Abstract
The defensive withdrawal reflexes of Aplysia californica have provided powerful behavioral systems for studying the cellular and molecular basis of memory formation. Among these reflexes the tail-elicited tail withdrawal reflex (T-TWR) has been especially useful. In vitro studies examining the monosynaptic circuit for the T-TWR, the tail sensory-motor (SN-MN) synapses, have identified the induction requirements and molecular basis of different temporal phases of synaptic facilitation that underlie sensitization in this system. They have also permitted more recent studies elucidating the role of synaptic and nuclear signaling during synaptic facilitation. Here we report the development of a novel, compartmentalized semi-intact T-TWR preparation that allows examination of the unique contributions of processing in the SN somatic compartment (the pleural ganglion) and the SN-MN synaptic compartment (the pedal ganglion) during the induction of sensitization. Using this preparation we find that the T-TWR is mediated entirely by central connections in the synaptic compartment. Moreover, the reflex is stably expressed for at least 24 h, and can be modified by tail shocks that induce sensitization across multiple temporal domains, as well as direct application of the modulatory neurotransmitter serotonin. This preparation now provides an experimentally powerful system in which to directly examine the unique and combined roles of synaptic and nuclear signaling in different temporal domains of memory formation.
The defensive reflexes of the marine mollusk, Aplysia californica, have provided a powerful model system in which to study the cellular and molecular mechanisms underlying memory formation. The three most commonly studied reflexes are the tail-elicited siphon withdrawal reflex (T-SWR), the tail-elicited tail withdrawal reflex (T-TWR), and the siphon-elicited siphon and gill withdrawal reflex (S-S/GWR) (Castellucci et al. 1970; Kupfermann et al. 1970; Pinsker et al. 1970; Carew et al. 1981a; Walters et al. 1983a,b; Scholz and Byrne 1987; Cohen et al. 1997; Sutton et al. 2001, 2002). All three reflexes exhibit a variety of forms of both nonassociative learning (sensitization, habituation, and dishabituation) (Pinsker et al. 1970, 1973b; Carew et al. 1971; Byrne et al. 1988; Sutton et al. 2001; Antonov et al. 2010) and associative learning (classical conditioning, and in the case of the S-S/GWR, operant conditioning) (Carew et al. 1981b, 1983; Antonov et al. 2001; Hawkins et al. 2006). The molecular mechanisms mediating these forms of learning are well conserved among other invertebrates and vertebrates (for reviews, see Silva et al. 1998; Barco et al. 2006; Reissner et al. 2006). The neural circuitry of these reflexes is relatively simple, often relying heavily on monosynaptic sensorimotor connections (Byrne et al. 1978; Walters et al. 1983a; Antonov et al. 1999). This simplicity facilitates detailed investigation of the cellular and molecular mechanisms underlying the induction and expression of memory, and permits the forging of direct links between these mechanisms and behavior.
At the cellular level, two monosynaptic connections have been particularly powerful in elucidating the mechanisms underlying memory formation in Aplysia: the siphon sensory neuron (LE)–siphon motor neuron (LFS) synapse that is a major contributor to the siphon withdrawal component of the S-S/GWR, and the tail sensory neuron (SN)–tail motor neuron (MN) synapse that is a predominant component of the T-TWR. Much of the LE-LFS research has been done in a reduced behavioral preparation that permits simultaneous intracellular recording from sensory and motor cells while monitoring siphon withdrawal behavior. This preparation has been used successfully to correlate changes in synaptic efficacy with changes in reflex responses (Antonov et al. 1999, 2001, 2003, 2007), and most recently, to examine the presynaptic and postsynaptic contributions to plasticity of the reflex during sensitization, habituation, and metaplasticity (experience-dependent plasticity) (Antonov et al. 2010).
Facilitation of the tail SN-MN monosynaptic connection is strongly correlated with sensitization of the T-TWR (Walters et al. 1983b; Walters 1987; Cleary et al. 1998; Wainwright et al. 2004), which makes it a useful synapse with which to link molecular and synaptic mechanisms to behavior. Investigations of plasticity at the tail SN-MN synapse have mainly focused on in vitro preparations of isolated pleural-pedal ganglia. These experiments elucidated a significant modulatory role of serotonin (5HT) in the induction of synaptic facilitation at the tail SN-MN synapse (Emptage and Carew 1993; Mauelshagen et al. 1996, 1998), and showed that 5HT is released in the CNS at the tail SN cell bodies and the SN-MN synapses by tail nerve shock (a proxy for sensitization training) (Marinesco and Carew 2002; Marinesco et al. 2004a, 2006. Three mechanistically distinct phases of synaptic facilitation can be differentially induced depending on the amount and pattern of training trials (sensitizing shocks to the animal, tail nerve shock, and 5HT exposure): Single training trials result exclusively in short-term facilitation (STF, <30 min), while repeated (≥4) training trials induce intermediate-term (ITF, <3 h), and long-term facilitation (LTF, ≥24 h) by engaging different combinations of signaling pathways (including the receptor tyrosine kinase pathway, PKA, PKC, and MAPK), as well as translational and transcriptional processes (Muller and Carew 1998; Sutton and Carew 2000; Purcell and Carew 2001; Purcell et al. 2003; Sharma et al. 2003; Sherff and Carew 2004; Ye et al. 2008). Moreover, different subcellular compartments of the SN-MN synapse are required for different forms of facilitation. In SN-MN cell cultures, STF is induced by 5HT puffed onto the synapse but not onto the SN soma, and LTF is induced by 5HT presented to either the synapse or the soma (Martin et al. 1997). Similar results were seen in pleural-pedal ganglion preparations in which 5HT presented to the pleural ganglion (compartment containing SN cell bodies) induced only LTF, but 5HT presented to the pedal ganglion (compartment containing SN-MN synapses) induced STF, ITF, and LTF (Emptage and Carew 1993; Sherff and Carew 1999, 2002, 2004). Thus, the tail SN-MN synapse has been informative concerning both the signaling mechanisms engaged by different patterns of training that support unique phases of plasticity, and the intracellular compartmentalization of that signaling.
What has been missing in these studies is a direct link from cellular and molecular mechanisms back to the reflex behavior. Mechanisms underlying the different forms of facilitation at the tail SN-MN synapse correspond to, and often predict, mechanisms underlying sensitization memory in the T-SWR (Sutton and Carew 2000; Sutton et al. 2001, 2002, 2004; Purcell et al. 2003; Sharma et al. 2003; Shobe et al. 2009). However, the tail SN-MN synapse is the monosynaptic component of the circuit underlying the T-TWR. A reduced behavioral preparation of the T-TWR was first developed by Walters and colleagues (Walters et al. 1983a, 1983b) and was used to identify and characterize the tail SNs and MNs, their synaptic contributions to the T-TWR, and their responses to sensitizing stimuli and to 5HT. However, the initial T-TWR preparation coexpressed both a centrally mediated T-TWR and a peripherally mediated T-TWR (∼25% of the response) (Walters et al. 1983a). To study more directly the links between the behavioral contribution of the SN-MN synapse with recent cellular and molecular studies, we have modified the reduced behavioral T-TWR preparation in two ways: First, we have surgically separated the tail into two regions so that the test site (where the reflex is elicited) and the response output site (attached to a tension transducer) are surgically isolated from each other (Fig. 1A,B), ensuring that the withdrawal response we measure is due entirely to centrally mediated processes and is not expressing local contractions initiated at the test site. Second, we have included a compartmentalized CNS chamber (Emptage and Carew 1993; Sherff and Carew 1999) which permits investigation of the separate contributions of the compartment containing the SN cell bodies (pleural ganglion) and the compartment containing the SN-MN synapses (pedal ganglion) to mechanistically and temporally distinct forms of memory, while simultaneously monitoring the T-TWR.
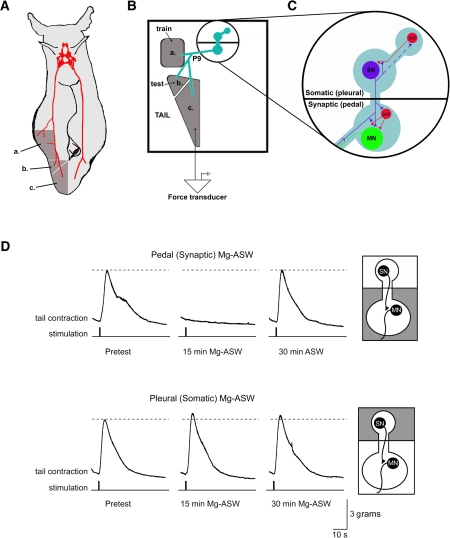
Figure 1.
Synaptic activity in the pedal but not the pleural CNS compartment is required for behavioral expression in the “split-bath” tail-elicited tail withdrawal reflex (T-TWR) reduced behavioral preparation. (A) Diagram of an intact Aplysia overlaid with critical CNS reflex circuitry (red). The ipsilateral pleural and pedal ganglia, tail nerve (P9), and exterior tissues (dark gray; a = training site, b = reflex initiation site, and c = withdrawal response output) preserved through surgery to produce the semi-intact behavioral preparation. (B) Arrangement within the experimental chamber of the training site, reflex initiation (test), and behavioral output sites of the isolated tail attached through a single P9 nerve to the CNS in an inner isolated chamber, and (C) the main circuit elements of the preserved pleural (SN soma) and pedal (SN-MN synapse, MN cell body) compartments. SN = sensory neuron, MN = motor neuron, 5HT = serotonergic neurons. (D) Bath exchange of artificial seawater (ASW) for high Mg2+ ASW (Mg-ASW) in the pedal chamber (containing the tail SN-MN synapses) reversibly blocks the T-TWR, which recovers when ASW is reintroduced (top). Bath exchange with Mg-ASW in the pleural chamber (containing the tail SN cell bodies, but not the tail SN-MN synapse) does not disrupt the tail withdrawal response (bottom). T-TWR traces were obtained using a force transducer which tracked tension (in grams of force) on the tail across time.
Here we report results from this novel T-TWR preparation, showing that the T-TWR is expressed entirely through central synapses located in the synaptic compartment (pedal ganglion). The reflex is stable over 24 h, and is modified by training shocks that induce sensitization across multiple temporal domains. We further show that serotonin is released within the central ganglia by sensitizing shocks, and that centrally applied exogenous 5HT can sensitize the T-TWR across similar temporal domains as sensitizing shock. This new preparation now affords the opportunity to forge direct links between synaptic plasticity observed at the tail SN-MN synapses and behavioral plasticity of the reflex mediated by these synapses.
Results
The essential synaptic contributions to the T-TWR reside in the pedal ganglion
The elemental reflex circuitry of the tail-elicited tail withdrawal reflex (T-TWR) of Aplysia is a very well-characterized central tail SN-MN synapse. The architecture of the T-TWR circuitry makes it possible to isolate the pleural ganglion, containing the tail SN cell bodies, from the pedal ganglion, containing the MNs and the SN synapses onto them (Fig. 1C; Emptage and Carew 1993; Sherff and Carew 1999). To identify the location of the synapses critical for expression of the T-TWR, we reversibly blocked synaptic transmission in one or the other of these compartments by exchanging normal ASW with high Mg2+ ASW (Mg-ASW, see Methods). Similar treatments have previously been used to block central synaptic transmission (Byrne et al. 1974; Walters et al. 1983a). A typical TWR following weak electrical stimulation to the test site of the tail is shown in Figure 1D, and was measured in grams of tension applied by the contracting tail to an attached force transducer. Perfusion of Mg-ASW to the pedal ganglion (SN-MN synapse compartment) completely abolished the T-TWR within 15 min (7/7 experiments, Fig. 1D). This block was reversible since the exchange of Mg-ASW for normal ASW returned the peak amplitude (amp) of the T-TWR to baseline response levels within 30 min (mean ± SEM, 83.9 ± 18% of baseline T-TWR amp, n.s., n = 7). Because Mg-ASW in the pedal ganglion completely blocked the T-TWR, these results demonstrate that the observed T-TWR is expressed entirely through a central synaptic pathway. These results differ from a previous T-TWR preparation (Walters et al. 1983a) in which only 75% of the response was eliminated after blocking central synaptic transmission. This discrepancy is most likely due to an important difference in the preparation. In the present studies, we physically separated the test site from the rest of the tail so that the tail reflex response would not be contaminated by local, direct muscle contraction due to stimulation of the test site.
We were able to further specify the site of reflex generation to the pedal ganglion by perfusing Mg-ASW to only the pleural ganglion (SN soma compartment). As shown in Figure 1D, the introduction of Mg-ASW to the pleural ganglion left the T-TWR intact (response amp after 15-min somatic Mg-ASW: 110 ± 16%, n = 7, n.s.; after 30-min somatic Mg-ASW: 85 ± 14%, n = 7, n.s.). These results demonstrate that synaptic activity in the pedal compartment, containing the critical SN-MN synapses, is required for generation of the T-TWR. Synapses within the pleural ganglion are not required for expression of the reflex. These data do not show that the SN-MN synapses are solely responsible for the reflex; specifically, they do not rule out a role for synaptic input from interneurons, which could contribute to a longer latency component of the withdrawal response (Cleary and Byrne 1993; White et al. 1993; see also Discussion).
The T-TWR is stable across 24 h
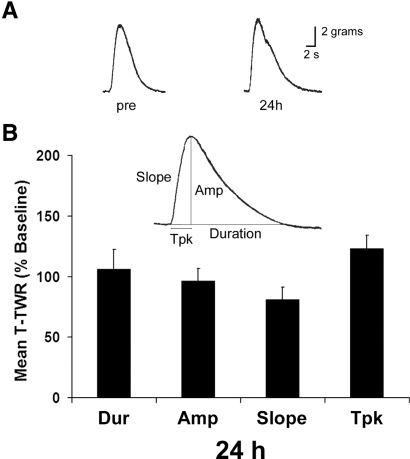
In order to use the semi-intact T-TWR preparation to explore memory formation into the long-term temporal domain, we first characterized the baseline T-TWR profile of reduced preparations (n = 6) across a 24-h period. Four T-TWR response parameters were monitored: peak amplitude (amp), time to peak amplitude (tpk), duration (dur), and slope (Fig. 2). We established a characteristic T-TWR for each preparation by averaging three initial tests of the T-TWR (pretests, intertrial interval [ITI] = 15 min; see Methods). Subsequent tests were conducted at 24 h (n = 6, three tests, ITI = 15 min). Average 24-h responding was compared to pretraining levels using paired sample t-tests. No significant effect of time was detected among the four parameters (dur [106 ± 16%], amp [97 ± 10%], slope [81 ± 10%], tpk [120 ± 9%], P > 0.05 in all comparisons). Thus we find the T-TWR to be stable across a 24-h period.
Figure 2.
The T-TWR remains stable across 24 h. (A) Representative traces and (B) group averages of the mean of three tests administered 24 h after determination of baseline T-TWR (n = 6). Data are presented as mean ± SEM. Duration (Dur), amplitude (Amp), time to peak amplitude (Tpk).
The T-TWR exhibits short-, intermediate-, and long-term memory for sensitization
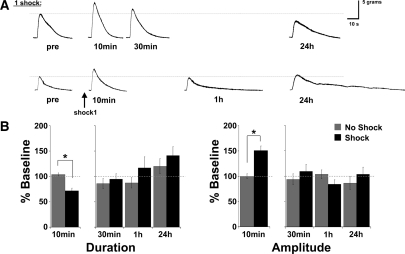
An essential feature of the T-TWR preparation is that it exhibits all three temporal domains of memory that have been identified in previous work (Pinsker et al. 1973a; Frost et al. 1985; Cleary et al. 1998; Sutton et al. 2001). Typically, for both intact T-SWR behavior and the SN-MN synapse, a single training session (one tail shock or pulse of 5HT) induces only short-term memory or synaptic facilitation, while multiple spaced training sessions induce intermediate-term and long-term memory or facilitation. We have recently identified a two-trial training pattern (two training sessions spaced by 45 min) that also induces short- and long-term sensitization memory in the T-SWR (Philips et al. 2007), as well as intermediate-term sensitization (GT Philips and TJ Carew, unpubl.). As a first test of the ability of the new T-TWR preparation to express memory across multiple temporal domains, we examined the T-TWR at short-term, intermediate-term, and long-term test times either after a single shock (Fig. 3) or after two spaced shocks (Fig. 4). Pilot studies indicated that training affected response duration (decrease), amplitude (increase), and slope (increase), but did not affect tpk. Since tpk did not change, we reasoned that the increased slope observed was likely driven by the increased response amplitude. Thus, we have restricted our final analysis to the two most informative parameters: T-TWR duration and amplitude. Trained (n = 21) and matched untrained animals (n = 13) were tested 10 min after a single shock and then divided into two groups: a subset of animals was tested in the absence of further training (“one shock” group) at 30 min, at 1 h, and at 24 h (average of three tests) and the remaining animals received a second spaced shock (“two shock” group) and were tested at 30 min, 1 h, and at 24 h following the second shock. In planned comparisons, we identified a significant effect of training at 10 min after a single shock on both T-TWR duration (one shock [n = 21]: 72 ± 5%; untrained [n = 13]: 104 ± 3%, P < 0.001) and amplitude (one shock: 151 ± 9%; untrained: 100 ± 5%, P < 0.001; Fig. 3). In the one shock group, a MANOVA of post-tests at 30 min, 1 h, and 24 h indicated no significant overall effect of training (F(2,29) = 0.988, P > 0.1). At 30 min, trained animals (n = 7, dur [85 ± 10%], amp [97 ± 7%]) had returned to control levels (n = 4, dur [87 ± 10%], amp [104 ± 9%]) and remained at control levels of responding at 1 h (one shock: n = 6, dur [117 ± 22%], amp [85 ± 8%]; untrained: n = 4, dur [87 ± 10%], amp [104 ± 9%]) and at 24 h (one shock: n = 9, dur [141 ± 17%], amp [104 ± 14%]; untrained: n = 6, dur [120 ± 15%], amp [87 ± 13%]). Thus, a single training shock gave rise to short-term sensitization of the T-TWR (at 10 min), which was reflected by a transient reduction in the withdrawal duration and increased peak withdrawal amplitude. In the absence of further training, this behavioral modification was not maintained.
Figure 3.
A single training shock induces a transient sensitization of the T-TWR. A single 1.5-sec shock induces a short-term sensitization at 10 min that is absent in subsequent post-tests at 30 min, 1 h, and 24 h. (A) Representative traces of animals receiving a single shock and tested at 10 min, 30 min, or 1 h and at 24 h, and (B) summary data. Asterisk (*) indicates P < 0.05.
Figure 4.
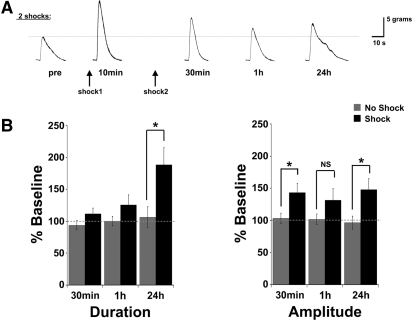
Two spaced training shocks (ISI = 45 min) induce intermediate- and long-lasting sensitization of the T-TWR. The T-TWR shows a similar sensitivity to trial number as previously described for the T-SWR reflex. (A) Representative traces from a single animal tested across all time points, and (B) summary data. Asterisk (*) indicates P < 0.05.
Whereas a single shock gave rise to only transient changes in the T-TWR, administration of a second shock (inter-shock interval [ISI] = 45 min) was sufficient to induce more persistent effects (Fig. 4). A MANOVA of 30-min, 1-h, and 24-h post-tests in the two shock group indicated a significant effect of training (F(2,37) = 12.887, P < 0.001). Subsequent univariate analysis revealed a significant effect on both T-TWR duration (F(1,42) = 9.777, P < 0.01) and amplitude (F(1,42) = 16.359, P < 0.001). Scheffe's multiple comparison procedure (Zar 2010) was used to conduct subsequent planned comparisons (critical F(1,42) = 20.36). At 30-min post-training, the average T-TWR amplitude was significantly enhanced (n = 8, 143 ± 14%) when compared to matched untrained control responses (n = 8, 103 ± 8%; Scheffe's adjusted F(1,42) = 21.84, P < 0.05), but duration was not significantly modified (two shock: 112 ± 9%; untrained: 94 ± 7%; F(1,42) = 2.29, P > 0.05). At 1 h, trained animal responding was not significantly different from matched controls (amp: [two shock: 132 ± 17%; untrained: 102 ± 8%; F(1,42) = 12.34, P > 0.05], dur: [two shock: 126 ± 15%; untrained: 100 ± 7%; F(1,42) = 4.69]). This “dip” in the retention profile is consistent with several other studies (see Discussion). Twenty-four hour post-tests revealed a significant enhancement of the T-TWR duration (two shock: n = 8, 189 ± 26%; untrained: n = 6, 106 ± 16%; F(1,42) = 48.16, P < 0.05) and amplitude (two shock: 148 ± 17%; untrained: 97 ± 10%; F(1,42) = 36.61, P < 0.05). Collectively, these data show that the induction of memory for sensitization in the T-TWR reduced preparation is sensitive to amount of training and is expressed within all three temporal domains that have been previously described in other reflex behaviors in Aplysia. Thus, these data illustrate the utility of this experimental system for the analysis of the cellular and molecular mechanisms recruited during memory formation across a range of temporal domains.
Sensitizing shock in the semi-intact T-TWR preparation releases serotonin at both the SN cell bodies and SN-MN synapses
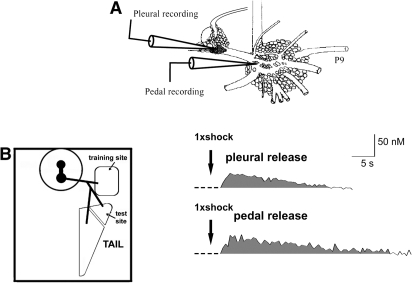
The release of the neuromodulatory transmitter serotonin (5HT) is associated with the induction of sensitization memory in Aplysia defensive reflexes, and with the heterosynaptic facilitation of Aplysia central synapses thought to underlie behavioral sensitization (Brunelli et al. 1976; Glanzman et al. 1989; Levenson et al. 1999; Marinesco et al. 2006). Using voltammetric techniques, we previously showed that peripheral nerve shock (head, siphon, and tail nerve shock used as proxies for shocks to the corresponding regions of the intact animal) releases 5HT at discrete sites throughout the Aplysia CNS, including the tail SN cell bodies and SN-MN synapses (Marinesco and Carew 2002; Marinesco et al. 2004a,b, 2006). To further confirm the utility of this preparation, we next explored the release of 5HT in the pleural and pedal ganglia (Fig. 5A) following body wall shocks in the semi-intact T-TWR preparation. Consistent with previous studies, 5HT release was detected at both pleural (tail SN cell bodies; average peak [5HT]: 36 ± 4 nM, n = 3) and pedal recording sites (tail SN-MN synapses; average peak [5HT]: 48 ± 9 nM, n = 4) immediately following a single shock to the training site (100 mA, 1.5 sec, AC; Fig. 5). Peak 5HT concentrations in the pleural and pedal recordings were reached on average at 6 sec and 10 sec after shock onset, and the average release duration was 34 ± 9 sec and 66 ± 20 sec, respectively. This release profile was similar to that evoked by tail nerve shock (38.5 ± 5 nM in the pleural and 58 ± 12.7 nM in the pedal) (Marinesco et al. 2004a). Taken together, these data support the conclusion that electric shock of the body wall, routinely used to induce sensitization in behavioral studies (Scholz and Byrne 1987; Goldsmith and Byrne 1993; Cleary et al. 1998), induces immediate 5HT release in regions containing the essential T-TWR circuitry, with a release profile consistent with that previously observed following peripheral nerve shock.
Figure 5.
Electric shocks to the reduced T-TWR preparation trigger immediate 5HT release within the vicinity of tail SN cell bodies and SN-MN synapses. (A) 5HT release was measured with carbon fiber electrodes inserted into the CNS near the tail SN cell bodies (pleural recording site) or at tail SN-MN synapses (pedal recording site). (B) Representative traces of 5HT release detected near the tail SN cell bodies (pleural release) and tail SN-MN synapses (pedal release) immediately after a 1.5-sec 100-mA shock to the training site. A stimulus artifact, caused by introduction of the shocking electrode to the preparations and the shock itself, has been removed in the representative traces.
5HT application to the CNS can sensitize the T-TWR across multiple temporal domains
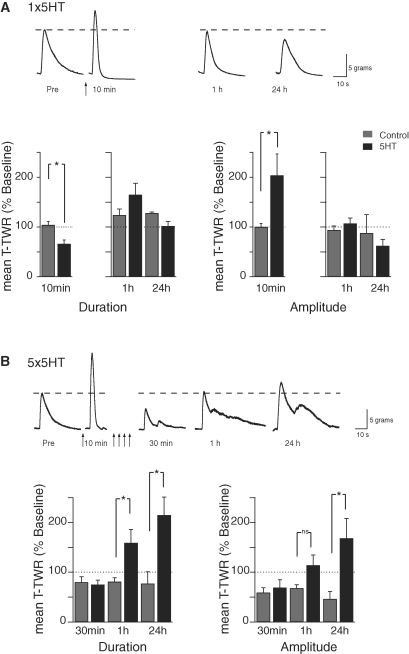
Exogenous 5HT can substitute for sensitizing shocks in the induction of facilitation at SN-MN synapses or sensitization of the T-TWR. Brief (5 min) exposure of the pleural-pedal ganglia to 5HT transiently facilitates the tail SN-MN synapses, whereas additional spaced pulses of 5HT (5 × 5 min 5HT pulses; 5 × 5HT) result in long-lasting facilitation (Mauelshagen et al. 1996, 1998). Moreover, 5HT exposure to the central ganglia can increase the T-TWR amplitude (Walters et al. 1983b). We have shown here that body wall shock induces both memory for sensitization (expressed in part by an increased T-TWR amplitude) and 5HT release in the CNS. These results thus predict that 5HT could serve as a proxy for sensitizing shock in the behavioral induction of memory in the current reduced behavioral preparation. As an initial step in exploring this overall prediction, we characterized the response of the T-TWR preparation in two conditions: (1) at 10 min, 1 h, and 24 h, following a single brief (5 min) exposure of 5HT to the CNS that transiently facilitates the tail SN-MN synapse (“1 × 5HT” group), and (2) at 10 min following the first 5HT pulse and at 30 min, 1 h, and 24 h following four additional spaced 5HT pulses (10-min inter-pulse interval), a training protocol which persistently strengthens tail SN-MN synapses (“5 × 5HT” group; Fig. 6). Planned between group comparisons with ASW-treated control preparations revealed that a single 5HT pulse to the CNS chamber induced a significant enhancement (observed at 10 min after 5HT onset) of the T-TWR amplitude (5HT: 204 ± 43%, n = 21; ASW: 100 ± 7%, n = 20; t-test P < 0.05), and a significant decrease in duration (5HT: 66 ± 8%, n = 20; ASW: 104 ± 7%, n = 20; t-test P < 0.01). These changes were not expressed an hour later in the absence of additional 5HT pulses to the CNS (amp: 1 × 5HT: 107 ± 11%, ASW: 94 ± 8%; dur: 1 × 5HT: 165 ± 23%, ASW: 124 ± 12%; n between 9 and 13 and P > 0.05 for all) or the following day (amp: 1 × 5HT: 62 ± 13%, ASW: 88 ± 37%; dur: 1 × 5HT: 102 ± 9%, ASW: 128 ± 2%; n between 6 and 9 and P > 0.05 for all). The short-term changes in reflex response (increased amplitude and shortened duration; Fig. 6A) exactly correspond to what we observed following a single shock (Fig. 3), further strengthening the notion that the effects of a training shock on the T-TWR are mediated through 5HT, and that 5HT can serve as a proxy for a single training shock.
Figure 6.
A single pulse of 5HT to the CNS results in a transient sensitization of the T-TWR, while multiple 5HT pulses induce intermediate- and long-term sensitization. Sample T-TWR recordings and summary data showing response amplitude and duration after a single 5-min pulse of 5HT (A) and five 5-min pulses of 5HT (B). Ten minutes after a single pulse of 5HT, or after the first of five pulses of 5HT (data from these two groups were combined for the 10-min analysis), the T-TWR showed an increase in amplitude and shortening of duration that was not present at 1 h or 24 h. After repeated 5HT pulses, the T-TWR expressed sensitization in the form of enhanced response amplitude and lengthened duration at 1 h and 24 h after 5HT. Asterisk (*) indicates P < 0.05.
Additional 5HT pulses to the CNS of reduced T-TWR behavioral preparations resulted in more persistent modifications to both T-TWR amplitude and duration as revealed by a significant effect of training in a MANOVA of the intermediate- and long-term post-tests (F(2,41) = 8.351, P < 0.01) and subsequent univariate tests (amp: F(1,46) = 10.926, P < 0.01; dur: F(1,46) = 12.522, P < 0.01). Planned between group comparisons (Scheffe's critical F(1,46) = 14.090, one-tail) revealed a significant effect of training at 1 h on T-TWR duration (n = 9, 5 × 5HT: 159 ± 27%, n = 8, 5 × ASW: 81 ± 8%, F(1,46) = 17.841, P < 0.05). T-TWR amplitude displayed a trend toward enhancement (5 × 5HT: 114 ± 21%, 5 × ASW: 68 ± 7%, F(1,46) = 7.829, P > 0.05) that paralleled a similar trend observed at 1 h following two sensitizing shocks (Fig. 4). At 24 h, however, both T-TWR amplitude (n = 8, 5 × 5HT: 168 ± 40%, n = 8, 5 × ASW: 46 ± 15%, F(1,46) = 54.922, P < 0.05) and duration (5 × 5HT: 215 ± 36%, 5 × ASW: 77 ± 24%, F(1,46) = 55.604, P < 0.05) were significantly modified above ASW-treated controls. Therefore, repeated 5HT exposures, which persistently strengthen tail SN-MN synapses at 1 h and at 24 h, also persistently modify behavior in the T-TWR in corresponding temporal intervals. Interestingly, there was no difference in the amplitude (n = 7, 5 × 5HT: 73 ± 20%, n = 8, 5 × ASW: 59 ± 10%, F(1,46) = 0.565, P > 0.05) or duration (n = 7, 5 × 5HT: 75 ± 9%, n = 8, 5 × ASW: 80 ± 11%, F(1,46) = 0.033, P > 0.05) of the T-TWR 30 min after 5 × 5HT relative to ASW controls. This result was unexpected, because at this time point both the T-TWR following sensitizing shocks (this study) and the SN-MN synapses following P9 shock (CM Sherff and TJ Carew, unpubl.) or 5 × 5HT (Sherff and Carew 2004) are enhanced. It will be interesting to examine in future studies the mechanisms underlying this temporal shift in behavioral plasticity elicited by direct 5HT exposure.
Discussion
A major strength of Aplysia as a model system has been the ability to make direct links between memory formation at the behavioral level and the molecular and synaptic changes directly contributing to the formation of that memory (Walters et al. 1983b; Byrne et al. 1991; Cohen et al. 1997; Antonov et al. 1999). The cellular and molecular mechanisms underlying facilitation of the tail SN-MN synapse, an essential monosynaptic component of the T-TWR circuit, are well characterized (Purcell and Carew 2003; Reissner et al. 2006; Stough et al. 2006). However, until the present study, no behavioral preparation existed which produced a centrally mediated T-TWR in the absence of a peripheral response component. We resolved this issue in a new semi-intact T-TWR preparation which exhibits a robust reflex response that is stable across a 24-h observation period and is mediated entirely by central synaptic transmission in the pedal ganglion. Memory formation in this preparation exhibits properties predicted by previous cellular and synaptic studies of the tail SN-MN synapse: (1) The T-TWR expresses sensitization in response to training shocks, paralleling the heterosynaptic facilitation seen at the synapse; (2) both memory for sensitization and heterosynaptic facilitation are expressed in short-, intermediate- and long-term temporal domains; (3) training shocks lead to 5HT release in the same regions of the T-TWR circuitry (at SN cell bodies and SN-MN synapses) as do nerve shocks in isolated ganglia preparations; and (4) 5HT exposure to the pleural and pedal ganglia induces an enhancement of the T-TWR amplitude across similar temporal domains as 5HT-induced facilitation of tail SN-MN synapses.
Central synapses mediate the T-TWR
The tail SNs form monosynaptic connections onto tail MNs located in the pedal ganglion (Walters et al. 1983a). In the present study, we reversibly abolished the T-TWR by disrupting synaptic transmission in the pedal ganglion, but saw no reflex disruption following a synaptic block in the pleural ganglion (the locus of the SN cell bodies). Our results demonstrate that the essential synapses mediating the reflex reside within the CNS, specifically in the pedal ganglion. These findings also demonstrate the importance of physically separating the tail into independent regions that mediate reflex initiation and response output (Fig. 1). In the current preparation, separation of the tail into physically independent reflex initiation and output sites prevented local muscle contraction initiated by the test stimulus from adding to the reflexive withdrawal observed at the response output region. In both the intact animal and semi-intact behavioral preparations that lack this physical separation, the expression of the T-TWR included a local muscle response from the test stimulus that is not completely disrupted by blocking central synaptic transmission (Walters et al. 1983a). Our ability to study the central mechanisms supporting learning and memory expression in the T-TWR is now facilitated by this preparation which, in combination with a compartmentalized CNS chamber, allows us to isolate the essential synaptic compartment for the reflex.
Memory expression in the T-TWR parallels facilitation at SN-MN synapses
A single sensitizing shock transiently alters the intrinsic properties of tail SNs and MNs, and transiently strengthens the tail SN-MN synapses heterosynaptically (Walters et al. 1983b). Repeated shocks are required to induce more persistent (24 h) facilitation of tail SN-MN synapses, and long-lasting modifications of the intrinsic properties of tail SNs (increased excitability and spike after depolarization) and MNs (hyperpolarization and decreased spike threshold) (Scholz and Byrne 1987; Walters 1987; Cleary et al. 1998). In vitro studies of the SN-MN synapse have shown that heterosynaptic facilitation is expressed in short-, intermediate-, and long-term temporal domains depending upon the amount and pattern of training (Mauelshagen et al. 1996, 1998). In the present study, training shocks induced sensitization memory in the T-TWR within similar temporal domains (Figs. 3, 4). Specifically, sensitization was expressed as an increase in the T-TWR amplitude following a single training shock at 10-min post-shock but was absent at 30 min, 1 h, and 24 h. Additional training (a second spaced training shock; Philips et al. 2007) was required to induce intermediate-term (30 min) and long-term (24 h) enhancement of the T-TWR amplitude. Earlier work identified a correlation between increasing T-TWR amplitude during training and the development of heterosynaptic facilitation at the tail SN-MN synapse (Walters et al. 1983b). Our data provide further support for the hypothesis that synaptic facilitation of the tail SN-MN synapse contributes significantly to memory expressed as an increase in the response amplitude of the T-TWR (Walters et al. 1983b; White et al. 1993).
The T-TWR also displayed another temporal feature that is characteristic of the sensitization of Aplysia reflexes and has been observed at the molecular, synaptic, and behavioral levels: a temporal discontinuity (a “dip”) that separates the expression of intermediate-term and long-term memory. The significant enhancement of the T-TWR amplitude following two shocks was present at 30 min, absent by 1 h, and was again expressed at 24 h. This dip was first described by Sutton et al. (2002), who found that the amplitude of the tail-elicited siphon withdrawal reflex returned to baseline after the expression of ITM and before the onset of LTM. A similar dip was previously observed in the temporal expression of heterosynaptic facilitation of the tail SN-MN synapse (Mauelshagen et al. 1996), as well as in the persistent activation of protein kinase A (Muller and Carew 1998), a signaling requirement in the induction of long-lasting forms of plasticity and memory. Thus this new preparation captures some of the fine-grained temporal features of synaptic and molecular plasticity previously identified in this reflex system.
Interneurons may help shape the reflex response
As described above, the T-TWR amplitude was enhanced in all three temporal domains of memory induced by sensitization training, which is consistent with strengthening of monosynaptic SN-MN connections, which exhibit facilitation in these same domains. However, the modulation of reflex duration was not uniform across all temporal domains and may reflect plasticity in a parallel polysynaptic pathway. Although the T-TWR can be expressed through the central tail SN-MN monosynaptic circuit (Walters et al. 1983a), there also exists a parallel polysynaptic circuit involving identified interneurons (INs: RPl4 and LP117) (Buonomano et al. 1992; Cleary and Byrne 1993; Xu et al. 1994). Whereas the monosynaptic circuit is believed to control the early response amplitude and slope of the T-TWR, the polysynaptic circuit may contribute to shaping the later portion of the response where it could be a major determinant of the T-TWR duration (see White et al. 1993; Cleary et al. 1995). A role for the recruitment of plasticity in INs for short-term modifications of behavior is well established (Frost et al. 1988; Trudeau and Castellucci 1992; Fischer and Carew 1995; Frost and Kandel 1995; Wright and Carew 1995; Xu et al. 1995).
In the short-term domain, the combination of an increase in response amplitude and a shortened duration suggests an early facilitatory monosynaptic component combined with a later inhibitory polysynaptic component (such as a decrease in excitatory drive onto the MNs or facilitation of an inhibitory connection). That the T-TWR duration had returned to baseline level by 30 min is consistent with a short-lasting contribution of interneuronal plasticity in sensitization (Fitzgerald and Carew 1991; Wright et al. 1991; Fischer and Carew 1993, 1995; Cleary et al. 1998).
The long-term broadening (longer duration) of the T-TWR with training is also suggestive of long-lasting changes in plasticity in the interneuronal pathway. Long-term sensitization effects have not yet been observed (see Cleary et al. 1998), but this does not rule out a role for the polysynaptic circuit, either by itself or in conjunction with persistent modification of intracellular properties of the tail SNs that would prolong SN firing. Importantly, the expression of centrally mediated changes in the T-TWR that cannot be completely accounted for by changes in the monosynaptic circuit suggests that the role of the T-TWR polysynaptic circuit in memory can be more fully explored in this new preparation.
The contribution of 5HT to the formation of memory for sensitization
In the current study we demonstrated that a training shock is associated with immediate release of the neuromodulatory transmitter 5HT onto critical components of the essential T-TWR reflex circuitry (Fig. 5). This is the first such demonstration of localized 5HT release during behavioral training in Aplysia (Levenson et al. [1999] described the global release of 5HT into the hemolymph during similar training), and supports the notion that 5HT-mediated heterosynaptic facilitation is responsible for the induction of sensitization memory by behaviorally relevant training shocks. It is informative that the 5HT release profile observed underneath the tail SN cell bodies and surrounding the tail SN-MN synapses following body wall shock is the same release profile that is generated by tail nerve shock (Marinesco and Carew 2002; Marinesco et al. 2004a, 2006). This provides further validation of the use of tail nerve shocks and 5HT exposure as in vitro training analogs.
As predicted from the earlier version of the reduced preparation (Walters et al. 1983b), we found that exogenous 5HT can substitute for sensitizing shock in the induction of sensitization of the T-TWR. 5HT can replicate the effect of a single training shock on the T-TWR not only with respect to the response amplitude of the T-TWR (Walters et al. 1983b), but also the transient decrease in duration that is observed with a single shock. Repeated, spaced pulses of 5HT to the CNS were also sufficient to persistently modify T-TWR amplitude and duration. These observations demonstrate the sufficiency of 5HT in mimicking the effects of training shock on the T-TWR and identify 5HT-induced contributions to sensitization that occur in parallel with the role for 5HT in the heterosynaptic facilitation of the SN-MN synapse.
Interestingly, both shock and 5HT produced a short-term shortening of the T-TWR duration. Although short-term sensitization can be expressed as an enhancement in the duration of some defensive reflexes (e.g., the T-SWR) (Sutton et al. 2002), our findings are similar to the results of studies of the short-term effect of tail shock and 5HT on the siphon withdrawal reflex (S-SWR) circuit. Following tail shock or 5HT exposure, the complex EPSP amplitude measured in siphon MNs is decreased and the siphon withdrawal response duration is shortened (Marcus et al. 1988; Fitzgerald and Carew 1991; Wright et al. 1991). Importantly, siphon SN-MN synapses are simultaneously facilitated, indicating that a parallel inhibitory pathway mediates the decreased input to siphon MNs and shortened siphon withdrawal duration. Indeed, tail shock-induced activation of interneurons within the S-SWR circuit is thought to underlie the short-term inhibition of the S-SWR (Frost and Kandel 1995; Wright and Carew 1995; Bristol et al. 2001). Thus, as in the S-SWR, the T-TWR appears to be modified by shock-induced 5HT at multiple sites within the circuit. The short-term strengthening of the response amplitude (reflecting strengthened tail SN-MN synapses) is expressed with a simultaneous shortening of the response duration (consistent with a parallel role for 5HT signaling within the polysynaptic circuit).
Somatic vs. synaptic signaling in memory formation
Finally, the new reduced preparation allows for independent manipulation of the molecular environment of the two ganglia contributing to the T-TWR: the pleural ganglion containing the SN cell bodies and the pedal ganglion containing SN-MN synapses. Use of this compartmentalization has facilitated our understanding of the induction of plasticity at the SN-MN synapse in in vitro studies (Sherff and Carew 1999, 2002, 2004). For example, we used this strategy to reveal that intermediate-term facilitation of the tail SN-MN is induced by synaptic serotonin signaling and requires local synaptic protein synthesis (Sherff and Carew 2004). The combination of this compartmentalization strategy with the reduced behavioral preparation has already demonstrated its utility in showing for the first time that the T-TWR is mediated exclusively by synaptic activity in the pedal (SN-MN synapse) compartment. Given that synaptic and nuclear signaling has been found to be critical for long-lasting plasticity in isolated cell cultures (Montarolo et al. 1986; Martin et al. 1997), and similarly, compartmental requirements have been observed in SN-MN connections in the pleural-pedal ganglion preparation (Emptage and Carew 1993; Sherff and Carew 1999, 2002, 2004), this new reduced preparation is well suited to identify the unique contributions of these intracellular signaling compartments while simultaneously measuring memory formation in the behaving animal.
Materials and Methods
Tail-elicited tail withdrawal reduced behavioral preparation
To study the tail-elicited tail withdrawal reflex (T-TWR), the tail and posterior body wall, attached via the tail (P9) nerves to the essential reflex circuitry of the central nervous system, were isolated from the anesthetized animal. Briefly, wild-caught Aplysia californica were obtained (Marinus Scientific; Santa Barbara Marine Bio.) and stored in 400-gallon circulating tanks of seawater (Reef Crystals) at 15°C. Animals were anesthetized with injections of MgCl2 (∼100 mL/100 g body weight) through the foot. In a dissection tray, animals were placed ventral side up and an incision was made longitudinally along the midline of the foot to expose the underlying gut and nervous system (this incision did not extend into the tail). Internal organs were removed and all peripheral nerves except for the two P9 nerves were cut. Next, lateral incisions were made to isolate the body wall and tail segments from the rest of the animal (care was taken to maintain the innervation of the segments by ipsilateral P9 nerve branches) (Fig. 1A). The tail was further bisected along its midline to permit the generation of two reduced T-TWR behavioral preparations; each preparation containing one tail hemi-segment, the adjacent body wall region, and one pleural-pedal ganglion pair containing the essential reflex circuitry (Fig. 1B,C). Importantly, in early versions of the preparation we explored the differential effect of also retaining the cerebral ganglia (the locus of serotonergic cell bodies, which send projections to the pleural and pedal ganglia) (Marinesco et al. 2004a). We identified no differences between preparations with or without cerebral ganglia in T-TWR stability, response to shock, response to 5HT treatment, or in 5HT release. Thus in the present study the cerebral ganglia are excluded. Each preparation (nervous system attached to body wall and tail segments via P9) was transferred to an experimental chamber filled with cold 50:50 MgCl2:artificial seawater (ASW) solution (ASW: 460 mM NaCl, 55 mM MgCl2, 11 mM CaCl2, 10 mM KCl, and 10 mM Tris, pH 7.6). The P9 nerve innervates the ipsilateral tail hemi-segment via two main branches. We further divided the tail hemi-segments with respect to the two main P9 collaterals into an anterior “input” region for eliciting the TWR, and a physically separate “output” region for monitoring the withdrawal response (Fig. 1A,B). This physical separation of input and output components was necessary to preclude the contribution of local muscle movements to the observed T-TWR (Walters et al. 1983a). The tail test site, body wall training segment, and the base of the output segment were pinned to the Sylgard floor of each experimental chamber. The distal portion of the tail output segment was connected by a fish hook and fine surgical thread to a strain gauge (Grass FT03), which allowed for quantification of the TWR (Fig. 1B), and the output of the strain gauge was fed through an amplifier (AD Instruments Bridge Amp), and recorded using customized computer software (PowerLab Chart v6, AD Instruments). The central ganglia were pinned to the Sylgard floor of an inner chamber and the sheath overlying the tail SNs and MNs was surgically removed. A Vaseline wall was then built on top of the tail nerve to isolate the inner CNS chamber from the peripheral tissues. In an alternate version of this preparation, the inner CNS chamber was subdivided (as in Fig. 1B,C) to allow compartmentalization of the monosynaptic tail SN-MN circuit (Emptage and Carew 1993; Sherff and Carew 1999). Anesthesia was relieved by the perfusion of ASW (15°C) through a combination of direct perfusion lines into the tail and body wall tissues as well as a continuous bath exchange. The direct tissue perfusion lines were removed 1 h before first testing the TWR (total perfusion time 2 h).
Behavioral procedures
Baseline TWR responses were determined for each preparation by applying a mild electric test shock (0.5 sec, 3–15 mA, AC, mimicking a weak tactile stimulus) to the tail test site via a handheld electrode (inter-test interval [ITI] = 15 min). The stimulus intensity was variable and was determined for each preparation by beginning at 3 mA and increasing the stimulus intensity until a stable response (amplitude ≥ 3 g of force) could be reliably obtained. Prior to each test, a modest amount of tension was applied to the tail (1.5 g). This tension permitted a consistent measurement of each response, including the beginning of each contraction. Between tests and throughout training, the tension on the tail was removed. T-TWR parameters (amplitude, duration, slope, and time to peak) were measured with PowerLab Chart software. To assess stable responding, we measured the peak amplitude of the TWR. Preparations with any single baseline response amplitude that varied more than 20% from the mean of three pretests were excluded. Only T-TWR preparations with stable tail withdrawal amplitudes averaging ≥3 g of force were included in experiments. ∼73% of T-TWR preparations fit this criterion.
T-TWR semi-intact preparations were trained with sensitizing shocks (1.5 sec, 100 mA, AC) to the training site (posterior body wall/anterior tail) beginning 10 min after the last pretest (nominally large current amplitudes were needed because the bulk of the current is shunted by seawater). Electrical shocks to this region (Fig. 1) reliably sensitize Aplysia defensive reflexes (Scholz and Byrne 1987; Goldsmith and Byrne 1993; Cleary et al. 1998) and release serotonin onto tail SN-MN synapses and in the vicinity of tail SN cell bodies (Fig. 5). Training shocks were not administered to the test site, ensuring that the behavioral modifications observed were nonassociative and relied on heterosynaptic facilitation of the underlying reflex circuitry (Walters et al. 1983a,b). Following a single shock, post-tests were administered at 10 min, 30 min, 1 h, and 24 h (average of three 24-h tests, inter-test interval = 15 min). In additional experiments we explored the contribution of a second sensitizing shock. In these experiments animals were given two shocks spaced by 45 min (a two-trial training interval that gave rise to both intermediate-term [ITM] and long-term memory [LTM] expression in the intact animal) (Philips et al. 2007; and GT Philips and TJ Carew, unpubl.) and post-tests were administered at 10 min following the first shock, and at 30 min, 1 h, and 24 h following the second shock. Control preparations received no training shocks, but were tested at matched time points.
In experiments examining the contribution of the CNS to the T-TWR, High Mg2+ (3x)/0 Ca2+ ASW (Mg-ASW: 295 mM NaCl, 176 mM MgCl2, 10 mM KCl, and 10 mM Trizma, pH 7.6) was perfused into the CNS chamber. To assess the effect of central application of the neuromodulator serotonin (5HT) on the T-TWR, a single 5-min pulse of 5HT (50 µM), or repeated 5HT pulses (5-min duration, inter-pulse interval = 15 min from onset to onset), were perfused (1.3 mL/min) into the CNS chamber and then washed out with ASW prior to testing. Although training shock induces 5HT release that lasts <1 min, we used a 5-min 5HT exposure because (1) this exposure has been shown to reliably induce facilitation at SN-MN synapses, and (2) chronoamperometry experiments have demonstrated that it takes approximately 3 min for bath applied 5HT to reach physiological levels in the neuropil (Marinesco and Carew 2002). Control preparations received the same number of CNS perfusions but in the absence of 5HT (ASW only).
Detection of 5HT release
To measure 5HT release following sensitizing shocks to the T-TWR semi-intact preparation, chronoamperometric techniques were employed as described previously (Marinesco and Carew 2002). Briefly, carbon fiber electrodes were prepared and placed directly underneath the tail SNs (pleural recording site) or into the neuropil containing tail SN-MN synapses (pedal recording site). 5HT oxidation currents were measured by chronoamperometry before, during, and after a single shock (1.5 sec, 100 mA, alternating current) was administered at the reduced preparation shock site via a handheld electrode. In all experiments, placement of the handheld electrode into the recording field, and the electrical shock itself, introduced a 3–5 sec artifact into the recordings which was removed in the representative traces in Figure 5. In order to quantify the maximum concentration of 5HT released, at the end of each experiment the carbon fiber electrodes were calibrated in a 500 nM 5HT/seawater solution.
Statistical analysis
The T-TWR was analyzed for training-specific changes using a number of response parameters, including peak amplitude of the withdrawal (grams of force), duration, rising slope, and time to peak. Data obtained following each experiment was first exposed to an outlier rule such that response values outside of two standard deviations from the mean were excluded. In practice, this excluded 6% of data points. Since the data were normally distributed, parametric statistics were used in all cases. Within-group comparisons were conducted on untrained responding preparations (Fig. 2) using paired sample t-tests of average pretraining and 24-h responses. All remaining experiments were designed with a priori planned comparisons between control and trained group responding at all post-tests. All trained animals were tested at 10-min post-training (short-term test) and then split into two general groups: one group was tested in the absence of continued training, and the other group received additional training. Both groups were then tested across 24 h. Planned comparisons conducted at 10 min were analyzed using t-tests for independent means at α = 0.05 (two-tail). Multivariate analysis of variance (MANOVA) was performed on control and trained behavioral responses across all subsequent post-tests (30 min, 1 h, 24 h). Rejection of the null hypothesis was followed up by univariate ANOVAs to test for effects on T-TWR duration and amplitude. Between group differences were subsequently explored with Scheffe's multiple comparison procedure (Zar 2010). All reported probabilities reflect two-tailed analyses (α = 0.05), except in the analysis of T-TWR following repeated 5HT pulses (one-tail). Previous experiments (5HT-induced synaptic facilitation, the 5HT-induced enhancement to T-TWR amp reported by Walters et al. [1983b], and the sensitizing shock experiments described in this study), as well as initial pilot experiments, provided an expectation that the T-TWR amp and dur would be increased by 5HT treatment. Statistical analyses were performed using SPSS v18.0 software and custom Excel spreadsheets.
Acknowledgments
We thank the Carew laboratory members and Aaron Mattfield for their thoughtful advice in both the early stages of this work, as well as on previous versions of this manuscript. This work was supported by NIH grant R01 MH-041083 and NSF grant IOB-0444762 to T.J.C., and NIH grant RO1 MH-081151 to T.J.C. and K.C. Martin.
References
- Antonov I, Kandel ER, Hawkins RD 1999. The contribution of facilitation of monosynaptic PSPs to dishabituation and sensitization of the Aplysia siphon withdrawal reflex. J Neurosci 19: 10438–10450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonov I, Antonova I, Kandel ER, Hawkins RD 2001. The contribution of activity-dependent synaptic plasticity to classical conditioning in Aplysia. J Neurosci 21: 6413–6422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonov I, Antonova I, Kandel ER, Hawkins RD 2003. Activity-dependent presynaptic facilitation and hebbian LTP are both required and interact during classical conditioning in Aplysia. Neuron 37: 135–147 [DOI] [PubMed] [Google Scholar]
- Antonov I, Ha T, Antonova I, Moroz LL, Hawkins RD 2007. Role of nitric oxide in classical conditioning of siphon withdrawal in Aplysia. J Neurosci 27: 10993–11002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonov I, Kandel ER, Hawkins RD 2010. Presynaptic and postsynaptic mechanisms of synaptic plasticity and metaplasticity during intermediate-term memory formation in Aplysia. J Neurosci 30: 5781–5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barco A, Bailey CH, Kandel ER 2006. Common molecular mechanisms in explicit and implicit memory. J Neurochem 97: 1520–1533 [DOI] [PubMed] [Google Scholar]
- Bristol AS, Fischer TM, Carew TJ 2001. Combined effects of intrinsic facilitation and modulatory inhibition of identified interneurons in the siphon withdrawal circuitry of Aplysia. J Neurosci 21: 8990–9000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli M, Castellucci V, Kandel ER 1976. Synaptic facilitation and behavioral sensitization in Aplysia: possible role of serotonin and cyclic AMP. Science 194: 1178–1181 [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Cleary LJ, Byrne JH 1992. Inhibitory neuron produces heterosynaptic inhibition of the sensory-to-motor neuron synapse in Aplysia. Brain Res 577: 147–150 [DOI] [PubMed] [Google Scholar]
- Byrne J, Castellucci V, Kandel ER 1974. Receptive fields and response properties of mechanoreceptor neurons innervating siphon skin and mantle shelf in Aplysia. J Neurophysiol 37: 1041–1064 [DOI] [PubMed] [Google Scholar]
- Byrne JH, Castellucci VF, Kandel ER 1978. Contribution of individual mechanoreceptor sensory neurons to defensive gill-withdrawal reflex in Aplysia. J Neurophysiol 41: 418–431 [DOI] [PubMed] [Google Scholar]
- Byrne JH, Eskin A, Scholz KP 1988. Neuronal mechanisms contributing to long-term sensitization in Aplysia. J Physiol (Paris) 83: 141–147 [PubMed] [Google Scholar]
- Byrne JH, Baxter DA, Buonomano DV, Cleary LJ, Eskin A, Goldsmith JR, McClendon E, Nazif FA, Noel F, Scholz KP 1991. Neural and molecular bases of nonassociative and associative learning in Aplysia. Ann N Y Acad Sci 627: 124–149 [DOI] [PubMed] [Google Scholar]
- Carew TJ, Castellucci VF, Kandel ER 1971. An analysis of dishabituation and sensitization of the gill-withdrawal reflex in Aplysia. Int J Neurosci 2: 79–98 [DOI] [PubMed] [Google Scholar]
- Carew TJ, Walters ET, Kandel ER 1981a. Associative learning in Aplysia: cellular correlates supporting a conditioned fear hypothesis. Science 211: 501–504 [DOI] [PubMed] [Google Scholar]
- Carew TJ, Walters ET, Kandel ER 1981b. Classical conditioning in a simple withdrawal reflex in Aplysia californica. J Neurosci 1: 1426–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew TJ, Hawkins RD, Kandel ER 1983. Differential classical conditioning of a defensive withdrawal reflex in Aplysia californica. Science 219: 397–400 [DOI] [PubMed] [Google Scholar]
- Castellucci V, Pinsker H, Kupfermann I, Kandel ER 1970. Neuronal mechanisms of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science 167: 1745–1748 [DOI] [PubMed] [Google Scholar]
- Cleary LJ, Byrne JH 1993. Identification and characterization of a multifunction neuron contributing to defensive arousal in Aplysia. J Neurophysiol 70: 1767–1776 [DOI] [PubMed] [Google Scholar]
- Cleary LJ, Byrne JH, Frost WN 1995. Role of interneurons in defensive withdrawal reflexes in Aplysia. Learn Mem 2: 133–151 [DOI] [PubMed] [Google Scholar]
- Cleary LJ, Lee WL, Byrne JH 1998. Cellular correlates of long-term sensitization in Aplysia. J Neurosci 18: 5988–5998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen TE, Kaplan SW, Kandel ER, Hawkins RD 1997. A simplified preparation for relating cellular events to behavior: mechanisms contributing to habituation, dishabituation, and sensitization of the Aplysia gill-withdrawal reflex. J Neurosci 17: 2886–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emptage NJ, Carew TJ 1993. Long-term synaptic facilitation in the absence of short-term facilitation in Aplysia neurons. Science 262: 253–256 [DOI] [PubMed] [Google Scholar]
- Fischer TM, Carew TJ 1993. Activity-dependent potentiation of recurrent inhibition: A mechanism for dynamic gain control in the siphon withdrawal reflex of Aplysia. J Neurosci 13: 1302–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer TM, Carew TJ 1995. Cutaneous activation of the inhibitory L30 interneurons provides a mechanism for regulating adaptive gain control in the siphon withdrawal reflex of Aplysia. J Neurosci 15: 762–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald K, Carew TJ 1991. Serotonin mimics tail shock in producing transient inhibition in the siphon withdrawal reflex of Aplysia. J Neurosci 11: 2510–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost WN, Kandel ER 1995. Structure of the network mediating siphon-elicited siphon withdrawal in Aplysia. J Neurophysiol 73: 2413–2427 [DOI] [PubMed] [Google Scholar]
- Frost WN, Castellucci VF, Hawkins RD, Kandel ER 1985. Monosynaptic connections made by the sensory neurons of the gill- and siphon-withdrawal reflex in Aplysia participate in the storage of long-term memory for sensitization. Proc Natl Acad Sci 82: 8266–8269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost WN, Clark GA, Kandel ER 1988. Parallel processing of short-term memory for sensitization in Aplysia. J Neurobiol 19: 297–334 [DOI] [PubMed] [Google Scholar]
- Glanzman DL, Mackey SL, Hawkins RD, Dyke AM, Lloyd PE, Kandel ER 1989. Depletion of serotonin in the nervous system of Aplysia reduces the behavioral enhancement of gill withdrawal as well as the heterosynaptic facilitation produced by tail shock. J Neurosci 9: 4200–4213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith JR, Byrne JH 1993. Bag cell extract inhibits tail-siphon withdrawal reflex, suppresses long-term but not short-term sensitization, and attenuates sensory-to-motor neuron synapses in Aplysia. J Neurosci 13: 1688–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RD, Clark GA, Kandel ER 2006. Operant conditioning of gill withdrawal in Aplysia. J Neurosci 26: 2443–2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfermann I, Castellucci V, Pinsker H, Kandel E 1970. Neuronal correlates of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science 167: 1743–1745 [DOI] [PubMed] [Google Scholar]
- Levenson J, Byrne JH, Eskin A 1999. Levels of serotonin in the hemolymph of Aplysia are modulated by light/dark cycles and sensitization training. J Neurosc i 19: 8094–8103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus EA, Nolen TG, Rankin CH, Carew TJ 1988. Behavioral dissociation of dishabituation, sensitization, and inhibition in Aplysia. Science 241: 210–213 [DOI] [PubMed] [Google Scholar]
- Marinesco S, Carew TJ 2002. Serotonin release evoked by tail nerve stimulation in the CNS of Aplysia: characterization and relationship to heterosynaptic plasticity. J Neurosci 22: 2299–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinesco S, Kolkman KE, Carew TJ 2004a. Serotonergic modulation in Aplysia. I. Distributed serotonergic network persistently activated by sensitizing stimuli. J Neurophysiol 92: 2468–2486 [DOI] [PubMed] [Google Scholar]
- Marinesco S, Wickremasinghe N, Kolkman KE, Carew TJ 2004b. Serotonergic modulation in Aplysia. II. Cellular and behavioral consequences of increased serotonergic tone. J Neurophysiol 92: 2487–2496 [DOI] [PubMed] [Google Scholar]
- Marinesco S, Wickremasinghe N, Carew TJ 2006. Regulation of behavioral and synaptic plasticity by serotonin release within local modulatory fields in the CNS of Aplysia. J Neurosci 26: 12682–12693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KC, Casadio A, Zhu H, Yaping E, Rose JC, Chen M, Bailey CH, Kandel ER 1997. Synapse-specific, long-term facilitation of Aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell 91: 927–938 [DOI] [PubMed] [Google Scholar]
- Mauelshagen J, Parker GR, Carew TJ 1996. Dynamics of induction and expression of long-term synaptic facilitation in Aplysia. J Neurosci 16: 7099–7108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauelshagen J, Sherff CM, Carew TJ 1998. Differential induction of long-term synaptic facilitation by spaced and massed applications of serotonin at sensory neuron synapses of Aplysia californica. Learn Mem 5: 246–256 [PMC free article] [PubMed] [Google Scholar]
- Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel ER, Schacher S 1986. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science 234: 1249–1254 [DOI] [PubMed] [Google Scholar]
- Muller U, Carew TJ 1998. Serotonin induces temporally and mechanistically distinct phases of persistent PKA activity in Aplysia sensory neurons. Neuron 21: 1423–1434 [DOI] [PubMed] [Google Scholar]
- Philips GT, Tzvetkova EI, Carew TJ 2007. Transient mitogen-activated protein kinase activation is confined to a narrow temporal window required for the induction of two-trial long-term memory in Aplysia. J Neurosci 27: 13701–13705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsker H, Kupfermann I, Castellucci V, Kandel E 1970. Habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science 167: 1740–1742 [DOI] [PubMed] [Google Scholar]
- Pinsker H, Carew TJ, Hening W, Kandel ER 1973a. Long-term sensitization of a defensive withdrawal reflex in Aplysia californica. Science 182: 1039–1042 [DOI] [PubMed] [Google Scholar]
- Pinsker HM, Hening WA, Carew TJ, Kandel ER 1973b. Long-term sensitization of a defensive withdrawal reflex in Aplysia. Science 182: 1039–1042 [DOI] [PubMed] [Google Scholar]
- Purcell AL, Carew TJ 2001. Modulation of excitability in Aplysia tail sensory neurons by tyrosine kinases. J Neurophysiol 85: 2398–2411 [DOI] [PubMed] [Google Scholar]
- Purcell AL, Carew TJ 2003. Tyrosine kinases, synaptic plasticity and memory: insights from vertebrates and invertebrates. Trends Neurosci 26: 625–630 [DOI] [PubMed] [Google Scholar]
- Purcell AL, Sharma SK, Bagnall MW, Sutton MA, Carew TJ 2003. Activation of a tyrosine kinase-MAPK cascade enhances the induction of long-term synaptic facilitation and long-term memory in Aplysia. Neuron 37: 473–484 [DOI] [PubMed] [Google Scholar]
- Reissner KJ, Shobe JL, Carew TJ 2006. Molecular nodes in memory processing: insights from Aplysia. Cell Mol Life Sci 63: 963–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz KP, Byrne JH 1987. Long-term sensitization in Aplysia: biophysical correlates in tail sensory neurons. Science 235: 685–687 [DOI] [PubMed] [Google Scholar]
- Sharma SK, Sherff CM, Shobe J, Bagnall MW, Sutton MA, Carew TJ 2003. Differential role of mitogen-activated protein kinase in three distinct phases of memory for sensitization in Aplysia. J Neurosci 23: 3899–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherff CM, Carew TJ 1999. Coincident induction of long-term facilitation in Aplysia: cooperativity between cell bodies and remote synapses. Science 285: 1911–1914 [DOI] [PubMed] [Google Scholar]
- Sherff CM, Carew TJ 2002. Coincident induction of long-term facilitation at sensory-motor synapses in Aplysia: presynaptic and postsynaptic factors. Neurobiol Learn Mem 78: 498–507 [DOI] [PubMed] [Google Scholar]
- Sherff CM, Carew TJ 2004. Parallel somatic and synaptic processing in the induction of intermediate-term and long-term synaptic facilitation in Aplysia. Proc Natl Acad Sci 101: 7463–7468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shobe JL, Zhao Y, Stough S, Ye X, Hsuan V, Martin KC, Carew TJ 2009. Temporal phases of activity-dependent plasticity and memory are mediated by compartmentalized routing of MAPK signaling in Aplysia sensory neurons. Neuron 61: 113–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S 1998. CREB and memory. Annu Rev Neurosci 21: 127–148 [DOI] [PubMed] [Google Scholar]
- Stough S, Shobe JL, Carew TJ 2006. Intermediate-term processes in memory formation. Curr Opin Neurobiol 16: 672–678 [DOI] [PubMed] [Google Scholar]
- Sutton MA, Carew TJ 2000. Parallel molecular pathways mediate expression of distinct forms of intermediate-term facilitation at tail sensory-motor synapses in Aplysia. Neuron 26: 219–231 [DOI] [PubMed] [Google Scholar]
- Sutton MA, Masters SE, Bagnall MW, Carew TJ 2001. Molecular mechanisms underlying a unique intermediate phase of memory in Aplysia. Neuron 31: 143–154 [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ide J, Masters SE, Carew TJ 2002. Interaction between amount and pattern of training in the induction of intermediate- and long-term memory for sensitization in Aplysia. Learn Mem 9: 29–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Bagnall MW, Sharma SK, Shobe J, Carew TJ 2004. Intermediate-term memory for site-specific sensitization in Aplysia is maintained by persistent activation of protein kinase C. J Neurosci 24: 3600–3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau LE, Castellucci VF 1992. Contribution of polysynaptic pathways in the mediation and plasticity of Aplysia gill and siphon withdrawal reflex: evidence for differential modulation. J Neurosci 12: 3838–3848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright ML, Byrne JH, Cleary LJ 2004. Dissociation of morphological and physiological changes associated with long-term memory in Aplysia. J Neurophysiol 92: 2628–2632 [DOI] [PubMed] [Google Scholar]
- Walters ET 1987. Multiple sensory neuronal correlates of site-specific sensitization in Aplysia. J Neurosci 7: 408–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters ET, Byrne JH, Carew TJ, Kandel ER 1983a. Mechanoafferent neurons innervating tail of Aplysia. I. Response properties and synaptic connections. J Neurophysiol 50: 1522–1542 [DOI] [PubMed] [Google Scholar]
- Walters ET, Byrne JH, Carew TJ, Kandel ER 1983b. Mechanoafferent neurons innervating tail of Aplysia. II. Modulation by sensitizing stimulation. J Neurophysiol 50: 1543–1559 [DOI] [PubMed] [Google Scholar]
- White JA, Ziv I, Cleary LJ, Baxter DA, Byrne JH 1993. The role of interneurons in controlling the tail-withdrawal reflex in Aplysia: a network model. J Neurophysiol 70: 1777–1786 [DOI] [PubMed] [Google Scholar]
- Wright WG, Carew TJ 1995. A single identified interneuron gates tail-shock induced inhibition in the siphon withdrawal reflex of Aplysia. J Neurosci 15: 790–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright WG, Marcus EA, Carew TJ 1991. A cellular analysis of inhibition in the siphon withdrawal reflex of Aplysia. J Neurosci 11: 2498–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Cleary LJ, Byrne JH 1994. Identification and characterization of pleural neurons that inhibit tail sensory neurons and motor neurons in Aplysia: correlation with FMRFamide immunoreactivity. J Neurosci 14: 3565–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Pieroni JP, Cleary LJ, Byrne JH 1995. Modulation of an inhibitory interneuron in the neural circuitry for the tail withdrawal reflex of Aplysia. J Neurophysiol 73: 1313–1318 [DOI] [PubMed] [Google Scholar]
- Ye X, Shobe JL, Sharma SK, Marina A, Carew TJ 2008. Small G proteins exhibit pattern sensitivity in MAPK activation during the induction of memory and synaptic facilitation in Aplysia. Proc Natl Acad Sci 105: 20511–20516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar JH 2010. Biostatistical analysis, 5th ed. Prentice Hall/Pearson, Upper Saddle River, NJ [Google Scholar]