Abstract
NSCs (neural stem cells) are undifferentiated neural cells endowed with a high potential for proliferation and a capacity for self-renewal with retention of multipotency to differentiate into neurons and glial cells. It has been recently reported that GD3, a b-series ganglioside, is a marker molecule for identifying and isolating mouse NSCs. However, the expression of gangliosides in human NSCs is largely unknown. In the present study, we analysed the expression of gangliosides, GD2 and GD3, in human NSCs that were isolated from human brains at gestational week 17 in the form of neurospheres, which are floating clonal aggregates formed by NSCs in vitro. Employing immunocytochemistry, we found that human NSCs were strongly reactive to anti-GD2 antibody and relatively weakly reactive to anti-GD3 antibody. Treatment of these cells with an organic solvent such as 100% methanol, which selectively removes glycolipids from plasma membrane, abolished the immunoreactivity with those antibodies, indicating that the reactivity was due to GD2 and GD3, but not to GD2-/GD3-like glycoproteins or proteoglycans. The immunoreactivity of human NSCs to antibody against SSEA-1 (stage-specific embryonic antigen-1), a well-known carbohydrate antigen of NSCs, was not decreased by the treatment with 100% methanol, indicating that SSEA-1 is mainly carried by glycoproteins and/or proteoglycans in human NSCs. Our study suggests that GD2 and GD3 can be marker gangliosides for identifying human NSCs.
Keywords: ganglioside, glycosphingolipid (GSL), neural stem cell (NSC), neurosphere, stage-specific embryonic antigen-1 (SSEA-1)
Abbreviations: bFGF, basic fibroblast growth factor; GSL, glycosphingolipid; LAMP-1, lysosome-associated membrane protein-1; NSC, neural stem cell; SSEA-1, stage-specific embryonic antigen-1. Abbreviations for gangliosides follow Svennerholm's nomenclature system (Svennerholm, 1963)
INTRODUCTION
NSCs (neural stem cells) are undifferentiated neural cells endowed with a high potential for proliferation and a capacity for self-renewal with retention of multipotency to differentiate into brain-forming cells such as neurons and glial cells (Weiss et al., 1996; McKay, 1997; Gage, 2000; Zhao et al., 2008). For studying their basic biological properties and clinical applications, it is important to characterize marker molecules for identifying and isolating NSCs from brain tissues. To date, a number of marker molecules have been found in NSCs, e.g., nestin, Sox2, Musashi-1, SSEA-1 (stage-specific embryonic antigen-1; also known as Lewis x antigen or CD15) and prominin-1 (CD133). To study NSCs more systematically and thoroughly, however, it is still essential to explore other cell-surface marker molecules of NSCs.
GSLs (glycosphingolipids) are lipids that contain at least one monosaccharide residue and either a sphingoid base or a ceramide (Hakomori, 1990; Yu et al., 2007, 2009). GSLs having at least one sialic acid residue in their carbohydrate chains are referred to as gangliosides. GSLs, including gangliosides, are ubiquitously expressed in vertebrate tissues, cells and body fluids, and are most abundant in the nervous system. The expression of gangliosides changes drastically during brain development. For instance, in rodents, simple GM3 and GD3 are predominant gangliosides in embryonic brains, but more complex gangliosides such as GM1, GD1a, GD1b and GT1b prevail in adult brains (Yu, 1994; Ngamukote et al., 2007). In cells, GSLs, including gangliosides, are localized in the outer leaflet of the plasma membrane and their carbohydrate moieties are exposed to the extracellular region. Thus, GSLs can be useful as stage-specific cell surface marker molecules of certain cell lineages (Yanagisawa and Yu, 2007; Yu et al., 2010; Yanagisawa, 2011). For instance, galactosylceramide (O1 antigen), sulfatide (O4 antigen) and c-series gangliosides (A2B5 antigens) have been used as marker molecules to identify and isolate cells of the glial lineage (Yanagisawa and Yu, 2007). In mouse NSCs, GD3 ganglioside is such a marker molecule.
GD3 (CD60a; NeuAcα2-8NeuAcα2-3Galβ1-4Glcβ1-1′Cer) is a b-series disialoganglioside that is frequently expressed in immature proliferative cells (Seyfried and Yu, 1985). In rodent brains, GD3 is heavily concentrated in the subventricular zone of the lateral ventricle (Goldman et al., 1984), where adult NSCs are localized (Doetsch et al., 1999). Also, GD3 has been reported to be expressed in mouse neuroepithelial cells that are rich in NSCs (Yanagisawa et al., 2004) and mouse radial glia, bipolar cells transiently appearing in the neuroepithelium and playing roles as NSCs at the embryonic stage (Cammer and Zhang, 1996). These observations suggest the expression of GD3 in rodent NSCs. In fact, we have recently provided direct evidence that GD3 is expressed in bulk-isolated mouse embryonic, postnatal and adult NSCs (Nakatani et al., 2010). Interestingly, GD3+ cells isolated from mouse brains by FACS have all the characteristics of NSCs (Nakatani et al., 2010). Our study clearly indicates the importance of GD3 as a biomarker of mouse NSCs. On the other hand, it has been reported that human NSCs were recognized by an antibody against GD2 [GalNAcβ1-4(NeuAcα2-8NeuAcα2-3)Galβ1-4Glcβ1-1′Cer], another b-series disialoganglioside (Klassen et al., 2001). However, the possibility that the immunoreactivity is attributed to other glycoconjugates such as proteoglycans and glycoproteins that have the epitopic structure of GD2 cannot be ruled out. In addition, it has not been shown whether GD3 is also expressed in NSCs prepared from human embryonic brain. In the present study, therefore, we analysed the expression of gangliosides (i.e., GD2 and GD3) in addition to SSEA-1 in NSCs prepared from human embryonic brains.
MATERIALS AND METHODS
Human NSC culture
NSCs isolated from human brains at gestational week 17 in the form of neurospheres and stored in liquid nitrogen (Poetic Normal Human Neural Progenitors; catalogue no. PT-2599; lot no. 0000089197; Lonza Walkersville, Walkersville, MD, U.S.A.; Tanikawa et al., 2003; Krathwohl and Kaiser, 2004; Zawlik et al., 2006) were revived and cultured in Neural Progenitor Maintenance medium that contained human recombinant bFGF (basic fibroblast growth factor) and human recombinant epidermal growth factor (Lonza Walkersville). These NSCs were collected into 1.5-ml tubes or re-plated on to Chamber Slides (Nalge Nunc International, Naperville, IL, U.S.A.) that were coated with 20 μg/ml Cultrex mouse laminin I (Trevigen, Gaithersburg, MD, U.S.A.) for immunocytochemistry.
Immunocytochemistry
Human NSCs, collected in 1.5-ml tubes or re-plated on to Chamber Slides, were fixed in PBS that contained 4% (w/v) paraformaldehyde. To wash out lipids that include gangliosides of the plasma membranes, cells were treated with 100% methanol for 10 min. The cells were treated for 2 h with PBS that contained 3% (v/v) FBS (fetal bovine serum) without detergent for blocking non-specific binding of antibodies. To stain nestin, PBS containing 3% FBS and 0.1% Triton X-100 was used for blocking and permeabilization. These NSCs were stained with isotype control mouse IgG (BD Biosciences, San Jose, CA, U.S.A.), isotype control mouse IgM (BD Biosciences), 10C2 anti-human nestin monoclonal antibody (Millipore, Billerica, MA, U.S.A.), AK97 anti-SSEA-1 monoclonal antibody (mouse IgM) (Yanagisawa et al., 1999), 14G2a anti-GD2 monoclonal antibody (mouse IgG; Millipore) (Mujoo et al., 1989) or R24 anti-GD3 monoclonal antibody (mouse IgG) (Pukel et al., 1982) and then with anti-mouse IgG antibody conjugated with Alexa Fluor® 488 (Invitrogen, Carlsbad, CA, U.S.A.) or anti-mouse IgM antibody conjugated with Alexa Fluor® 488 (Invitrogen). Nuclei were stained with 2 μg/ml Hoechst 33258 (Sigma–Aldrich, St. Louis, MO, U.S.A.) in PBS. The stained cells were mounted with Fluoro-Gel (Electron Microscopy Sciences, Hatfield, PA, U.S.A.), covered with a coverslip and then photographed under a Nikon Eclipse TE300 fluorescent microscope (Nikon Instruments, Melville, NY, U.S.A.) equipped with a Magnafire digital charge-coupled device camera (Optronics, Goleta, CA, U.S.A.).
Mouse NSC culture
Mouse NSCs were prepared from brains of ICR mice (embryonic day 14.5; Harlan, Indianapolis, IN, U.S.A.) in the form of neurospheres as previously described (Reynolds et al., 1992; Nakatani et al., 2010; Yagi et al., 2010b). Neurospheres formed in Neurobasal A medium (Invitrogen) that contained B27 supplement (Invitrogen), 2 mM l-glutamine (Invitrogen), 20 ng/ml human recombinant bFGF (Peprotech, Rocky Hill, NJ, U.S.A.) and 20 ng/ml human recombinant epidermal growth factor (Peprotech) were collected into 1.5-ml tubes or plated on to Chamber Slides that were coated with poly-l-ornithine (Sigma–Aldrich) and bovine fibronectin (Sigma–Aldrich). These mouse NSCs in 1.5-ml tubes or on Chamber Slides were fixed and immunostained as described above. Mice used in this study were treated according to the guidelines of the Institutional Animal Care and Use Committee of Georgia Health Sciences University.
RESULTS
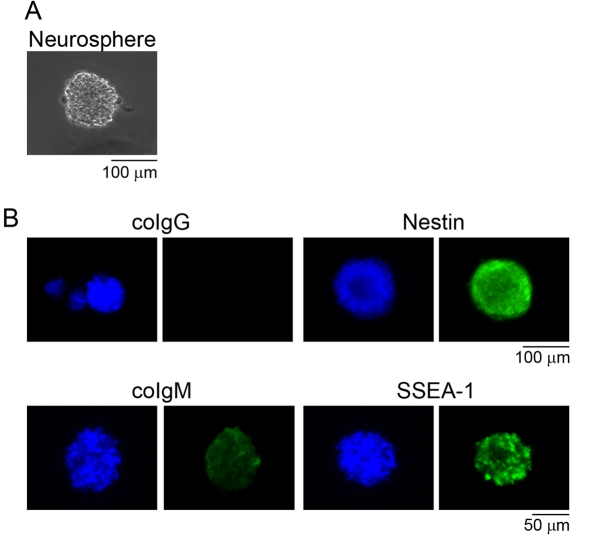
In the present study, we analysed commercially available human NSCs, Poetic Normal Human Neural Progenitors (Tanikawa et al., 2003; Krathwohl and Kaiser, 2004; Zawlik et al., 2006), prepared from human brains at gestational week 17 in the form of neurospheres, which are floating clonal aggregates formed by NSCs in vitro (Figure 1A). These human NSCs were confirmed to be positive for marker molecules of NSCs, nestin and SSEA-1, as Klassen et al., (2001) have previously reported (Figure 1B).
Figure 1. Marker expression of human NSCs.
Human NSCs, Poetic Normal Human Neural Progenitors, prepared from human brains at gestational week 17 in the form of neurospheres (A) were immunostained with anti-human nestin antibody (mouse IgG) and anti-SSEA-1 antibody (mouse IgM) (B). Human NSCs were positive for nestin (green) and SSEA-1 (green), marker molecules of NSCs. coIgG and coIgM indicate human NSCs stained with isotype control mouse IgG and IgM respectively. Nuclei were stained with Hoechst 33258 (blue).
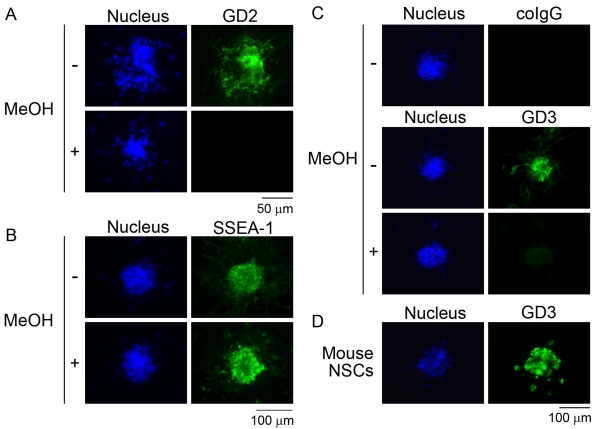
First, we immunostained these human NSCs with antibody against a b-series ganglioside, GD2. As shown in Figure 2(A), human NSCs were immunoreactive with anti-GD2 antibody as Klassen et al. (2001) have previously reported. However, there was a possibility that the anti-GD2 antibody cross-reacted with glycoproteins or proteoglycans that have carbohydrate structures similar to those of GD2 in human NSCs. It is well known that lipids, including glycolipids in the plasma membrane, are easily washed out by treatment of the cells with an organic solvent before immunocytochemistry (Kinoshita et al., 2009). To confirm that the immunoreactivity of human NSCs with anti-GD2 antibody was attributed to GD2, we treated these cells with 100% methanol for 10 min to wash out plasma membrane lipids, including gangliosides. As shown in Figure 2(A), human NSCs thus treated lost completely their immunoreactivity to anti-GD2 antibody. This result indicates that the immunoreactivity against anti-GD2 antibody was attributed to GD2, but not to glycoproteins or proteoglycans having the same epitopic structure of GD2. In contrast, the immunoreactivity of human NSCs to anti-SSEA-1 antibody was resistant to the treatment with 100% methanol (Figure 2B). This clearly indicates that the SSEA-1 epitope in human NSCs is mainly carried by glycoproteins and/or proteoglycans, but not by glycolipid antigens. As shown in Figure 2(C), human NSCs were also reactive with an antibody against GD3, a predominant ganglioside in mouse NSCs (Nakatani et al., 2010) (Figure 2D). The immunoreactivity of human NSCs to anti-GD3 antibody was relatively weaker than that to anti-GD2 antibody (results not shown). The sensitivity of the GD3 immunoreactivity to methanol treatment (Figure 2C) indicates that GD3, but not glycoproteins or proteoglycans having carbohydrate structures of GD3, is expressed in human NSCs.
Figure 2. Effect of methanol on GD2, SSEA-1 and GD3 immunoreactivity in human NSCs.
Human NSCs treated with or without methanol (MeOH) were immunostained with antibodies against GD2 (A), SSEA-1 (B) and GD3 (C). Human NSCs were positive for GD2 (green; A) and relatively weakly positive for GD3 (green; C). No immunoreactivity of human NSCs to antibodies against GD2 (A) and GD3 (C) after treatment with 100% methanol for 10 min to wash out lipids of plasma membranes indicates that the immunoreactivity is attributed to GD2 and GD3 gangliosides, but not to glycoproteins or proteoglycans. (B) Human NSCs treated with methanol were still positive for SSEA-1 (green), indicating that SSEA-1 is mainly carried by glycoproteins and/or proteoglycans in these cells. (D) Mouse embryonic NSCs prepared from mouse brains at embryonic day 14 in the form of neurospheres were stained with anti-GD3 antibody (green) as a positive control. Nuclei were stained with Hoechst 33258 (blue).
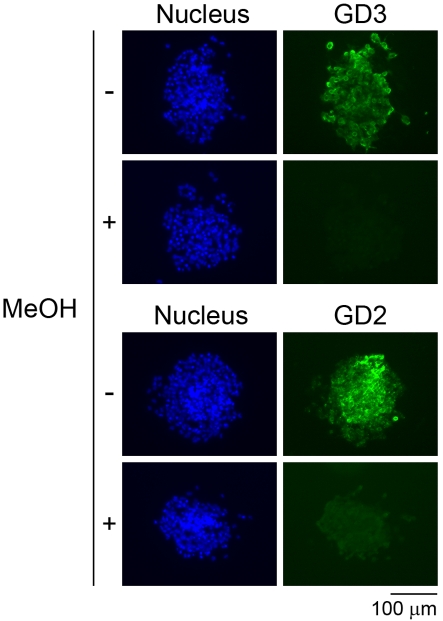
From mouse NSCs and neural precursor cells, certain gangliosides other than GD3 such as GT1b, GQ1b, GT1aα [originally designated GTx (Nakamura et al., 1988); Chol-1α antigen] and GQ1bα (Chol-1α antigen) have been identified so far (Yanagisawa et al., 2004; Ngamukote et al., 2007; Nakatani et al., 2010; Yanagisawa, 2011). GD2, however, has not been biochemically detected in mouse NSCs. Then, we evaluated whether mouse NSCs express GD2 ganglioside as human NSCs. As shown in Figure 3, mouse NSCs were positive for not only GD3 but also GD2. As previously reported (Yanagisawa et al., 2005), the GD3 immunoreactivity disappeared in mouse NSCs treated with methanol (Figure 3). Similarly, the GD2 immunoreactivity disappeared in mouse NSCs treated with methanol (Figure 3), suggesting that GD2 immunoreactivity in mouse NSCs is also attributed to GD2, but not to glycoproteins or proteoglycans having the same epitopic structures of GD2. There have been results suggesting that the expression of GD2 in mouse NSCs is less than the amount required for chemical detection based on TLC (Nakatani et al., 2010). The finding in the previous study and the result in the present study establish that GD2 is unequivocally expressed in mouse NSCs, albeit at a lower concentration.
Figure 3. Effect of methanol on GD2 and GD3 immunoreactivity in mouse embryonic NSCs.
Mouse embryonic NSCs were stained with anti-GD3 antibody (green) and anti-GD2 antibody (green). No immunoreactivity to antibodies against GD2 and GD3 in cells treated with 100% methanol (MeOH) for 10 min indicates that the GD3 and GD2 immunoreactivity is attributed to GD3 and GD2 gangliosides, but not to glycoproteins or proteoglycans. Nuclei were stained with Hoechst 33258 (blue).
DISCUSSION
In the present study, we analysed the expression of gangliosides in human NSCs. As Klassen et al. (2001) have previously reported, human NSCs were reactive with antibody against GD2. In addition, these human NSCs were relatively weakly reactive with antibody against GD3, a predominant ganglioside in mouse NSCs (Nakatani et al., 2010). No immunoreactivity of the human NSCs to antibodies against GD2 and GD3 after treatment with methanol to wash out lipids of plasma membranes confirmed that GD2 and GD3 gangliosides were expressed in human NSCs.
SSEA-1, which is expressed in mouse NSCs (Klassen et al., 2001; Capela and Temple, 2002), was also found in human NSCs. The carbohydrate determinant of this antigen [Galβ1-4(Fucα1-3)GlcNAcβ1-] is usually carried by proteoglycans, glycoproteins and glycolipids. The nature of the carrier molecules of this epitope in human NSCs, however, has not been fully characterized. The immunoreactivity of SSEA-1 in human NSCs was resistant to the methanol treatment, suggesting that SSEA-1 is mainly carried by glycoproteins and/or proteoglycans, but not by glycolipids. This result is consistent with the analysis of SSEA-1 in mouse neuroepithelial cells (Yanagisawa et al., 2005). Recently, LAMP-1 (lysosome-associated membrane protein-1) was identified as a major SSEA-1 carrier protein in mouse NSCs (Yagi et al., 2010a). There is a possibility that LAMP-1 is also a major SSEA-1 carrier protein in human NSCs.
Recently, we have reported that GD3 can be a marker molecule for isolating NSCs from mouse embryonic, postnatal and adult brains (Nakatani et al., 2010). Also, some researchers have found that GD2 is useful as a marker molecule for isolating mesenchymal stem cells, multipotent stromal cells that can differentiate into cells of the mesodermal lineage such as myocytes, osteocytes, adipocytes and chondrocytes, from human bone marrow (Martinez et al., 2007) and umbilical cord blood (Xu et al., 2009; Jin et al., 2010). Similar to these, it is anticipated that GD2 and GD3 confirmed to be expressed in human NSCs in the present study also can be marker molecules available for identifying and isolating NSCs in human brains especially in adulthood; in adult human brains, GM1, GD1a, GD1b and GT1b, but not GD2 and GD3, are predominantly expressed (Svennerholm, 1963). Further systematic studies of gangliosides in human NSCs should be important for the progress of stem cell biology.
ACKNOWLEDGEMENTS
We thank Dr Mutsumi Yamamoto and Dr Yoichi Seki (Loyola University Chicago, Maywood, IL, U.S.A.) for their technical advice.
Footnotes
This work was supported by the United States Public Health Service [grant numbers NS11853-34 and NS26994-20], a grant from the Children's Medical Research Foundation (Chicago, IL, U.S.A.) to RKY, and a start-up fund awarded from the Medical College of Georgia (Augusta, GA, U.S.A.) to MY.
REFERENCES
- Cammer W, Zhang H. Ganglioside GD3 in radial glia and astrocytes in situ in brains of young and adult mice. J Neurosci Res. 1996;46:18–23. doi: 10.1002/(SICI)1097-4547(19961001)46:1<18::AID-JNR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX/SSEA-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Goldman JE, Hirano M, Yu RK, Seyfried TN. GD3 ganglioside is a glycolipid characteristic of immature neuroectodermal cells. J Neuroimmunol. 1984;7:179–192. doi: 10.1016/s0165-5728(84)80017-x. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J Biol Chem. 1990;265:18713–18716. [PubMed] [Google Scholar]
- Jin HJ, Nam HY, Bae YK, Kim SY, Im IR, Oh W, Yang YS, Choi SJ, Kim SW. GD2 expression is closely associated with neuronal differentiation of human umbilical cord blood-derived mesenchymal stem cells. Cell Mol Life Sci. 2010;67:1845–1858. doi: 10.1007/s00018-010-0292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita MO, Furuya S, Ito S, Shinoda Y, Yamazaki Y, Greimel P, Ito Y, Hashikawa T, Machida T, Nagatsuka Y, Hirabayashi Y. Lipid rafts enriched in phosphatidylglucoside direct astroglial differentiation by regulating tyrosine kinase activity of epidermal growth factor receptors. Biochem J. 2009;419:565–575. doi: 10.1042/BJ20081896. [DOI] [PubMed] [Google Scholar]
- Klassen H, Schwartz MR, Bailey AH, Young MJ. Surface markers expressed by multipotent human and mouse neural progenitor cells include tetraspanins and non-protein epitopes. Neurosci Lett. 2001;312:180–182. doi: 10.1016/s0304-3940(01)02215-7. [DOI] [PubMed] [Google Scholar]
- Krathwohl MD, Kaiser JL. Chemokines promote quiescence and survival of human neural progenitor cells. Stem Cells. 2004;22:109–118. doi: 10.1634/stemcells.22-1-109. [DOI] [PubMed] [Google Scholar]
- Martinez C, Hofmann TJ, Marino R, Dominici M, Horwitz EM. Human bone marrow mesenchymal stromal cells express the neural ganglioside GD2: a novel surface marker for the identification of MSCs. Blood. 2007;109:4245–4248. doi: 10.1182/blood-2006-08-039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay R. Stem cells in the central nervous system. Science. 1997;276:66–71. doi: 10.1126/science.276.5309.66. [DOI] [PubMed] [Google Scholar]
- Mujoo K, Kipps TJ, Yang HM, Cheresh DA, Wargalla U, Sander DJ, Reisfeld RA. Functional properties and effect on growth suppression of human neuroblastoma tumors by isotype switch variants of monoclonal antiganglioside GD2 antibody 14.18. Cancer Res. 1989;49:2857–2861. [PubMed] [Google Scholar]
- Nakamura K, Inagaki F, Tamai Y. A novel ganglioside in dogfish brain. Occurrence of a trisialoganglioside with a sialic acid linked to N-acetylgalactosamine. J Biol Chem. 1988;263:9896–9900. [PubMed] [Google Scholar]
- Nakatani Y, Yanagisawa M, Suzuki Y, Yu RK. Characterization of GD3 ganglioside as a novel biomarker of mouse neural stem cells. Glycobiology. 2010;20:78–86. doi: 10.1093/glycob/cwp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngamukote S, Yanagisawa M, Ariga T, Ando S, Yu RK. Developmental changes of glycosphingolipids and expression of glycogenes in mouse brains. J Neurochem. 2007;103:2327–2341. doi: 10.1111/j.1471-4159.2007.04910.x. [DOI] [PubMed] [Google Scholar]
- Pukel CS, Lloyd KO, Travassos LR, Dippold WG, Oettgen HF, Old LJ. GD3, a prominent ganglioside of human melanoma. Detection and characterisation by mouse monoclonal antibody. J Exp Med. 1982;155:1133–1147. doi: 10.1084/jem.155.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfried TN, Yu RK. Ganglioside GD3: structure, cellular distribution, and possible function. Mol Cell Biochem. 1985;68:3–10. doi: 10.1007/BF00219383. [DOI] [PubMed] [Google Scholar]
- Svennerholm L. Chromatographic separation of human brain gangliosides. J Neurochem. 1963;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Tanikawa C, Matsuda K, Fukuda S, Nakamura Y, Arakawa H. p53RDL1 regulates p53-dependent apoptosis. Nat Cell Biol. 2003;5:216–223. doi: 10.1038/ncb943. [DOI] [PubMed] [Google Scholar]
- Weiss S, Reynolds BA, Vescovi AL, Morshead C, Craig CG, van der Kooy D. Is there a neural stem cell in the mammalian forebrain? Trends Neurosci. 1996;19:387–393. doi: 10.1016/s0166-2236(96)10035-7. [DOI] [PubMed] [Google Scholar]
- Xu J, Liao W, Gu D, Liang L, Liu M, Du W, Liu P, Zhang L, Lu S, Dong C, Zhou B, Han Z. Neural ganglioside GD2 identifies a subpopulation of mesenchymal stem cells in umbilical cord. Cell Physiol Biochem. 2009;23:415–424. doi: 10.1159/000218188. [DOI] [PubMed] [Google Scholar]
- Yagi H, Yanagisawa M, Kato K, Yu RK. Lysosome-associated membrane protein 1 is a major SSEA-1-carrier protein in mouse neural stem cells. Glycobiology. 2010a;20:976–981. doi: 10.1093/glycob/cwq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi H, Yanagisawa M, Suzuki Y, Nakatani Y, Ariga T, Kato K, Yu RK. HNK-1 epitope-carrying Tenascin-C spliced variant regulates the proliferation of mouse embryonic neural stem cells. J Biol Chem. 2010b;285:37293–37301. doi: 10.1074/jbc.M110.157081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M. Stem cell glycolipids. Neurochem Res. 2011 doi: 10.1007/s11064-010-0358-1. doi:10.1007/ s11064-010-0358-1. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Yu RK. The expression and functions of glycoconjugates in neural stem cells. Glycobiology. 2007;17:57R–74R. doi: 10.1093/glycob/cwm018. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kojima H, Kawakami Y, Iriko H, Nakamura T, Nakamura K, Uchida A, Murata Y, Tamai Y. A monoclonal antibody against a glycolipid SEGLx from Spirometra erinaceieuropaei plerocercoid. Mol Biochem Parasitol. 1999;102:225–235. doi: 10.1016/s0166-6851(99)00102-4. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Nakamura K, Taga T. Roles of lipid rafts in integrin-dependent adhesion and gp130 signalling pathway in mouse embryonic neural precursor cells. Genes Cells. 2004;9:801–809. doi: 10.1111/j.1365-2443.2004.00764.x. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Taga T, Nakamura K, Ariga T, Yu RK. Characterization of glycoconjugate antigens in mouse embryonic neural precursor cells. J Neurochem. 2005;95:1311–1320. doi: 10.1111/j.1471-4159.2005.03452.x. [DOI] [PubMed] [Google Scholar]
- Yu RK. Development regulation of ganglioside metabolism. Prog Brain Res. 1994;101:31–44. doi: 10.1016/s0079-6123(08)61938-x. [DOI] [PubMed] [Google Scholar]
- Yu RK, Yanagisawa M, Ariga T. Glycosphingolipid structures. In: Kamerling J P, editor. Comprehensive Glycoscience. Oxford, U.K: Elsevier; 2007. pp. 73–122. [Google Scholar]
- Yu RK, Nakatani Y, Yanagisawa M. The role of glycosphingolipid metabolism in the developing brain. J Lipid Res. 2009;50:S440–S445. doi: 10.1194/jlr.R800028-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu RK, Suzuki Y, Yanagisawa M. Membrane glycolipids in stem cells. FEBS Lett. 2010;584:1694–1699. doi: 10.1016/j.febslet.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawlik I, Zakrzewska M, Witusik M, Golanska E, Kulczycka-Wojdala D, Szybka M, Piaskowski S, Wozniak K, Zakrzewski K, Papierz W, Liberski PP, Rieske P. KCTD11 expression in medulloblastoma is lower than in adult cerebellum and higher than in neural stem cells. Cancer Genet Cytogenet. 2006;170:24–28. doi: 10.1016/j.cancergencyto.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]