Priming rice seeds (soaking followed by drying) or soaking just before sowing improved emergence from flooded soil, reduced membrane damage from ROS and hastened carbohydrate mobilization. Most benefit was to lines with a superior ability to germinate in flooded soil even when untreated.
Abstract
Background and aims
Early flooding helps control weeds but reduces seedling establishment in direct-seeded rice (Oryza sativa). When combined with appropriate management practices, the use of genotypes that better tolerate flooding during emergence can enhance crop establishment in flood-prone areas. Management options include seed pre-treatment and we tested the influence of pre-soaking for 24 h prior to sowing or of priming (soaking for 24 or 48 h followed by drying).
Methodology
The effects on seedling establishment after 21-day flooding of pre-soaking seeds for 24 h before sowing and/or of priming seeds were examined together with physiological responses connected with reactive oxygen scavenging. Seeds of four lines with contrasting abilities to tolerate flooding at the germination stage were compared. Seeds were primed using KCl solutions (48 h) or water (24 h) and pre-soaked using water. Lipid peroxidation and activities of reactive oxygen-scavenging enzymes were measured in seeds before sowing. Carbohydrate mobilization in germinating seeds and seedling growth were also monitored at intervals.
Principal results
Seed pre-treatment by pre-soaking or by priming increased survival of flooding and accelerated and improved seedling establishment, especially in tolerant genotypes. Primed seeds had less lipid peroxidation and higher superoxide dismutase (SOD) and catalase (CAT) activities than non-primed seeds. Amylase activities and starch breakdown were also hastened in primed seeds. Survival after flooding was positively correlated with amylase activity but negatively correlated with the extent of lipid peroxidation.
Conclusions
Pre-soaking and priming improved seedling establishment in flooded soil, enhanced the capacity to scavenge reactive oxygen species in seeds by increasing SOD and CAT activities, and hastened carbohydrate mobilization. Tolerant genotypes responded better to these treatments, emphasizing the effectiveness of combining genetic tolerance with appropriate seed pre-treatment to improve seedling establishment of rice sown in flooded soils.
Introduction
There are three principal methods for establishing a crop of rice. These are (i) dry seeding, where seeds are sown (broadcast, drilled or dibbled) on unsaturated soils; (ii) wet seeding, where pre-germinated seeds are sown in saturated puddled soils; and (iii) transplanting, which involves replanting rice seedlings grown in nurseries into puddled soils. Dry- and wet-seeding methods, often referred to as direct seeding, are becoming more popular with rice farmers because they require less labour and time than transplanting (Tuong et al. 2000). Where there is good control over the water supply (irrigated systems), farmers commonly practise wet seeding, using pre-germinated seeds. In areas where water is scarce or the supply is unpredictable, as in rainfed areas, dry seeding is usually adopted (Chin and Mortimer 2002).
Direct seeding is more attractive than transplanting because it is cheaper and can result in an earlier harvest (Balasubramanian and Hill 2002). However, poor germination, uneven stand establishment and high weed infestation are some of the constraints that restrict its large-scale adoption in flood-prone rainfed areas (Du and Tuong 2002; Ismail et al. 2009). Weed infestation can however be lessened by shallow flooding immediately after sowing. Although rice seed can germinate under such flooded conditions, subsequent growth is mostly limited to the coleoptile and only tolerant genotypes have the ability to elongate and produce healthy seedlings that emerge above the water surface (Ismail et al. 2009). Earlier screening identified several rice genotypes that are more tolerant of flooding during germination and emergence (Angaji et al. 2010). These tolerant types have the ability to mobilize and use stored carbohydrates even under the low oxygen stress caused by flooding, through mechanisms associated with carbohydrate metabolism (Ismail et al. 2009).
Knowledge and adoption of practices that prolong seed longevity through proper storage and handling, and produce optimal seedbed conditions can further improve the performance of tolerant cultivars to ensure good crop establishment in flooded soils. Our recent studies described some of these seed-handling strategies and seedbed management options, and established the need to combine good agronomic practices with genetic tolerance for better crop establishment following direct seeding in soils prone to early-season floods (Ella et al. 2010).
Seed priming through soaking followed by partial dehydration to trigger the germination processes and further drying before radicle emergence (Bradford 1986) can ensure rapid and uniform seed germination in various cereals (Lee et al. 1998; Basra et al. 2002; Farooq et al. 2006a) and vegetable species such as tomato (Mitra and Basu 1979) and onion (Ilbi and Eser 2002). In most of these studies, seeds were grown under aerated conditions and priming was reported to increase seed vigour and extend seed longevity. In rice sown in aerated soils, seed priming enhances seedling emergence, kernel quality and yield (Farooq et al. 2006a). Activities of several enzymes associated with the germination process have been observed to change in response to seed priming. These include increases in activities of α-amylase in rice (Farooq et al. 2006b), acid phosphatase and esterase in lettuce (Khan et al. 1978), and antioxidant enzymes in lucerne (Zhang et al. 2007). A rapid resumption of DNA synthesis and initiation of cell division was observed in wheat soon after hydration (Dell'Aquila and Taranto 1986) while repair of DNA and other cellular components (e.g. membranes), which may be damaged during seed maturation, dehydration and storage, has been suggested to take place during seed priming. It has also been suggested that the onset or completion of DNA repair may be a major contributing factor to the improvement in germination after osmopriming (Burgass and Powell 1984).
Generation of reactive oxygen species (ROS) is known to occur during the dehydration of various plant tissues, including seeds during maturation (Smirnoff 1993). Oxidative damage results from the reaction of ROS with cellular components and membrane phospholipids, causing their breakdown to malondialdehyde (MDA) and simple alkanes. These reactions could impair various metabolic functions by changing the physicochemical properties of cell membranes through disruption of lipid bilayers. This in turn can promote leakage of solutes, leading to cell death (Scandalios 1993). The peroxidation of polyunsaturated fatty acids is likely to be one of the major causes of deterioration of stored seeds (Wilson and McDonald 1986). Previous studies reported the presence of several antioxidative and hydrolytic enzymes in dry seeds, and a rise in their activities after the start of imbibition. Any pre-treatment that can enhance the activities of antioxidants can be expected to enhance seed germination and early seedling growth, particularly when seeds are sown under suboptimal conditions such as flooded soils. Achieving this will benefit direct seeding in flood-prone areas.
The present study evaluates the effectiveness of seed pre-treatments before sowing on seedling establishment under flooded conditions. Seeds of contrasting genotypes were either pre-soaked in water for 24 h prior to sowing or subjected to the priming process. Priming involved soaking in water for 24 h or in KCl solution for 48 h and then drying and storing prior to sowing. The effects of these treatments on growth and survival, and the capacity of seeds to scavenge ROS and mobilize and use carbohydrate reserves were investigated in lines with inherently different abilities to tolerate flooding at the germination stage.
Materials and methods
Plant material and experimental set-up
Four rice genotypes selected from earlier screenings (Ismail et al. 2009; Angaji et al. 2010; Ella et al. 2010) were used. Two of the genotypes are tolerant of flooding during germination and early establishment (‘Khaiyan’ and ‘Khao Hlan On’) and two are relatively intolerant of flooding (‘FR13A’ and ‘IR42’). Two experiments were conducted using seeds stored in a desiccator in cold storage (5–8 °C). Before each experiment, seed dormancy was broken at 55 °C for 5 days. Seedling trays with grids of 34 rows were used, each row comprising 17 cells of 1 cm × 1 cm × 2.5 cm filled with finely ground field soil. Seeds of each genotype were sown dry at ∼0.5 cm soil depth in six rows per replication (one seed per cell, and a total of 102 seeds) with two rows left vacant between genotypes. Trays were submerged in 8–10 cm of tap water in concrete tanks immediately after sowing. Another set of trays was maintained under control (non-flooded) conditions in an adjacent area. A randomized complete block design was used with four replications in each experiment. The set-up was maintained inside a greenhouse under ambient temperature and irradiance.
Experiment I tested the effects of two seed treatments: (i) pre-soaking seeds for 24 h before sowing and (ii) seed priming, in which seeds were soaked for 24 h and then dried and stored before sowing. In both treatments, tap water was used, and effects on emergence, seedling survival and growth in flooded soil were monitored. Additional trays were set up and seedlings were harvested from two rows daily for the first 9 days after sowing and flooding, for assessing emergence and growth. Pre-soaking of seeds before sowing is relevant in irrigated areas where farmers can use seeds that were hydrated before sowing, while priming is more likely to be used in rainfed areas where rainfall is unpredictable and dry seeding is preferred.
In Experiment II, seeds were primed for 48 h using three concentrations of KCl solution with water potentials of −0.75, −1.50 and −2.25 MPa (0.15, 0.30 and 0.45 M, respectively). In preliminary trials, 48 h was more effective than 24 h when KCl was used as the priming solution. Additional trays allowed seedlings from two rows of cells to be harvested daily for the first 9 days after flooding for measuring root and shoot emergence and growth, and two further rows for assessing non-structural carbohydrates and amylase activity.
Seed pre-treatments
Uniformly sized seeds were selected and 100-grain weight was recorded. To minimize contamination during priming, seeds were surface sterilized with 2.63 % NaOCl solution (household bleach diluted to 1:1 with sterile water) for 30 min and then rinsed three times with sterile dH2O. The seeds were then soaked in aerated priming solution (1 g of seeds per 5 mL of solution) at room temperature (26.0±0.1 °C) in the dark. The priming solution was decanted after the specified duration of priming, and seeds were placed in plastic dishes lined with a few layers of paper towels that were frequently replaced during the first hours to accelerate drying of primed seeds. After blotting dry, seeds were transferred to a laminar airflow cabinet with an air flow rate of 880 m3 min−1 (measured using a heavy-duty Vane anemometer model 407113; Extech Instrument Corporation, Waltham, MA, USA). The air flow temperature was 26.2±0.2 °C and the relative humidity was 63.5±1.0 %. Air drying of seeds in the laminar airflow cabinet was terminated after a week, when the seeds reached a moisture content similar to the original level of 12.9±0.1 %. The dried seeds were kept in labelled plastic containers inside a desiccator at 5–8 °C and used within a month. Pre-soaking of the seeds before sowing was achieved following the same methods used during priming, using aerated water (1 g of seeds per 5 mL of water) at room temperature in the dark.
Seedling growth and survival
The number of surviving seedlings was counted in both the control and flooded treatments in both experiments 21 days after sowing and flooding. In flooded treatments, seedling survival was measured as the percentage of seedlings that emerged above the water surface. Seedlings were harvested during the first 9 days following sowing, germinating seeds were counted, and lengths of shoot and root were measured.
Lipid peroxidation
Lipid peroxidation was measured before sowing from all treatments in Experiment II, as the concentration of MDA determined using the thiobarbituric acid method described in Ella et al. (2003). Fifty dry seeds were weighed and ground to a powder in liquid N2 and then extracted with 10 mL of 50 mM potassium phosphate buffer (pH 7.0). The extracts were centrifuged at 10 000×g and 4 °C for 20 min and the supernatant was used for the assay. The reaction mixture contained 1 mL of supernatant and 1 mL of 0.5 % (w/v) thiobarbituric acid solution containing 20 % (w/v) trichloroacetic acid. After mixing, the reaction mixture was incubated at 95 °C for 30 min and then placed in an ice bath. The cooled mixture was centrifuged at 10 000×g and 4 °C for 10 min. The absorbance of the supernatant layer was measured at 532 and 600 nm and, after subtracting the non-specific absorbance measured at 600 nm, the MDA concentration was calculated using an extinction coefficient of 155 mM−1 cm−1.
Extraction of reactive oxygen-scavenging enzymes and activity assessment
Extraction and enzyme activity assays were carried out as described in Ella et al. (2003). In Experiment II, reactive oxygen-scavenging enzymes were extracted from non-primed seeds and seeds primed with KCl solution of −2.25 MPa water potential (0.45 M). Fifty seeds were weighed and ground to a fine powder in liquid N2 and extracted with 10 mL of ice-cold potassium phosphate buffer containing 1 mM ascorbic acid (pH 7.8). The extract was centrifuged at 10 000×g and 4 °C for 20 min and the supernatant was used for the assay. Ascorbate peroxidase (APX) and catalase (CAT) activities were assayed using the fresh supernatant layer containing the crude enzyme extract. About 1 mL of the supernatant was dialysed against Viskase Membra-Cel (MWCP 7000, Chicago, IL, USA) overnight with potassium phosphate buffer (pH 7.8). A 10 % increase in extract volume was observed after dialysis. The dialysed extract was used for the activity assay of glutathione reductase (GR) and superoxide dismutase (SOD). A total assay volume of 1 mL was maintained in all measurements of enzyme activities. All assays were done at published optimum conditions (temperature, pH and substrate concentration) and with recovery checks using (i) purified enzymes manufactured by Sigma Chemicals, (ii) a mixture of purified enzyme and sample, and (iii) sample mixtures. Percentage recovery was high (within a maximum uncertainty limit of 5.0 %) and activity values observed were very close to the additive value predicted in a given mixture of purified enzyme and sample, and also in sample mixtures. The activity values reported here were corrected according to their respective recovery. Enzyme activity assays were measured following Nakano and Asada (1987) for APX, Beers and Sizer (1952) for CAT, Halliwell and Foyer (1978) for GR, and Asada et al. (1973) for SOD. Enzyme activities are expressed in units per milligram of protein. Protein concentration was measured following Bradford (1976).
Non-structural carbohydrate analysis
A subsample of the same seeds treated in Experiment II was used for carbohydrate analysis. Germinating non-primed seeds and seeds primed with a KCl solution of −2.25 MPa were collected daily after sowing and frozen in liquid N2, freeze-dried and weighed to obtain their dry weights. The method for non-structural carbohydrate analysis is described in Ismail et al. (2009). Dried samples were extracted in 80 % ethanol (v/v) and analysed for total soluble sugar concentration. The residue was then washed several times and analysed for starch concentration.
Amylase activity
Amylase activity was assayed following the method described in Ismail et al. (2009). Seedlings harvested 1 and 3 days after sowing were extracted with 0.02 M sodium phosphate buffer (pH 6.9) with 0.006 M NaCl. The crude extract was used in the assay at room temperature. Starch was first converted to maltose as catalysed by amylase. The maltose produced was reacted with 3,5-dinitrosalicylic acid, which was reduced to form a coloured product with maximum absorption at 540 nm. The absorption values were read on a standard curve established with increasing amounts of maltose. One unit of amylase activity is defined as micromoles of maltose produced per minute and specific activity was expressed in units per milligram of protein. Protein concentration was determined following Bradford (1976).
Statistical analysis
Data from each experiment were subjected to analysis of variance using CROPTAT for Windows version 7.2 (International Rice Research Institute 2007) based on a randomized complete block design model with four replications. Relationships between different attributes were studied using linear regression.
Results
Effect of seed priming and pre-soaking on growth and survival under flooded conditions
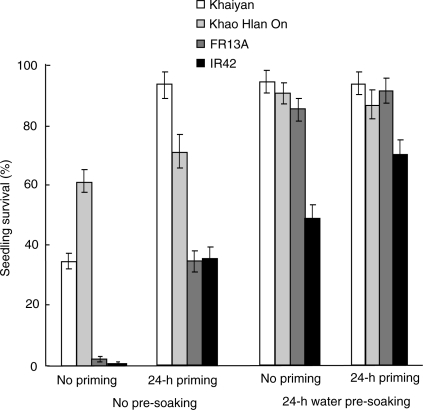
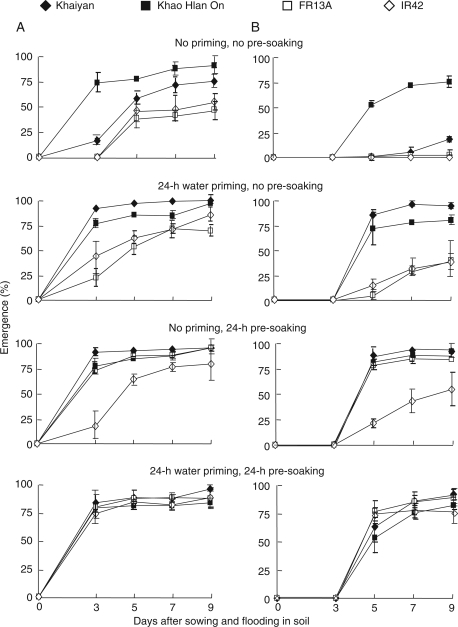
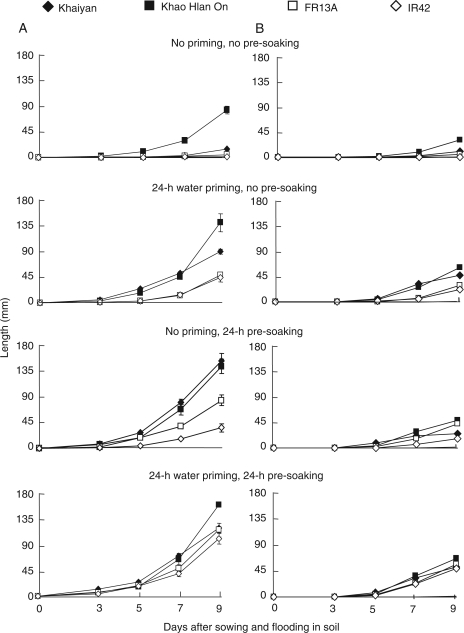
Priming with water for 24 h in the absence of pre-soaking significantly improved seedling survival of all four genotypes under flooded conditions compared with no priming. The two tolerant genotypes, ‘Khaiyan’ and ‘Khao Hlan On’, survived flooding better than intolerant genotypes in the absence of priming, and this advantage was enhanced by priming (Fig. 1). Pre-soaking seeds in water for 24 h before sowing had greater positive effects than priming in the absence of pre-soaking except with ‘Khaiyan', where both priming alone and pre-soaking had similar effects. In flooding-intolerant ‘IR42’ only, combining seed priming and seed pre-soaking resulted in better survival than either treatment alone. Seed pre-treatment also enhanced the emergence of both shoot and root (Fig. 2). Both priming and pre-soaking enhanced shoot emergence, the effect being more evident in flooding-intolerant genotypes ‘FR13A’ and ‘IR42’ (Fig. 2A). Root emergence was delayed by ∼1–2 days in all genotypes compared with shoot emergence and was enhanced in both primed and pre-soaked seeds, with greater effects in the two flooding-intolerant lines (Fig. 2B). Post-germination shoot and root elongation also benefited from the pre-treatment. Both grew longer in seedlings from primed and pre-soaked seeds, and were enhanced most in seeds subjected to both priming and pre-soaking before sowing (Fig. 3). The flooding-intolerant genotypes ‘FR13A’ and ‘IR42’ gave the largest response. In both Experiment I and Experiment II, seedlings of the flooding-tolerant lines derived from primed seeds reached the soil surface earlier (∼5–6 days after sowing and flooding) than those from the control seeds (∼8–9 days). Also, a more uniform growth was visually apparent in seedlings from primed seeds (data not shown). Similar positive effects on seedling growth and survival were observed in seeds primed with tap water for 24 h or with KCl solution for up to 48 h (data not shown). Correlations of survival with emergence and root and shoot growth were strong and positive (Table 1).
Fig. 1.
Seedling survival of four rice genotypes after priming or pre-soaking of seeds for 24 h before sowing in flooded soil. Data are means of four replicates, each of 102 seeds (Experiment I). Vertical bars indicate ±SE. Genotypes ‘Khaiyan’ and ‘Khao Hlan On’ are classified as more flooding tolerant than ‘FR13A’ and ‘IR42’ at the germination stage.
Fig. 2.
Effect of seed pre-treatments on emergence of (A) shoot and (B) root of four rice genotypes during flooding with 10 cm of water (Experiment I). Data are means of four replicates, each of 34 seeds. Vertical bars indicate ±SE. Genotypes ‘Khaiyan’ and ‘Khao Hlan On’ are classified as more flooding tolerant than ‘FR13A’ and ‘IR42’ at the germination stage.
Fig. 3.
Effect of seed pre-treatments on elongation of (A) shoot and (B) root of four rice genotypes during flooding with 10 cm of water (Experiment I). Data are means of four replicates, each of 34 seeds. Vertical bars indicate ±SE. Genotypes ‘Khaiyan’ and ‘Khao Hlan On’ are classified as more flooding tolerant than ‘FR13A’ and ‘IR42’ at the germination stage.
Table 1.
Correlation coefficients for associations between different traits of seeds and seedlings germinated and grown under flooded conditions.
| Parameters in association | r value |
|---|---|
| Experiment I | |
| Seedling survival and | |
| Shoot emergence 3 days after sowing | 0.87* |
| Root emergence 5 days after sowing | 0.91* |
| Shoot length 5 days after sowing | 0.89* |
| Root length 7 days after sowing | 0.83* |
| Experiment II | |
| Lipid peroxidation in seeds before sowing and | |
| SOD activity | −0.73* |
| CAT activity | −0.76* |
| Total amylase activity 1 day after sowing and | |
| Shoot length 5 days after sowing | 0.89* |
| Root length 5 days after sowing | 0.48* |
| Seedling survival and | |
| Total amylase activity 1 day after sowing | 0.84* |
| α-Amylase activity 1 day after sowing | 0.83* |
| β-Amylase activity 1day after sowing | 0.85* |
| Shoot length 5 days after sowing | 0.88* |
| Root length 5 days after sowing | 0.63* |
*Significant at the P < 0.001 level.
Lipid peroxidation and activities of reactive oxygen-scavenging enzymes in dry seeds before sowing
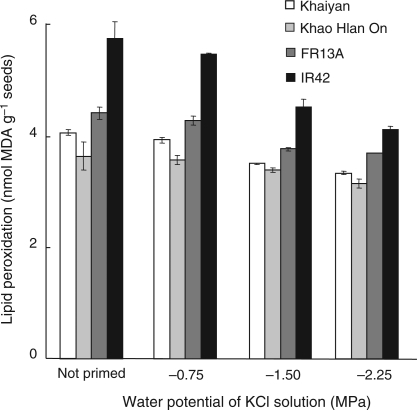
Lipid peroxidation was measured before sowing in non-primed seeds and in seeds primed with different concentrations of KCl solution. Priming significantly reduced the extent of lipid peroxidation, with stronger reduction in seeds of ‘IR42’ (Fig. 4). Seeds primed for 48 h with the most concentrated KCl solution (−2.25 MPa) had the lowest concentration of MDA, indicating the least amount of lipid peroxidation.
Fig. 4.
Effect of seed priming for 48 h in KCl solutions of different water potentials on lipid peroxidation in dry seeds before sowing (Experiment II). Data are means of four replicates, each of 50 seeds. Vertical bars indicate ±SE. Genotypes ‘Khaiyan’ and ‘Khao Hlan On’ are classified as more flooding tolerant than ‘FR13A’ and ‘IR42’ at the germination stage.
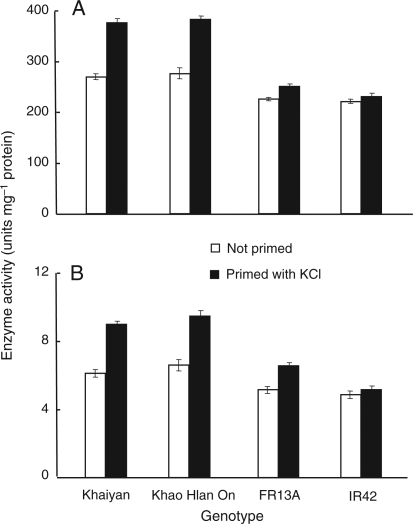
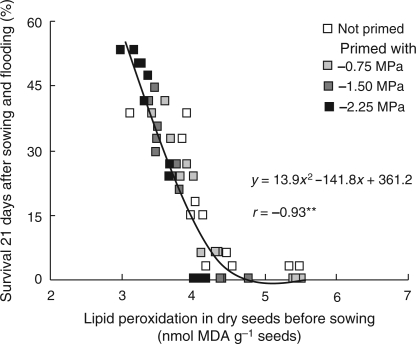
Activities of selected reactive oxygen-scavenging enzymes were assessed before sowing in non-primed seeds and in seeds primed with −2.25 MPa KCl solution for 48 h. Priming substantially increased the activity of SOD and CAT in seeds of the tolerant genotypes ‘Khaiyan’ and ‘Khao Hlan On’. Smaller positive effects were seen in flooding-intolerant ‘FR13A’ and ‘IR42’ (Fig. 5). No APX or GR activity was found in any of the seeds. Strong negative correlations between the extent of lipid peroxidation in seeds before sowing and activity of SOD (r = −0.73, P< 0.001) and CAT (r = −0.76, P< 0.001; Table 1) suggest their involvement in reducing membrane degradation during germination. The strong negative correlation between lipid peroxidation in seeds before sowing and seedling survival 21 days after sowing and flooding (r = −0.93, P< 0.001; Fig. 6) also suggested the importance of maintaining lipid membrane integrity for better germination and emergence during flooding.
Fig. 5.
Effect of seed priming for 48 h in KCl solution with −2.25 MPa water potential on the activities of (A) SOD and (B) CAT in dry seeds before sowing (Experiment II). Data are means of four replicates, each of 50 seeds. Vertical bars indicate ±SE. Genotypes ‘Khaiyan’ and ‘Khao Hlan On’ are classified as more flooding tolerant than ‘FR13A’ and ‘IR42’ at the germination stage.
Fig. 6.
Association between lipid peroxidation in dry seeds before sowing and survival 21 days after sowing and flooding in non-primed seeds and seeds primed with KCl solutions of different water potentials (Experiment II). Data are individual values of four genotypes in four replicates, each with 102 seeds used for measurements of survival and 50 seeds for lipid peroxidation.
Non-structural carbohydrates and amylase activity in seeds germinating under flooding
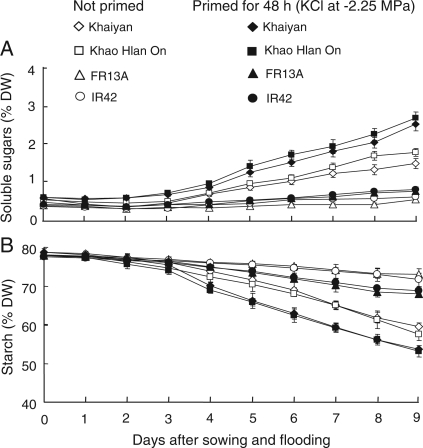
Changes in the concentrations of soluble sugars and starch in germinating non-primed and primed seeds were assessed during the first 9 days after sowing and flooding in the two tolerant and two intolerant genotypes. Seed priming significantly affected the concentrations of soluble sugars and starch in germinating seeds. Starting 3 days after sowing and the start of flooding, concentrations of soluble sugars increased with time. Over the 9 days flooding, sugar concentrations increased most strongly in the two flooding-tolerant genotypes. They also give a larger response to priming (Fig. 7). Starch showed a reversed trend starting from Day 4 when concentrations decreased with time, with faster reductions in tolerant genotypes and in seedlings from primed seeds.
Fig. 7.
Effect of seed priming on concentrations of (A) soluble sugars and (B) starch in germinating seeds of four rice genotypes under flooded conditions (Experiment II). Data are means of four replicates, each of 34 seeds. Vertical bars indicate ±SE. Genotypes ‘Khaiyan’ and ‘Khao Hlan On’ are classified as more flooding tolerant than ‘FR13A’ and ‘IR42’ at the germination stage.
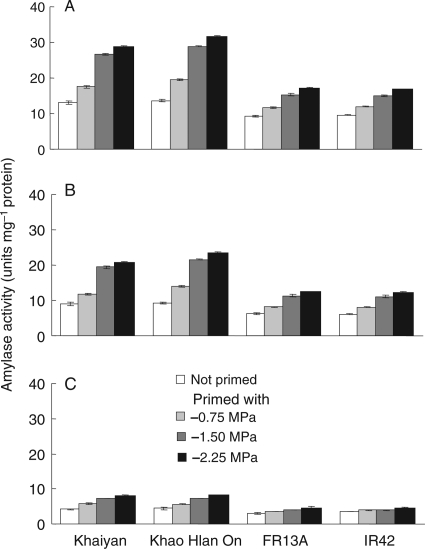
Amylase activity in germinating seeds was measured 1 and 3 days after the start of the experiment, and similar trends were observed. Data collected at Day 1 are presented in Fig. 8. Total amylase activity was significantly higher in primed seeds than in non-primed seeds and tolerant genotypes showed substantially greater responsiveness to priming (Fig. 8A). In all genotypes, α-amylase activity was increased by priming and, again, the two flooding-tolerant lines were more responsive than the intolerant lines. β-Amylase activities were lower than those for α-amylase in all genotypes but were increased by priming, particularly in the tolerant lines. Correlations between amylase activity and growth and survival were strong and positive. Total amylase activity 1 day after sowing correlated positively with shoot (r = 0.89, P < 0.001) and root (r = 0.48, P < 0.001) length measured 5 days after sowing, and with seedling survival 21 days after sowing (r = 0.84; Table 1). Correlations of survival with α- and β-amylase activities, as well as with shoot and root lengths, were high and statistically significant (Table 1).
Fig. 8.
Effect of seed priming for 48 h in different concentrations of KCl of different water potentials on (A) total, (B) α- and (C) β-amylase activity 1 d after sowing in flooded soil (Experiment II). Data are means of four replicates, each of 34 seeds. Vertical bars indicate ± SE. Genotypes ‘Khaiyan’ and ‘Khao Hlan On’ are classified as more flooding tolerant than ‘FR13A’ and ‘IR42’ at the germination stage.
Discussion
In rainfed areas, direct-seeded rice is frequently affected by waterlogging and shallow floods when heavy rain falls soon after sowing. Even in irrigated areas, irregular crop establishment is common when the land is uneven or when shallow flooding is used to suppress weed growth. This damage arises because of the high sensitivity of rice to flooding during these stages. Genetic variation in the ability of rice seeds to germinate in flooded soils has been reported before (Yamauchi et al. 1993; Angaji et al. 2010), and the basis of the tolerance was recently investigated (Ismail et al. 2009; Angaji et al. 2010). Our recent studies showed that germination and seedling establishment under flooded conditions could be affected by numerous seed and seedbed management practices before sowing. These include controlling seed age, storage conditions and the conditions of the floodwater during germination (Ella et al. 2010). In the present paper we report on pre-sowing treatments that could further enhance the expression of tolerance under field conditions. In direct-seeded farming systems, these could be used to advantage in conjunction with new flood-tolerant lines currently being developed in a breeding programme. In particular, we studied pre-soaking or soaking followed by drying and storage before sowing on the emergence and growth of seedlings under flooded conditions. Furthermore, we attempted to unravel the physiological bases for the enhancement in seed germination and seedling establishment resulting from these treatments.
Higher seedling survival under flooded conditions was observed in pre-soaked or primed seeds than in non-primed seeds (Fig. 1). Priming increased the percentage of seeds with emerging shoots and roots under flooded conditions (Fig. 2), and also the rate of shoot and root growth (Fig. 3). This was also observed in other crops but under aerated conditions, as in lucerne (Zhang et al. 2007), muskmelon (Nascimento 2003) and sorghum (Tiryaki and Buyukcingil 2009). Similar effects were also observed when seeds were pre-soaked with water for 24 h before sowing. However, further increases in seedling growth and survival were observed in the intolerant genotypes when seeds were primed and then pre-soaked before sowing. This suggests that these seed treatments could substantially enhance crop establishment in flooded soils particularly when using seeds of less tolerant genotypes.
Priming decreased lipid peroxidation (Fig. 4) and increased SOD and CAT activities in dry seeds before sowing (Fig. 5). This positive effect on reducing the extent of lipid peroxidation was also observed in lucerne seedlings (Zhang et al. 2007). Lipid peroxidation is one major consequence of free radical-mediated injury to cellular membranes, and peroxidation of fatty acyl groups occurs mostly in membrane phospholipids, which can greatly alter the physicochemical properties of lipid bilayers, resulting in severe cellular dysfunction. This damage leads to leakage of cellular contents and affects the respiratory activity of the mitochondria and the carbon-fixing ability of the chloroplast (Scandalios 1993). The improved emergence and enhanced growth in rice seedlings from primed seeds under flooded conditions may, to a degree, be attributed to the reduced lipid peroxidation caused by higher SOD and CAT activities in primed seeds.
Priming enhanced the activities of amylases in germinating rice seeds (Fig. 8), probably in part, through advancing the germination process. This suggests that priming and pre-soaking of seeds before sowing could accelerate carbohydrate mobilization under low oxygen stress. Amylases are known to play a major role in starch breakdown in cereal seeds (Guglielminetti et al. 1995) and, during germination under low oxygen stress, rice seeds are capable of partial degradation of starch into readily fermentable carbohydrates to generate the minimum energy required for growth of the coleoptiles. Starch is a major energy source for developing rice embryos and previous studies have established the importance of α-amylases in starch degradation even when O2 is limiting (Guglielminetti et al. 1995; Ismail et al. 2009). The enhanced capacity of primed seeds to (i) scavenge ROS and (ii) mobilize carbohydrates during germination under low oxygen stress could at least partially explain the enhanced seedling growth and survival in flooded soils observed in this study.
We observed that seed priming, pre-soaking seeds before sowing, or the combination of both is more effective in enhancing germination under flooded conditions than under control conditions (data not shown), which highlights the importance of seed pre-treatment when the conditions for crop establishment are suboptimal (e.g. flooded soil), and also when seeds are old or have reduced viability because of poor storage conditions (Ella et al. 2010). The effect of seed priming on seedling survival under flooded conditions is more apparent in tolerant genotypes, suggesting the importance of combining genetic tolerance with proper management when sowing conditions are unfavourable. Hence, farmers are more likely to benefit most from seed priming and other management technologies when tolerant varieties become available.
We also studied the effect of priming with water for 24 h, of pre-soaking for 24 h before sowing, and the combination of priming and pre-soaking. Both priming with water and pre-soaking for 24 h before sowing improved germination, growth and survival, with greater enhancement when seeds were primed and then pre-soaked before sowing. However, the combined positive effect of priming and pre-soaking was more evident in the intolerant genotype ‘IR42’. Pre-soaking seeds for 24 h before sowing may serve as an alternative to seed priming in areas where farmers could have control over water, as in irrigated areas, because pre-soaked seeds can no longer be stored. However, under rainfed conditions, seed priming still remains the obvious choice for farmers, as they can prime and store their seeds in order for them to be ready for sowing any time when sufficient rainwater accumulates in the soil.
Conclusions and forward look
This study established the importance of pre-soaking or priming seeds (soaking and then drying and storing them before use) before sowing in improving emergence and seedling establishment in flooded soils. The enhancement in survival and seedling establishment was associated with (i) lower lipid peroxidation in dry seeds before sowing and maintenance of higher activity of SOD and CAT, two key reactive oxygen-scavenging enzymes, and (ii) higher catabolic activities of amylase enzymes associated with greater starch mobilization to provide energy for the growing embryos under low oxygen stress. The effect of seed priming on carbohydrate mobilization could, in some way, be exerted through advancing the germination process. The positive effect of priming and/or pre-soaking is more evident in the intolerant genotypes. To our knowledge, this is the first study to establish the effectiveness of these seed treatments in improving emergence from flooded soils. Seed priming will be more effective in areas that are prone to uncontrolled flooding. However, pre-soaking seeds for 24 h before sowing could be used instead of, or in combination with, seed priming when water resources are secure and controlled at the time of sowing. Combining genetic tolerance with these seed pre-treatments is more effective when sowing conditions are less favourable.
Sources of funding
This research was partly funded by the German Federal Ministry for Economic Cooperation and Development (BMZ) and the Bill and Melinda Gates Foundation.
Contributions by the authors
E.S.E. conducted the trials, collected the data and performed initial analysis. A.M.I. and E.S.E. planned the research and wrote up the manuscript. M.L.D.-S. contributed to the discussion of the work and editing of the manuscript.
Conflict of interest statement
None declared.
Acknowledgements
We thank Lamberto V. Licardo, Melencio J. Apostol and Mary Louise C. Mendoza for technical assistance.
References
- Angaji SA, Septiningsih EM, Mackill DJ, Ismail AM. Identification of QTLs associated with tolerance of anaerobic conditions during germination in rice (Oryza sativa L.) Euphytica. 2010;172:159–168. doi:10.1007/s10681-009-0014-5. [Google Scholar]
- Asada K, Urano M, Takahashi M. Subcellular location of superoxide dismutase in spinach leaves and preparation and properties of crystalline spinach superoxide dismutase gene sodA. European Journal of Biochemistry. 1973;36:257–266. doi: 10.1111/j.1432-1033.1973.tb02908.x. doi:10.1111/j.1432-1033.1973.tb02908.x. [DOI] [PubMed] [Google Scholar]
- Balasubramanian V, Hill JE. Direct seeding of rice in Asia: emerging issues and strategic research needs for the 21st century. In: Pandey S, Mortimer M, Wade L, Tuong TP, Lopez K, Hardy B, editors. Direct seeding: research strategies and opportunities. Los Baños, Philippines: International Rice Research Institute; 2002. pp. 15–39. [Google Scholar]
- Basra SMA, Zia MN, Mehmood T, Afzal I, Khaliq A. Comparison of different invigoration techniques in wheat (Triticum aestivum L.) seeds. Pakistan Journal of Arid Agriculture. 2002;5:11–16. [Google Scholar]
- Beers RF, Sizer I. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. Journal of Biological Chemistry. 1952;195:133–140. [PubMed] [Google Scholar]
- Bradford KJ. Manipulation of seed water relations via osmotic priming to improve germination under stress conditions. HortScience. 1986;21:1105–1112. [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. doi:10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burgass RW, Powell AA. Evidence for repair processes in invigoration of seeds. Annals of Botany. 1984;53:753–757. [Google Scholar]
- Chin DV, Mortimer M. Weed management in direct-seeded rice in Southern Vietnam. In: Pandey S, Mortimer M, Wade L, Tuong TP, Lopez K, Hardy B, editors. Direct seeding: research strategies and opportunities. Los Baños, Philippines: International Rice Research Institute; 2002. pp. 349–356. [Google Scholar]
- Dell'Aquila A, Taranto G. Cell division and DNA synthesis during osmopriming treatments and following germination in aged wheat embryos. Seed Science Technology. 1986;14:333–341. [Google Scholar]
- Du LV, Tuong TP. Enhancing performance of dry-seeded rice: effects of seed priming, seeding rate, and time of seeding. In: Pandey S, Mortimer M, Wade L, Tuong TP, Lopez K, Hardy B, editors. Direct seeding: research strategies and opportunities. Los Baños, Philippines: IRRI; 2002. pp. 241–256. [Google Scholar]
- Ella ES, Kawano N, Ito O. Importance of active oxygen-scavenging system in the recovery of rice seedlings after submergence. Plant Science. 2003;165:85–93. doi:10.1016/S0168-9452(03)00146-8. [Google Scholar]
- Ella ES, Dionisio-Sese ML, Ismail AM. Proper management improves seedling survival during early flooding in contrasting rice genotypes. Crop Science. 2010;50:1997–2008. doi:10.2135/cropsci2009.09.0492. [Google Scholar]
- Farooq M, Basra SMA, Tabassum R, Afzal I. Enhancing the performance of direct-seeded fine rice by seed priming. Plant Production Science. 2006a;9:446–456. doi:10.1626/pps.9.446. [Google Scholar]
- Farooq M, Basra SMA, Wahid A. Priming of field-sown rice seed enhances germination, seedling establishment, allometry and yield. Plant Growth Regulation. 2006b;49:285–294. doi:10.1007/s10725-006-9138-y. [Google Scholar]
- Guglielminetti L, Yamaguchi J, Perata P, Alpi A. Amylolytic activities in cereal seeds under aerobic and anaerobic conditions. Plant Physiology. 1995;109:1069–1076. doi: 10.1104/pp.109.3.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Foyer CH. Properties and physiological function of a glutathione reductase purified from spinach leaves by affinity chromatography. Planta. 1978;139:9–17. doi: 10.1007/BF00390803. doi:10.1007/BF00390803. [DOI] [PubMed] [Google Scholar]
- Ilbi H, Eser B. The effects of pre-storage treatments on ageing in onion seeds. Acta Horticulturae. 2002;579:613–618. [Google Scholar]
- International Rice Research Institute. CROPSTAT for Windows, version 7.2. Los Banños, Philippines: International Rice Research Institute; 2007. [Google Scholar]
- Ismail AM, Ella ES, Vergara GV, Mackill DJ. Mechanisms associated with tolerance for flooding during germination and early seedling growth in rice (Oryza sativa) Annals of Botany. 2009;103:197–209. doi: 10.1093/aob/mcn211. doi:10.1093/aob/mcn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AA, Tao KL, Knypl JS, Borkowska B, Powell LE. Osmotic conditioning of seeds: physiological and biochemical changes. Acta Horticulturae. 1978;83:267–282. [Google Scholar]
- Lee SS, Kim JH, Hong SB, Yun SH. Effect of humidification and hardening treatment on seed germination of rice. Korean Journal of Crop Science. 1998;43:157–160. [Google Scholar]
- Mitra R, Basu RM. Seed treatment for viability, vigor and productivity of tomato. Scientia Horticulturae. 1979;11:368–373. doi: [Google Scholar]
- Nakano YK, Asada K. Purification of ascorbate peroxidase in spinach chloroplast: inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiology. 1987;28:131–214. [Google Scholar]
- Nascimento WM. Muskmelon seed germination and seedling development in response to seed priming. Scientia Agricola. 2003;60:71–75. [Google Scholar]
- Scandalios JG. Oxygen stress and superoxide dismutases. Plant Physiology. 1993;101:7–12. doi: 10.1104/pp.101.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytologist. 1993;125:27–58. doi: 10.1111/j.1469-8137.1993.tb03863.x. doi:10.1111/j.1469-8137.1993.tb03863.x. [DOI] [PubMed] [Google Scholar]
- Tiryaki I, Buyukcingil Y. Seed priming combined with plant hormones: influence on germination and seedling emergence of sorghum at low temperature. Seed Science and Technology. 2009;37:303–315. [Google Scholar]
- Tuong TP, Pablico PP, Yamauchi M, Confesor R, Moody K. Increasing water productivity and weed suppression of wet-seeded rice: effect of water management and rice genotypes. Experimental Agriculture. 2000;36:71–89. doi:10.1017/S0014479700361099. [Google Scholar]
- Wilson DO, McDonald MB. The lipid peroxidation model of seed ageing. Seed Science Technology. 1986;14:269–300. doi: [Google Scholar]
- Yamauchi M, Aguilar AM, Vaughan DA, Seshu DV. Rice (Oryza sativa L.) germplasm suitable for direct sowing under flooded soil surface. Euphytica. 1993;67:177–183. doi:10.1007/BF00040619. [Google Scholar]
- Zhang S, Hu J, Zhang Y, Xie XJ, Knapp A. Seed priming with brassinolide improves lucerne (Medicago sativa L.) seed germination and seedling growth in relation to physiological changes under salinity stress. Australian Journal of Agricultural Research. 2007;58:811–815. doi:10.1071/AR06253. [Google Scholar]