Abstract
Background
Children with intellectual disability (ID) are at heightened risk for behaviour problems and diagnosed mental disorder. Likewise, mothers of children with ID are more stressed than mothers of typically-developing children. Research on behavioural phenotypes suggests that different syndromes of ID may be associated with distinct child behavioural risks and maternal well-being risks. In the present study, maternal reports of child behaviour problems and maternal well-being were examined for syndrome-specific differences.
Methods
The present authors studied the early manifestation and continuity of syndrome-specific behaviour problems in 215 preschool children belonging to 5 groups (typically-developing, undifferentiated developmental delays, Down syndrome, autism, cerebral palsy), as well as the relation of syndrome group to maternal well-being.
Results
At age 3, children with autism and cerebral palsy showed the highest levels of behaviour problems, and children with Down syndrome and typically-developing children showed the lowest levels. Mothers of children with autism reported more parenting stress than all other groups. These syndrome-specific patterns of behaviour and maternal stress were stable across ages 3, 4 and 5 years, except for relative increases in behaviour problems and maternal stress in the Down syndrome and cerebral palsy groups. Child syndrome contributed to maternal stress even after accounting for differences in behaviour problems and cognitive level.
Conclusions
These results, although based on small syndrome groups, suggest that phenotypic expressions of behaviour problems are manifested as early as age 3. These behavioural differences were paralleled by differences in maternal stress, such that mothers of children with autism are at elevated risk for high stress. In addition, there appear to be other unexamined characteristics of these syndromes, beyond behaviour problems, which also contribute to maternal stress.
Keywords: autism, behavioural phenotypes, cerebral palsy, Down syndrome, intellectual disability, syndrome specificity
Introduction
Individuals with intellectual disability (ID) are at heightened risk for also developing behaviour problems and mental disorder, a phenomenon known as dual diagnosis (Dykens et al. 2000; Gath & Gumley, 1986; Reiss, 1990). This increased risk is well-documented in adulthood, and most researchers estimate that between 20% and 35% of adults with ID have psychiatric disorders (Nezu et al. 1992). Dual diagnosis presents particular challenges for individuals and their caregivers, resulting in greater perceived family caregiving burden, increasing the likelihood of being placed out of the home in residential treatment settings, and putting individuals at greater risk for social isolation, failed attempts at community living, and poor academic and vocational outcomes (Borthwick-Duffy & Eyman, 1990; Bruininks et al. 1988; Maes et al. 2003; Pearson et al. 2000; Pfeiffer & Baker, 1994).
The increased risk for behavioural and psychiatric disorders appears to extend to children and adolescents with ID, thus bringing similar challenges to these children and their caregivers as to adults with ID (e.g. Emerson, 2003). Significant research efforts have been concentrated on measuring the nature and prevalence of behaviour and psychiatric problems among children with ID (Dykens, 2000). However, less attention has been given to the reasons behind dual diagnosis and to predicting the increased risk for psychopathology and behaviour problems among children with ID. Researchers are beginning to highlight the fact that specific syndromes associated with ID may have direct effects on children’s behaviour and psychiatric problems as well as indirect effects on the adjustment of their caregivers and family (Dykens, Hodapp, & Finucane, 2000; Hodapp, 1997).
The present study aimed to improve our understanding of the increased risk for psychopathology among children with developmental delays. We examined how specific syndromes of ID relate to the emergence of behaviour problems among young children and to the psychological well-being of their mothers. We studied preschool-aged children and their mothers longitudinally, from child ages 3 through 5. Prior research on syndrome-specific differences in behaviour has focused on older children; although some studies have wide age ranges that include some preschoolers, to our knowledge no study has focused on behavioural differences across these syndromes among children as young as 3 years of age. Included in our sample were children with specific syndromes associated with ID (Down syndrome, autism, cerebral palsy), as well as undifferentiated developmental delays and children without developmental delays.
Direct effects of syndrome: Psychopathology among children with ID
Research on school-age children and adolescents clearly demonstrates that those with ID are at heightened risk for psychiatric disorders (Merrell & Holland, 1997). In a recent epidemiological study, Emerson (2003) analyzed a national dataset of diagnostic information on over 10,000 children aged 5 to 15 years in Great Britain; 39% of children with ID met DSM-IV and ICD-10 criteria for at least one psychiatric disorder, compared to only 8.1% of children without ID. In particular, children with ID appear to be at greater risk for Attention Deficit Hyperactivity Disorder, conduct disorders, anxiety disorders, and pervasive developmental disorders (Einfeld & Tonge, 1996b; Emerson, 2003; Stromme & Diseth, 2000).
Although preschoolers rarely present diagnosable mental disorders, increased rates of psychopathology may be evident at an early age in the form of heightened behaviour problems. Feldman and colleagues (2000) found that two-year old children with, or at risk for, developmental delays did not have more behaviour problems than their typically-developing peers. On the other hand, previous work with the Collaborative Family Study (CFS), from which the current sample is derived, found that, by age 3, children with developmental delays already showed greater internalizing, externalizing, and total behaviour problems than typically-developing children (Baker et al. 2002). Considering Child Behaviour Checklist total behaviour problems T scores within the clinical range (Achenbach, 2000), the ratio was 3 or 4:1 (Baker et al. 2002). A subsequent longitudinal examination revealed stability in this ratio from age 3 to age 4 (Baker et al. 2003).
There are over 750 known genetic syndromes that cause ID, as well as other distinct syndromes that are not purely genetic, such as autism or cerebral palsy, but that are frequently associated with ID. Recent research suggests that important behavioural and psychiatric differences exist between individuals with specific aetiologies of ID, and researchers have begun to outline the behavioural phenotypes characterizing specific syndromes (Dykens et al. 2000). This syndrome-specific approach attempts to link behaviours and psychopathology to specific genetic or biological syndromes, with the goal of identifying behavioural phenotypes, or clusters of characteristic developmental and behavioural features, for these syndromes (Dykens, 1995; Dykens, 2000).
Considerable research has been conducted regarding the behavioural characteristics of two common syndromes: autism and Down syndrome. Much of this work has compared children with autism or Down syndrome to each other (Bieberich & Morgan, 1998; Loveland & Kelley, 1991), or to a control group of children with mixed aetiologies of ID (Gath & Gumley, 1986; Stores et al. 1998), or to typically-developing children (Rodrigue et al. 1991; Stores et al. 1998). Most studies compared no more than two or three groups. These studies typically have indicated that children with autism have heightened and wide-ranging difficulties (e.g. more negative affect, less positive affect, and less compliant, self-regulated, and socialized) while children with Down syndrome adjust better than children with other ID diagnoses and on some measures are similar to typically developing children (e.g. Bieberich & Morgan, 1998; Hodapp et al. 2001). Some studies have examined the behavioural phenotypes of less common, genetic syndromes of ID, such as Prader-Willi, Smith-Magenis, and Williams syndromes (e.g. Dykens & Kasari, 1997). It appears that many genetic syndromes bring together certain behavioural and physical features in ways that set the stage for specific, highly characteristic ways of behaving and interacting.
Research on syndrome-specific differences in behaviour is in its early stages, however, and many gaps remain in our understanding of these differences. For instance, very little research has examined the behavioural characteristics associated with cerebral palsy, especially in comparison to other types of ID. Children with cerebral palsy are often examined only as part of a mixed sample including children with spina bifida, cystic fibrosis, and other physical impairments (e.g., Breslau, 1985). Further, to our knowledge, researchers have not examined the early emergence of syndrome-specific behavioural differences among very young children and the continuity of these behavioural patterns over time. Finally, few studies have compared behaviour problems across several different syndromes of ID in contrast to control groups of children with undifferentiated developmental delays and children without developmental delays. The inclusion of such control groups is crucial for understanding the extent to which variation in a specific syndrome group is due to unique behavioural characteristics of the syndrome, rather than simply to relative differences between syndromes or to trends reflected by the general population.
Indirect effects of syndrome: Well-being of mothers of children with ID
Raising a child with ID also impacts parents’ well-being and is associated with increased parenting-related stress (e.g. Donenberg & Baker, 1993; Hauser-Cram et al. 2001; Rodrigue et al. 1990). Baker et al. (2002) found negative impact on mothers and fathers that was manifest as early as child age 3 years. Other researchers have found that parents of school age children with ID spend significantly more time issuing commands and working to gain compliance, and they experience more behaviour management struggles and coercive parent-child interactions (Floyd & Phillippe, 1993). Interestingly, greater stress and negative interactions appear to be more attributable to the increased levels of behaviour problems among children with developmental delay than to the presence of developmental delay itself (Baker et al. 2002; Baker et al. 2003; Floyd & Phillippe, 1993). This pattern appears in young adulthood as well; among mothers of young adults with moderate or severe ID, more behavioural or mental health issues in their offspring were associated with greater maternal stress, as well as a greater tendency to seek out-of-home placement (McIntyre et al. 2002).
Given the role of child behaviour problems in influencing parent stress and the likely differences in behaviour problems across syndromes, we expected that the impact, both positive and negative, of raising a child with ID would vary as a function of the child’s syndrome to the extent that behavioural challenges vary. There is some evidence that behavioural phenotypes are associated with the way children interact with and impact their environment, caregivers, family members, and friends. For instance, parents of school-age children with autism seem to experience particular adjustment difficulties, including increased depression, greater stress, and less marital satisfaction and intimacy (Fisman et al. 1989; Hoppes & Harris, 1990; Wolf et al. 1989), whereas mothers of school-age children with Down syndrome report higher perceived parenting competence than mothers of children with autism (Rodrigue et al. 1990).
The present study, then, addressed three central questions across child ages 3–5 years: (1) Are there syndrome-related differences in behaviour problems? (2) Are there syndrome-related differences in maternal well-being? and (3) Is there syndrome-specific variance in maternal well-being, even after cognitive delay and behaviour problems are accounted for?
Materials and Methods
Participants
Participants were 215 families with a 3-year-old with or without developmental delay recruited between 30 and 40 months of age. Families were from rural Pennsylvania (24%) and southern California, USA (76%). Families of children with developmental delays were primarily recruited through regional agencies that provide diagnostic and early intervention services for individuals with ID, and also purchase additional client services. In California, practically all families with a young child with ID register with the Regional Centres in order to receive services. All children were ambulatory and not diagnosed with autism at time of recruitment. They scored between 30 and 75 on the Bayley Scales of Infant Development II (BSID-II) at age 3, and children with undifferentiated developmental delays were only retained if they also scored below 85 on the Stanford-Binet Intelligence Scale IV (Stanford-Binet) at age 5. Children in the typically-developing group were recruited primarily through preschools and day care centers. They scored at or above 85 on the BSID-II at age 3 and the Stanford-Binet at age 5, were not born prematurely, and did not have developmental disabilities. Children scoring between 76 and 84 on the BSID-II (n = 11) were not included in the present sample, with the exception of one child with cerebral palsy who scored 79.
Children were classified in five groups: Typically-developing (n=136); undifferentiated developmental delay (n=43), Down syndrome (n=12), autism spectrum disorders (n=14) and cerebral palsy (n=10). Four children with other specific syndromes of ID--Soto syndrome, Smith-Magenis syndrome, cerebral migrational disorder, and Trisomy 18-P chromosomal disorder--were excluded from this sample. One child with a postnatal aetiology of ID, brain injury due to near drowning, was also excluded. One child who was diagnosed with both autism and cerebral palsy was excluded. As children with confirmed diagnoses of autism at intake were initially excluded from the larger sample at the time of recruitment, our group of children that we have labelled autism spectrum disorders (and, for simplicity will call “autism”) included any child with a developmental delay whose parents reported a diagnosis on the autism spectrum at any time subsequent to the age 3 assessment and continued to confirm this diagnosis at later assessment points. No single standardized diagnostic tool was used to diagnose autism; this classification was based on diagnoses given by service agencies that specialize in identifying and serving children with MR/DD. All of the children in the autism group had age 3 IQ scores in the mentally retarded range, and none of these children had a diagnosis of Asperger’s Disorder.
Table 1 shows the demographic characteristics of the sample by syndrome group status. Children’s age at intake averaged 35.3 months (SD = 3.1); 97% of the children were between the ages of 30 and 40 months at intake. Fifty-five percent of the children were boys. Fifty-nine percent of the children were Caucasian, 16% were Latino, 9% were African-American, 3% were Asian-American, and 13% were other ethnicities. Because recruitment initially focused on intact families, 84% of participating parents were married (defined here as legally married or living together for at least six months). Overall, about half of mothers (48%) and fathers (46%) graduated from college, and about half of families (53%) earned more than $50,000 annually.
Table 1.
Demographics by Syndrome Group at child age three (n=215)
| TD (n = 136) |
UDD (n = 43) |
DS (n = 12) |
Autism (n = 14) |
CP (n = 10) |
χ2 or F | |
|---|---|---|---|---|---|---|
| Children | ||||||
| Mean age (SD) at testing (months) | 35.0 (3.1) | 36.0 (2.5) | 34.7 (3.6) | 36.5 (3.7) | 34.7 (2.4) | F=1.70 |
| Gender (% boys) | 50.0a | 58.1a | 58.3a | 100b | 40.0a | χ2=113.96** |
| Race (% Caucasian) | 59.6 | 55.8 | 58.3 | 78.6 | 40.0 | χ2=3.91 |
| Siblings (% only children) | 30.1 | 20.9 | 33.3 | 42.9 | 30.0 | χ2 =2.84 |
| Mean BSID-III MDI score (SD) | 105.1a (11.4) | 61.1b (8.8) | 45.1c (9.3) | 58.1b (8.6) | 55.6bc (16.0) | F=243.56*** |
| Mean Stanford-Binet score (SD) | 105.1a (11.2) | 67.3b (13.2) | 44.0c (8.4) | 65.8b (20.4) | 61.5b (21.0) | F=125.92*** |
| Parent and family | ||||||
| Marital status (% married) | 88.2a | 69.8b | 91.7ab | 85.7ab | 70.0ab | χ2 =10.16* |
| Mother’s education (% college degree) | 61.0a | 11.6b | 75.0a | 42.9ab | 10.0b | χ2 =41.45*** |
| Mother’s employment (% employed) | 60.3 | 55.8 | 66.7 | 50.0 | 70.0 | χ2 =1.52 |
| Mothers’ mean age (SD) | 34.1a (5.6) | 31.8a (6.7) | 33.6a (5.6) | 35.6a (5.6) | 30.7a (5.5) | F=2.34 |
| Father’s education (% college degree) | 50.0a | 38.2b | 41.7a | 21.4b | 55.6b | χ2 =5.43 |
| Father’s employment (% employed) | 96.0 | 97.3 | 91.7 | 92.9 | 88.9 | χ2 =1.86 |
| Fathers’ mean age (SD) | 36.8 (6.5) | 37.3 (9.1) | 36.3 (7.1) | 35.4 (4.9) | 32.4 (4.0) | F=.98 |
| Family income (% $50K +) | 60.7a | 32.6b | 58.3ab | 42.9ab | 40.0ab | χ2 =11.85* |
Means designated by different letters (a, b, c) are significantly different, P<0.05.
P<0.05,
P<0.01,
P<0.001.
TD: typically developing; UDD: undifferentiated developmental delays; DS = Down syndrome; CP = cerebral palsy. BSID: Bayley Scales of Infant Development; MDI: Mental Development Index.
As shown in Table 1, the five groups did not differ on any child attributes except for the gender ratio and their BSID-II and Stanford-Binet scores. Whereas most groups showed approximately equal numbers of boys and girls, all children with autism were boys, consistent with the population of children diagnosed with autism. Children with Down syndrome scored lower on the IQ measures than other ID groups. On parent attributes, mothers’ level of education varied by group, and thus was entered as a covariate in all subsequent analyses. Marital status and family income also varied significantly by syndrome group, though less strongly than maternal education. After maternal education was accounted for, neither marital status nor family income related significantly to syndrome group. Thus, marital status and family income were not entered as covariates in subsequent analyses.
Assessments
Data were obtained through home visits, laboratory sessions, and mailed questionnaires. The initial measures of the child’s behaviours and level of cognitive development were obtained at a home assessment when the child was approximately 36 months old. Prior to this initial visit, parents received project descriptions and the informed consent form, and completed a telephone interview with project staff. Two trained examiners visited the family’s home for two hours. After reviewing study procedures, answering questions, and obtaining parental informed consent, they administered the BSID-II to the child. During this testing, the child’s mother, and father if present, completed the Child Behaviour Checklist (CBCL) and a demographic questionnaire, including information about the child’s health and development. Measures of parental well-being were obtained at 36 months and again, along with the CBCL, at child age 48 and 60 months. At child age 60 months, each family came to a laboratory session, where a trained examiner administered the Stanford-Binet to the child.
Measures
Bayley Scales of Infant Development (2nd Edition)
The BSID-II (Bayley, 1993) is a widely used measure of mental and motor development in children ages one month to 42 months. Only the mental development items were administered. The Mental Development Index (MDI) is normed with a mean score of 100 and a standard deviation of 15. Bayley (1993) reported high short-term test-retest reliability for the MDI, r = .91. With children ages 36 to 42 months, the MDI related to the Full Scale IQ of the Wechsler Preschool and Primary Scale of Intelligence-Revised (WPPSI-R), r = .73 (Bayley, 1993).
Stanford-Binet Intelligence Scale – 4th Edition (Stanford-Binet)
The Stanford-Binet (Delaney & Hopkins, 1987) is a widely used assessment of current cognitive functioning in children and adults ages two to 85 years. The Stanford-Binet yields a Composite Score of overall cognitive functioning, as well as Standard Area Scores. Only the Composite Score was used in the present analyses. The Composite Score is normed with a mean score of 100 and a standard deviation of 15. The Stanford-Binet has been shown to be a reliable and valid measure of cognitive functioning among children (Glutting, 1989).
Child Behaviour Checklist for Ages 1.5–5
The CBCL (Achenbach, 2000) is a new version of the widely used CBCL (Achenbach, 1991), designed for preschool-age children aged 1.5 to 5 years. The questionnaire has 99 items indicating child problems, listed in alphabetical order (from “aches and pains without medical cause” to “worries”). For each item, the respondent indicates whether it is not true (0), somewhat or sometimes true (1), or very true or often true (2) now or within the past two months. The CBCL produces a total behaviour problems T score with a mean of 50 and a standard deviation of 10, broadband externalizing problems and internalizing problems T scores, and seven narrowband scale scores. Total score alpha for the present sample was .95 for mothers; scale alphas are shown in Table 2.
Table 2.
Mothers’ Child Behaviour Checklist (CBCL) score means by syndrome group at age 3. Analyses were run as univariate analyses of covariance, covarying for mother’s education.
| CBCL scale (# of items) | TD | UDD | DS | Autism | CP | F-value for syndrome | Alpha |
|---|---|---|---|---|---|---|---|
| Total T (99) | 50.2a (9.3) | 57.0bc (10.9) | 49.4ab (11.0) | 62.3c (10.8) | 63.0c (7.9) | 8.06*** | .95 |
| Externalizing T (24) | 50.1a (9.9) | 55.7ab (11.1) | 49.4ab (9.3) | 60.2b (12.6) | 60.0ab (5.7) | 4.96** | .91 |
| Internalizing T (36) | 48.5a (9.9) | 56.2bc (10.8) | 46.5ab (12.5) | 59.8c (10.6) | 61.6c (10.2) | 7.27*** | .88 |
| Aggressive behaviour (19) | 10.7a (6.5) | 13.8a (7.8) | 8.5a (5.7) | 15.6a (8.4) | 15.5a (5.0) | 3.00* | .89 |
| Anxious/Depressed (8) | 2.3a (2.0) | 3.7ab (2.6) | 1.4a (1.2) | 2.9ab (3.2) | 4.9b (2.6) | 4.28** | .68 |
| Attention problems (5) | 2.2a (1.9) | 3.9b (2.5) | 3.8ab (2.2) | 5.2b (2.6) | 5.6b (1.6) | 12.97*** | .73 |
| Emotionally reactive (9) | 2.2a (1.9) | 3.1ab (2.9) | 1.6a (1.6) | 4.9bc (4.7) | 5.6c (2.7) | 6.85*** | .71 |
| Sleep problems (7) | 3.6(2.8) | 3.5(3.1) | 2.4(2.9) | 3.9(3.8) | 4.4(2.6) | .71 | .76 |
| Somatic Complaints (11) | 1.6a (1.9) | 2.9b (2.5) | 1.7ab (2.9) | 3.2ab (2.7) | 3.2ab (3.3) | 3.67** | .66 |
| Withdrawn (8) | 1.6a (1.8) | 3.4b (2.5) | 2.8ab (3.3) | 5.2b (3.1) | 3.9b (2.6) | 12.39*** | .74 |
Means designated by different letters (a, b, c) are significantly different, P<0.05.
P<0.05,
P<0.01,
P<0.001.
TD: typically developing; UDD: undifferentiated developmental delays; DS = Down syndrome; CP = cerebral palsy.
Family Impact Questionnaire
The Family Impact Questionnaire (FIQ; Donenberg & Baker, 1993) is a 50-item questionnaire that measures parents’ perceptions of the “child’s impact on the family compared to the impact other children his/her age have on their families” (e.g. item 1: “My child is more stressful”). Parents endorse items on a four-point scale ranging from not at all to very much. There are five subscales measuring perceptions of the child’s negative impact on their feelings about parenting (9 items), social relationships (11), finances (7), and, if applicable, siblings (9) and marriage (7). A sixth subscale measures perceptions of the child’s impact on positive feelings about parenting (7 items). We examined the positive impact score and a combined negative impact score, created by combining the social relationships subscale and the negative feelings about parenting subscale. Alphas for mothers in the present sample were .92 for the combined negative impact score and .81 for positive impact.
Centre for Epidemiological Studies Depression Scale
The Centre for Epidemiological Studies Depression Scale (CES-D; Radloff, 1977) is a 20-item questionnaire that was designed to measure depressive symptoms in the general adult population. With adults ages 18 to 54 years, the CES-D related to the Beck Depression Inventory (BDI), r = .75 (Skorikov & Vandervoort, 2003). The total score alpha for the present sample of mothers was .88.
Results
Child behaviour problems at age 3 by syndrome
Our first question asked whether there are syndrome-related differences in behaviour problems; we examined the syndrome group ranking, percentages in the borderline or clinical range, and mean scores at age 3 and across the preschool years. Table 2 shows means and standard deviations for the mother-reported CBCL T scores at child age 3 across the five syndrome groups (typically-developing, undifferentiated developmental delays, Down syndrome, autism, and cerebral palsy). The autism and cerebral palsy groups each ranked first or second on nine of the 10 CBCL scales (total, 2 broadband and 7 narrowband). At the other extreme, the Down syndrome group ranked lowest among the groups with developmental delay on all CBCL subscales. Also, children with Down syndrome ranked lowest overall on 7 of the 10 CBCL scales, showing even fewer behaviour problems than typically-developing children.
Children with behaviour problems in the borderline or clinical range
Mother-reported CBCL total T scores at age 3 were in the borderline or clinical range (≥ 60; Achenbach, 2000) for 10.3% of typically-developing children, 41.5% of children with undifferentiated developmental delays, 8.3% of children with Down syndrome, 46.2% of children with autism, and 50% of children with cerebral palsy. A Pearson Chi-square test showed a highly significant relationship between syndrome group and CBCL clinical status (borderline or clinical range vs. non-clinical range) [χ2 (4, N = 212) = 31.67, p < .001]. Follow-up Pearson Chi-square tests compared the typically-developing group to each delay group. Typically-developing children did not differ from children with Down syndrome, but were significantly less likely to have behaviour problems in the clinical or borderline range than children with undifferentiated delays [χ2 (1, N = 177] = 21.19, p < .001], autism [χ2 (1, N = 149] = 13.13, p < .001], or cerebral palsy [χ2 (1, N = 146] = 12.97, p < .001].
Analyses of child behaviour problems at age 3 by syndrome
ANCOVAs were conducted, with syndrome as the independent variable, mothers’ level of education as a covariate, and CBCL score as the dependent variable. As shown in Table 2, there was a significant main effect of syndrome group on mother-reported CBCL total behaviour problems, externalizing problems, internalizing problems, and all subscales except for sleep problems. Planned comparisons were conducted to identify differences between specific syndrome groups, with a Sidak adjustment for multiple tests. Table 2 reflects specific between-group differences on all CBCL scales by superscript letters. To summarize total and broadband score differences, children with undifferentiated developmental delays had significantly more total behaviour problems and more internalizing problems than typically-developing children. Children with autism or cerebral palsy had more total behaviour problems and more internalizing problems than children with Down syndrome or typically-developing children, and children with autism had significantly more externalizing behaviour problems than typically-developing children.
Continuity of child behaviour problems
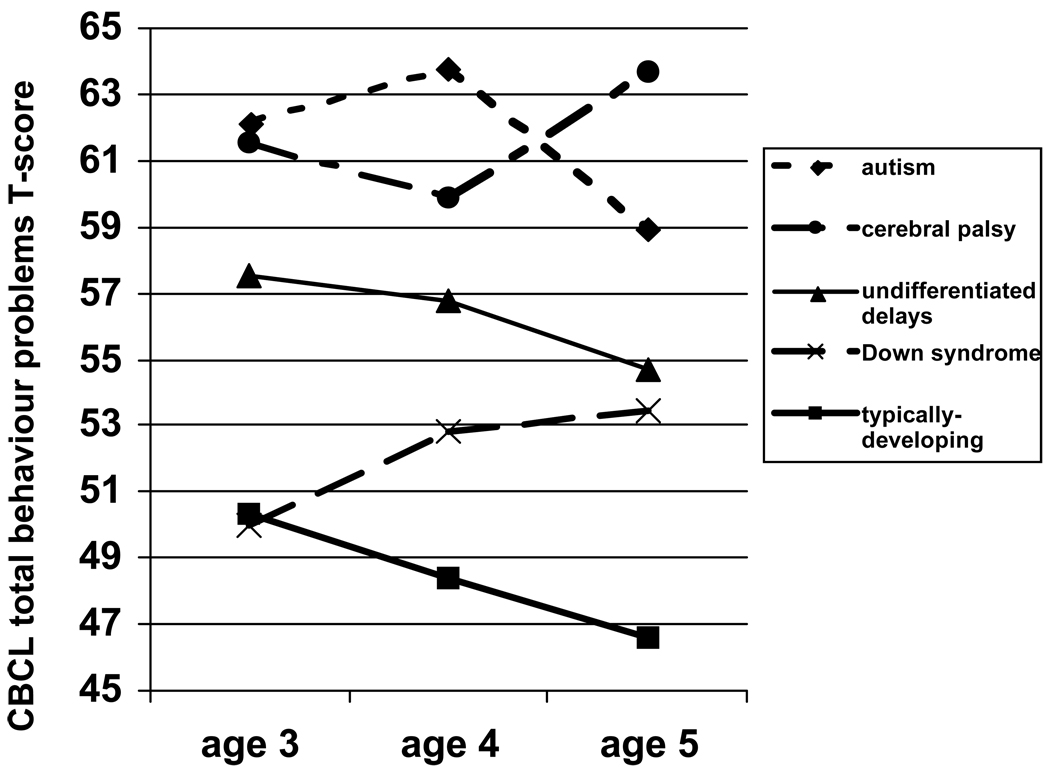
The stability of the relationships between syndrome group and behaviour problems was examined across child ages 3, 4, and 5 years. A repeated measures ANCOVA was conducted, with CBCL total behaviour problem T scores as the dependent variable, syndrome group and child age as independent variables, and mother education as a covariate. Figure 1 shows a significant main effect of syndrome [F (4, 171) = 8.29, p ≤ .001], a non-significant main effect of child age [F (2, 170) = 2.02, p = .14], and a significant age by syndrome interaction [F (8, 342) = 2.11, p < .05]. The rank order pattern across ages remained generally consistent, with two exceptions. First, while the autism and cerebral palsy remained the most elevated in total behaviour problem scores, the autism group was highest in behaviour problems at ages 3 and 4, but the cerebral palsy group emerged as even higher in behaviour problems at age 5. Second, while the Down syndrome group and typically-developing group showed comparable total behaviour problems at age 3, behaviour problems increased in the Down syndrome group and decreased in the typically developing group over time. Further examination of these changes revealed that children with Down syndrome showed a significant increase in their externalizing behaviour problems from age 3 to age 5 [t (10) = −2.93, p< .05], specifically on the aggression subscale [t (10) = −2.54, p < .05]. On the other hand, the other four groups of children as a whole showed a significant decrease from age 3 to 5 in aggression [t (169) = 4.00, p < .001], externalizing problems [t (170) = 4.73, p < .001] and total behaviour problems [t (170) = 5.43, p < .001].
Figure 1.
Mother-reported Child Behaviour Checklist (CBCL) Total T scores by syndrome group and child age. A repeated measures analysis of covariance was conducted, covarying for mother’s education.
Maternal well-being at child age 3 by syndrome
Our second question asked whether there are syndrome-related differences in maternal well-being; we examined whether syndrome group means differed in maternal stress, depression, and positive impact, at age 3 and across the preschool years. Table 3 shows mother-reported positive and negative impact on the FIQ and symptoms of depression on the CES-D, across syndrome groups when children were 3 years old. The autism group ranked highest in negative impact and maternal depression and lowest in positive impact. Similarly, the cerebral palsy group ranked second only to the autism group on negative impact and depression, although they ranked highest in positive impact. Mothers of children with undifferentiated developmental delays fell in the middle of the five groups on all three well-being measures. The Down syndrome group was ranked lowest in maternal depression and second lowest to typically-developing children in negative impact, although they were ranked second lowest in positive impact. We conducted ANCOVAs, with syndrome group as the independent variable, mothers’ level of education as a covariate, and family impact or depression scores as the dependent variable. There was a highly significant group difference in negative impact scores, with the autism group reporting the highest negative impact, significantly greater than the typically developing, Down syndrome, and undifferentiated delay groups and marginally greater than the cerebral palsy group (P = 0.07).1 Depression and positive impact scores did not differ significantly across syndrome groups.
Table 3.
Mothers’ Family Impact (FIQ) and Depression (CES-D) by syndrome group at age three. Analyses were run as univariate analyses of covariance, covarying for mother’s education.
| TD | UDD | DS | Autism | CP | F-value for syndrome | |
|---|---|---|---|---|---|---|
| FIQ Negative Impact combined score | 11.1a (8.0) | 18.3b (11.5) | 12.6ab (8.8) | 29.7c (15.5) | 18.8abc (8.4) | 13.14*** |
| CES-D total depression | 9.6 (9.0) | 10.4 (7.6) | 7.3 (4.3) | 14.9 (11.3) | 10.9 (8.6) | 1.14 |
| FIQ Positive Impact | 16.3 (4.7) | 16.0 (5.1) | 15.1 (4.1) | 13.3 (5.6) | 18.2 (3.4) | 1.92 |
Means designated by different letters are significantly different, P<0.05.
P<0.001.
TD: typically developing; UDD: undifferentiated developmental delays; DS = Down syndrome; CP = cerebral palsy. FIQ: Family Impact Questionnaire; CES-D: Centre for Epidemiological Studies Depression Scale.
Continuity of maternal well-being
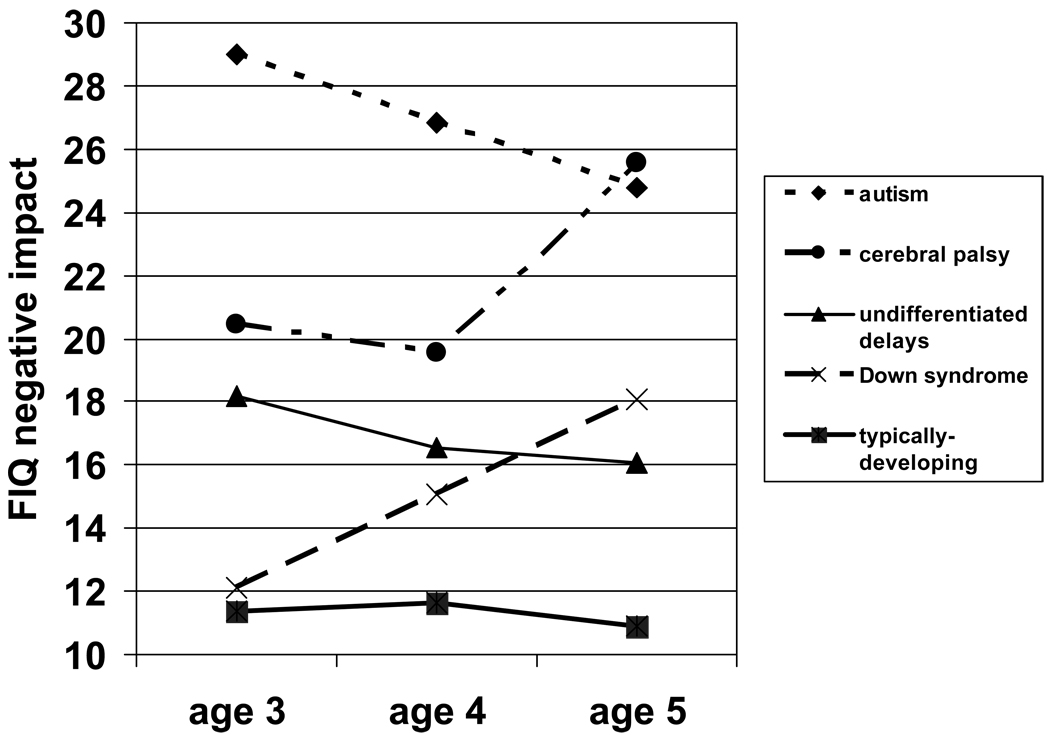
For the relationship between syndrome group and maternal stress over time, a repeated measures ANCOVA was conducted, with scores on mother-reported negative impact as the dependent variables, syndrome group and child age as independent variables, and mother education as the covariate. Figure 2 shows a significant main effect of syndrome [F (4, 172) = 10.15, p < .001], a non-significant main effect of child age [F (2, 171) = 1.24, p = .29], and a significant age by syndrome interaction [F (8, 344) = 2.95, p < .01]. The rank-order pattern in maternal stress observed at age 3 remained generally consistent across ages 4 and 5 with two exceptions, mirroring the changes observed in child behaviour problems. Taken together, Figures 1 and 2 demonstrate the extent to which trends in child behaviour problems over time were paralleled by similar patterns in maternal stress. As with reports of child behaviour problems, mothers’ reports of negative impact in the Down syndrome group increased at ages 4 and 5, surpassing mothers of the undifferentiated delay group by age 5. Secondly, paralleling child behaviour problem levels, mothers of the cerebral palsy group reported less stress than mothers of the autism group at ages 3 and 4, but reported more stress than mothers of the autism group by age 5.
Figure 2.
Mothers’ Family Impact Questionnaire (FIQ) negative impact scores by syndrome group and child age. A repeated measures analysis of covariance was conducted, covarying for mother’s education.
Contributions of child behaviour problems, cognitive level, and syndrome to maternal well-being
Our third question asked whether there is syndrome-specific variance in maternal well-being, even after cognitive delay and behaviour problems are accounted for. The group differences observed in mothers’ reports of negative impact seemed to parallel syndrome group differences in child behaviour problems. Thus we employed hierarchical linear regression analysis to determine whether significant variance in FIQ negative impact at child age 3 was explained by child syndrome group even after controlling for child behaviour problems and cognitive level. Mothers’ level of education was entered in Step 1, child behaviour problems (CBCL total T score) and cognitive level (Bayley MDI) were entered in Step 2, and child syndrome group was entered in Step 3, with mothers’ FIQ negative impact as the dependent variable. The final model accounted for 52.1% of variance. As shown in Table 4, Step 1, mothers’ education, accounted for 2.6% of the variance. Step 2, child behaviour problems and cognitive level, accounted for an additional 45.8% of the variance; both behaviour problems (t = 11.63, p < .001) and cognitive level (t = −3.05, p < .01) contributed significantly to negative impact in Step 2. Step 3, child syndrome group, accounted for an additional 3.7% of the variance. Thus child syndrome group contributed to maternal stress (negative impact) above and beyond the contributions of behaviour problems and cognitive level. Standardized betas for the final model were significant only for behaviour problems and the autism syndrome group.
Table 4.
Summary of hierarchical regression analysis for variables predicting mother - reported negative impact (FIQ) at child age 3 (N=199)
| Variable | Mean Square |
d.f. | Unstandardiz ed beta (SE) |
Standardi zed beta |
R2 | R2 chg |
F R2 change |
P |
|---|---|---|---|---|---|---|---|---|
| Step 1 | 573.2 | 1, 198 | .026 | .026 | 5.23 | .023 | ||
| Mother’s level of education | −1.02 (.45) | −0.16* | ||||||
| Step 2 | 3590.7 | 2, 196 | .484 | .458 | 87.01 | < .001 | ||
| Mother’s level of education | 0.32 (0.35) | 0.05 | ||||||
| Child total behaviour problems | 0.63 (0.05) | 0.64*** | ||||||
| Child cognitive level (BSID MDI) | −0.07 (0.02) | −0.17** | ||||||
| Step 3 | 1656.2 | 4, 192 | .521 | .037 | 3.70 | .006 | ||
| Mother’s level of education | 0.34 (0.35) | 0.05 | ||||||
| Total behaviour problems | 0.60 (0.06) | 0.60*** | ||||||
| Child cognitive level (BSID MDI) | −0.04 (0.05) | −0.10 | ||||||
| Syndrome (undifferentiated delays vs. all other groups) | −1.47 (2.52) | −0.06 | ||||||
| Syndrome (Down syndrome vs. all other groups) | −0.59 (3.71) | 0.01 | ||||||
| Syndrome (autism vs. all other groups) | −8.85 (3.13) | −0.21*** | ||||||
| Syndrome (cerebral palsy vs. all other groups) | .87 (3.48) | 0.02 |
P<0.05,
P<0.01,
P<0.001.
FIQ: Family Impact Questionnaire; BSID: Bayley Scales of Infant Development; MDI: Mental Development Index.
To further understand the role of cognitive level, this regression was repeated including only the children in the delay groups. In Step 2, child behaviour problems continued to contribute to negative impact (t = 8.02, p < .001), but child cognitive level no longer contributed to negative impact (t = .08, p = ns). In Step 3, child syndrome continued to make a marginally significant contribution to maternal reports of negative impact [F-value change (3, 65) = 2.47, p = .07]. This effect of syndrome was again carried by the autism grouping variable, for which there was a significant t-value (t = −2.19, p < .05) in Step 4.
Hierarchical linear regressions were repeated on data from child ages 4 and 5 years, including all groups. BSID Mental Development Index was again used to approximate cognitive level at age 4, and Stanford-Binet IQ score, assessed at age 5, was used to approximate cognitive level at age 5. Since the autism group accounted for the effect of syndrome at age 3, only this variable was entered in Step 4. The final model accounted for 49.9% of variance at age 4 and 52.6% at age 5. The autism grouping variable continued to contribute significantly to maternal negative impact even after controlling for maternal education, child behaviour problems, and child cognitive level [F-value change (1, 173) = 5.89, p <.05 at age 4; F-value change (1, 176) = 6.30, p < .05 at age 5].
Discussion
The first question of interest was whether preschool children with specific syndromes of ID manifested phenotypic expressions of behavioural problems, in comparison to each other as well as to children who have undifferentiated developmental delays or those who are typically-developing. Overall rates of problem behaviour in the ID groups were high, with 38.2% of these 3-year-old children scoring in the borderline or clinical range on CBCL total behaviour problems, compared to 10.3% of the typically-developing children. Behaviour problems differed by syndrome, with the highest levels found among children with autism or cerebral palsy. Children with Down syndrome were similar to typically-developing children, with these two groups generally showing the lowest levels of behaviour problems. The group with undifferentiated delays generally fell between these extremes. Similar differences were found across an array of behaviour problem measures, including the CBCL total, externalizing, internalizing, and six sub-scale scores. These findings lend support to a growing body of literature that highlights behavioural differences across syndromes and emphasizes the importance of a syndrome-specific understanding of children’s development of behavioural and psychiatric problems (e.g. Dykens, Hodapp, & Finucane, 2000). The inclusion of a typically-developing comparison group allowed more balanced interpretation of behavioural aspects of syndrome phenotypes (Abbeduto et al. 2003.)
The present results are consistent with those from studies of behaviour problems in school-age children, where children with Down syndrome were more compliant, with better self-regulation, than those with autism (e.g. Bieberich & Morgan, 1998). Also, our findings that children with Down syndrome were less emotionally reactive, with fewer internalizing or total behaviour problems than children with autism or cerebral palsy, are consistent with findings with older children and young adults showing that those with Down syndrome had fewer behavioural and psychiatric problems than persons with other ID syndromes. (e.g. Blacher & McIntyre, 2003; Stores et al. 1998). However, the elevated behaviour problems in our sample of young children with cerebral palsy were contrary to Blacher and McIntyre’s (2003) finding that young adults with cerebral palsy were lower in behaviour problems than those with autism. This discrepancy may be attributable to the lower functioning level in Blacher and McIntyre’s sample of individuals with moderate or severe mental retardation, where many individuals with cerebral palsy were non-ambulatory.
Our second hypothesis concerned syndrome group differences in maternal stress and well-being. Extensive literature has established the increased risk for adjustment problems among families of children with ID (e.g. Donenberg & Baker, 1993; Hauser-Cram et al. 2001), and some previous work has found this negative impact on families to be associated with the level of psychiatric or behaviour problems in the child (Baker et al. 2003). We further found that mothers’ reports of negative impact (stress) differed significantly by syndrome group at child age 3, with mothers in the autism group reporting higher negative impact than all other groups except cerebral palsy. This finding is consistent with the elevated stress reported by mothers of older children with autism (e.g. Hoppes & Harris, 1990; Wolf et al. 1989).
Interestingly, mothers of children in the cerebral palsy group did not report significantly more negative impact at age three than mothers of other groups, even though their children showed elevated levels of behaviour problems comparable to those of the autism group. These findings suggest that there are other aspects of the autism and cerebral palsy phenotypes, beyond behaviour problems, that differentially impact mothers’ experiences of stress.
We found that maternal depression did not differ significantly by syndrome group, although the rank order of syndrome groups was generally consistent with negative impact. This is consistent with previous studies in which measures that were not directly related to child-rearing did not suggest lower well-being for parents raising young children with disabilities (Baker et al., in press; Donenberg & Baker, 1993; Dyson, 1997). Thus, raising a child with disabilities may at first only affect child-related domains of well-being but may, as the child grows older, affect mood and other domains as well.
We also found that mothers’ reports of the positive impact of the child did not differ by syndrome group. Positive impact may be related more to parental personality and cultural perspectives than to actual child behaviour (Baker & Blacher, 2004). These results support the current assertion that researchers should examine positive as well as negative outcomes (Taunt & Hastings, 2002; Stainton & Besser, 1998), given that the specific syndrome pattern of positive impact did not mirror the pattern of negative impact.
We also examined the continuity of syndrome group differences in behaviour problems and maternal well-being over time. As expected, specific syndromes continue to relate significantly to children’s expression of behaviour problems across the preschool years. There was, however, an interaction between child age and syndrome, accounted for by the increase over time in behaviour problems among the Down syndrome group relative to other groups. Also, the relation between syndrome groups and maternal experience of stress was maintained across the preschool years. Here, too, there was an interaction between child age and syndrome, accounted for by an increase over time in negative impact among the Down syndrome and cerebral palsy groups relative to other groups. These increases in behaviour problems and negative impact among children with Down syndrome may, in part, reflect their characteristic stubbornness (Dykens et al. 2000).
These findings demonstrate much continuity in relative levels of behaviour problems and negative impact across syndrome groups. However, they also suggest that the protective effects of Down syndrome against behaviour problems and maternal stress in comparison with other syndromes may be most evident among very young children and may already be diminishing by age 5. This observed trend among preschool children with Down syndrome may complement the cross-sectional results of Dykens et al. (2002), who found that children aged 4 to 6 years with Down syndrome showed fewer externalizing and internalizing problems than those aged 10 to 13 years. A better understanding of developmental trajectories of behavioural and psychiatric risks related to Down syndrome or other syndromes will most likely come from longitudinal studies.
Behaviour problems in children with cerebral palsy also increased from age 3 to 5, at which point they surpassed the autism group’s behaviour problems, which had decreased from age 3 to 5. This discrepancy may reflect the differences in services available to the two groups. Whereas intensive, behavioural interventions are available to many young children with autism in the early school years, services targeting children with cerebral palsy may be more likely to focus on physical, speech, or occupational therapy. These may be less likely to address behaviour problems of children with cerebral palsy with the intensity found in many autism treatment programs.
Lastly, we examined the relative contributions of syndrome, cognitive level, and behaviour problems to maternal stress. Regression analyses revealed that while child behaviour problems accounted for considerable variance in maternal stress, child syndrome contributed to maternal stress after controlling for behaviour problems and cognitive level. This remaining contribution of syndrome group was primarily accounted for by the autism group, which contributed significantly to maternal stress at ages 3, 4, and 5. One possible explanation is that there are other behaviours characteristic of autism that are not included in the CBCL listing of behaviour problems (e.g. self-injury, insistence on routine, social avoidance, dysregulated sleep and waking cycles) which appear to cause distress for caretakers. For instance, Hoppes and Harris (1990) found that the lower interpersonal responsiveness of children with autism may be an added source of distress for their mothers. Characteristics beyond cognitive level and behaviour problems appear to affect parents raising children with other syndromes as well. Researchers have hypothesized that the physical limitations of children with cerebral palsy may be a unique source of stress for parents of these children (Pisula, 1998), and the characteristically sociable nature of children with Down syndrome may protect against stress in parents (Hodapp et al. 2001).
The current study has several limitations in the sample and method that should be considered in interpreting results. First, as the original sample was not specifically recruited to include children with specific syndromes of ID, our syndrome group sizes are small, limiting statistical power to detect group differences in behaviour problems and maternal stress. Second, parents in our sample had somewhat above average education; 48% of mothers had a college degree compared to 27.2% of adults in the general population (U.S. Census Bureau, 2004). Third, diagnoses of syndromes were based on parent report of diagnoses from agencies serving children with ID as well as, in some cases, independent assessments; they were not otherwise verified for the purposes of this study. In particular, the autism diagnoses may be less valid than if they were based on a standardized assessment. Finally, the present analyses did not include fathers, so we do not know if the syndrome specific effects we have found hold for both parents.
We chose not to control for children’s mental age in this study. A strength of our design is that it provides a typically-developing baseline group against which to compare the syndrome groups. Removing the variance associated with mental age in our sample would, effectively, eliminate any differences between typically-developing children and children with ID on our variables of interest.
In another potential drawback of the study, mothers in our sample reported on both behaviour problems and maternal stress; therefore, the shared method variance between these measures may partially account for the relationship found between behaviour problems and maternal stress in the regression analysis. In order to assess this possibility, we repeated the regression analysis replacing mother-reported CBCL total T scores with father-reported CBCL total T scores as our measure of behaviour problems, and continuing to use mother-reported FIQ scores of negative impact. In our original analysis we established that child behaviour problems and cognitive ability contributed significantly to variance in negative impact in Step 2 of the regression, and that there was still remaining variance accounted for by syndrome group in Step 3. Using father-reported CBCL scores we found similar results. In Step 2, child behaviour problems and cognitive level accounted for 31.0% of the variance, and father-reported behaviour problems alone contributed significantly to mothers’ negative impact (t = 7.44, p < .001). Step 3, child syndrome group, also continued to contribute significantly to mothers’ negative impact, accounting for an additional 5.8% of the variance. These findings are consistent with the findings in the original regression analysis. Thus, the relationship between child behaviour problems and maternal stress persists even after removing the shared method variance.
In sum, syndrome specific behavioural patterns were manifested in children at 3 years of age and were relatively stable across the preschool years. Syndrome made a significant contribution to maternal stress above and beyond the contribution of cognitive ability and behaviour problems. This finding underscores the need for the identification and examination of additional factors beyond behavioural problems that may differ by syndrome. For instance, personality characteristics, availability of intervention services, occupational or physical limitations, and other developmental features of specific syndromes should be explored for their impact on parent well-being. This research also highlights the importance of understanding syndrome-specific age-related patterns of behaviour problems, as evidenced by the increase in such problems among children with Down syndrome over time. Finally, these findings suggest that interventions that are targeted to specific syndromes of ID, and their associated phenotypic expressions of behavioural and psychiatric problems, may be particularly effective, not only for children but also for the adjustment of their mothers and other family members.
Acknowledgments
This article is based on the activities of the Collaborative Family Study (CFS), supported by NICHD grant 34879-1459 (Dr Keith Crnic, Principal Investigator (PI), and Drs Bruce L. Baker, Jan Blacher and Craig Edelbrock, co-PIs). The CFS is conducted at three sites: Pennsylvania State University, State College, PA, the Fernald Child Study Center at the University of California, Los Angeles, CA, and the Vernon Eady Center at the University of California, Riverside, CA, USA. We are indebted to our co-workers on the CFS, Jason Baker, Shannon Bekman, Rachel Fenning, Catherine Gaze, Casey Hoffman, Sandra Minassian, Emilie Paczkowski, and Araksia Trmrian.
Footnotes
As child gender is likely related to both behaviour problems and maternal stress, the question arises as to whether child gender may account for the syndrome specific differences obtained. Child gender could not be included as a factor in the analyses because the autism group contained all boys; this disorder is strongly gender-linked. To explore gender effects, we reran the ANCOVA analyses excluding the autism group. Gender as a covariate contributed significantly to child behaviour problems (p < .05) and to maternal stress (p < .01); however, child syndrome group continued to relate significantly and strongly to child behaviour problems (p < .001) and maternal stress (p < .001). So, while boys, as expected, had higher behaviour problems and more stressed mothers, the ANCOVA results for syndrome are similar to those when gender is not controlled. We can infer that the observed differences between the autism group and other groups are not entirely due to gender, even though we cannot test this directly.
References
- Abbeduto L, Murphy MM, Cawthon SW, Richmond EK, Weissman MD, Karadottir S, O’Brien A. Receptive language skills of adolescents and young adults with Down syndrome or fragile X syndrome. American Journal on Mental Retardation. 2003;108:149–160. doi: 10.1352/0895-8017(2003)108<0149:RLSOAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Achenbach TM. Manual for the Child Behavior Checklist. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Achenbach TM. Manual for the Child Behavior Checklist 1 1/2 – 5. Burlington, VT: University of Vermont, Department of Psychiatry; 2000. [Google Scholar]
- Baker BL, Blacher J, Crnic KA, Edelbrock C. Behavior problems and parenting stress in families of three year old children with and without developmental delays. American Journal on Mental Retardation. 2002;107:433–444. doi: 10.1352/0895-8017(2002)107<0433:BPAPSI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Baker BL, McIntyre LL, Blacher J, Crnic K, Edelbrock C, Low C. Preschool children with and without developmental delay: behaviour problems and parenting stress over time. Journal of Intellectual Disability Research. 2003;47:217–230. doi: 10.1046/j.1365-2788.2003.00484.x. [DOI] [PubMed] [Google Scholar]
- Baker BL, Blacher J. Positive impact of children with intellectual disabilities: the Family Impact Questionnaire. Journal of Intellectual Disability Research. 2004;48:372. [Google Scholar]
- Baker BL, Blacher J, Olsson MB. Preschool children with and without developmental delay: behavior problems, parents’ optimism, and Well being. Journal of Intellectual Disability Research. doi: 10.1111/j.1365-2788.2005.00691.x. (in press) [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of Infant Development. 2nd ed. San Antonio, TX: Manual. Psychological Corporation; 1993. [Google Scholar]
- Bieberich AA, Morgan SB. Brief report: affective expression in children with autism or Down syndrome. Journal of Autism and Developmental Disorders. 1998;28:333–338. doi: 10.1023/a:1026016804357. [DOI] [PubMed] [Google Scholar]
- Blacher J, McIntyre LL. Behavior disorders and syndrome specificity in young adults with intellectual disability: cultural differences in family impact; Gatlinburg Conference on Research and Theory in Intellectual and Developmental Disabilities; Annapolis, MD. 2003. [DOI] [PubMed] [Google Scholar]
- Borthwick-Duffy SA, Eyman RK. Who are the dually diagnosed? American Journal on Mental Retardation. 1990;94:586–595. [PubMed] [Google Scholar]
- Breslau N. Psychiatric disorder in children with physical disabilities. Journal of the American Academy of Child Psychiatry. 1985;24:87–94. doi: 10.1016/s0002-7138(09)60415-5. [DOI] [PubMed] [Google Scholar]
- Bruininks R, Hill B, Morreau L. Prevalence and implications of maladaptive behaviors and dual diagnosis in residential and other service programs. In: Stark J, Menolascino F, Albarelli M, Gray V, editors. Mental retardation and mental health: Classification, diagnosis, treatment services. New York: Springer-Verlag; 1988. pp. 3–29. [Google Scholar]
- Delaney EA, Hopkins TF. Examiner's Handbook. 4th ed. Chicago, IL: Riverside Publishing; 1987. The Stanford-Binet Intelligence Scale. [Google Scholar]
- Donenberg G, Baker BL. The impact of young children with externalizing behaviours on their families. Journal of Abnormal Child Psychology. 1993;21:179–198. doi: 10.1007/BF00911315. [DOI] [PubMed] [Google Scholar]
- Dykens EM. Measuring behavioral phenotypes: provocations from the “New Genetics.”. American Journal on Mental Retardation. 1995;99:522–532. [PubMed] [Google Scholar]
- Dykens EM. Annotation: psychopathology in children with intellectual disability. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2000;41:407–417. [PubMed] [Google Scholar]
- Dykens EM, Hodapp RM, Finucane BM. Genetics and Mental Retardation Syndromes: A New Look at Behavior and Interventions. Baltimore, MD: Paul H. Brookes Publishing Co.; 2000. [Google Scholar]
- Dykens EM, Kasari C. Maladaptive behavior in children with Prader-Willi Syndrome, Down Syndrome, and nonspecific mental retardation. American Journal on Mental Retardation. 1997;102:228–237. doi: 10.1352/0895-8017(1997)102<0228:MBICWP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Shah B, Sagun J, Beck T, King BH. Maladaptive behaviour in children and adolescents with Down’s syndrome. Journal of Intellectual Disability Research. 2002;46:484–492. doi: 10.1046/j.1365-2788.2002.00431.x. [DOI] [PubMed] [Google Scholar]
- Dyson LL. Fathers and mothers of school-age children with developmental disabilities: parental stress, family functioning, and social support. American Journal on Mental Retardation. 1997;102:267–279. doi: 10.1352/0895-8017(1997)102<0267:FAMOSC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Einfeld SL, Tonge BJ. Population prevalence of psychopathology in children and adolescents with intellectual disability: II epidemiological findings. Journal of Intellectual Disability Research. 1996;40:99–109. doi: 10.1046/j.1365-2788.1996.768768.x. [DOI] [PubMed] [Google Scholar]
- Emerson E. Prevalence of psychiatric disorders in children and adolescents with and without intellectual disability. Journal of Intellectual Disability Research. 2003;47:51–58. doi: 10.1046/j.1365-2788.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- Feldman MA, Hancock CL, Reilly N, Minnes P, Cairns C. Behavior problems in young children with or at risk for developmental delay. Journal of Child and Family Studies. 2000;9:247–261. [Google Scholar]
- Fisman SN, Wolf LC, Noh S. Marital intimacy in parents of exceptional children. Canadian Journal of Psychiatry. 1989;34:519–525. doi: 10.1177/070674378903400607. [DOI] [PubMed] [Google Scholar]
- Floyd FJ, Phillippe KA. Parental interactions with children with and without mental retardation: behavior management, coerciveness, and positive exchange. American Journal on Mental Retardation. 1993;97:673–684. [PubMed] [Google Scholar]
- Gath A, Gumley D. Behaviour problems in retarded children with special reference to Down's syndrome. The British Journal of Psychiatry. 1986;149:156–161. doi: 10.1192/bjp.149.2.156. [DOI] [PubMed] [Google Scholar]
- Glutting JJ. Introduction to the structure and application of the Stanford-Binet Intelligence Scale--Fourth Edition. Journal of School Psychology. 1989;27:69–80. [Google Scholar]
- Hauser-Cram P, Warfield ME, Shonkoff JP, Krauss MW. Children with disabilities: a longitudinal study of child development and parent well-being. Monographs of the Society for Research in Child Development. 2001;66:1–131. [PubMed] [Google Scholar]
- Hodapp RM. Direct and indirect behavioral effects of different genetic disorders of mental retardation. American Journal on Mental Retardation. 1997;102:67–79. doi: 10.1352/0895-8017(1997)102<0067:DAIBEO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hodapp RM, Ly TM, Fidler DJ, Ricci LA. Less stress, more rewarding: parenting children with Down Syndrome. Parenting: Science and Practice. 2001;1:317–337. [Google Scholar]
- Hoppes K, Harris SL. Perceptions of child attachment and maternal gratification in mothers of children with autism and Down syndrome. Journal of Clinical Child Psychology. 1990;19:365–370. [Google Scholar]
- Loveland KA, Kelley ML. Development of adaptive behavior in preschoolers with autism or Down syndrome. American Journal on Mental Retardation. 1991;96:13–20. [PubMed] [Google Scholar]
- Maes B, Broekman TG, Dosen A, Nauts J. Caregiving burden of families looking after persons with intellectual disability and behavioural or psychiatric problems. Journal of Intellectual Disability Research. 2003;47:447–455. doi: 10.1046/j.1365-2788.2003.00513.x. [DOI] [PubMed] [Google Scholar]
- McIntyre LL, Blacher J, Baker BL. Behaviour/mental health problems in young adults with intellectual disability: the impact on families. Journal of Intellectual Disability Research. 2002;46:239–249. doi: 10.1046/j.1365-2788.2002.00371.x. [DOI] [PubMed] [Google Scholar]
- Merrell KW, Holland ML. Social-emotional behavior of preschool-age children with and without developmental delays. Research in Developmental Disabilities. 1997;18:393–405. doi: 10.1016/s0891-4222(97)00018-8. [DOI] [PubMed] [Google Scholar]
- Nezu CM, Nezu AM, Gill-Weiss MJ. Psychopathology in persons with mental retardation: clinical guidelines for assessment and treatment. Champaign, IL: Research Press; 1992. [Google Scholar]
- Pearson DA, Lachar D, Loveland KA, Santos CW, Faria LP, Azzam PN, Hentges BA, Cleveland LA. Patterns of behavioral adjustment and maladjustment in mental retardation: comparison of children with and without ADHD. American Journal on Mental Retardation. 2000;105:236–251. doi: 10.1352/0895-8017(2000)105<0236:POBAAM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Pfeiffer SI, Baker BL. Residential treatment for children with dual diagnoses of mental retardation and mental disorder. In: Blacher J, editor. When there's no place like home: Options for children living apart from their natural families. Baltimore, MD: Paul H. Brookes Publishing Co.; 1994. pp. 273–298. [Google Scholar]
- Pisula E. Stress in mothers of children with developmental disabilities. Polish Psychological Bulletin. 1998;29:305–311. [Google Scholar]
- Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Reiss S. Prevalence of dual diagnosis in community-based day programs in the Chicago metropolitan area. American Journal on Mental Retardation. 1990;94:578–585. [PubMed] [Google Scholar]
- Rodrigue JR, Morgan SB, Geffken G. Families of autistic children: psychological functioning of mothers. Journal of Clinical Child Psychology. 1990;19:371–379. [Google Scholar]
- Rodrigue JR, Morgan SB, Geffken G. A comparative evaluation of adaptive behaviour in children and adolescents with autism, Down syndrome, and normal development. Journal of Autism and Developmental Disorders. 1991;21:187–196. doi: 10.1007/BF02284759. [DOI] [PubMed] [Google Scholar]
- Skorikov VB, Vandervoort DJ. Relationships between the underlying constructs of the Beck Depression Inventory and the Center for Epidemiological Studies Depression Scale. Educational & Psychological Measurement. 2003;63:319–335. [Google Scholar]
- Stainton T, Besser H. The positive impact of children with an intellectual disability on the family. Journal of Intellectual and Developmental Disability. 1998;23:57–70. [Google Scholar]
- Stores R, Stores G, Fellows B, Buckley S. Daytime behaviour problems and maternal stress in children with Down’s syndrome, their siblings, and non-intellectually disabled and other intellectually disabled peers. Journal of Intellectual Disability Research. 1998;42:228–237. doi: 10.1046/j.1365-2788.1998.00123.x. [DOI] [PubMed] [Google Scholar]
- Stromme P, Diseth TH. Prevalence of psychiatric diagnoses in children with mental retardation: data from a population-based study. Developmental Medicine and Child Neurology. 2000;42:266–270. doi: 10.1017/s0012162200000451. [DOI] [PubMed] [Google Scholar]
- Taunt HM, Hastings RP. Positive impact of children with developmental disabilities on their families: a preliminary study. Education & Training in Mental Retardation & Developmental Disabilities. 2002;37:410–420. doi: 10.1352/0895-8017(2002)107<0116:PPIFOC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- United States Census Bureau (2004) Educational attainment in the United States: 2003. [Retrieved November 21, 2004]; from http://www.census.gov/prod/2004pubs/p20-550.pdf.
- Wolf LC, Noh S, Fisman SN, Speechley M. Brief report: psychological effects of parenting stress on parents of autistic children. Journal of Autism and Developmental Disorders. 1989;19:157–166. doi: 10.1007/BF02212727. [DOI] [PubMed] [Google Scholar]