Abstract
Resolving the specific cell of origin for prostate cancer is critical to define rational targets for therapeutic intervention and requires the isolation and characterization of both normal human prostate stem cells and prostate cancer-initiating cells (CIC). Single epithelial cells from fresh normal human prostate tissue and prostate epithelial cell (PrEC) cultures derived from them were evaluated for the presence of subpopulations expressing stem cell markers and exhibiting stem-like growth characteristics. When epithelial cell suspensions containing cells expressing the stem cell marker CD133+ are inoculated in vivo, regeneration of stratified human prostate glands requires inductive prostate stromal cells. PrEC cultures contain a small subpopulation of CD133+ cells, and fluorescence-activated cell sorting–purified CD133+ PrECs self-renewand regenerate cell populations expressing markers of transit-amplifying cells (ΔNp63), intermediate cells (prostate stem cell antigen), and neuroendocrine cells (CD56). Using a series of CD133 monoclonal antibodies, attachment and growth of CD133+ PrECs requires surface expression of full-length glycosylated CD133 protein. Within a series of androgen receptor–positive (AR+) human prostate cancer cell lines, CD133+ cells are present at a low frequency, self-renew, express AR, generate phenotypically heterogeneous progeny negative for CD133, and possess an unlimited proliferative capacity, consistent with CD133+ cells being CICs. Unlike normal adult prostate stem cells, prostate CICs are AR+ and do not require functional CD133. This suggests that (a) AR-expressing prostate CICs are derived from a malignantly transformed intermediate cell that acquires “stem-like activity” and not from a malignantly transformed normal stem cell and (b) AR signaling pathways are a therapeutic target for prostate CICs.
Introduction
The normal prostate is composed of a stratified epithelium, which is functionally organized in stem cell units and subject to strict paracrine control via stromally derived growth and survival factors (1–3). Adult prostate epithelial stem cells reside within the basal layer at a very low frequency, possess high self-renewal capacity, proliferate infrequently to renew themselves, and simultaneously generate progeny for two distinct cell lineages (2, 4, 5). The much less frequent lineage commitment is to differentiate into proliferatively quiescent CD56+ neuroendocrine cells, which secrete a series of peptide growth factors (6, 7). The more common lineage commitment is to differentiate into ΔNp63+ transit-amplifying (TA) epithelial cells. TA epithelial cells undergo a limited number of proliferative replications before maturing into intermediate cells, characterized by a loss of ΔNp63 coupled with gain of expression of prostate stem cell antigen (PSCA; refs. 8, 9). These TA cells do not express androgen receptor (AR) protein and are dependent for proliferation, but not survival, on AR signaling in the stroma (4, 5). Intermediate epithelial cells migrate upward to form the luminal-secretory layer, where they express and engage the AR and undergo terminal differentiation characterized by proliferative quiescence and expression of prostate-specific antigen (PSA) and other prostate luminal-secretory specific markers (2, 4, 10, 11). Unlike their proliferating precursors, luminal-secretory cells depend on stromally derived andromedins for survival, and hence, androgen ablation or specific inactivation of AR function in prostate stroma induces apoptosis of the luminal-secretory cells (4, 12).
Although it is clear that prostate cancer arises within the epithelial compartment, the identification of the specific epithelial cell subtype in which the carcinogenic process is initiated has been the focus of intense study. There is a growing literature supporting that cancer lethality is the result of the hierarchical expansion of “cancer-initiating cells” (CIC), which function as stem-like cells to maintain malignant growth (13). Defining characteristics of such CICs include cells that are present at low frequency, possess an unlimited proliferative capacity, undergo self-renewal, and produce phenotypically heterogeneous progeny with only a limited proliferative potential. This has raised the issue of whether these CICs are derived from a malignantly transformed normal adult stem cell or from a more differentiated progeny that has acquired stem-like abilities. Resolving the specific cell of origin for prostate cancer is critical to appropriately define rational targets for therapeutic intervention because there are major differences in the growth regulatory pathways, particularly those involved in the AR axis for stem cells versus their more differentiated progeny. As a consequence, it is critical to develop experimental systems to isolate and characterize both the human normal prostate stem cells and the prostate CICs.
Along these lines, it has been suggested that CD133 is a marker for both of these cell types (14). CD133 (a.k.a. prominin-1 or AC133) is a membrane glycoprotein with an NH2-terminal extracellular domain, five transmembrane loops with two large extracellular loops containing eight putative N-linked glycosylation sites and a cytoplasmic tail (14). Very little is known about the biological function of CD133 except that it is localized to membrane protrusions where it interacts with membrane cholesterol and marks cholesterol-based lipid microdomains (15). Adult stem cells often express CD133 as a surface marker (14, 16, 17), and it is thought that CD133-marked cholesterol microdomains function to maintain stem cell properties by suppressing differentiation (18). CD133 was identified as the target of two monoclonal antibodies, AC133 and AC141, and both monoclonal antibodies bind to uncharacterized glycosylated epitopes on the extracellular loops of the CD133 protein (19). However, there are discordant observations about the expression and modulation of CD133 binding using these carbohydrate-specific antibodies among various cell types, and antibodies are now available that bind specifically to peptide epitopes in the extracelluar loops of human CD133 (19).
In the adult human prostate, CD133 expression is thought to be restricted to stem-like populations based on their expression of α2β1 integrins (20), rapid attachment to type I collagen (21), and high clonogenic ability when grown in low-calcium serum-free defined (SFD) medium (22, 23). Furthermore, CD133 expression has been reported to mark putative prostate CICs (24). In the present study, we document (a) that single-cell suspensions from freshly dissociated human prostate tissue contain a small population of CD133+ cells and that the unfractionated single-cell suspension regenerates prostate glands when recombined with rodent embryonic urogenital sinus mesenchyme (UGSM) and grown as xenografts in a host mouse; (b) that from such dissociated single-cell suspensions, in vitro epithelial [prostate epithelial cell (PrEC)] cultures can be established, which contain a subpopulation of CD133+ cells that retain the stem-like ability to regenerate progeny containing neuroendocrine, TA, and intermediate cells; and (c) that human prostate cancer cell lines contain subpopulations of CD133+ cells that are clonogenic but, unlike normal CD133+ prostate stem cells, coexpress AR. Using different monoclonal antibodies, we discovered that CD133 has a critical role in the attachment and subsequent growth of CD133+ normal prostate stem cells, which was not observed with prostate cancer cells.
Materials and Methods
Cells and materials
Primary prostate cells were isolated from patients undergoing radical prostatectomy at our institution according to an Institutional Review Board–approved protocol. Dissociation of prostate tissue has been previously described (25). Briefly, 18-gauge biopsy needle cores (Bard) of prostate tissue were digested overnight at 37°C in collagenase solution [0.28% collagenase I (S igma-Aldrich), 1% DNase I (Sigma), 10% FCS, 1× antibiotic/antimycotic (Life Technologies-Invitrogen), in RPMI 1640]. The following day, the cell suspension was washed in PBS, and epithelial organoids were isolated by density sedimentation, whereby cells in 10 mL PBS were allowed to settle for 10 min at room temperature and the top 9 mL of medium (containing fibroblasts) were removed; this was repeated two more times. Prostate epithelial organoids were further dissociated into single cells via treatment with DTT (1 mmol/L for 30 min at 37°C), a PBS wash, and trypsin/EDTA (0.25% for 30 min at 37°C). The trypsin was neutralized with RPMI 1640 + 10% FCS, and the cells were washed twice in PBS. Cells were subsequently passed through a cell strainer to ensure a single-cell suspension (BD Falcon). Ten biopsy cores yield ~100,000 single PrECs with >90% cell viability as scored by trypan blue exclusion. Nonfractionated single-cell suspensions of prostate tissue (i.e., containing both stromal and epithelial cells) were obtained by a similar enzymatic dissociation without the density sedimentation separation.
PrEC cultures were established and grown in serum-free defined PrEGM growth medium (Lonza/Cambrex) as previously described (5). Commercially available PrEC cultures, obtained from young men, were additionally used (Lonza/Cambrex). All prostate cancer cell lines were grown as previously described (23). The human colon cancer cell line CaCo-2 was a generous gift from Dr. Fred Bunz (Johns Hopkins University) and was cultured in DMEM + 10% FCS. All chemicals were purchased from JT Baker or Sigma-Aldrich.
Flow cytometry and cell sorting
All antibody incubations, washes, and flow cytometric analyses were performed in cell sorting buffer [1× PBS, 0.5% bovine serum albumin (BSA), 2 mmol/L EDTA]. Analysis was conducted on a Becton Dickinson LSR, and a minimum of 10,000 counts was acquired for each experimental condition. Fluorescence-activated cell sorting (FACS) was performed on a BD FACSAria, and cells were sorted into HBSS (without calcium or magnesium). Primary antibody labeling for flow cytometry and cell sorting was conducted using a 20-min cold incubation with a 1:10 dilution of antibody in a volume of 100 µL per 1 million cells in cell sorting buffer. The cells were washed in 1 mL cold cell sorting buffer, resuspended in 0.5 to 1.0 cell sorting buffer, and analyzed. For secondary antibody labeling, cells were incubated for 20 min with a 1:1,000 dilution of Alexa Fluor 488 F(ab′)2 fragment of goat anti-rabbit IgG (Invitrogen) in cell sorting buffer and similarly washed before analysis. The AC141 (293C3)-PE–conjugated mouse monoclonal antibody (Miltenyi Biotec) was used for all flow cytometric analyses and the peptide-derived CD133 rabbit monoclonal antibody (C24B9; Cell Signaling Technology) for Western blotting and FACS. Additional antibodies used for flow cytometry and sorting were PSCA (H83; Santa Cruz Biotechnology), FITC-conjugated mouse monoclonal CD56 (NCAM16.2; BD Biosciences), FITC-conjugated mouse monoclonal EpCAM (CD326;Miltenyi Biotec), FITC-conjugated mouse monoclonal ABCG2 (5D3; Chemicon-Millipore), and isotype control antibodies (Miltenyi Biotec). Enrichment of CD133+ PrECs was performed using the CD133 Cell Isolation kit according to the manufacturer’s specifications (Miltenyi Biotec).
Dual-variable flow cytometric analysis of AR and CD133 was conducted on fixed cells. Ice-cold methanol (1 mL) was added to 1.5 × 106 cells in 350 µL PBS, and the cells were incubated on ice for 15 min, washed with 10 mL cold PBS, and passed through a cell strainer (BD Falcon). Cells were incubated in blocking buffer (PBS, 0.5% FCS, 2 mmol/L EDTA) for 30 min on ice, and all subsequent antibody incubations were carried out in blocking buffer for 30 min followed by three washes in blocking buffer. The antibodies used were the rabbit polyclonal anti-AR (PG-21; Upstate Biotechnology) followed by a goat anti-rabbit IgG-FITC secondary antibody (Santa Cruz Biotechnology), and mouse monoclonal anti-CD133/2 (AC141, clone 293C3; Miltenyi Biotec) followed by an anti-mouse IgG-F(ab′)2-PE/Cy5 secondary antibody (Santa Cruz Biotechnology). Rabbit and mouse IgG antibodies (Santa Cruz Biotechnology) were used as isotype controls.
Immunoblotting, immunoprecipitation, and immunohistochemistry
Western blotting was performed as previously described (5). Whole-cell lysates collected from 100,000 cells were used per lane. Antibodies used were anti-β-actin (Cell Signaling Technology), anti-ΔNp63 (4A4; Santa Cruz Biotechnology), and anti-CD133 (Cell Signaling Technology). All secondary horseradish peroxidase–conjugated antibodies and chemiluminescent detection reagents (ECL) were purchased from Amersham Biosciences.
For CD133 immunoprecipitation, cells were lysed in immunoprecipitation lysis buffer [20 mmol/L Tris (pH 7.5), 140 mmol/L NaCl, 1 mmol/L EDTA, 1% Triton X-100, 1× protease inhibitor, 1× phosphatase inhibitor (Sigma)]. Protein lysate (1.0 mg) was incubated overnight with rocking at 4°C using either 4 µg of the AC141 antibody or a mouse IgG control antibody. Subsequently, 40 µL of protein A–conjugated Dynabeads (Invitrogen) were washed in immunoprecipitation lysis buffer and added to protein/antibody mixture and incubated with rocking for 3 h at 4°C. Immunoprecipitation was performed using a magnetic stand (Millipore). The beads were washed with immunoprecipitation lysis buffer, resuspended in 40 µL gel loading buffer, and incubated for 5 min at 80°C to release the CD133 protein from the protein A Dynabeads. The Dynabeads were removed using the magnetic stand, and the supernatant was collected and analyzed according to the Western blotting protocol.
Immunostaining for ΔNp63 (LabVison/NeoMarkers) and AR (N-20; Santa Cruz Biotechnology) was done as previously described (23, 26). Immunostaining for human Nkx3.1 was conducted as described previously (27).
UGSM isolation and tissue recombination
All animal studies were performed under the guidance of Institutional Animal Care and Use Committee–approved protocols. UGSM was isolated from E17 embryos of timed pregnant Sprague-Dawley rats (Harlan) according to previously reported protocols (28, 29). UGSM was dissociated into single cells using 0.1% collagenase B (Boehringer Mannheim) in DMEM + 10% FCS at 37°C for 2 h. The single-cell suspensions of UGSM cells were washed thrice in DMEM + 10% FCS and counted. UGSM cells were recombined with human epithelial cells at a ratio of 2:1 in accordance with previous reports (40,000 UGSM cells to 20,000 prostate cells; ref. 30). Tissue recombinants were embedded in 10 µL of type I collagen (BD Biosciences) that consisted of 88% collagen solution, 2% of 1 N NaOH, and 10% of 10× PBS solution, which hardened on warming to room temperature. The recombinant implants were overlaid with DMEM + 10% FCS and 1 nmol/L R1881 and incubated at 37°C overnight and then implanted under the renal capsule of 4- to 6-wk-old male athymic nude mice. At various times up to 3 mo, the renal tissue was harvested, fixed in formalin, and serially sectioned for histologic analysis.
Centromere/telomere fluorescence in situ hybridization
Specimens underwent routine neutral-buffered formalin fixation followed by paraffin embedding. Slide preparation was performed without protease digestion as previously described (31). Briefly, 5-µm tissue sections were deparaffinized, hydrated through a graded ethanol series, and underwent heat-induced antigen retrieval for 14 min in citrate buffer (target unmasking solution, Vector Laboratories, Inc.) using a vegetable steamer and placed into PBS + 0.1% Tween (Sigma) for 5 min. Fluorescence in situ hybridization (FISH) was performed in the dark on sections by cohybridization of a custom-made NH2-terminal Cy3-labeled peptide nucleic acid (PNA) probe that recognizes all mammalian telomeres (N-CCCTAACCCTAACCCTAA-C) and a NH2-terminal FITC-labeled PNA probe that recognized both human and mouse centromeres (N-ATTCGTTGGAAACGGGA-C) but does not recognize rat centromeres (Applied Biosystems). PNA probes were incubated at 300 ng/mL in diluent [70% formamide, 10 mmol/L Tris (pH 7.5), 0.5% B/M blocking reagent (Boehringer-Mannheim)] at 83°C for 4 min followed by 2 h at room temperature. Sections were washed twice for 15 min with PNA wash buffer [70% formamide, 10 mmol/L Tris (pH 7.5), 0.1% BSA] followed by three 5-min washes in PBS + 0.1% Tween. Nuclei were counterstained with 0.05% 4′,6-diamidino-2-phenylindole (Sigma). Sections were mounted using Prolong Anti-Fade Mounting Media solution (Invitrogen) and imaged with a Nikon 50i epifluorescence microscope equipped with an X-Cite series 120 illuminator and an attached Photometrics CoolsnapEZ digital camera.
Results
Single-cell suspensions of human adult PrECs contain gland-regenerating stem cells
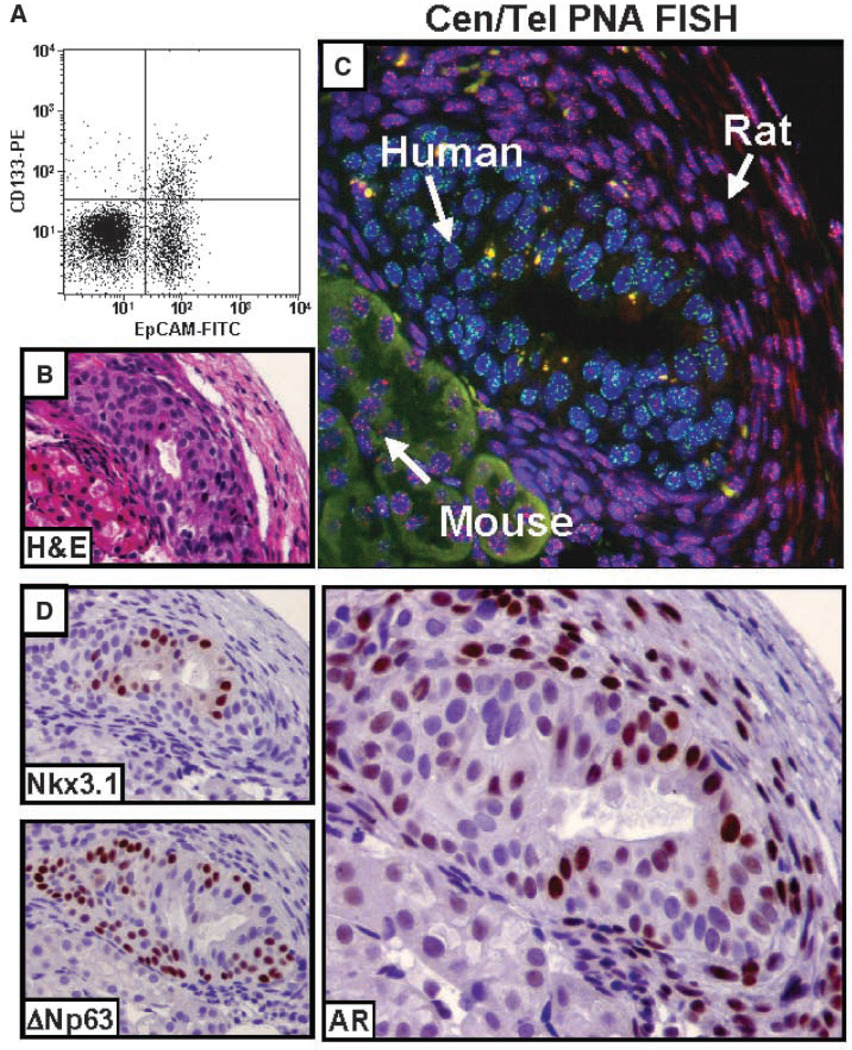
Collagenase digestion of whole human prostate tissue liberates epithelial aggregates, known as organoids, which can be separated from supporting stromal cells. Previous data documented that when these human prostatic epithelial organoids are injected s.c. with Matrigel into nude mice, a population of stem cells proliferates and gives rise to progeny regenerating stratified glands in which the luminal cells terminally differentiate and secrete PSA (25, 32). To evaluate whether the CD133-expressing cells are the prostate stem cells responsible for this regenerative ability, the organoid must be dissociated into single cells and the CD133 subpopulation was isolated and tested for its regenerative ability. In rodent prostates, stem cells are a minor fraction of the adult epithelium (33). This raises the issue of whether the available antibodies are sensitive enough to detect the potentially low number of CD133+ putative prostate stem cells. To evaluate this, the proportion of CD133+ cells was analyzed in nonfractionated (i.e., containing both stroma and epithelia) dissociated cells from fresh human prostate tissue using flow cytometry. As a second marker, the pan-epithelial surface antigen EpCAM was used to discern epithelial cells from stromal cells. Such analyses reveal a minor (i.e., <10%) subpopulation of CD133+ cells present of which >80% are of epithelial origin (i.e., EpCAM+; Fig. 1A). Notably, the percentage of CD133+ cells is higher in donors under the age of 30 versus those older than 50 years (i.e., 10–15% versus 1–5%, respectively).
Figure 1.
Glandular regeneration in vivo from single-cell dissociations of adult human prostatic epithelia. A, detection of CD133+ cells in single-cell suspensions of whole prostate tissue. Fresh prostate tissue was dissociated into single cells and contained both stroma and epithelial cells. A FITC-conjugated pan-epithelial antibody (EpCAM) marked epithelial cells and a PE-conjugated anti-CD133 antibody (AC141, CD133-PE) was used to detect CD133+ cells. The majority of CD133+ cells are EpCAM+. B to D, recombination of rat UGSM with dissociated human epithelial cells regenerates human glandular structures when implanted under the renal capsule of a nude mouse. Single cells from dissociated human prostate epithelial organoids (devoid of stroma cells) were combined with rat UGSM cells isolated from day E17 rat embryos and placed under the renal capsule of male nude mice. B, glandular structures were observed in as few a 2 wk. C, centromere-telomere PNA FISH (Cen/Tel PNA FISH) showing human cells comprising the gland, with surrounding rodent stroma. A human- and mouse-specific FITC-labeled centromeric FISH probe (green) was combined with a Cy3-labeled telomere FISH probe (red), which highlights the longer rodent telomeres. Such a method allowed unambiguous discernment between cells of human (Cen-positive; Tel-dim), mouse (Cen-positive; Tel-bright), and rat (Cen-negative; Tel-bright) origin. D, top left, luminal expression of prostate-restricted Nkx3.1; bottom left, expression of ΔNp63 in the basal epithelial cells. Right, AR expression in the luminal epithelial cells and the surrounding rat stroma. Adjacent mouse renal parenchymal cells were AR−.
To eliminate the population of CD133+ stromal cells, prostate tissue was collagenase digested and the epithelial organoids were collected. The prostate epithelial organoids were dissociated into single cells and implanted in vivo under the renal capsule of host nude mouse. No glandular formation was observed up to 3 months; these negative results are consistent with the known stromal requirement for prostate glandular morphogenesis (34). To provide such stromal support, coinoculation with UGSM was tested based on the established ability of UGSM to induce prostate epithelial organogenesis of human embryonic tissues and single cells in vivo (34, 35). Such single-cell recombination results in the regeneration of stratified prostate glands detectable as early as 2 weeks after inoculation (Fig. 1B).
To discern the cellular contribution of human, mouse, and rat cells to the regenerated glands, a novel technique was used, which takes advantage of unique genomic differences between all three species (rat, human, and mouse). Specifically, telomeres in commonly used inbred laboratory rodent strains are significantly longer than human telomeres (50–150 kb in rodent versus 5–10 kb in human), and this difference in length results in a notable difference in the intensity of telomeric FISH signals (36). Thus, rodent cells (mouse and rat) are easily distinguished from human cells by virtue of their very bright telomeres. The centromere-specific PNA probe used hybridizes to DNA repeats in human and mouse centromeres but does not hybridize to rat centromeres (37, 38). Thus, simultaneous staining with these centromere and telomere PNA FISH probes (Cen/Tel FISH) allows for rapid and unequivocal identification of species origin (human versus rat versus mouse) at the single-cell level in tissue hetero-recombinants. In these rat UGSM/human recombinants, Cen/Tel FISH confirmed the presence of human epithelial glands surrounded by rat stroma and adjacent to renal parenchymal cells of mouse origin (Fig. 1C).
Such human-derived glandular structures are positive for Nkx3.1, a PrEC-restricted protein (39), and contain a ΔNp63+ basal layer and an AR+ luminal layer (Fig. 1D). The stroma surrounding the human-derived glands is derived from the rat UGSM (Fig. 1C) and contains AR+ stromal cells, providing androgen-dependent paracrine signaling (Fig. 1D). These data document that within single-cell suspensions of human adult epithelial cells, there are prostate stem cells that are capable of regenerating complete prostatic glandular structures and that the recombination of human prostate tissue with rodent UGSM is a useful assay for detecting such prostate stem cell capabilities. Thus, the presence of CD133+ cells is present within such single-cell dissociates, consistent with their being putative stem cells. However, their frequency within fresh tissue is so low that a culture method is needed to obtain sufficient numbers to test their stem cell ability in such an in vivo assay.
Phenotypic characteristics of CD133+ cells isolated from human prostate epithelial cultures
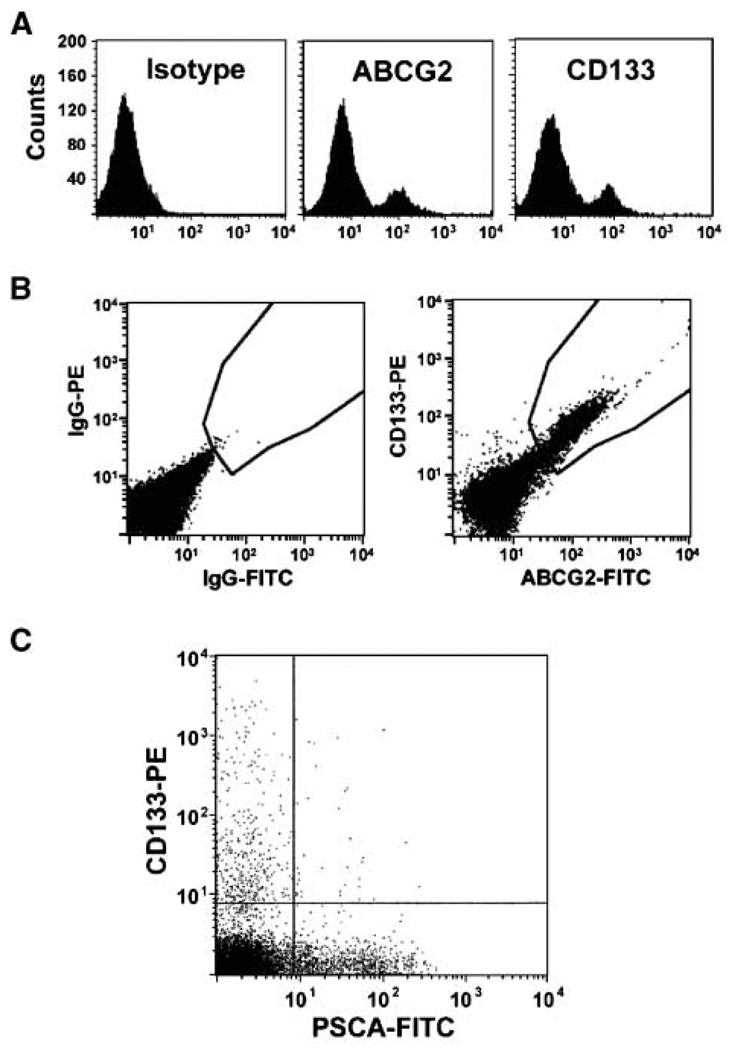
From freshly dissociated tissue, human PrECs can be routinely cultured and propagated for 8 to 10 serial passages using low-calcium, serum-free defined medium (i.e., either keratinocyte serum-free medium with ~100 µmol/L calcium or PrEGM medium with ~300 µmol/L calcium; ref. 23). By the second serial passage, such cultures are devoid of prostate fibroblasts and smooth muscle cells (5, 23). Early-passage cultures from a series (n = 12) of different commercial and in-house donors were analyzed for the expression of CD133, ABCG2, β1-integrin, ΔNp63, PSCA, AR, CD56, and chromogranin A. These analyses consistently documented that these cultures are phenotypically heterogeneous being composed of at least four discernable subpopulations: (a) a minor population of small-sized putative stem cells (CD133+/ABCG2+/β1-integrin+/ΔNp63−/PSCA−/AR−/CD56−; Fig. 2A–C), (b) a major population (~80%) of small- to intermediate-sized TA cells (ΔNp63+/CD133−/PSCA−/AR−/CD56−), (c) a minor population (~10%) of larger-sized intermediate cells (PSCA+/AR+/CD133−/ΔNp63−/CD56−), and (d) a minor population (~2–5%) of dendritic-shaped neuroendocrine cells (CD56+/chromogranin A+/CD133−/ΔNp63−/PSCA−/AR−; refs. 5, 23).
Figure 2.
Expression of stem cell markers in PrEC cultures. A, ABCG2-FITC and CD133-PE (AC141) expression in commercially available PrECs. A nonspecific IgG was used as an isotype control. B, dual-label flow cytometry showing that CD133+ cells are also ABCG2+. C, CD133+ PrECs do not express the intermediate cell marker PSCA.
Glycosylation-specific monoclonal antibodies prevent attachment and survival of CD133+ cells
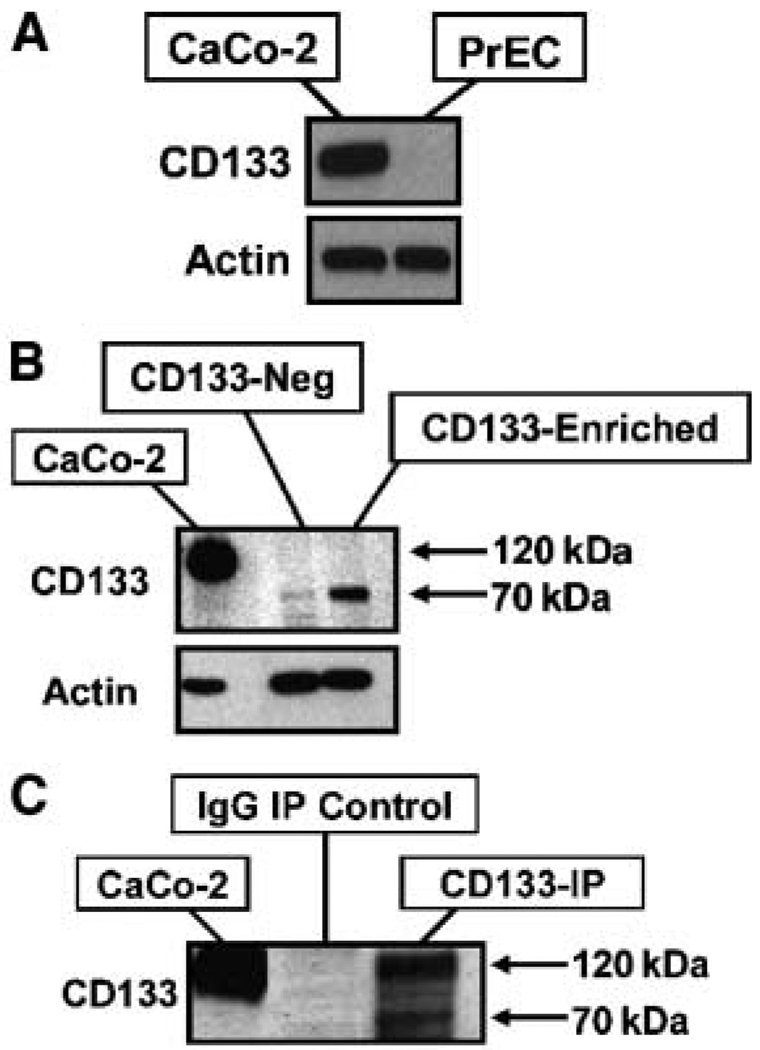
Western blot analyses were unable to detect CD133 protein expression in unsorted PrECs (Fig. 3A). As a positive control for these CD133 Western blots, the CaCo-2 human colon cancer cell line was used because it is uniformly CD133+ (40). To increase the sensitivity of Western blot analysis, CD133+ PrECs were enriched using live cell magnetic-activated cell sorting (MACS) with the AC133 antibody. This monoclonal antibody binding is glycosylation dependent, recognizing a carbohydrate-specific epitope on the extracellular loop of CD133 and has been used previously to isolate stem cells from a variety of nonprostate tissues (19). The AC133-enriched cells were subsequently lysed and subjected to Western blotting using an anti-CD133 rabbit monoclonal antibody (C24B9), which specifically recognizes a peptide epitope in the second extracellular loop of CD133 (19). This documented that AC133-enriched PrECs have a detectable level of a truncated and glycosylated 70-kDa form of CD133 but not full-length glycosylated 120- to 130-kDa CD133 protein detected in CaCo-2 cells (Fig. 3B). Full-length CD133 protein is expressed, however, by a subset of PrECs before binding the AC133 antibody. This is documented by the observation that lysis of unsorted PrECs followed by immunoprecipitation using a second glycosylation-specific antibody (i.e., AC141) revealed a full-length form of CD133 in addition to the smaller 70-kDa form (Fig. 3C).
Figure 3.
Molecular weight of CD133 in PrECs. A, Western blot of nonfractionated PrECs compared with the CD133+ CaCo-2 colon cancer cell line. Actin was used as a loading control. B, Western blot of CD133-enriched PrECs and CaCo-2 cells. PrECs and CaCo-2 cells were subjected to magnetic enrichment of CD133 (AC133)–positive cells and the expression of CD133 compared with non–CD133-expressing PrECs (CD133-Neg). Actin was used as a loading control. Western blotting using the peptide-derived CD133 antibody (293C3) reveals a lower molecular weight form of CD133 expressed in the AC133-enriched PrECs compared with CaCo-2 cells. C, immunoprecipitation (IP) of AC141 from lysed PrECs and CaCo-2 cells and Western blotting using the peptide-derived anti-CD133 antibody reveal the expression of CD133 at the correct molecular weight in addition to the smaller 70-kDa form.
Binding of the glycosylation-specific AC133 antibody results not only in truncation of the glycosylated 120-kDa full-length CD133 protein but also in the inability of antibody-associated CD133+ PrECs to attach and grow in vitro. This was initially observed when CD133+ PrECs were isolated via FACS using the anti-CD133-PE–conjugated AC141 mouse monoclonal antibody and consistently exhibited an inability to attach and spread when replated in culture, resulting in their eventual death. A variety of culture conditions were used in an attempt to improve the survival and growth of AC141-isolated PrECs cells, including poly-d-lysine or type I collagen coating, adding either conditioned medium from unfractionated PrEC cultures or culturing on an irradiated feeder layer (i.e., mouse STO cells). All conditions failed to increase the viability and growth of the AC141-sorted PrECs. A series of controls were used to document that specific binding of the AC141 antibody uniquely inhibits PrECs attachment and growth after sorting. First, sham-sorted or EpCAM-sorted PrECs attach and proliferate in a manner similar to nonmanipulated PrECs; second, FACS isolation using the same carbohydrate-specific AC141 monoclonal antibody to isolate CD133+ cells from the CaCo-2 human colon cancer line yielded viable cells, which attach and grow equally well as unsorted CaCo-2 cells. In addition, to test whether this inhibition is unique to the AC141 monoclonal antibody or is a general property of antibodies that bind the carbohydrate portion of CD133 on PrECs, MACS isolation using the AC133 antibody yielded similar results, whereby CD133+ PrECs failed to attach and grow. These combined results document that the glycosylation-specific AC141 and AC133 anti-CD133 antibodies inhibit the attachment and growth of CD133+ PrECs in a cell context–dependent manner.
CD133+ cells regenerate phenotypically heterogeneous PrEC cultures
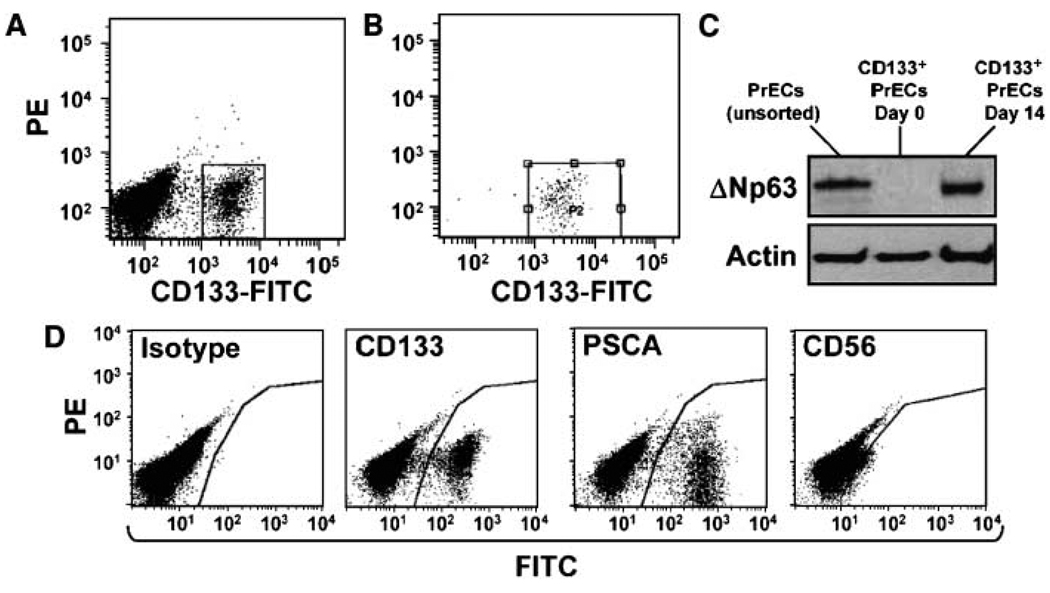
In contrast to the negative results using the glycosylation-specific AC141 and AC133 antibodies, FACS sorting and subsequent growth of CD133+ PrECs was possible using the peptide-specific C24B9 rabbit monoclonal antibody. CD133+ PrECs were sorted by flow cytometry to generate a >98% pure population (Fig. 4A and B). Western blot analysis of flow-sorted CD133+ PrECs revealed that the expression of ΔNp63 is below the level of detection (Fig. 4C). This CD133+ population was placed back into culture and tested for its ability to regenerate all the cell populations present within heterogeneous PrEC cultures. By 2 weeks, the cultures had undergone approximately six population doublings and were morphologically identical to those of unsorted parental PrEC cultures, being composed of a heterogeneous mixture of small-, medium-, and large-sized epithelial as well as dendritic-shaped cells. CD133-derived cultures maintain a CD133+ population (Fig. 4D) and also regenerate a ΔNp63+ population of TA cells (Fig. 4C), a population of PSCA+ intermediate cells, and a population of CD56+ neuroendocrine cells (Fig. 4D). Flow cytometry documented that by 2 weeks, the number of CD133+ cells is approximately six times greater than the initial number of CD133+ cells plated, indicating that the CD133+ cells not only renew themselves but also give rise to progeny of two distinct cell lineages, the neuroendocrine cell lineage and the TA cell lineage, where a subset of ΔNp63+ TA cells matures to form PSCA+ intermediate cells. In contrast to the ability of CD133+ cells to regenerate PrEC cultures, flow-sorted PSCA+ and CD56+ PrECs CD133 attached but did not grow when placed back into culture. These data document that CD133+ PrECs are both self-renewing and capable of generating progeny of two distinct cell lineages (neuroendocrine and TA) and are thus bona fide prostate stem cells.
Figure 4.
Pure populations of CD133+ cells regenerate PrEC cultures. A, flow cytometric analysis of PrECs using the peptide-derived 293C3 anti-CD133 antibody. CD133+ cells (box) were isolated via FACS. B, FACS yields a population of CD133+ cells, which is >98% pure. CD133+ cells were either replated for growth or lysed for Western blotting. C, expression of the basal marker ΔNp63 in CD133+ PrECs is below the level of detection but is restored after 14 d in culture. Actin was used as a loading control. D, cultures established from pure CD133+ cells contain an expanded number of CD133+ stem cells as well as PSCA+ intermediate cells and CD56+ neuroendocrine cells. A nonspecific IgG was used as an isotype control. Thus, cultures derived from pure populations of CD133+ cells contain the original progenitor population (CD133+ cells) and two distinct cell lineages (basal-intermediate and neuroendocrine).
CD133+ human prostate cancer cells have cancer-initiating ability
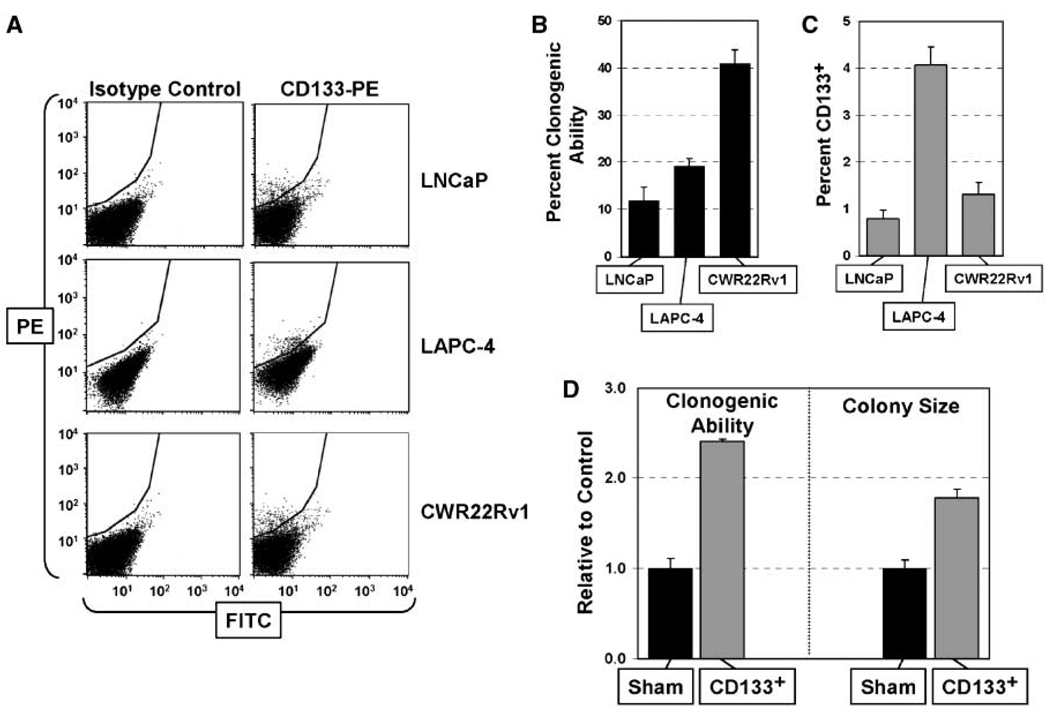
Similar to normal prostate epithelial cultures, the frequency of CD133+ cells within a series of human prostate cancer cell lines is so low that the protein is below detection in mass culture by Western blot analysis. Using more sensitive flow cytometry, however, prostate cancer lines do contain a minor population (~1–5%) of CD133+ malignant cells (Fig. 5A). These results raise the issue of whether the CD133+ cancer cells have cancer-initiating ability. Therefore, to test if CD133+ prostate cancer cells have these abilities, three androgen ablation refractory prostate cancer cell lines (LNCaP, LAPC-4, and CWR22Rv1) were analyzed for their clonogenic ability, percent CD133+ cells, and their ability to generate progeny with a different phenotype. These lines were chosen to be representative of the range of lethal metastatic prostate cancers observed clinically because LAPC-4 expresses wild-type AR (41), LNCaP expresses point mutated AR (42), and CWR22Rv1 expresses exon 3 duplicated AR and exon 2 truncated isoform of AR protein (43). Initially, the clonogenic ability of unfractionated cultures for all three cell lines was determined based on formation of colonies (>10 cells) within 10 days (Fig. 5B). These clonogenic abilities were 5- to 40-fold higher than the percentage of CD133+ cells in the three lines (Fig. 5C). These results are explainable by the fact that either (a) CD133+ cells are not CICs or (2) CD133+ cells are CICs with unlimited ability to self-renew but the majority of their progenies become CD133− with a sufficient proliferative ability to form the vast majority of colonies in primary clonogenic assay but not unlimited proliferative ability to form colonies in serial clonogenic assays. If the first possibility is true and CD133+ cells are present at <5% (Fig. 5C), then 5 to 10 individual clones derived from each of these lines should be negative for CD133 expression. In contrast, if CD133+ cells are the CICs, then serially passaged cultures initially derived from single-cell clones should always be heterogeneous, containing mostly CD133− cells and a small fraction of CD133+ cells. Thus, to resolve between these possibilities, multiple clones containing >200 cells were isolated from LNCaP, LAPC-4, and CWR22Rv1 lines and the clones were serially propagated for >25 population doublings (i.e., >50 days) before being analyzed by flow cytometry. These analyses documented that all of the clones from LNCaP (n = 5 clones), LAPC-4 (n = 8 clones), and CWR22Rv1 (n = 6 clones) contain about 1% to 5% CD133+ cells consistent with their CIC ability.
Figure 5.
CD133 marks prostate CICs. A, flow cytometric analyses revealed minor populations of CD133+ cells in three androgen-responsive prostate cancer cell lines: LNCaP, LAPC-4, and CWR22Rv1. A PE-conjugated CD133 antibody (CD133-PE) and a nonspecific IgG were used as controls. B, clonogenic ability of LNCaP, LAPC-4, and CWR22Rv1 cells to form microscopic clones over 10 d. Clones were stained and counted at ×10 magnification. C, percentage of CD133+ cells in LNCaP, LAPC-4, and CWR22Rv1 cultures. These data represent the average of six individual analyses. D, CD133+ CWR22Rv1 prostate cancer cells are more clonogenic and form larger clones compared with the sham-sorted control cells.
To directly test whether CD133+ cells give rise to CD133− progeny with limited proliferation ability, CWR22Rv1 cells were flow sorted using the AC141-PE antibody. In contrast to the poor survival of PrECs after sorting with the AC141 antibody, CWR22Rv1 cells exhibited no differences in viability after sorting using the AC141 antibody (7 days after inoculating 2,000 cells, there were 81,500 cells in the CD133-sorted versus 84,800 cells in the mock-sorted control group). Further propagation of the CD133-derived CWR22Rv1 cultures revealed that the percentage of CD133+ cells was only 6.15% after 2 weeks in culture. Thus, although these cultures were initiated from sorted cells, which were >98% CD133+, the population of CD133+ cells is maintained at the same low level as that of the unsorted cultures, with the majority (93.85%) of the progeny in the expanded cultures no longer expressing CD133. The clonogenic ability of flow-sorted CD133+ CWR22Rv1 cells is 2.4 times greater than the unsorted population and the average colony size was two times larger by 10 days (Fig. 5D). These combined results document that the CD133+ prostate cancer cells have the defining characteristics of CICs because they are present at low frequency, self-renew, exhibit unlimited proliferative capacity, and give rise to phenotypically different progeny with a lower growth potential.
CD133+ human prostate cancer cells express and respond to AR
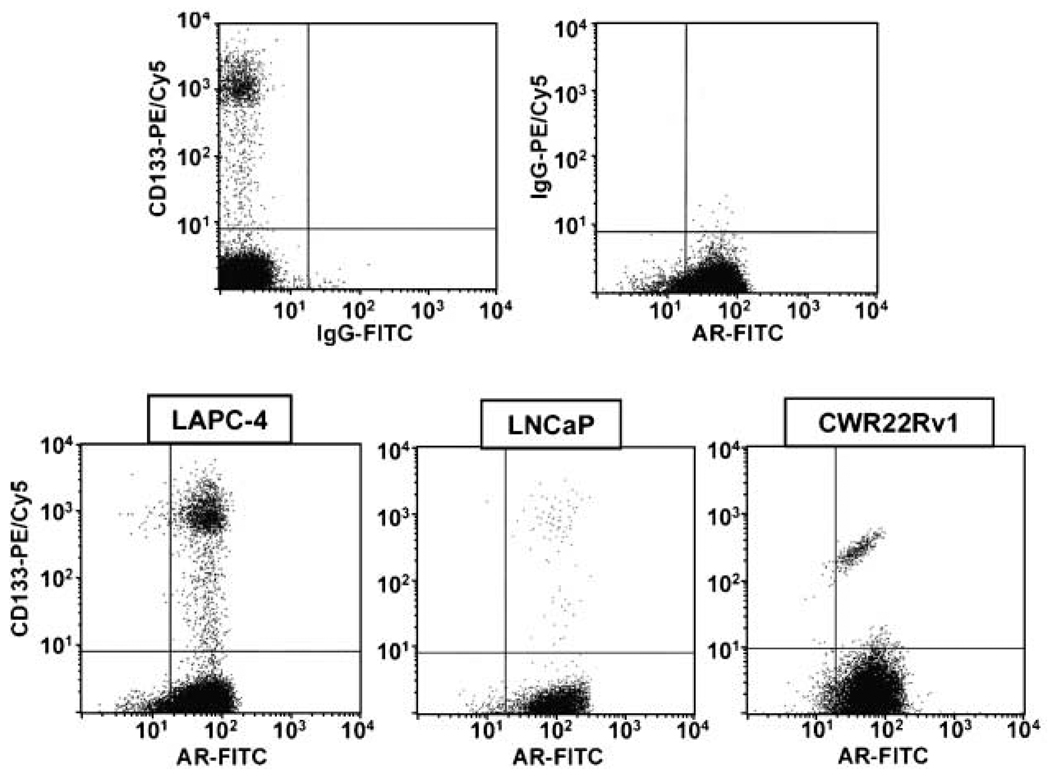
To determine whether the CD133+ CICs in human prostate cancer express AR protein, two-variable flow cytometry was used. These results document that ~98% of the CD133+ populations in the LAPC-4, LNCaP, and CWR22Rv1 cell lines are positive for the AR (Fig. 6). As expected, there is a very small (>2%) population of AR− cells present within these exponentially growing cultures, which is consistent with our previous observation that prostate cancer cells degrade AR during mitosis (26). As a functional test to evaluate the signaling ability of the AR expressed in CD133+ cells, the clonogenic ability of LNCaP cells was tested using a growth-inhibitory dose of androgen (i.e., 10 nmol/L of the synthetic androgen R1881; ref. 44). This is based on the rationale that if AR signaling is not occurring in the cancer-initiating CD133+ LNCaP cells, then there will be no effect of high-dose androgen on the clonogenic ability of these cells. In contrast, high-dose androgen decreases by >95% the clonogenic ability of LNCaP cells (2.3 ± 0.8% versus 0.18 ± 0.1% clonogenic ability of untreated versus androgen-treated cells).
Figure 6.
CD133+ prostate cancer cells express the AR. Dual-variable flow cytometry of CD133 (AC141) and AR in the LAPC-4, LNCaP, and CWR22Rv1 prostate cancer cell lines. Top, control staining in LAPC-4 using IgG antibodies (top left, CD133 versus IgG; top right, IgG versus AR). Similar controls were documented in the LNCaP and CWR22Rv1 cell lines. Bottom, dual labeling for CD133 and AR in LAPC-4, LNCaP, and CWR22Rv1 prostate cancer cells showing that CD133+ prostate cancer cells also express AR.
Discussion
The concept of adult prostate stem cells first emerged to explain the immense capacity of the epithelial compartment for cyclic regeneration. Previous studies document that the prostate can undergo >30 successive cycles of androgen deprivation and replacement without diminishing its ability for continued epithelial regeneration (45). In the present study, we document that CD133 marks both normal prostate epithelial stem cells as well as malignant prostate CICs.
We identified CD133+ cells in PrEC cultures and showed that pure populations of CD133+ cells are able to regenerate PrEC cultures and exhibit characteristics of stem cells by their ability to self-renew and regenerate PrEC cultures with two distinct cell lineages. A disadvantage of the low-Ca2+ SFD culture conditions used to establish and propagate PrEC cultures is that luminal differentiation (i.e., AR expression, terminal differentiation, and PSA expression) does not occur within these cultures. This is due to the activation of Notch signaling and the inhibition of E-cadherin signaling, both of which prevent terminal differentiation (23, 46). As such, using our current culture conditions, the prostate stem cell compartment is unable to complete its full differentiation potential and progress only to the intermediate cell stage.
The expanding use of CD133 as a human stem cell marker has yielded a variety of antibodies for the isolation and characterization of CD133+ stem cell populations. The glycosylation-specific AC133 and AC141 anti-CD133 antibodies were the first to be developed and aided the identification of the CD133 gene (19). However, the binding of such antibodies to normal prostate stem cells profoundly inhibited their attachment and growth after cell sorting. The same antibodies, however, did not affect the attachment and growth of CD133+ prostate cancer cells. These observations document that CD133 functions differently between normal and malignant prostate cells and that the glycosylation sites of CD133 play a significant role in the function of normal prostate stem cells.
If prostate cancer were derived from a transformed normal stem cell, one would expect that prostate CICs do not express AR, give rise to ΔNp63+ progenies that differentiate into AR+ cells, and do not respond to androgen-mediated growth inhibition. Prostate cancers exhibit characteristics of intermediate and luminal-secretory epithelial cells because they express AR, PSA, and PSCA (47, 48). Furthermore, a hallmark of prostate cancer is the loss of the basal cell marker ΔNp63 (49), and normal prostate stem cells do not express AR and are thus not dependent on androgen for their survival. We document that CD133+ prostate CICs express AR and are subject to AR-mediated growth inhibition. The data presented here are consistent with prostate CICs being derived from a malignantly transformed intermediate cell, which has gained the expression of the stem cell marker CD133. Such an observation shows that prostate CICs are valid targets for the continued development of improved antiandrogen therapies.
Acknowledgments
Grant support: NIH grant R01DK52645 and Maryland Stem Cell Research Fund MSCRFII-0428-00. D.J. Vander Griend was supported by a Urology Training Grant (NIH T32DK07552) and is now supported by a Department of Defense Postdoctoral Training Award (PC060843).
We thank Leslie Meszler and Lillian Dasko-Vincent (Johns Hopkins Cell Imaging Core Facility) and Lee Blosser and Ada Tam (Johns Hopkins School of Medicine Flow Sorting Facility) for their expert assistance and Jessica Hicks and Yuko Konishi (Johns Hopkins Department of Pathology) for the immunostaining and help with the Cen/Tel FISH, respectively.
Footnotes
Reprints and Subscriptions To order reprints of this article or to subscribe to the journal, contact the AACR Publications Department at pubs@aacr.org.
Disclosure of Potential Conflicts of Interest
J.T. Isaacs: commercial research grant, Active Biotech; ownership interest, Genspera and Protox; consultant/advisory board, Wiley Publishing. The other authors disclosed no potential conflicts of interest.
References
- 1.Bonkhoff H, Remberger K. Differentiation pathways and histogenetic aspects of normal and abnormal prostatic growth: a stem cell model. Prostate. 1996;28:98–106. doi: 10.1002/(SICI)1097-0045(199602)28:2<98::AID-PROS4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 2.Isaacs JT, Coffey DS. Etiology and disease process of benign prostatic hyperplasia. Prostate Suppl. 1989;2:33–50. doi: 10.1002/pros.2990150506. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Hayward S, Cao M, Thayer K, Cunha G. Cell differentiation lineage in the prostate. Differentiation. 2001;68:270–279. doi: 10.1046/j.1432-0436.2001.680414.x. [DOI] [PubMed] [Google Scholar]
- 4.Litvinov IV, De Marzo AM, Isaacs JT. Is the Achilles’ heel for prostate cancer therapy a gain of function in androgen receptor signaling? J Clin Endocrinol Metab. 2003;88:2972–2982. doi: 10.1210/jc.2002-022038. [DOI] [PubMed] [Google Scholar]
- 5.Uzgare AR, Xu Y, Isaacs JT. In vitro culturing and characteristics of transit amplifying epithelial cells from human prostate tissue. J Cell Biochem. 2004;91:196–205. doi: 10.1002/jcb.10764. [DOI] [PubMed] [Google Scholar]
- 6.Yao JL, Madeb R, Bourne P, et al. Small cell carcinoma of the prostate: an immunohistochemical study. Am J Surg Pathol. 2006;30:705–712. doi: 10.1097/00000478-200606000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Rumpold H, Heinrich E, Untergasser G, et al. Neuroendocrine differentiation of human prostatic primary epithelial cells in vitro. Prostate. 2002;53:101–108. doi: 10.1002/pros.10129. [DOI] [PubMed] [Google Scholar]
- 8.Tran CP, Lin C, Yamashiro J, Reiter RE. Prostate stem cell antigen is a marker of late intermediate prostate epithelial cells. Mol Cancer Res. 2002;1:113–121. [PubMed] [Google Scholar]
- 9.van Leenders G, Dijkman H, Hulsbergen-van de Kaa C, Ruiter D, Schalken J. Demonstration of intermediate cells during human prostate epithelial differentiation in situ and in vitro using triple-staining confocal scanning microscopy. Lab Invest. 2000;80:1251–1258. doi: 10.1038/labinvest.3780133. [DOI] [PubMed] [Google Scholar]
- 10.Garraway LA, Lin D, Signoretti S, et al. Intermediate basal cells of the prostate: in vitro and in vivo characterization. Prostate. 2003;55:206–218. doi: 10.1002/pros.10244. [DOI] [PubMed] [Google Scholar]
- 11.Bonkhoff H, Stein U, Remberger K. The proliferative function of basal cells in the normal and hyperplastic human prostate. Prostate. 1994;24:114–118. doi: 10.1002/pros.2990240303. [DOI] [PubMed] [Google Scholar]
- 12.Kyprianou N, Isaacs JT. Quantal relationship between prostatic dihydrotestosterone and prostatic cell content: critical threshold concept. Prostate. 1987;11:41–50. doi: 10.1002/pros.2990110106. [DOI] [PubMed] [Google Scholar]
- 13.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea—a paradigm shift. Cancer Res. 2006;66:1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- 14.Mizrak D, Brittan M, Alison MR. CD133: molecule of the moment. J Pathol. 2008;214:3–9. doi: 10.1002/path.2283. [DOI] [PubMed] [Google Scholar]
- 15.Corbeil D, Roper K, Fargeas CA, Joester A, Huttner W. Prominin: a story of cholesterol, plasma membrane protrusions and human pathology. Traffic. 2001;2:82–91. doi: 10.1034/j.1600-0854.2001.020202.x. [DOI] [PubMed] [Google Scholar]
- 16.Hess DA, Wirthlin L, Craft TP, et al. Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood. 2006;107:2162–2169. doi: 10.1182/blood-2005-06-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kania G, Corbeil D, Fuchs J, et al. Somatic stem cell marker prominin-1/CD133 is expressed in embryonic stem cell-derived progenitors. Stem Cells. 2005;23:791–804. doi: 10.1634/stemcells.2004-0232. [DOI] [PubMed] [Google Scholar]
- 18.Bauer N, Fonseca AV, Florek M, et al. New insights into the cell biology of hematopoietic progenitors by studying prominin-1 (CD133) Cells Tissues Organs. 2008;188:127–138. doi: 10.1159/000112847. [DOI] [PubMed] [Google Scholar]
- 19.Bidlingmaier S, Zhu X, Liu B. The utility and limitations of glycosylated human CD133 epitopes in defining cancer stem cells. J Mol Med. 2008;86:1025–1032. doi: 10.1007/s00109-008-0357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins AT, Habib FK, Maitland NJ, Neal DE. Identification and isolation of human prostate epithelial stem cells based on α(2)β(1)-integrin expression. J Cell Sci. 2001;114:3865–3872. doi: 10.1242/jcs.114.21.3865. [DOI] [PubMed] [Google Scholar]
- 21.Hudson DL, O’Hare M, Watt FM, Masters JR. Proliferative heterogeneity in the human prostate: evidence for epithelial stem cells. Lab Invest. 2000;80:1243–1250. doi: 10.1038/labinvest.3780132. [DOI] [PubMed] [Google Scholar]
- 22.Richardson GD, Robson CN, Lang SH, Neal DE, Maitland NJ, Collins AT. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci. 2004;117:3539–3545. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- 23.Litvinov IV, Vander Griend DJ, Xu Y, Antony L, Dalrymple SL, Isaacs JT. Low-calcium serum-free defined medium selects for growth of normal prostatic epithelial stem cells. Cancer Res. 2006;66:8598–8607. doi: 10.1158/0008-5472.CAN-06-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 25.Gao J, Arnold JT, Isaacs JT. Conversion from a paracrine to an autocrine mechanism of androgen-stimulated growth during malignant transformation of prostatic epithelial cells. Cancer Res. 2001;61:5038–5044. [PubMed] [Google Scholar]
- 26.Litvinov IV, Vander Griend DJ, Antony L, et al. Androgen receptor as a licensing factor for DNA replication in androgen-sensitive prostate cancer cells. Proc Natl Acad Sci U S A. 2006;103:15085–15090. doi: 10.1073/pnas.0603057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bethel CR, Faith D, Li X, et al. Decreased NKX3.1 protein expression in focal prostatic atrophy, prostatic intraepithelial neoplasia, and adenocarcinoma: association with Gleason score and chromosome 8p deletion. Cancer Res. 2006;66:10683–10690. doi: 10.1158/0008-5472.CAN-06-0963. [DOI] [PubMed] [Google Scholar]
- 28.Staack A, Donjacour AA, Brody J, Cunha GR, Carroll P. Mouse urogenital development: a practical approach. Differentiation. 2003;71:402–413. doi: 10.1046/j.1432-0436.2003.7107004.x. [DOI] [PubMed] [Google Scholar]
- 29.Xin L, Ide H, Kim Y, Dubey P, Witte ON. In vivo regeneration of murine prostate from dissociated cell populations of postnatal epithelia and urogenital sinus mesenchyme. Proc Natl Acad Sci U S A. 2003;100 Suppl 1:11896–11903. doi: 10.1073/pnas.1734139100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Sudilovsky D, Zhang B, et al. A human prostatic epithelial model of hormonal carcinogenesis. Cancer Res. 2001;61:6064–6072. [PubMed] [Google Scholar]
- 31.Meeker AK, Hicks JL, Iacobuzio-Donahue CA, et al. Telomere length abnormalities occur early in the initiation of epithelial carcinogenesis. Clin Cancer Res. 2004;10:3317–3326. doi: 10.1158/1078-0432.CCR-0984-03. [DOI] [PubMed] [Google Scholar]
- 32.Hayward SW, Haughney PC, Rosen MA, et al. Interactions between adult human prostatic epithelium and rat urogenital sinus mesenchyme in a tissue recombination model. Differentiation. 1998;63:131–140. doi: 10.1046/j.1432-0436.1998.6330131.x. [DOI] [PubMed] [Google Scholar]
- 33.Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci U S A. 2007;104:181–186. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunha GR, Sekkingstad M, Meloy BA. Heterospecific induction of prostatic development in tissue recombinants prepared with mouse, rat, rabbit and human tissues. Differentiation. 1983;24:174–180. doi: 10.1111/j.1432-0436.1983.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 35.Taylor RA, Cowin PA, Cunha GR, et al. Formation of human prostate tissue from embryonic stem cells. Nat Methods. 2006;3:179–181. doi: 10.1038/nmeth855. [DOI] [PubMed] [Google Scholar]
- 36.Kipling D, Cooke HJ. Hypervariable ultra-long telomeres in mice. Nature. 1990;347:400–402. doi: 10.1038/347400a0. [DOI] [PubMed] [Google Scholar]
- 37.Meeker AK, Gage WR, Hicks JL, et al. Telomere length assessment in human archival tissues: combined telomere fluorescence in situ hybridization and immunostaining. Am J Pathol. 2002;160:1259–1268. doi: 10.1016/S0002-9440(10)62553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meeker AK, Hicks JL, Platz EA, et al. Telomere shortening is an early somatic DNA alteration in human prostate tumorigenesis. Cancer Res. 2002;62:6405–6409. [PubMed] [Google Scholar]
- 39.Gelmann EP, Bowen C, Bubendorf L. Expression of NKX3.1 in normal and malignant tissues. Prostate. 2003;55:111–117. doi: 10.1002/pros.10210. [DOI] [PubMed] [Google Scholar]
- 40.Shmelkov SV, Jun L, St Clair R, et al. Alternative promoters regulate transcription of the gene that encodes stem cell surface protein AC133. Blood. 2004;103:2055–2061. doi: 10.1182/blood-2003-06-1881. [DOI] [PubMed] [Google Scholar]
- 41.Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5:280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 42.Veldscholte J, Berrevoets CA, Ris-Stalpers C, et al. The androgen receptor in LNCaP cells contains a mutation in the ligand binding domain which affects steroid binding characteristics and response to antiandrogens. J Steroid Biochem Mol Biol. 1992;41:665–669. doi: 10.1016/0960-0760(92)90401-4. [DOI] [PubMed] [Google Scholar]
- 43.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–5477. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langeler EG, van Uffelen CJ, Blankenstein MA, van Steenbrugge GJ, Mulder E. Effect of culture conditions on androgen sensitivity of the human prostatic cancer cell line LNCaP. Prostate. 1993;23:213–223. doi: 10.1002/pros.2990230304. [DOI] [PubMed] [Google Scholar]
- 45.Isaacs JT. Control of cell proliferation and cell death in the normal and neoplastic prostate: a stem cell model. Bethesda (MD): U.S. Department of Health and Human Services; 1987. pp. 85–94. NIH Publication #87-2881. [Google Scholar]
- 46.Dalrymple S, Antony L, Xu Y, et al. Role of notch-1 and E-cadherin in the differential response to calcium in culturing normal versus malignant prostate cells. Cancer Res. 2005;65:9269–9279. doi: 10.1158/0008-5472.CAN-04-3989. [DOI] [PubMed] [Google Scholar]
- 47.De Marzo AM, Nelson WG, Meeker AK, Coffey DS. Stem cell features of benign and malignant prostate epithelial cells. J Urol. 1998;160:2381–2392. doi: 10.1097/00005392-199812020-00004. [DOI] [PubMed] [Google Scholar]
- 48.van Leenders GJ, Gage WR, Hicks JL, et al. Intermediate cells in human prostate epithelium are enriched in proliferative inflammatory atrophy. Am J Pathol. 2003;162:1529–1537. doi: 10.1016/S0002-9440(10)64286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parsons JK, Gage WR, Nelson WG, De Marzo AM. p63 protein expression is rare in prostate adenocarcinoma: implications for cancer diagnosis and carcinogenesis. Urology. 2001;58:619–624. doi: 10.1016/s0090-4295(01)01311-5. [DOI] [PubMed] [Google Scholar]