Abstract
Sexual receptivity induced in ovariectomized rats by the long-term administration of estradiol benzoate (EB) can be inhibited by concurrent administration of androgens. Experiment 1 examined the role of time course and dose of androgens in the inhibition of estrogen-induced sexual receptivity. Ovariectomized rats were treated with EB (2.0 µg per rat per day) for 6 days and tested for sexual receptivity (Test Day I). EB treatment continued for 15 days concomitant with daily administration of one of three doses of dihydrotestosterone propionate (DHTP; 7.5, 0.75, 0.075 mg/kg) or 3α-androstanediol (3α-Adiol; 3.75, 1.0, 0.375 mg/kg). Four tests for sexual receptivity were conducted on days 3, 6, 14, and 15 of the androgen/vehicle treatment period (Test Days II – V). On Day 15 (Test Day V), the rats received progesterone (1.0 mg per rat) 4 h before testing. Using the same experimental design, Experiment 2 examined the effect of increasing the dose of estrogen on the androgenic inhibition of sexual receptivity. Ovariectomized rats were treated with one of two doses of EB (2.0 or 10.0 µg per rat per day) concomitant with daily administration of DHTP (7.5 mg/kg) or 3α-Adiol (3.75 mg/kg). In Experiment 1, the highest doses of both DHTP and 3α-Adiol significantly inhibited estrogen-induced sexual receptivity. Data from Experiment 2 indicate that the inhibitory effects of DHTP but not 3α-Adiol can be moderated by an increased dose of EB.
Keywords: Sexual behavior, Androgens, Estrogen, Lordosis, Inhibition
1. Introduction
Androgens antagonize the actions of estrogens in both central [1,2] and peripheral tissues [3,4] to influence behavioral and endocrine responses. Substantial evidence has shown that both naturally-occurring [5,6,7,8] and synthetic [9] androgens suppress estrogen-induced receptivity in ovariectomized rats. The effect of androgens on female sexual behavior appears to be mediated, at least in part, by actions at the androgen receptor. Specifically, the administration of flutamide, an androgen receptor antagonist, reverses the inhibition of sexual receptivity by dihydrotestosterone propionate (DHTP) and an androgenic metabolite, 3α-Androstanediol (3α-Adiol) [6,10]. Flutamide has also been shown to modulate male-directed sexual preference in female rats by a selective androgen receptor modulator [11]. An unequivocal role for the androgen receptor in mediating androgenic effects on female sexual behavior is challenged, however, by data showing that flutamide fails to block the inhibitory effects of dihydrotestosterone (DHT) on sexual receptivity induced by short-term treatment with unesterified estradiol-17β in combination with progesterone (P) [8]. Additionally, DHT has been shown to inhibit estrogen-mediated increases in progesterone receptor content in breast tissue from tfm/Y mice that lack functional androgen receptors [12]. These results suggest that androgens may affect female sexual behavior through more than one mechanism.
The androgenic inhibition of sexual receptivity may occur as a result of disruption of critical estrogenic processes in the ventromedial nucleus of the hypothalamus (VMN) [13,14]. Delivery of antiestrogenic compounds into the VMN inhibits the normal display of female receptive behavior [15,16]. Similarly, analysis of the VMN has also shown a downregulation of estrogen receptor expression and a decrease in estrogen receptor binding following 4 days of DHT treatment [17,18]. Therefore, androgenic inhibition of female sexual behavior may reflect the ability of androgens to interfere with necessary estrogen priming in the hypothalamus.
If androgens inhibit female sexual behavior by interfering with estrogenic activity, then the dose of estrogen or duration of treatment used in a given study may modulate the inhibitory actions of androgens, a possibility that has not yet been explored. Inhibition of estrogen-induced lordosis by cholinergic antagonists is reduced by supplemental estrogen [19,20] and extending the duration of estrogen treatment attenuates the inhibition of lordosis in response to 5HT1A agonists [21,22]. Increasing the duration of treatment and/or the dose of estrogen could maximize possible occupation of available central estrogen receptors, therefore optimizing the chance to elicit the estrogen-dependent behavior. However, if the behavior remains inhibited despite presumably maximal estrogen receptor occupation, it is likely that androgens are acting through another mechanism (independent of the estrogen receptor) to inhibit behavior. The present study tested directly the ability of an increased estrogen dose and extended treatment period to override the inhibition of sexual receptivity by DHTP and 3α-Adiol.
2. Methods
2.1 Animals
Adult female Long-Evans rats, approximately 3–4 months old and derived originally from stock obtained from Harlan (Indianapolis, IN), were maintained in the vivarium of the Department of Psychological and Brain Sciences at Dartmouth College. Sexually experienced adult male Long-Evans rats were used as stimulus rats in behavioral tests. Male and female rats were housed individually in hanging metal cages under temperature-controlled conditions on a 14L:10D (Lights ON at 2030 h) light cycle. Food and water were freely available. Rats were ovariectomized (OVX) under sodium methohexital (50.0 mg/kg) anesthesia 1 week before the start of the experiment. The Institutional Animal Care and Use Committee at Dartmouth College approved the procedures using rats in these experiments.
2.2 Behavioral testing
Tests for sexual receptivity were conducted during the early portion of the dark period under dim red light in rectangular Plexiglas chambers (38.5 × 21.7 × 30.4 cm) with wood shavings covering the floor. OVX stimulus rats were primed with 10.0 µg estradiol benzoate (EB) 48 h before testing and 1.0 mg progesterone (P) 4 h before testing. Experimental female rats were placed with stimulus male rats until each female rat received 10 mounts. Lordosis responses were scored on a 4-point scale [23], and the percent of times a female rat exhibited lordosis (lordosis quotient; LQ) were calculated for each female rat. Experimenters unaware of the treatment conditions of individual female rats conducted the behavioral tests.
2.3 Hormone treatments
Experiment 1: Effect of androgen dose on estrogen-induced receptivity
Three doses of dihydrotestosterone propionate (DHTP; 5α-androstane-17β-OL-3-one propionate; 7.5, 0.75, and 0.075 mg/kg) were chosen based on previous studies and represent a range of doses shown to inhibit estrogen-induced sex behavior [6,24,25,26]. Three doses of 3α-androstanediol (3α-Adiol; 5α-androstane-3-α, 17β-diol; 3.75, 0.375, and 0.0375 mg/kg) were chosen based on previous studies and represent a range of doses shown to inhibit estrogen-induced sex behavior [25,27,28,29]. DHTP was purchased from Sigma (St. Louis, MO) and delivered daily in a volume of 1.0 ml/kg in a sesame oil vehicle. 3α-Adiol was purchased from Steraloids Inc. (Newport, RI) and was delivered daily in a 1.0 ml/kg volume in a 10% ethanol in propylene glycol (PG) [18].
The rats received daily injections of EB (2.0 µg/rat) for the duration of the study and a single injection of P (1.0 mg/rat) on Day 15. The dose of EB was chosen based on previous experiments examining the inhibition of estrogen-induced sexual receptivity by androgens [6,25,30]. These supraphysiological doses have been shown to reliably induce high levels of sexual receptivity that are appropriate for examining inhibitory effects on a robust behavior. The EB and P were purchased from Sigma (St. Louis, MO) and were delivered in a volume of 0.1 ml/rat. All hormones were administered via subcutaneous injection and EB/P and the androgen were administered on opposite sides of the body. Androgen and EB injections were given 20–24 h before behavioral testing and P was given 4 h before behavioral testing.
Experiment 2: Effect of estrogen dose on androgenic inhibition
Based on the results of Experiment 1, the highest doses of each androgen (7.5 mg/kg DHTP and 3.75 mg/kg 3α-Adiol) were examined in Experiment 2. Rats received daily injections of the androgen or vehicle concomitant with either 2.0 or 10.0 µg /rat of EB.
2.4 Testing schedule
Four independent experiments were conducted. Separate groups of rats (n ≥ 7 per group) were used in each experiment. Approximately 1 week following ovariectomy, rats were tested for sexual receptivity in response to a single injection of EB (10.0 µg/rat) 48 h and P (1.0 mg/rat) 4 h before the test. Rats exhibiting an LQ ≥ 60 continued in the experiment. Within 2 weeks, rats were tested for sexual receptivity after six daily injections of EB (Experiment 1: 2.0 µg/rat/day; Experiment 2: 2.0 or 10.0 µg/rat/day). After the EB alone test (Test Day I), rats continued to receive daily injections of the specified dose of EB along with the androgen or the vehicle for 15 days. On Day 15, all rats received an injection of 1.0 mg P 4 h before the test. Tests for sexual receptivity were conducted on Days 3, 6, 14, and 15 (Test Days II, III, IV and V, respectively), in accord with other published studies [6,9].
2.5 Data analysis
LQs were calculated for each rat on each of the sexual receptivity tests (Test Days I–V). Statistical analyses focused on the data from Test Days II, III and IV. The data from Test Day I served to confirm high levels of receptivity before initiating androgen treatment and the data from Test Day V served as a positive control to verify high levels of receptivity in response to P. In both experiments, maximal levels of sexual receptivity were seen on Test Days I and V and therefore the data are not discussed further. In both Experiments 1 and 2, data were subjected to repeated-measures ANOVAs (Test Day X Androgen/Estrogen Dose). In Experiment 2, data were also subjected to a two-factor ANOVA (Estrogen Dose X DHTP/3α-Adiol Treatment). ANOVAs were followed by post-hoc comparisons (if appropriate) using the Tukey HSD test and a P < 0.05 significance level.
3. Results
In agreement with previous studies, estrogen-induced sexual receptivity was inhibited in response to DHTP and to 3α-Adiol. In addition, the influence of the dose of estrogen on the androgen inhibition of sexual receptivity varied as a function of the specific androgen being tested and the test day.
3.1 Experiment 1: Effect of androgen dose on estrogen-induced receptivity
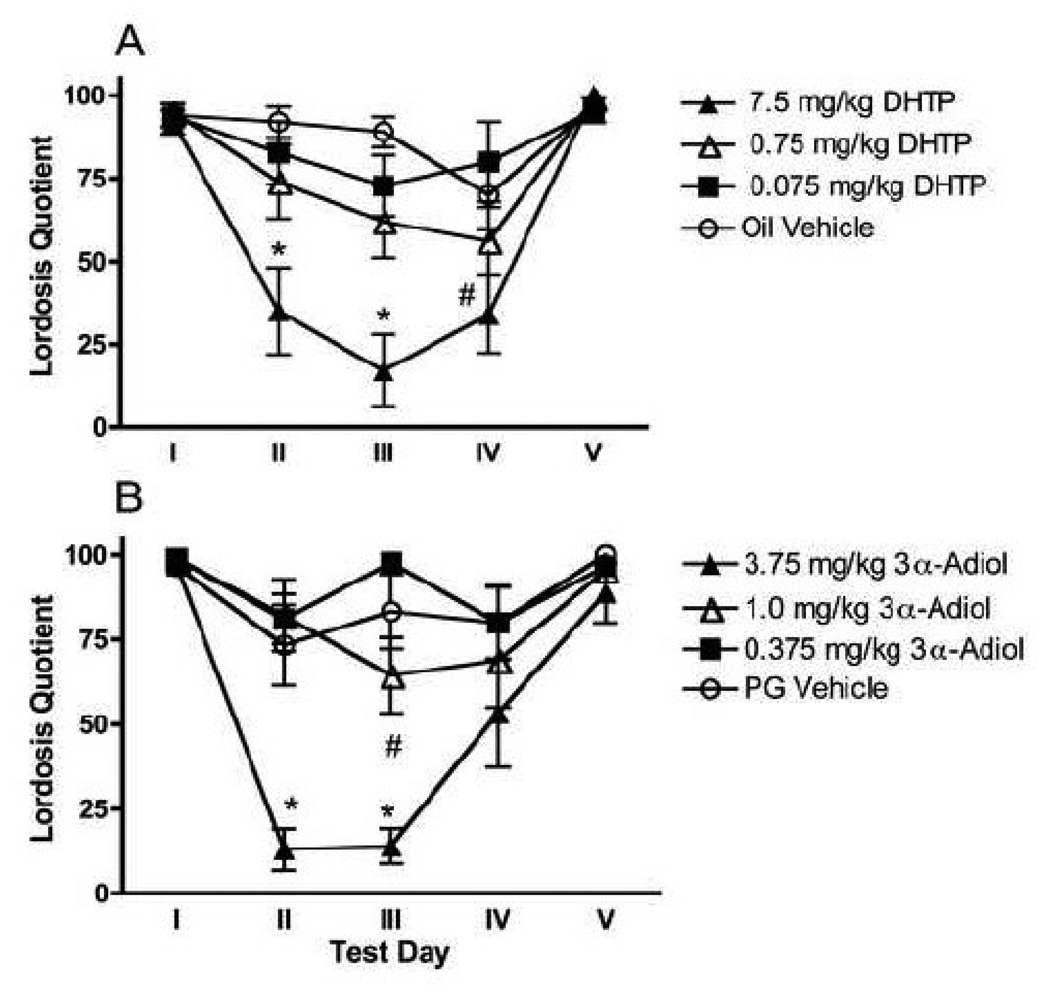
On Test Days II, III, and IV, there was an overall effect of DHTP administration on estrogen-induced sexual receptivity [F (3, 37) = 8.0], a significant effect of test day [F (2, 36) = 3.7] but there was no significant DHTP by Test Day interaction. As shown in Figure 1A, on Test Days II and III, the animals receiving 7.5 mg/kg DHTP displayed significantly lower LQs as compared to all other groups (Tukey, P < 0.05). On Test Day IV, the animals receiving 7.5 mg/kg displayed significantly lower LQs as compared to animals receiving 0.75 mg/kg (Tukey, P < 0.05).
Figure 1.
Mean (±SEM) Lordosis quotients (LQs) were determined on sexual receptivity tests conducted after 6 days of estradiol benzoate (EB; 2.0 µg/day; Test Day I), and again following 3, 6, 14, and 15 days (Test Days II, III, IV, and V, respectively) of continued EB treatment concurrent with dihydrotestosterone propionate (DHTP; 7.5 mg/kg, 0.75 mg/kg or 0.075 mg/kg) or the oil vehicle or 5α-androstane-3-α, 17β-diol (3α-Adiol; 3.75 mg/kg, 1 mg/kg, or 0.375 mg/kg) or the propylene glycol (PG) vehicle. On Day 15 (Test Day V), all female rats received progesterone (1.0 mg/rat) 4 h before testing.
N ≥ 9 per group.
* P < 0.05 vs. all other groups on the same Test Day
# P < 0.05 vs. 0.075 mg/kg DHTP or 0.375 mg/kg DHTP group on the same Test Day
On Test Days II, III, and IV, there was an overall effect of 3α-Adiol administration on estrogen-induced sexual receptivity [F (3, 32) = 11.4]. There was no effect of test day but there was a significant 3α-Adiol by Test Day interaction [F (6, 64) = 3.5]. As shown in Figure 1B, on Test Day II, animals receiving 3.75 mg/kg displayed significantly lower LQs as compared to all other groups (Tukey, P < 0.05). On Test Day III, the animals receiving 3.75 mg/kg 3α-Adiol displayed significantly lower LQs than all other groups, and the animals receiving 1.0 mg/kg displayed significantly lower LQs as compared animals receiving 0.375 mg/kg (for both Tukey, P < 0.05). Although animals receiving 3.75 mg/kg continued to display low levels of sexual receptivity, there were no significant group differences on Test Day IV.
3.2 Experiment 2: Effect of estrogen dose on androgenic inhibition
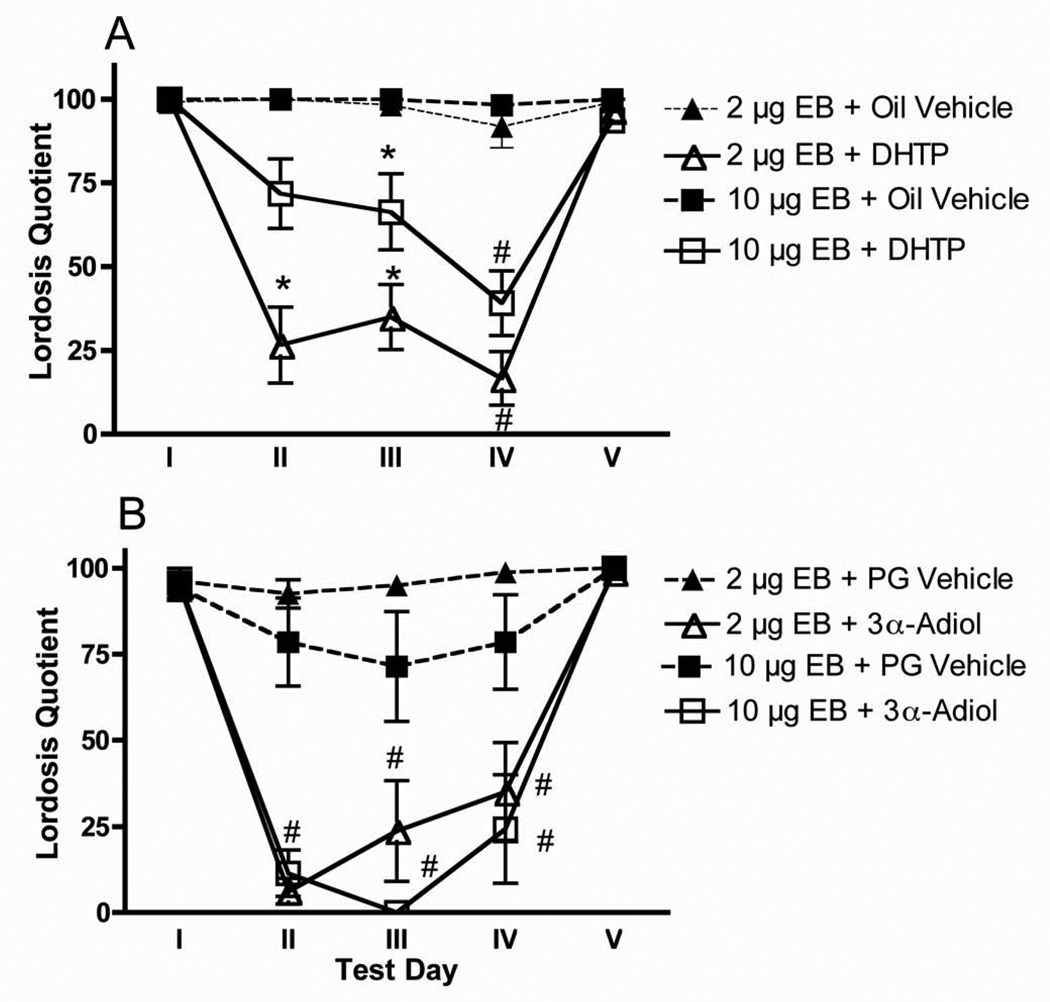
Inhibition of estrogen-induced sexual receptivity by DHTP was modulated by the dose of estrogen. On Test Days II, III, and IV, there was a significant effect of test day [F (2, 40) = 5.9] but there was no significant Estrogen Dose by Test Day interaction. There was a significant overall effect of estrogen dose on sexual receptivity [F (1, 41) = 8.95], a significant overall effect of DHTP [F (1, 41) = 86.3] and a significant Estrogen Dose by DHTP Treatment interaction [F (1, 41) = 6.4]. There was a main effect of estrogen dose on Test Days II, III, and IV [for all, F (3, 44) > 4.1]. As shown in Figure 2A, on each test day, animals receiving 10.0 µg EB displayed higher levels of sexual receptivity as compared to animals receiving 2.0 µg EB (Tukey, P < 0.05). There was also a significant main effect of DHTP on sexual receptivity on Test Days II, III, and IV [for all, F (3, 44) > 40.4]. On each test day, the animals receiving DHTP displayed lower levels of sexual receptivity as compared to animals receiving the oil vehicle. On Test Days II and III, the 2.0 µg EB dose plus DHTP group displayed significantly lower levels of sexual receptivity as compared to all other groups (Tukey, P < 0.05). On Test Day III, the 10.0 µg EB dose plus DHTP group was significantly different from all other groups (Tukey, P < 0.05).
Figure 2.
Mean (±SEM) Lordosis quotients (LQs) were determined on sexual receptivity tests conducted after 6 days of estradiol benzoate (EB; 2.0 or 10.0 µg/day; Test Day I), and again following 3, 6, 14, and 15 days (Test Days II, III, IV, and V, respectively) of continued EB treatment concurrent with dihydrotestosterone propionate (DHTP; 7.5 mg/kg) or the oil vehicle or 5α-androstane-3-α, 17β-diol (3α-Adiol; 3.75 mg/kg) or the propylene glycol (PG) vehicle. On Day 15 (Test Day V), all female rats received progesterone (1.0 mg/rat) 4 h before testing.
N ≥ 7 per group.
* P < 0.05 vs. all other groups on the same Test Day
# P < 0.05 vs. both EB + oil/PG treated groups on same Test Day
Inhibition of estrogen-induced sexual receptivity by 3α-Adiol was not modulated by the dose of estrogen. There was no significant effect of test day or any Estrogen Dose by Test Day interaction. There was a significant overall effect of 3α-Adiol [F (1, 26) = 97.9] and no significant Estrogen Dose by 3α-Adiol Treatment interaction. As shown in Figure 2B, on Test Days II, III, and IV, the animals receiving 3α-Adiol displayed lower levels of sexual receptivity as compared to animals receiving the PG vehicle, regardless of EB dose [for all, F (3, 29) ≥ 22.6].
4. Discussion
The present results illustrate that both DHTP and 3α-Adiol have potent inhibitory effects on estrogen-induced sexual receptivity. In addition, this report is the first to demonstrate that androgenic inhibition of estrogen-induced sexual receptivity by DHTP can be reversed by additional estrogen treatment. In Experiment 1, the highest doses of DHTP and 3α-Adiol inhibited sexual receptivity induced by EB (2.0 µg) within three days of treatment. This time course is similar to that reported in studies examining the inhibitory effects of anabolic-androgenic steroids on estrogen-induced sexual receptivity [9,10] and the downregulation of estrogen receptor binding in the VMN following DHT administration [17,18]. However, there was some variation in behavior over the course of the 14-day treatment period, suggesting that short-term androgen treatment (3 or 6 days) may have more consistent inhibitory effects on estrogen-induced sexual receptivity. When treatment is extended (i.e., 14-days), the inhibitory effects of DHTP and 3α-Adiol become less consistent, even at higher doses. Earlier reports that prolonged estradiol treatment down-regulates estrogen receptor levels in forebrain areas relevant for sexual receptivity may explain some of the variability observed after 14 days of treatment (Test IV) in animals receiving EB only [17]. Furthermore, chronic estrogen treatment (15– 30 days) has been shown to decrease androgen receptor protein expression in testis, prostate and pituitary tissues in adult male rats [31,32]. The extended 14-day EB and androgen treatment may yield inconsistent results due to changes in estrogen and androgen receptor levels as a result of the extended treatment period. Therefore, the results observed on Test Day IV may reflect interactions between time-course and hormone treatment that merit further investigation.
The consistently high levels of sexual receptivity observed on Test V suggest that while androgens are able to interfere with estrogen priming of sexual receptivity, subsequent administration of progesterone was able to reverse androgenic inhibition such that maximal levels of lordosis were observed. Previous studies have shown that DHT does not inhibit induction of progestin receptors in the hypothalamus following pulses of estradiol despite a decrease in receptivity [7]. A similar dissociation between estrogen priming of sexual behavior and induction of progestin receptors was observed following treatment with certain antiestrogens [33]. In addition, we have demonstrated that administration of progesterone can reverse the inhibitory effects of anabolic-androgenic steroids, DHTP and 3α-Adiol on female sexual behavior, even after 15 days of daily androgen and estradiol benzoate (EB) treatment [9,10]. The results of these studies suggest that androgenic inhibition of female sexual behavior may be due to effects on estrogenic actions in the hypothalamus but is not related to induction or function of progestin receptors.
We hypothesized that a higher dose of estrogen might reverse the inhibitory effects of androgens on receptivity. The inhibitory effects of DHTP were attenuated by a 10.0 µg dose of EB and sexual receptivity was increased compared to rats receiving 2.0 µg EB and DHTP on both Test Days II and III. In contrast, the inhibitory effects of 3α-Adiol on sexual receptivity persisted in animals receiving 10.0 µg EB. To explore this result further, we examined sexual receptivity in animals receiving a higher dose of EB (50.0 µg/rat) in combination with 3.75 mg/kg 3α-Adiol (Clark, unpublished results). Surprisingly, sexual receptivity was inhibited even in these animals receiving an extremely high dose of EB. Thus, the inhibitory effects of 3α-Adiol on sexual receptivity appear to be more resistant to override by EB than observed for DHTP.
These new data on the override of DHTP by supplemental EB are consistent with previous findings that increased doses of estrogen have been shown to prevent the inhibition of sexual receptivity by certain pharmacological treatments [19,20] and by large doses of progesterone [34,35]. Our results are also consistent with a model in which androgen regulation of sexual receptivity is mediated by down-regulation of estrogen receptors in the VMN [17,18]. We predicted that increasing the dose of EB might also decrease the inhibitory effects of DHTP on sexual receptivity based on reports that increasing the dose of estrogen attenuates the ability of DHT to reduce estrogen receptor binding in the brain [18]. While a decrease in estrogen receptor binding was still observed in the VMN following a larger dose of estrogen, the overall inhibitory effect of DHT was diminished in all of the brain areas examined [18]. Maximizing estrogen receptor binding in the VMN and other brain areas by increasing the amount of circulating EB may allow for greater estrogen receptor occupation, resulting in an increase in sexual receptivity. As predicted, the results of Experiment 2 describe a significant increase in receptivity in groups given the larger EB dose (10.0 µg/rat) in combination with DHTP (Figure 2A), yet the levels of receptivity were not equal to the groups treated with EB alone. These results are consistent given the reported effects of DHT on estrogen receptor binding in the hypothalamus [18]. While it is likely that androgen administration can affect estrogen receptor actions, it is unlikely that estrogen treatment may have an effect on androgen receptor expression.
Substantial evidence supports a role for the androgen receptor in mediating the effects of DHT on estrogen-induced sexual receptivity. Flutamide partially blocked the reduction in estrogen receptor binding in the VMN induced by DHT [18], and reversed the inhibitory effects of DHT on female sexual behavior in ovariectomized rats [6,10]. The androgen receptor also appears to mediate the effects of endogenous androgens on sexual behavior observed during the estrous cycle. Endogenous androgens reach peak circulating levels at approximately the same time as estrogen and progesterone [36,37,38]. However, androgens do not appear to be necessary for the induction of sexual receptivity (Erskine, 1983). Daily administration of the androgen receptor blocker, flutamide, to female rats over three consecutive estrous cycles did not prevent the display of sexual receptivity [39]. However, female rats treated with flutamide displayed significantly elevated levels of sexual receptivity for 3 hours longer that untreated female rats [39]. The findings of Erskine (1983) suggest that suggesting androgens may act via the androgen receptor to modulate estrous termination but not initiation of the behavior. Although the present studies examined the behavioral effects of supraphysiological hormone levels, the results suggest that changes in relative levels of estrogens and androgens over the course of the estrous cycle may serve to regulate timing of receptivity in intact rats. Future studies might examine the effect of physiological levels of DHTP and 3α-Adiol on estrous behavior in intact rats.
We have shown previously that the inhibition of sexual receptivity by 3α-Adiol is mediated by the androgen receptor [10], however the effectiveness of supplemental EB in overriding the inhibitory effects of DHTP, but not 3α-Adiol, observed in Experiment 2 raises the possibility of a role for an additional androgen receptor- independent mechanism. Recently described non-genomic actions of another androgen metabolite, 3β-Adiol, at estrogen receptor β (ERβ) opens up the possibility that the behavioral actions of androgens may not be mediated through the androgen receptor [40,41]. An alternative mechanism is also suggested by findings showing that 3α-Adiol interacts allosterically with the GABAA receptor to affect female sexual behavior [28,29,42,43]. Future studies might examine the effects of ERβ or GABAA agonists and/or antagonists on the inhibition of female sexual behavior observed following androgen administration to test directly these possibilities. In closing, the results of the present study contribute to an expanding literature documenting increased complexity in the ways in which androgens and estrogens interact to mediate behavior.
Research Highlights.
DHTP and 3α-Adiol inhibit estrogen-induced sexual receptivity.
Inhibitory effects of DHTP but not 3α-Adiol are moderated by greater dose of EB.
Androgenic inhibition may involve androgen receptor-dependent/independent pathways.
Acknowledgements
Grant No. 08574 from the National Institute of Drug Abuse to A.S. Clark supported this research. We thank Christian A. Oberle, Andrew Whitney, and Lauren Zipse for assistance with behavioral testing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brawer J, Schipper H, Robaire B. Effects of long term androgen and estradiol exposure on the hypothalamus. Endocrinology. 1983;112:194–199. doi: 10.1210/endo-112-1-194. [DOI] [PubMed] [Google Scholar]
- 2.Erskine MS, Miller S. Ultrastructural effects of estradiol and 5 alpha-androstane-3 alpha, 17 beta-diol on neurons within the ventromedial nucleus of the hypothalamus. Neuroendocrinology. 1995;61:669–679. doi: 10.1159/000126894. [DOI] [PubMed] [Google Scholar]
- 3.Poulin R, Simard J, Labrie C, Petitclerc L, Dumont M, Lagace L, et al. Down-regulation of estrogen receptors by androgens in the ZR-75-1 human breast cancer cell line. Endocrinology. 1989;125:392–399. doi: 10.1210/endo-125-1-392. [DOI] [PubMed] [Google Scholar]
- 4.Cardenas H, Pope WF. Attenuation of estrogenic effects by dihydrotestosterone in the pig uterus is associated with downregulation of the estrogen receptors. Biol Reprod. 2004;70:297–302. doi: 10.1095/biolreprod.103.022384. [DOI] [PubMed] [Google Scholar]
- 5.Butler PC, Mills RH, Bloch GJ. Inhibition of lordosis behavior in male and female rats by androgens and progesterone. Horm Behav. 2001;40:384–395. doi: 10.1006/hbeh.2001.1703. [DOI] [PubMed] [Google Scholar]
- 6.Dohanich GP, Clemens LG. Inhibition of estrogen-activated sexual behavior by androgens. Horm Behav. 1983;17:366–373. doi: 10.1016/0018-506x(83)90046-6. [DOI] [PubMed] [Google Scholar]
- 7.Erskine MS, MacLusky NJ, Baum MJ. Effect of 5 alpha-dihydrotestosterone on sexual receptivity and neural progestin receptors in ovariectomized rats given pulsed estradiol. Biol Reprod. 1985;33:551–559. doi: 10.1095/biolreprod33.3.551. [DOI] [PubMed] [Google Scholar]
- 8.Erskine MS. Effect of 5 alpha-dihydrotestosterone and flutamide on the facilitation of lordosis by LHRH and naloxone in estrogen-primed female rats. Physiol Behav. 1989;45:753–759. doi: 10.1016/0031-9384(89)90290-4. [DOI] [PubMed] [Google Scholar]
- 9.Blasberg ME, Clark AS. Anabolic-androgenic steroid effects on sexual receptivity in ovariectomized rats. Horm Behav. 1997;32:201–208. doi: 10.1006/hbeh.1997.1422. [DOI] [PubMed] [Google Scholar]
- 10.Blasberg ME, Robinson S, Henderson LP, Clark AS. Inhibition of estrogen-induced sexual receptivity by androgens: role of the androgen receptor. Horm Behav. 1998;34:283–293. doi: 10.1006/hbeh.1998.1484. [DOI] [PubMed] [Google Scholar]
- 11.Kudwa AE, Lopez FJ, McGivern RF, Handa RJ. A selective androgen receptor modulator enhances male-directed sexual preference, proceptive behavior, and lordosis behavior in sexually experienced, but not sexually naive, female rats. Endocrinology. 2010;151:2659–2668. doi: 10.1210/en.2009-1289. [DOI] [PubMed] [Google Scholar]
- 12.Casey RW, Wilson JD. Antiestrogenic action of dihydrotestosterone in mouse breast. Competition with estradiol for binding to the estrogen receptor. J Clin Invest. 1984;74:2272–2278. doi: 10.1172/JCI111654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pleim ET, Brown TJ, MacLusky NJ, Etgen AM, Barfield RJ. Dilute estradiol implants and progestin receptor induction in the ventromedial nucleus of the hypothalamus: correlation with receptive behavior in female rats. Endocrinology. 1989;124:1807–1812. doi: 10.1210/endo-124-4-1807. [DOI] [PubMed] [Google Scholar]
- 14.Rubin BS, Barfield RJ. Priming of estrous responsiveness by implants of 17 beta-estradiol in the ventromedial hypothalamic nucleus of female rats. Endocrinology. 1980;106:504–509. doi: 10.1210/endo-106-2-504. [DOI] [PubMed] [Google Scholar]
- 15.Etgen AM. Antiestrogens: effects of tamoxifen, nafoxidine, and CI-628 on sexual behavior, cytoplasmic receptors, and nuclear binding of estrogen. Horm Behav. 1979;13:97–112. doi: 10.1016/0018-506x(79)90050-3. [DOI] [PubMed] [Google Scholar]
- 16.Howard SB, Etgen AM, Barfield RJ. Antagonism of central estrogen action by intracerebral implants of tamoxifen. Horm Behav. 1984;18:256–266. doi: 10.1016/0018-506x(84)90015-1. [DOI] [PubMed] [Google Scholar]
- 17.Brown TJ, Scherz B, Hochberg RB, MacLusky NJ. Regulation of estrogen receptor concentrations in the rat brain: effects of sustained androgen and estrogen exposure. Neuroendocrinology. 1996;63:53–60. doi: 10.1159/000126935. [DOI] [PubMed] [Google Scholar]
- 18.Brown TJ, Adler GH, Sharma M, Hochberg RB, MacLusky NJ. Androgen treatment decreases estrogen receptor binding in the ventromedial nucleus of the rat brain: a quantitative in vitro autoradiographic analysis. Mol Cell Neurosci. 1994;5:549–555. doi: 10.1006/mcne.1994.1067. [DOI] [PubMed] [Google Scholar]
- 19.Menard CS, Hebert TJ, Ross SM, Dohanich GP. The effect of estrogen treatment on scopolamine inhibition of lordosis. Horm Behav. 1992;26:364–374. doi: 10.1016/0018-506x(92)90006-h. [DOI] [PubMed] [Google Scholar]
- 20.Hebert TJ, Cashion MF, Dohanich GP. Effects of hormonal treatment and history on scopolamine inhibition of lordosis. Physiol Behav. 1994;56:835–839. doi: 10.1016/0031-9384(94)90312-3. [DOI] [PubMed] [Google Scholar]
- 21.Jackson A, Uphouse L. Prior treatment with estrogen attenuates the effects of the 5-HT1A agonist, 8-OH-DPAT, on lordosis behavior. Horm Behav. 1996;30:145–152. doi: 10.1006/hbeh.1996.0018. [DOI] [PubMed] [Google Scholar]
- 22.Jackson A, Etgen AM. Estrogen modulates 5-HT(1A) agonist inhibition of lordosis behavior but not binding of [(3)H]-8-OH-DPAT. Pharmacol Biochem Behav. 2001;68:221–227. doi: 10.1016/s0091-3057(00)00455-x. [DOI] [PubMed] [Google Scholar]
- 23.Hardy DF, DeBold JF. The relationship between levels of exogenous hormones and the display of lordosis by the female rat. Horm Behav. 1971;2:287–297. [Google Scholar]
- 24.Baum MJ, Sodersten P, Vreeburg JT. Mounting and receptive behavior in the ovariectomized female rat: influence of estradiol, dihydrotestosterone, and genital anesthetization. Horm Behav. 1974;5:175–190. doi: 10.1016/0018-506x(74)90042-7. [DOI] [PubMed] [Google Scholar]
- 25.Baum MJ, Vreeburg JT. Differential effects of the anti-estrogen MER-25 and of three 5alpha-reduced androgens on mounting and lordosis behavior in the rat. Horm Behav. 1976;7:87–104. doi: 10.1016/0018-506x(76)90007-6. [DOI] [PubMed] [Google Scholar]
- 26.Dohanich GP, Cada DA. Reversal of androgen inhibition of estrogen-activated sexual behavior by cholinergic agents. Horm Behav. 1989;23:503–513. doi: 10.1016/0018-506x(89)90038-x. [DOI] [PubMed] [Google Scholar]
- 27.Erskine MS, Hippensteil M, Kornberg E. Metabolism of dihydrotestosterone to 3 alpha-androstanediol in brain and plasma: effect on behavioural activity in female rats. J Endocrinol. 1992;134:183–195. doi: 10.1677/joe.0.1340183. [DOI] [PubMed] [Google Scholar]
- 28.Frye CA, Duncan JE, Basham M, Erskine MS. Behavioral effects of 3 alpha-androstanediol. II: Hypothalamic and preoptic area actions via a GABAergic mechanism. Behav Brain Res. 1996;79:119–130. doi: 10.1016/0166-4328(96)00005-8. [DOI] [PubMed] [Google Scholar]
- 29.Frye CA, Van Keuren KR, Erskine MS. Behavioral effects of 3 alpha-androstanediol. I: Modulation of sexual receptivity and promotion of GABA-stimulated chloride flux. Behav Brain Res. 1996;79:109–118. doi: 10.1016/0166-4328(96)00004-6. [DOI] [PubMed] [Google Scholar]
- 30.Menniti FS, Erskine MS, Tobet SA, Baum MJ. Dihydrotestosterone-induced inhibition of lordosis in estrogen-primed ovariectomized rats following 6-hydroxydopamine or electrolytic septal lesions. Pharmacol Biochem Behav. 1982;16:211–216. doi: 10.1016/0091-3057(82)90150-2. [DOI] [PubMed] [Google Scholar]
- 31.Kaushik MC, Misro MM, Sehgal N, Nandan D. Effect of chronic oestrogen administration on androgen receptor expression in reproductive organs and pituitary of adult male rat. Andrologia. 2010;42:193–205. doi: 10.1111/j.1439-0272.2009.00979.x. [DOI] [PubMed] [Google Scholar]
- 32.Kaushik MC, Misro MM, Sehgal N, Nandan D. AR versus ER (α) expression in the testis and pituitary following chronic estrogen administration in adult rat. Syst Biol Reprod Med. 2010 doi: 10.3109/19396368.2010.501891. [DOI] [PubMed] [Google Scholar]
- 33.Etgen AM, Shamamian P. Regulation of estrogen-stimulated lordosis behavior and hypothalamic progestin receptor induction by antiestrogens in female rats. Horm Behav. 1986;20:166–180. doi: 10.1016/0018-506x(86)90015-2. [DOI] [PubMed] [Google Scholar]
- 34.Blaustein JD, Wade GN. Sequential inhibition of sexual behavior by progesterone in female rats: comparison with a synthetic antiestrogen. J Comp Physiol Psychol. 1977;91:752–760. doi: 10.1037/h0077365. [DOI] [PubMed] [Google Scholar]
- 35.Blaustein JD, Wade GN. Concurrent inhibition of sexual behavior, but not brain [3H] estradiol uptake, by progesterone in female rats. J Comp Physiol Psychol. 1977;91:742–751. doi: 10.1037/h0077366. [DOI] [PubMed] [Google Scholar]
- 36.Dunlap KD, Sridaran R. Plasma levels of dihydrotestosterone in the cycling rat: implications for the regulation of lordosis behavior. Physiol Behav. 1988;42:199–202. doi: 10.1016/0031-9384(88)90298-3. [DOI] [PubMed] [Google Scholar]
- 37.Meijs-Roelofs HM, Kramer P, van Cappellen WA, Gribling-Hegge L, Woutersen PJ. Periovulatory changes in serum concentration and ovarian content of 5 alpha-androstane-3 alpha, 17 beta-diol in the adult rat. Biol Reprod. 1986;35:890–896. doi: 10.1095/biolreprod35.4.890. [DOI] [PubMed] [Google Scholar]
- 38.Toorop AI, Gribling-Hegge L. Androgen and oestrogen concentrations in the ovary of the cyclic rat. J Endocrinol. 1982;93:25–30. doi: 10.1677/joe.0.0930025. [DOI] [PubMed] [Google Scholar]
- 39.Erskine MS. Effects of an anti-androgen and 5 alpha-reductase inhibitors on estrus duration in the cycling female rat. Physiol Behav. 1983;30:519–524. doi: 10.1016/0031-9384(83)90214-7. [DOI] [PubMed] [Google Scholar]
- 40.Handa RJ, Weiser MJ, Zuloaga DG. A role for the androgen metabolite, 5alpha-androstane-3beta, 17beta-diol, in modulating oestrogen receptor beta-mediated regulation of hormonal stress reactivity. J Neuroendocrinol. 2009;21:351–358. doi: 10.1111/j.1365-2826.2009.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5alpha-androstane-3beta, 17beta-diol. Horm Behav. 2008;53:741–752. doi: 10.1016/j.yhbeh.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Front Neuroendocrinol. 2008;29:169–181. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jorge-Rivera JC, McIntyre KL, Henderson LP. Anabolic steroids induce region- and subunit-specific rapid modulation of GABA(A) receptor-mediated currents in the rat forebrain. J Neurophysiol. 2000;83:3299–3309. doi: 10.1152/jn.2000.83.6.3299. [DOI] [PubMed] [Google Scholar]