfully weaned from inotropes prior to implant (PI; n=124) and those on inotropes at the time of implant (II; n=50). CRT recipients never on inotropes (NI; n = 585) and a separate group of patients who met eligibility criteria for CRT-D but failed transvenous left ventricular lead placement and did not receive a surgically implanted epicardial lead (SD; n=94) comprised comparative cohorts. Patients in the SD group received a standard defibrillator, and the decision to forgo surgical epicardial lead placement was primarily made by the patients involved.
Device Implantation and Follow Up
CRT-D implantation was performed under moderate conscious sedation by electrophysiologists using transvenous techniques. Patients received a standard pacemaker lead in the right atrium, a high-voltage lead in the right ventricular apex, and a left ventricular lead preferentially placed in a lateral or posterolateral branch of the coronary sinus. Ventricular fibrillation was induced routinely by the shock-on-T method or rapid ventricular burst pacing to ensure at least a 10-J safety margin of defibrillation below the maximum output of the implanted device. Patients were regularly followed in device clinic, with programming changes made as deemed clinically appropriate. Pharmacologic means, device reprogramming, or ablation of the atrioventricular junction were utilized to ensure that biventricular pacing occurred ≥90% of the time among CRT recipients. Right ventricular pacing was minimized in SD patients, either by programming long atrioventricular delays or utilizing proprietary pacing algorithms.
Heart Failure Therapy
Optimal pharmacologic therapy included β-adrenergic antagonists, angiotensin-converting enzyme-inhibitors or angiotensin receptor-blockers, and aldosterone antagonists. Efforts were made to maximize doses of neurohormonal antagonists without inducing symptomatic hypotension or renal dysfunction. Patients were free to receive follow-up care at our institution and/or with local practitioners for HF management. The decision to undergo cardiac transplant or mechanical circulatory support with a ventricular assist device (VAD) was made by cardiologists specializing in HF/transplant and transplant surgeons.
Endpoints
The primary endpoint was death or the need for either transplant or VAD, reflecting a failure of CRT to reverse the clinical HF syndrome. The secondary endpoint was death from any cause. Follow-up was limited to 5 years in all patients. Events after 5 years from CRT implant were censored. Mortal status was determined using the United States Social Security Death Index (http://ssdi.rootsweb.ancestry.com/). Patients requiring transplant or VAD implant were identified in the electronic medical record.
Statistical Analysis
Baseline characteristics across inotrope groups were compared using the chi-square test for dichotomous variables and ANOVA for continuous variables, which are reported as means ± standard deviation. When necessary, Kruskal-Wallis and Fisher's exact tests were used when there were violations of normality. Because this is observational data and subjects were not randomized to one of the four groups, a multiple propensity score analysis was used to control for underlying bias. (12, 13) The multiple propensity score is an extension of basic twotreatment propensity score described by Rosenbaum & Rubin. (14) First, a multinomial logistic regression was fit with treatment group as the outcome and all of the baseline variables in Table 1 as predictor variables except for left ventricular end-diastolic diameter, left ventricular end-systolic diameter, and cardiomyopathy duration. These variables had nearly half of all subjects missing and were not related to outcome. The model was then used to estimate predicted probabilities of being in each treatment group. Since the predicted probabilities sum to one, only three of them were needed. We investigated the distribution of each propensity score across inotrope groups to see if there was considerable overlap. The distribution of baseline variables across the four groups was recalculated adjusting for subjects' multiple propensity scores. Finally, all primary and secondary analyses were conducted with multiple propensity scores as covariates.
Table 1.
Baseline Characteristics of the Study Population.
| NI (n=585) | PI (n=124) | II (n=50) | SD (n=94) | p-value | adjusted p-value | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, y | 66.1 ± 12.1 (68) | 67.9 ± 10.6 (70) | 68.3 ± 9.2 (69) | 65.0±14.4 (66) | 0.34# | >0.999 |
| Male, n | 426 (72.8%) | 89 (71.8%) | 36 (72.0%) | 66 (70.2%) | 0.96 | >0.999 |
| HF duration, mos | 61± 64(41) | 69 ± 67(55) | 76 ± 73 (59) | 72 ± 81(50) | 0.45 | 0.999 |
| ECG Findings | ||||||
| QRS duration, ms | 171 ± 30 (169) | 168 ± 30 (160) | 172 ± 34 (170) | 170 ± 32 (170) | 0.67 | 0.998 |
| RBBB, n | 59 (10.3%) | 9 (7.4%) | 6 (12.0%) | 17 (18.9%) | 0.05 | 0.986 |
| Comorbidities | ||||||
| Diabetes, n | 204 (34.9%) | 53 (43.1%) | 27 (54.0%) | 35 (37.6%) | 0.03 | >0.999 |
| GFR, mL/min | 62 ± 24 (60) | 55 ± 20 (54) | 52 ± 26 (48) | 59 ± 28 (59) | 0.001# | 0.992 |
| Known atrial fibrillation, n | 274 (46.8%) | 71 (57.7%) | 29 (58.0%) | 44 (48.4%) | 0.09 | >0.999 |
| Ischemic HF, n | 317 (54.3%) | 83 (66.9%) | 32 (64.0%) | 56 (60.2%) | 0.04 | 0.995 |
| NYHA class 4, n | 12 (2.1%) | 10 (8.1%) | 20 (40.0%) | 3 (3.2%) | <0.001 | - |
| Echocardiography | ||||||
| LVEF, % | 22.3 ± 6.9 (22) | 20.6 ± 6.4 (21) | 20.3 ± 7.4 (22) | 23.1±10.3 (22) | 0.07# | 0.999 |
| LVEDD, cm | 6.27 ± 0.88 (6.2) | 6.26 ± 0.95 (6.2) | 6.23 ± 1.00 (6.2) | 6.12±0.77 (6.0) | 0.65 | 0.895 |
| LVESD, cm | 5.34 ± 0.98 (5.3) | 5.32 ± 0.98 (5.4) | 5.43 ± 1.16 (5.6) | 4.98±0.99 (4.9) | 0.09 | 0.454 |
| Pharmacologic Therapy | ||||||
| β-blocker, n | 488 (83.4%) | 88 (71.0%) | 27 (54.0%) | 72 (77.4%) | <0.001 | 0.996 |
| ACE-I/ARB, n | 500 (85.5%) | 104 (83.9%) | 37 (74.0%) | 76 (81.7%) | 0.17 | >0.999 |
| Aldosterone antagonist, n | 157 (26.8%) | 22 (17.7%) | 14 (28.0%) | 18 (19.4%) | 0.10 | >0.999 |
RBBB: right bundle-branch block. GFR: glomerular filtration rate. LVESD: left ventricular end-systolic diameter. LVEDD: left ventricular end-diastolic diameter. ACE-I: angiotensin-converting enzyme-inhibitor. ARB: angiotensin receptor blocker. Numbers in parentheses are median values except if followed by %. P-values denoted with # are based on Kruskal-Wallis or Fisher's Exact tests. P-values reflect 4-way comparison. Adjusted p-values reflect adjustment for propensity scores.
Comparisons of time-dependent outcomes across the four groups were made using Cox proportional hazard models for multivariate analyses. For multiple comparisons, Bonferroni post-hoc analyses were used to compare the II group to the remaining three groups. NYHA class 4 HF was not included in multivariate analyses because it largely segregates with the defined cohorts; II patients are much more likely to be classified as NYHA class 4, whereas NI and PI patients are more likely to be NYHA class 3. As an exploratory analysis, subjects in the PI and II groups were collapsed into the EI group and compared to the SD group. A p-value ≤ 0.05 was considered statistically significant. All statistical analyses were performed using STATA/SE 11.0 (StataCorp, College Station, TX).
Results
Baseline Characteristics
Baseline characteristics of the study groups are listed in Table 1. The four primary cohorts (NI, PI, II, and SD) differed in baseline renal function, HF etiology, and incidence of diabetes, right bundle branch-block, NYHA class 4 HF, and β-blocker use. The higher incidence of NYHA class 4 HF and lower β-blocker use in the II group was expected, given the inability to wean these patients from inotropes. After adjusting for the multiple propensity scores, all baseline differences across cohorts disappeared.
Long-Term Survival
Mean follow-up time was 42.3 ± 17.5, 37.1 ± 20.5, 30.5 ± 24.0 and 24.5 ± 18.9 months for the NI, PI, II, and SD groups, respectively (p<0.001). Differences in follow-up reflected a higher incidence of death, transplant, and need for VAD in the II cohort. Among NI patients, 226 (38.6%) met the primary endpoint of death (n=196), VAD (n=9), or transplant (n=21). The PI cohort had 59 patients (48.0%) meeting the primary endpoint (44 deaths, 3 VADs, and 12 transplants), while the II cohort had 35 patients (70.0%) who fulfilled this endpoint (26 deaths, 1 VAD, and 8 transplants). Among SD patients, 43 (45.7%) met the primary endpoint (35 deaths, 1 VAD, and 7 transplants).
Before adjusting for subjects' multiple propensity scores, survival curves were compared among the 4 cohorts. Due to the violation of the proportional hazards assumption, an interaction between time and group was included in the model. There was a significant difference in transplant- and VAD-free survival among the groups (p<0.001), holding time constant (Figure 1a). After adjusting for multiple comparisons, II subjects had worse survival at 12 months than NI (HR 3.70, 95% CI 2.05-6.68) and PI (HR 2.03, 95% CI 1.11-3.69) groups, respectively. At 48 months, survival free from transplant and VAD continued to be worse in the II cohort compared to NI (HR 2.56, 95% CI 1.55-4.26) and PI patients (HR 1.40, 95% CI 0.74-2.67). Findings for overall survival were similar (Figure 1b).
Figure 1.
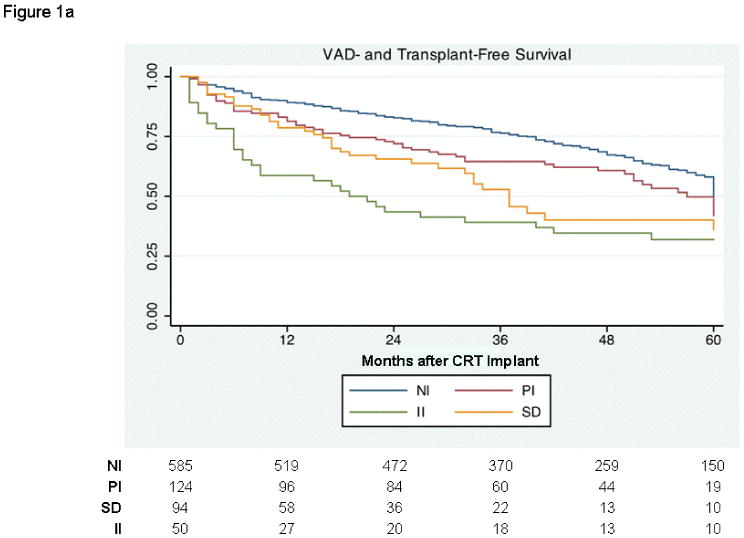
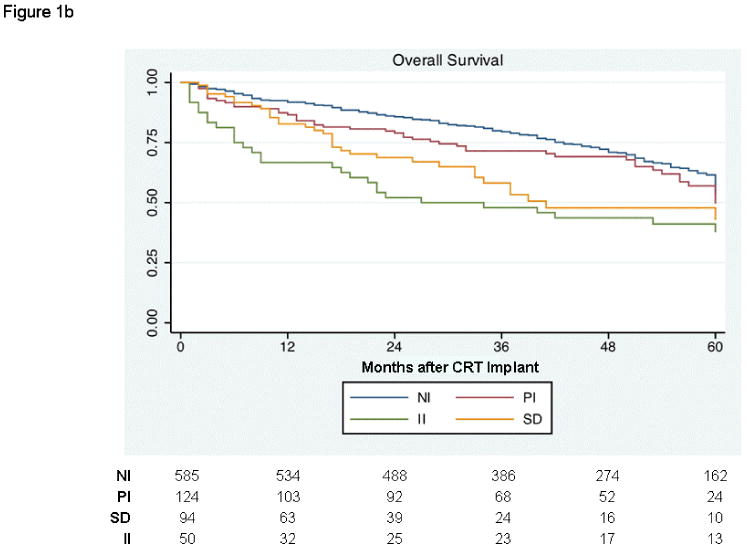
Kaplan-Meier curves depicting outcomes among patients never on inotropes (NI), those weaned from inotropes prior to CRT (PI), those on inotropes at the time of implant (II), and those with (SD). (a) Survival free from transplant or ventricular assist device. (b) Overall survival.
After adjusting for their multiple propensity scores, there was a significant difference in VAD- and transplant-free survival among the cohorts (Figure 2a; p=0.002). With adjustment for multiple comparisons, however, the II cohort did not differ statistically from any of the other groups at either 12 or 48 months. There was, again, asignificant difference in overall survival among the 4 groups (Figure 2b; p<0.001), and at 12 months, II subjects had worse survival than NI subjects (HR 2.95, 95% CI 1.05-8.35). This difference was not observed at 48 months.
Figure 2.


Propensity score adjusted Kaplan-Meier curves depicting outcomes among patients never on inotropes (NI), those weaned from inotropes prior to CRT (PI), those on inotropes at the time of implant (II), and those with unsuccessful left ventricular lead placement who received a standard defibrillator (SD). (a) Survival free from transplant or ventricular assist device. (b) Overall survival.
Receiver-operator characteristic analysis among II patients demonstrated that >8 days of pre-CRT inotrope days was the best predictor of 6-month mortality, transplant, or VAD (sensitivity 50%, specificity 69%), with an area-under-the-curve of 0.63 (95% CI 0.45-0.82). The number of pre-CRT inotrope days did not correlate with the ability to wean inotropes prior to hospital discharge.
Among the 94 SD patients, the 17 subjects who were treated with inotropes prior to or during CRT implant comprised the SDI subgroup. This cohort was similar at baseline to CRT recipients who had ever been on inotropes (EI), which comprised both PI and II patients (Table 2). Neither the primary nor the secondary endpoint differed between the EI and SDI groups (Figure 3).
Table 2.
Baseline characteristics of the study patients who had ever received inotropes prior to CRT implant.
| EI (n=174) | SDI (n=17) | p-value | |
|---|---|---|---|
| Demographics | |||
| Age, y | 68 ± 10 (70) | 60 ± 18 (60) | 0.11# |
| Male, y | 125 (71.8%) | 10 (58.8%) | 0.27# |
| HF duration, mo | 71 ± 68 (57) | 127 ± 127 (85) | 0.33# |
| ECG Findings | |||
| QRS duration, ms | 169 ± 31 (160) | 171 ± 29 (165) | 0.77 |
| RBBB, n | 15 (8.7 %) | 4 (23.5%) | 0.07# |
| Comorbidities | |||
| Diabetes, n | 80 (46.2 %) | 5 (29.4%) | 0.18 |
| GFR, mL/min | 54 ± 22 (53) | 62 ± 24 (66) | 0.11 |
| Known atrial fibrillation, n | 100 (57.8 %) | 8 (47.1%) | 0.39 |
| Ischemic HF, n | 115 (66.1 %) | 11 (64.7%) | 0.91 |
| NYHA class 4, n | 30 (17.2 %) | 3 (17.6%) | >0.99# |
| Echocardiography | |||
| LVEF, % | 20.5 ± 6.7 (22) | 17.6 ± 5.3 (17) | 0.09 |
| LVEDD, cm | 6.25 ± 0.96 (6.2) | 6.28 ± 0.72 (6.2) | 0.92 |
| LVESD, cm | 5.36 ± 1.04 (5.4) | 5.16 ± 0.81 (5.1) | 0.55 |
| Pharmacologic Therapy | |||
| β-blocker, n | 115 (66.1 %) | 11 (64.7%) | 0.91 |
| ACE-I/ARB, n | 141 (81.0 %) | 15 (88.2%) | 0.74# |
| Aldosterone antagonist, n | 36 (20.7 %) | 3 (17.6%) | >0.99# |
Abbreviations are as per Table 1. Numbers in parentheses are median values except if followed by %. P-values denoted with # are based on Kruskal-Wallis or Fisher's Exact tests.
Figure 3.


Kaplan-Meier curves comparing outcomes between CRT recipients who had ever received inotropes and patients with unsuccessful left ventricular lead placement who received a standard defibrillator and had ever received inotropes. (a) Survival free from transplant or ventricular assist device. (b) Overall survival.
Periprocedural Morbidity and Mortality
Death, VAD implant, or transplant at 30 days post-CRT was considered separately as a surrogate for potential sub-clinical implantation-associated morbidity. The PI group had 5 patients (4.1%) who met this endpoint, compared to 4 patients (8.0%) in the II group and 2 patients (0.3%) in the NI group (p<0.001). The number of ventricular fibrillation inductions did not affect early survival.
There were 2 adverse events among II patients that were attributable to their inotrope-dependent status during CRT implant. One patient required mechanical ventilation because of worsening hypoxemia from decompensated HF compounded by sedation and orthopnea, and the other developed contrast-induced nephropathy in the setting of low cardiac output and a modest intravenous contrast load.
Factors Associated With Successful Weaning From Inotropes
Predictors of successful inotrope weaning among II patients after CRT was assessed by investigating the significance of each of the baseline variables as a predictor of hospital discharge off inotropes at the 0.10 level. Age, gender, ischemic HF, NYHA class 4 HF, and β-blocker use were independently associated with successful weaning. These five variables were then entered into a single model, in which older age, male gender, and β-blocker use independently correlated with inotrope weaning.
Discussion
We have described the outcomes of a large, modern cohort of inotrope-treated CRT recipients at an academic hospital and placed these findings into perspective by comparison to CRT recipients never treated with inotropes and to a group of patients with unsuccessful CRT implant who received a standard defibrillator. Propensity score analysis was utilized to control for baseline differences among the groups; the II cohort, in particular, was associated with more baseline comorbid conditions. Both survival free from VAD and transplant and overall survival differed significantly among the NI, PI, II, and SD cohorts, in that NI patients had the best outcomes, II patients had the worse outcomes, and the PI and SD groups had intermediate survival. Pairwise adjusted comparisons demonstrated that the only statistically significant difference was overall survival between NI and II patients at 12 months, however. In addition, not only did CRT not obviate the excess mortality risk associated with inotropes when the PI and II groups were compared together to SD patients ever treated with inotropes, but the ability to tolerate β-blockers was a predictor of successful inotrope-free hospital discharge among II patients.
There are few data addressing the role of CRT in HF patients requiring inotrope support despite the significant morbidity and mortality experienced by these patients. A retrospective review of 38 inotrope-dependent HF patients showed mortality rates of 26% and 29% at 6 months and 1 year, respectively, after CRT. (9) In the present study, we observed similar 6-month mortality but somewhat higher 1-year mortality (Figure 1b). Konstantino et al. analyzed the role of urgent CRT implantation in 10 patients with ischemic cardiomyopathy and intraventricular conduction delay presenting with decompensated HF requiring inotrope therapy. (15) While CRT was associated with symptomatic improvement in 8 patients, mortality was 50% over a median follow-up of 9.5 months. In comparison, the REMATCH trial, which studied a cohort of NYHA class 4 HF patients who were ineligible for transplant, demonstrated 1-year mortality of 48% in those receiving mechanical circulatory support and 75% in those on medical-therapy alone. (2) Mortality was lower in a retrospective, observational study of 10 inotrope-dependent HF patients by Cowburn et al., in which 3 patients died and 1 was transplanted post-CRT during a follow up of 1 year after CRT. (16) Taken altogether, these data show poor survival outcomes regardless of treatment modality in inotrope-dependent HF patients; therefore, current guidelines do not advocate using CRT in this population despite a paucity of randomized clinic trial data. (10, 11) The MIRACLE study demonstrated that in addition to improving NYHA functional class, 6-minute walk time, and LVEF, CRT decreased the need for intravenous vasodilator or inotropic agents. (3) Other major CRT trials (5-7), however, have excluded patients recently treated with inotropes. In fact, the unscheduled administration of inotropes or other intravenous vasoactive agents for >4 hours in the emergency department or outpatient setting was a primary endpoint (i.e. CRT failure) in COMPANION. (6)
The shorter survival of II patients observed in the present study may reflect a more advanced degree of pump failure compared to NI patients, suggesting that there is a threshold of decompensated HF beyond which CRT is ineffective. The use of neurohormonal antagonists, such as β-adrenergic antagonists or angiotensin-converting enzyme-inhibitors, in these patients is often limited by hypotension and/or renal dysfunction, yet blockade of the renin-angiotensinaldosterone axis and reduction in sympathetic tone are known to benefit HF patients. (17-19) By improving hemodynamics, CRT may also diminish neurohormonal activation. For example, studies have demonstrated that CRT decreases circulating levels of brain natriuretic peptide and reverses derangements in adrenergic cardiac enervation. (21, 22) Patients who cannot be weaned from inotropes have already demonstrated that they require augmented adrenergic tone to maintain cardiac output. Therefore, CRT may not attenuate neurohormonal activation, and as a consequence, these patients may not derive benefit from CRT. The intermediate survival within the PI group may reflect the fact that it represents a mixed cohort of NYHA class 3-4 HF patients who have been intermittently treated with inotropes. Some patients may require more neurohormonal activation to maintain hemodynamic stability than others. Long term prospective follow-up and comparison with a larger group of SDI patients would provide further insight into whether CRT benefits this cohort.
It is difficult to ascertain whether CRT provides additional survival benefit above that conferred by a defibrillator among patients ever treated with inotropes, particularly the II subgroup, in the absence of randomized data. We addressed this question in the comparison of EI and SDI patients. Because all SD patients received defibrillators, sudden cardiac death rates were presumably equal between this cohort and the CRT-D groups. Non-cardiac deaths would also be expected to be randomly distributed between EI and SDI patients, given their similar baseline characteristics. Thus, the lack of any observed survival difference suggests similar rates of HF death from progressive pump failure.
Limitations
This analysis was limited by its retrospective nature. Propensity score adjustment was therefore used to provide less biased comparisons among the cohorts. We acknowledge that while mortality data were complete, mode of death was not known. Other outcomes, including HF hospitalizations and quality of life measures, were also not ascertained. However, including transplant or VAD implant in the primary endpoint does incorporate quality of life and HF morbidity; patients undergoing these procedures presumably did not derive functional benefit from CRT. Another limitation was the size of the SDI group; while survival among EI patients was not statistically different from SDI patients, these comparisons may be relatively underpowered.
Conclusion
In this retrospective, single-center analysis of HF patients receiving CRT while on modern background medical therapy, survival free from cardiac transplant or VAD and overall survival differed significantly according to whether patients had never been on intravenous inotropes, had received inotropes in the past, were on inotropes at implant, or did not receive a left ventricular lead. Patients who were inotrope-dependent at implant had particularly poor survival, and this was significantly worse at 12 months than inotrope-naïve patients. CRT recipients who had ever been on inotropes had similar survival outcomes as patients who failed left ventricular lead placement but did receive a defibrillator and had ever been on inotropes. Based upon these findings, “rescue” CRT may be of limited clinical benefit in decompensated HF patients who cannot be weaned from inotropes. Aggressive efforts should be made to wean these patients from inotropes and initiate neurohormonal antagonists, with consideration of CRT-D implantation when HF is better compensated. If weaning is poorly tolerated, implantation of a conventional defibrillator combined with mechanical circulatory support as a bridge to transplant may be an alternative to CRT.
Acknowledgments
We would like to thank the Electrophysiology and Advanced Heart Failure/Transplant physicians and staff at Presbyterian University Hospital for their efforts in providing clinical care for the patients involved in this study.
This publication was made possible by Grant Number UL1 RR024153 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH.
Footnotes
Disclosures: Drs. Bhattacharya, Abebe, Simon, and Adelstein have no financial disclosures. Dr. Saba receives research support from Boston Scientific, Medtronic, and St. Jude Medical and serves as a consultant for St. Jude Medical.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stevenson LW. Clinical Use of Inotropic Therapy for Heart Failure: Looking Backward or Forward? Part II: Chronic Inotropic Therapy. Circulation. 2003;108:492–7. doi: 10.1161/01.CIR.0000078349.43742.8A. [DOI] [PubMed] [Google Scholar]
- 2.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 3.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 4.Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003;289:2685–94. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 5.Higgins SL, Hummel JD, Niazi IK, Giudici MC, Worley SJ, Saxon LA, et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol. 2003;42:1454–59. doi: 10.1016/s0735-1097(03)01042-8. [DOI] [PubMed] [Google Scholar]
- 6.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac Resynchronization Therapy with or without an Implantable Defibrillator in Advanced Chronic Heart Failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 7.Cleland JGF, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The Effect of Cardiac Resynchronization on Morbidity and Mortality in Heart Failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 8.Herweg B, Ilercil A, Cutro R, Dewhurst R, Krishnan S, Weston M, Barold SS. Cardiac Resynchronization Therapy in Patients with End-Stage Inotrope-dependent Class IV Heart Failure. Am J Cardiol. 2007;100:90–3. doi: 10.1016/j.amjcard.2007.02.058. [DOI] [PubMed] [Google Scholar]
- 9.James KB, Militello M, Gus B, Wilkoff BL. Biventricular Pacing for Heart Failure Patients on Inotropic Support. A Review of 38 Consecutive Cases. Texas Heart Institute Journal. 2006;33:19–22. [PMC free article] [PubMed] [Google Scholar]
- 10.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults. J Am Coll Cardiol. 2009;53:e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NAM, III, Freedman RA, Gettes LS, Gillinov MA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. J Am Coll Cardiol. 2008;51:e1–e62. [Google Scholar]
- 12.Imbens GW. The role of the propensity score in estimating dose-response functions. Biometrika. 2000;87:706–710. [Google Scholar]
- 13.Spreeuwenberg M, Bartak A, Croon MA, Hagenaars JA, Busschbach JJV, Andrea H, Twisk J, Stijnen T. The Multiple Propensity Score as Control for Bias in the Comparison of More Than Two Treatment Arms: An Introduction From a Case Study in Mental Health. Med Care. 2010;48:166–174. doi: 10.1097/MLR.0b013e3181c1328f. [DOI] [PubMed] [Google Scholar]
- 14.Rosenbaum P, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 15.Konstantino Y, Iakobishvili Z, Arad O, Beg-Gal T, Kusniec J, Mazur A, et al. Urgent cardiac resynchronization therapy in patients with decompensated chronic heart failure receiving inotropic therapy. A case series. Cardiology. 2006;106:59–62. doi: 10.1159/000092616. [DOI] [PubMed] [Google Scholar]
- 16.Cowburn PJ, Patel H, Jolliffe RE, Wald RW, Parker JD. Cardiac resynchronization therapy: an option for inotrope-supported patients with end-stage heart failure? Eur J Heart Fail. 2005;7:215–7. doi: 10.1016/j.ejheart.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Agostini D, Belin A, Amar MH, Darlas Y, Hamon M, Grollier G, et al. Improvement of cardiac neuronal function after carvedilol treatment in dilated cardiomyopathy: A 123I-MIBG scitigraphic study. J Nucl Med. 2000;41:845–51. [PubMed] [Google Scholar]
- 18.Kawai H, Fan TH, Dong E, Siddiqui RA, Yatani A, Stevens SY, Liand CS. ACE inhibition improves cardiac NE uptake and attenuates sympathetic nerve terminal abnormalities in heart failure. Am J Physiol. 1999;277:H1609–17. doi: 10.1152/ajpheart.1999.277.4.H1609. [DOI] [PubMed] [Google Scholar]
- 19.Anand IS, Fisher LD, Chiang YT, Latini R, Masson S, Maggioni AP, et al. Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT) Circulation. 2003;107:1278–83. doi: 10.1161/01.cir.0000054164.99881.00. [DOI] [PubMed] [Google Scholar]
- 20.Boriani G, Regoli F, Saporito D, Martignani C, Toselli T, Biffi M, et al. Neurohormones and inflammatory mediators in patients with heart failure undergoing cardiac resynchronization therapy: time courses and prediction of response. Peptides. 2006;27:1776–86. doi: 10.1016/j.peptides.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Erol-Yilmaz A, Verberne HJ, Schrama TA, Hrudova J, De Winter RJ, Van Eck-Smit BLF, et al. Cardiac resynchronization induces favorable neurohumoral changes. PACE. 2005;28:304–10. doi: 10.1111/j.1540-8159.2005.09508.x. [DOI] [PubMed] [Google Scholar]


