Abstract
Objective
To examine the association between cardiovascular reactivity to a set of psychological stressors and carotid artery intima-media thickness, a marker of subclinical cardiovascular disease in healthy adolescents.
Methods
Participants were 25 boys and 23 girls age 14.2±0.9 years who were measured for heart rate (HR), systolic (SBP) and diastolic (DBP) blood pressure reactivity to mirror-tracing, reaction time, speech preparation and ad lib speech tasks and for common carotid artery intima-media thickness. Sequential regression analyses were used to establish the incremental increase in R2 (R2inc) for the prediction of intima-media thickness due to cardiovascular reactivity independent of age, BMI percentile, sex, socioeconomic status, and resting HR or BP.
Results
SBP reactivity while preparing (β= 0.0019, R2inc = 0.09) and giving the speech (β = 0.0014, R2inc = 0.10) and an aggregate reactivity score based on all 4 tasks (β= 0.0026, R2inc = 0.11) independently predicted (p ≤ 0.05) mean carotid artery intima-media thickness. Neither DBP reactivity nor HR reactivity during any task were independent predictors of intima-media thickness.
Conclusion
Stress-induced cardiovascular reactivity, and especially SBP reactivity, is associated with carotid intima-media thickness and the early pathogenesis of cardiovascular disease. The use of an aggregate stress reactivity index provides a more reliable reflection of trait SBP reactivity to psychological stress and increases the confidence that youth with greater cardiovascular stress reactivity may indeed have greater progression of subclinical cardiovascular disease.
Keywords: psychological stress, cardiovascular disease, atherosclerosis, carotid artery intima media thickness
INTRODUCTION
Although the clinical manifestations of cardiovascular disease are realized in adulthood, its pathogenesis occurs during childhood.1 Traditional risk factors (e.g., family history, dyslipidemia, hypertension, diabetes) do not fully predict future cardiovascular disease.2 Thus, there is a great need to understand other factors associated with the early development of atherosclerosis. Stress reactivity may be one of these factors. Daily stress is associated with a >2.1 fold increased risk for developing cardiovascular disease and myocardial infarction,3 which similar to the relative risks of diabetes, hypercholesterolemia, and hypertension.4 Psychosocial stress accounts for 30% of the population attributable risk of acute myocardial infarction.5 Only smoking and serum lipid concentrations account for greater population attributable risk, while hypertension, diabetes, and abdominal obesity account for lower risk.
In addition to the magnitude of daily stress, the initial cardiovascular reactivity to a stressor is also associated with intermediate markers of cardiovascular pathophysiology,6 atherosclerosis and cardiovascular events.7, 8 The carotid artery intima-media thickness is a valid index of generalized subclinical atherosclerosis in other vascular beds such as the coronary, aortic, and peripheral arteries.9 Until recently the relationships between psychological stress-induced cardiovascular reactivity and intima-media thickness had not been investigated in youth. Roemmich and colleagues10 presented initial evidence that systolic blood pressure (SBP) reactivity, but not diastolic BP (DBP) or heart rate (HR) reactivity, was associated with carotid artery intima-media thickness in children. A more recent study found that children who displayed greater DBP reactivity increases over the course of 3 years exhibited greater intima-media thickness.11 Given that there are only two studies in this area and that the studies do not have concordant results, further work is needed to determine the association of stress-induced cardiovascular reactivity and early atherogenesis.
A reason for the variability in results may be inter-study differences in the stressors used to measure cardiovascular reactivity. Autonomic and cardiovascular responses differ according to the stressor being experienced.12 A set of stress tasks that produce predominately α-, β-, and mixed α- and β-adrenergic cardiovascular response patterns allows for the calculation of an aggregate stress reactivity score. This approach provides a more reliable reflection of a child’s ‘trait’ cardiovascular reactivity.13 Thus, the purpose was to measure adolescent’s cardiovascular reactivity to stress tasks that differed in their ability to activate α-and β-adrenergic responses.
METHODS
Subjects
Subjects included 25 boys and 23 girls, 13 to 16 years of age. Two boys were of more than one race, all others were White. Inclusion criteria included no history of diagnosed psychiatric disorder, no current illness or pregnancy, and no current use of medications that would alter baseline stress or stress reactivity. Participants ranged in weight status from the 10th to 85th body mass index (BMI) percentile. Socioeconomic status was assessed by the parent completing a questionnaire. Parents provided written consent and children provided assent to participate in the study. The study was approved by the University at Buffalo Social and Behavioral Sciences Institutional Review Board. An investigator debriefed children and parents after finishing the experiment.
Procedures
Youth were tested on two days; a stress reactivity measurement day and an intima-media thickness measurement day. The visits were separated by no more than one week. All visits occurred during the mid- to late-afternoon. On the stress reactivity day, the adolescent’s height and weight were measured. A parent completed a demographic questionnaire about their own education, employment and family income. Youth were fitted with a HR transmitter strapped around the torso and a watch receiver (Polar Vantage, Port Washington, New York) placed out of view. Youth were also fitted with a cuff and surface electrodes to measure BP with an automated monitor (Suntech Tango+, Morrisville, North Carolina). Adolescents were told not to eat anything at least 1 h prior to the appointment and not to participate in any intense physical activity the day before or the day of the lab visit. For the first 20 min, youth rested by reading magazines and then baseline SBP, DBP, and HR measures were collected. The adolescents then participated in a computerized mirror star tracing task, a reaction time task, speech preparation task, and speech task. The order of the mirror tracing and reaction time tasks was counterbalanced across subjects. Due to the length of the tasks, the speech preparation and speech tasks always occurred last. Adolescents rested by reading magazines for 5 min between the mirror tracing, reaction time and speech preparation/speech tasks. There was no rest between preparing and giving the speech.
Stress Reactivity Tasks
Mirror star tracing task
The mirror tracing task produces predominately α-adrenergic activation resulting in vasoconstriction and increased BP with smaller increase in HR.14 Youth sat at a table with a computer monitor in-front of them and attempted to slide a computer mouse to control a cursor to trace a 5-sided star. To produce a mirror-tracing effect, both horizontal and vertical movements of the cursor were programmed to be reversed relative to movement of the mouse. Youth were instructed to trace the star as quickly as possible without allowing the cursor to stray out-of-bounds in either the center or the area surrounding the external border of the star. Out-of-bounds errors are noted with a large red X flashing on the screen. Adolescents completed as many trials as they could in 5 minutes. The width of the in-bounds portion of the star adjusted between trials to maintain the time out-of-bounds at 40%.
Reaction time task
Reaction time tasks elicit predominately enhanced β-adrenergic activity resulting in increases in HR and SBP, with little change in DBP.15 Using the stop-signal paradigm, youth engaged in a primary choice reaction time task that presents a 5-digit number in black font on a computer screen that disappeared and was followed by another 5-digit number. The randomly generated 5-digit numbers appear for 600 ms, once every 2 s (600 ms on, 1400 ms off). A “go” signal occurs when the second 5-digit number is also in black font and exactly matches the original number. This is a No-Stop trial and participants were told to respond when the number they see was identical to the previous number. A Stop trial occurs when the color of the matching numerals change from black to red (“stop” signal) between 25 to 350 ms after presentation. Youth were instructed to respond to the identically matching numbers before the number disappears from the screen, but not to respond to a number that turns red. No-stop and stop trials each occur 25% of the time. The remaining 50% of the trials are Novel trials and consist of numbers that differ randomly from the previous number. An adjusting procedure was used for the Stop trials. The initial interval that the number remains black (go) before turning red (stop) was set at 200 ms duration and adjusted by 25 ms. Each time an adolescent failed to inhibit their response during a Stop trial, the next stop signal onset-delay was reduced by 25 ms. Each time they successfully inhibited and did not respond to a Stop trial, the onset-delay increased by 25 ms. The onset-delay adjusted so that the inhibition rate approached 50%. Trials were presented for 4 min. Points were earned or lost depending on task performance. Not responding to a Stop trial earned a 5 point reward while responding to a Stop trial incurred a 5 point penalty. Responding to a No-Stop trial earned a 5 point reward while not-responding to a No-Stop trial incurred a 5 point penalty. To encourage quick responding, late responses during No-Stop trial incurred a 10 point penalty. Responding to a Novel trial resulted in a 5 point penalty. Youth were instructed to earn as many points as possible, but told that no rewards would be tied to their point total.
Speech preparation and speech tasks
Speech tasks produce a mix of α- and β-adrenergic activation, but modulate cardiovascular function predominantly through β-adrenergic mechanisms.16–18 Youth were given 5 min to prepare and 5 min to deliver a speech about why they are a good friend and were informed that their speeches would be recorded and judged for honesty, believability, and confidence. If they stopped speaking before the 5 min were completed, they were encouraged to continue talking by adding any additional information that others should know about them, or restating or summarizing their main qualities that make them a good friend.
Measurement
Anthropometrics
Body weight was measured to the nearest 0.01 kg with the subjects wearing light clothing. Height was measured with a stadiometer to the nearest 0.1 cm. BMI (weight in kg/height in m2) percentile was calculated in relationship to 50th BMI percentile based on their sex and age.19
Stress Reactivity
During the stress tasks, HR was measured using a Polar (Port Washington, NY) HR monitor. BP was measured with a Suntech Tango+ monitor (Morrisville, North Carolina). The Suntech Tango uses the auscultatory method aided by electrocardiographic R-wave gating, and an oscillometric transducer to determine systolic BP and diastolic BP and is a valid and reliable measure of BP during rest and exercise.20 Measurement of BP followed published guidelines21 regarding cuff length and width, placement of the cuff around the arm 2.5 cm above the antecubital space, and seating of the cuff of the arm by inflating and deflating the cuff before taking any measurements. SBP and DBP were measured 2 times during the last 5 min of baseline and during the last 2 min of each stress task and rest period. HR was measured continuously and averaged across the last 2 min of baseline and each stress task. Perceived stress reactivity to the laboratory tasks was measured by having youth assess their current degree of psychological stress using a 10 point Likert scale, anchored by “no stress”/“very stressed”.
High Resolution Carotid Ultrasonography
Carotid ultrasounds were performed with the Biosound Esaote (MyLab25 Gold) ultrasound imaging machine (Biosound Esaote, Inc., Indianapolis, IN) and with a 7.5MHz transducer. The right and left extracranial carotid arteries were scanned by trained and carotid intima-media thickness -certified ultrasound technicians.22 A preliminary exploratory transverse scan was performed to assess the participant’s anatomy and determine the optimum angle for viewing the intima-media thickness. Once the angle was determined, an exploratory longitudinal scan was performed of the common carotid artery. Beginning at the determined optimal angle, standardized longitudinal images were acquired of 1.0 cm segments of the near and far walls of the distal common carotid. The sonographer obtained images at three different scanning angles (optimal angle, anterior, latero-posterior) on each side of the neck. Images were recorded on videotape using a super videocassette recorder and analyzed offline using ImagePro Plus software. During reading, digitized frames were captured to measure the intima-media thickness of each of the 1.0 cm segments imaged. The mean common carotid artery intima-media thickness was measured in 12 common carotid artery segments approximately 1.0 cm in length: the near and far wall, from 3 interrogation angles, on both the right and left side (2 × 3 × 2=12). The common carotid measurements for each side were averaged to obtain the mean common carotid artery intima-media thickness. The reader was blind to the magnitude of stress reactivity of the adolescent.
Data Reduction
Mean HR, SBP, and DBP were calculated during each of the baseline, stress tasks, and inter-stress task rest periods. The mean HR, SBP, and DBP were used to calculate cardiovascular reactivity to each stress task and to calculate aggregate HR, SBP, and DBP stress reactivity across all four (star tracing, reaction time, speech preparation, speech) stress tasks. The speech preparation and speech tasks were considered separate tasks because they differ in whether the subject is speaking, which alters cardiovascular responses.23 Stress reactivity scores for each stress task were calculated as the mean value during each stress task minus the mean of all of the rest periods (baseline and inter-task rest values). Averaging the rest periods provided an overall index of the resting measures. This approach was taken because there were no significant differences between baseline and either of the inter-task rest interval values for SBP (p > 0.12), DBP (p > 0.09), or HR (p > 0.26). Similar procedures were used to calculate perceived stress reactivity except there was no need to average the scores as perceived stress was collected just once per stress or rest period. The reactivity scores were then averaged across the four stressor tasks to yield aggregate indices of cardiovascular and psychological reactivity.
Analytic Plan
Separate one-way analysis of variance models were used to test sex differences in demographics, subject characteristics and carotid artery intima-media thickness. SBP, DBP, HR and perceived stress reactivity to each of the four stress tasks were evaluated using separate two-way analysis of variance with sex (male, female) as a between variable and stress task (star tracing, reaction time, speech preparation, speech) as a within variable. Separate sequential regression models were used to determine whether DBP, SBP, or HR reactivity or perceived stress reactivity significantly added to the prediction of carotid artery intima-media thickness after accounting for individual differences in sex, SES, race, percentage overweight, and baseline BP or HR (step 1 covariates). All p values are two tailed.
RESULTS
Physical characteristics and demographics are shown in Table 1. The boys were taller (p < 0.05) and had a greater socioeconomic status (p = 0.053) and mean carotid artery intima-media thickness (p = 0.053) than the girls. There were no other sex differences.
Table 1.
Subject physical characteristics and demographics
| Boys (n = 25) |
Girls (n = 23) |
|
|---|---|---|
| Age (years) | 14.2 ± 1.1 | 14.2 ± 0.7 |
| Height (cm)* | 169.1 ± 8.8 | 161.4 ± 6.6 |
| Weight (kg) | 56.0 ± 7.6 | 53.5 ± 8.9 |
| Body mass index (kg/m2) | 19.5 ± 1.5 | 20.4 ± 2.3 |
| Body mass index percentile | 48.7 ± 19.4 | 57.5 ± 25.2 |
| Percent overweight | 0.5 ± 7.6 | 5.1 ± 11.8 |
| Mean carotid artery IMT (mm)* | 0.52 ± 0.04 | 0.49 ± 0.04 |
| Socioeconomic status | 51.0 ± 7.9 | 46.8 ± 6.4 |
Data are mean ± SD
Boys significantly different than girls (p ≤ 0.05)
SES: socioeconomic status. An SES of 40 through 50 is equivalent to medium size business owners, minor professionals and technical jobs, such as computer programmers, real estate agents, sales managers, social workers and teachers.
IMT: intima-media thickness
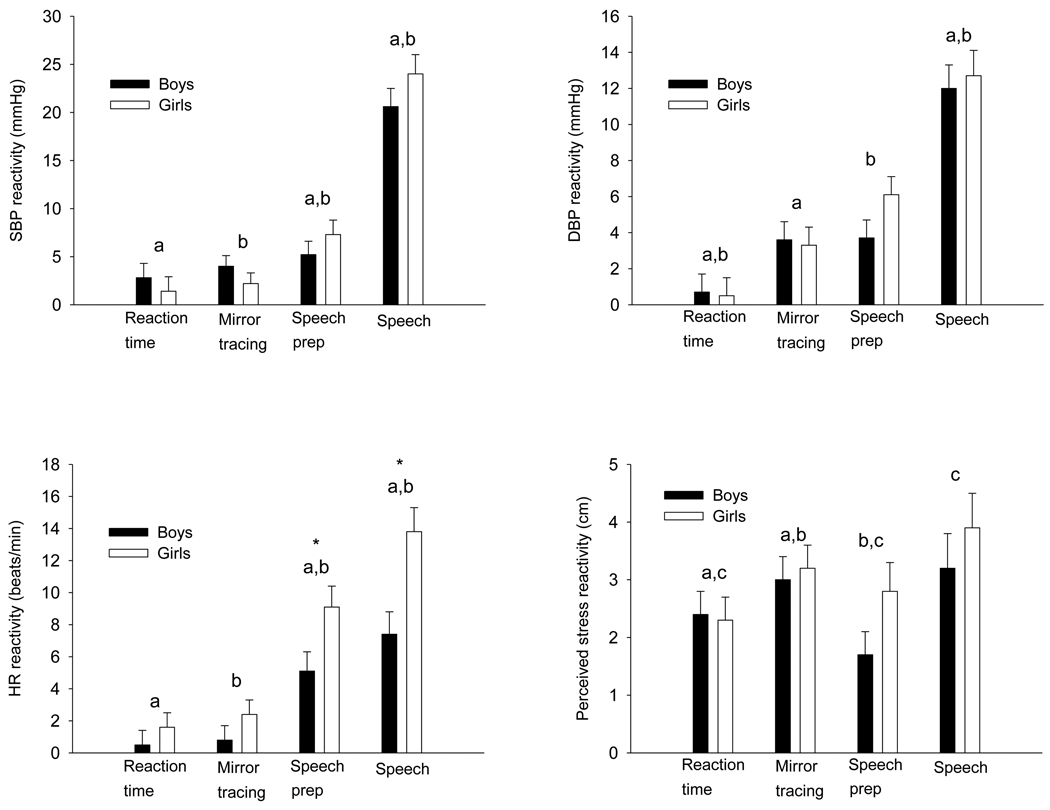
There was a main effect (p < 0.001) of stress task for both SBP and DBP reactivity (Figure 1). SBP reactivity was greater (p < 0.002) during speech preparation than the reaction time or mirror tracing tasks. SBP reactivity during the speech was greater (p < 0.001) than all other tasks. DBP reactivity was greater during mirror tracing (p < 0.01) and speech preparation (p < 0.001) than the reaction time task. DBP reactivity during the speech was greater (p < 0.001) than all other tasks. There were no significant main effects of sex (p ≥ 0.55) for SBP or DBP reactivity or sex differences in SBP or DBP reactivity to any of the four stress tasks. There was a significant (p < 0.001) main effect of stress task for HR reactivity which was greater (p < 0.001) during speech preparation than during the reaction time and mirror tracing tasks. HR reactivity during the speech was greater (p < 0.001) than all other tasks. There was a main effect of sex for HR reactivity in that it was greater (p < 0.006) in the girls and there was a significant (p < 0.05) sex by stress task interaction. HR reactivity was greater in the girls during the speech preparation (p < 0.03) and speech tasks (p < 0.004), but there was no (p ≥ 0.10) sex difference in HR reactivity to the reaction time or mirror tracing tasks. There was a significant (p < 0.001) main effect of stress task for perceived stress reactivity. Perceived stress reactivity was greater during the mirror tracing (p < 0.006) and speech (p < 0.005) tasks than the reaction time task and greater during the mirror tracing (p < 0.02) and speech (p < 0.002) tasks than the speech preparation task. The main effect of sex for perceived stress was not significant (p ≥ 0.40).
Figure 1.
Systolic blood pressure (SBP) reactivity, diastolic blood pressure (DBP) reactivity, heart rate (HR) reactivity, and perceived stress reactivity of the boys and girls during the reaction time, mirror tracing, speech preparation, and speech tasks. Means with the same letter are significantly (p < 0.05) different based on a significant main effect of stress task (i.e., data from boys and girls are combined). An * denotes a significant (p < 0.05) sex difference.
Sequential regression results to predict carotid artery intima-media thickness from SBP reactivity of various stress tasks and the aggregate SBP stress reactivity score are shown in Table 2. Age, BMI percentile, sex, socioeconomic status and average resting SBP were entered as a block of variables in step 1 and produced an R2 of 0.12. Addition of individual SBP reactivity scores in step 2 incrementally increased R2 by 0.03 (p ≥ 0.29), 0.08 (p < 0.06), 0.09 (p < 0.05), 0.10 (p < 0.05), and 0.11 (p < 0.03) for reaction time, mirror tracing, speech preparation, speech, and aggregate SBP reactivity, respectfully. Neither DBP reactivity to a specific stress task (p ≥ 0.14) nor the aggregate DBP reactivity (p ≥ 0.43) predicted carotid artery intima-media thickness (data not shown). Likewise none of the HR reactivities (p ≥ 0.22) or perceived stress (p ≥ 0.09) reactivities predicted carotid artery intima-media thickness (data not shown).
Table 2.
Sequential regression to predict mean common carotid artery intima-media thickness from systolic blood pressure reactivity during the speech
| B | β | R2 (unique) | P | |
|---|---|---|---|---|
| Step 1 | 0.12 | |||
| Age (y) | −0.0012 | −0.0268 | 0.86 | |
| BMI percentile | 0.0001 | 0.0215 | 0.89 | |
| Sex | −0.0294 | −0.3691 | 0.03 | |
| SES | −0.0002 | −0.0310 | 0.84 | |
| Resting SBP (mmHg) | 0.0001 | 0.0128 | 0.93 | |
| Step 2: Individually tested SBP reactivity predictors (mmHg) | ||||
| Reaction time | 0.0010 | 0.1680 | 0.03 | 0.29 |
| Mirror tracing | 0.0023 | 0.2922 | 0.08 | 0.06 |
| Speech preparation | 0.0019 | 0.3200 | 0.09 | <0.05 |
| Speech | 0.0014 | 0.3208 | 0.10 | <0.05 |
| Aggregate reactivity | 0.0026 | 0.3568 | 0.11 | <0.05 |
Each step 2 predictor was tested singularly as part of a separate model.
Sex: 0 = boys, 1 = girls
SES: socioeconomic status, SBP: systolic blood pressure
DISCUSSION
This study evaluated the association of cardiovascular and psychological reactivity to a set of stressors with greater carotid artery intima-media thickness in a sample of healthy adolescents. SBP reactivity to preparing and giving a speech and an aggregate SBP reactivity score predicted greater carotid artery intima-media thickness. These results replicate previous research that stress-induced SBP reactivity to preparing and then given an interpersonal speech is associated with subclinical cardiovascular disease in children.10 Moreover, the present study extends earlier work by demonstrating that an aggregate stress reactivity measure that provides a more reliable reflection of ‘trait’. cardiovascular reactivity,13 predicts greater intima-media thickness. In toto, the present results provide further evidence that youth with greater SBP reactivity to stress may be at increased risk for developing cardiovascular disease in adulthood.
The speech task produces a mix of α- and β-adrenergic activation, but modulates cardiovascular function predominantly through β-adrenergic activation.16–18 The importance of stress-related increases in β-adrenergic tone for promoting atherogenesis has been recognized for some time in that blocking β-adrenergic activity significantly reduces the development of atherosclerosis in stressed monkeys.24 However, SBP reactivity during the reaction time task, which produces β-adrenergic responses, did not significantly predict carotid artery intima-media thickness. Perhaps the α-adrenergic component of the speech task is important for producing atherogenic cardiovascular responses. For the mirror tracing task, which produces α-adrenergic activation, there was a strong trend (p = 0.06) for SBP reactivity to predict intima-media thickness. Alpha-adrenoceptors stimulate vasoconstriction via contraction of the vascular smooth muscle. Repeated increases in α-adrenergic activity and blood catecholamine, especially norepinephrine, concentrations are risk factors for carotid arterial wall hypertrophy and cardiovascular disease in small animal models and 25 and humans.26
It is not yet clear how psychological stress promotes the development of cardiovascular disease. Acute psychological stress may result in recurrent turbulent blood flow and sheer stress resulting in mechanical injury to the endothelial lining.24 At the same time there is activation of procoagulants and development of a procoagulant state that results in occlusive effects within the micro-circulation and a proinflammatory response.27, 28 If acute stress occurs on a frequent basis, these responses acting alone and especially in concert with one another could promote the formation of atherosclerotic plaque.
In agreement with previous research,10 DBP reactivity was not predictive of intima-media thickness, though others have found that adolescents with greater DBP reactivity increases over 3 years had greater intima-media thickness.11 As expected, HR reactivity was not associated with increased carotid artery intima-media thickness.7, 10, 11
When youth in the present study were categorized as high or low for SBP reactivity based on a median split of the aggregate SBP score, the reactive group (0.52 mm) had a 6.1% greater carotid intima-media thickness than the low reactive group (0.49 mm). For comparison, youth with primary hypertension have a 5.9% greater carotid intima-media thickness.29 In agreement with previous research,10 SBP reactivity accounted for approximately 10% of the variability in carotid artery intima-media thickness in youth and each 1 mmHg increase in SBP reactivity was associated with a 0.002 to 0.003 mm increase in carotid artery intima-media thickness. Although the clinical implications of an association of this magnitude remain to be fully elucidated, repeated exposures to stressful situations on a daily basis from adolescence through adulthood could have important implications for atherogenesis and cardiovascular events later in life. Although the association between SBP reactivity and greater carotid artery intima-media thickness in youth may have much clinical importance for the development of cardiovascular disease, stronger evidence of this presumption would require a long-term prospective research design. Other cardiovascular risk factors measured during childhood do predict greater carotid artery intima-media thickness when the children reach adulthood.30
The present study has the limitations of a smaller sample size and the cross-sectional design prohibits conclusions regarding cause and effect. However, the results are in close agreement with previous cross-sectional studies of youth10 and with larger prospective studies.7
In conclusion, SBP reactivity to a variety of stressors was associated with greater carotid artery intima-media thickness in adolescents. This study extends previous research in youth by replicating the association reported between SBP reactivity and carotid artery intima-media thickness in younger children and increasing confidence in the results by demonstrating that an aggregate stress reactivity index that provides a more reliable reflection of ‘trait’. SBP reactivity to psychological stress predicts greater intima-media thickness. These results provide further evidence that psychological stress reactivity may play a role in the pathogenesis of cardiovascular disease in youth.
Acknowledgments
We thank Vivian Boyd, R.N., M.A. and Debbie Saltino, B.S. for their technical assistance in completing the intima-media thickness measurements. This study was supported by the University at Buffalo UB 2020 Interdisciplinary Research Development Fund and RO1 HD42766, both to Dr. Roemmich. The funding sources had no role in study design, collection, analysis or interpretation of the results, or in the writing of the report or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.McGill HC, Jr, McMahan CA, Zieske AW, Sloop GD, Walcott JV, Troxclair DA, Malcom GT, Tracy RE, Oalmann MC, Strong JP. Associations of coronary heart disease risk factors with the intermediate lesion of atherosclerosis in youth. The Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb Vasc Biol. 2000;20:1998–2004. doi: 10.1161/01.atv.20.8.1998. [DOI] [PubMed] [Google Scholar]
- 2.Flaa A, Eide IK, Kjeldsen SE, Rostrup M. Sympathoadrenal stress reactivity is a predictor of future blood pressure: an 18-year follow-up study. Hypertension. 2008;52:336–341. doi: 10.1161/HYPERTENSIONAHA.108.111625. [DOI] [PubMed] [Google Scholar]
- 3.Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi-amorn C, Sato H, Yusuf S. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:953–962. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- 4.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 6.Gottdiener JS, Kop WJ, Hausner E, McCeney MK, Herrington D, Krantz DS. Effects of mental stress on flow-mediated brachial arterial dilation and influence of behavioral factors and hypercholesterolemia in subjects without cardiovascular disease. Am J Cardiol. 2003;92:687–691. doi: 10.1016/s0002-9149(03)00823-3. [DOI] [PubMed] [Google Scholar]
- 7.Jennings JR, Kamarck TW, Everson-Rose SA, Kaplan GA, Manuck SB, Salonen JT. Exaggerated blood pressure responses during mental stress are prospectively related to enhanced carotid atherosclerosis in middle-aged Finnish men. Circulation. 2004;110:2198–2203. doi: 10.1161/01.CIR.0000143840.77061.E9. [DOI] [PubMed] [Google Scholar]
- 8.Leor J, Kloner RA. The Northridge earthquake as a trigger for acute myocardial infarction. Am J Cardiol. 1996;77:1230–1232. doi: 10.1016/s0002-9149(96)00169-5. [DOI] [PubMed] [Google Scholar]
- 9.Sutton KC, Wolfson SK, Jr, Kuller LH. Carotid and lower extremity arterial disease in elderly adults with isolated systolic hypertension. Stroke. 1987;18:817–822. doi: 10.1161/01.str.18.5.817. [DOI] [PubMed] [Google Scholar]
- 10.Roemmich JN, Lobarinas CL, Joseph PN, Lambiase MJ, Archer Iii FD, Dorn J. Cardiovascular reactivity to psychological stress and carotid intima-media thickness in children. Psychophysiology. 2009;46:293–299. doi: 10.1111/j.1469-8986.2008.00776.x. [DOI] [PubMed] [Google Scholar]
- 11.Low CA, Salomon K, Matthews KA. Chronic life stress, cardiovascular reactivity, and subclinical cardiovascular disease in adolescents. Psychosom Med. 2009;71:927–931. doi: 10.1097/PSY.0b013e3181ba18ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen MT. Laboratory Tasks for Cardiovascular Reactivity Research, 1999. 2008 http://www.macses.ucsf.edu/Research/Psychosocial/notebook/reactivitytable.html.
- 13.Kamarck TW, Lovallo WR. Cardiovascular reactivity to psychological challenge: conceptual and measurement considerations. Psychosom Med. 2003;65:9–21. doi: 10.1097/01.psy.0000030390.34416.3e. [DOI] [PubMed] [Google Scholar]
- 14.Sherwood A, Turner JR. Hemodynamic responses during psychological stress: implications for studying disease processes. Int J Behav Med. 1995;2:193–218. doi: 10.1207/s15327558ijbm0203_1. [DOI] [PubMed] [Google Scholar]
- 15.Mills PJ, Dimsdale JE, Ziegler MG, Berry CC, Bain RD. Beta-adrenergic receptors predict heart rate reactivity to a psychosocial stressor. Psychosom Med. 1990;52:621–623. doi: 10.1097/00006842-199011000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Hurwitz BE, Nelesen RA, Saab PG, Nagel JH, Spitzer SB, Gellman MD, McCabe PM, Phillips DJ, Schneiderman N. Differential patterns of dynamic cardiovascular regulation as a function of task. Biol Psychol. 1993;36:75–95. doi: 10.1016/0301-0511(93)90082-j. [DOI] [PubMed] [Google Scholar]
- 17.Sherwood A, Turner RJ. A conceptual and Methodological Overview of Cardiovascular Reactivity Research. In: Turner JR, Sherwood A, Light KC, editors. Individual Differences in Cardiovascular Response to Stress. New York, NY: Plenum Publishing; 1992. pp. 3–27. [Google Scholar]
- 18.Al'Absi M, Bongard S, Buchanan T, Pincomb GA, Licinio J, Lovallo WR. Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology. 1997;34:266–275. doi: 10.1111/j.1469-8986.1997.tb02397.x. [DOI] [PubMed] [Google Scholar]
- 19.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC Growth Charts for the United States: Methods and Development. Hyattsville,MD: National Center for Health Statistics; 2000. p. 201. [PubMed] [Google Scholar]
- 20.Taylor RS, Gallen I. Evaluation of SunTech 4240 during rest and during exercise:A novel automated blood pressure device. J Cardiopulm Rehabil. 1994;14:330–334. [Google Scholar]
- 21.Shapiro D, Jamner LD, Lane JD, Light KC, Myrtek M, Sawada Y, Steptoe A. Blood pressure publication guidelines. Society for Psychophysical Research. Psychophysiology. 1996;33:1–12. doi: 10.1111/j.1469-8986.1996.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 22.Riley W. Brachial Ultrasound - B-Mode Image Reading Protocol. Draft 1.0. Buffalo, NY: Department of Social and Preventive Medicine, State University of New York at Buffalo; 2004. [Google Scholar]
- 23.Siegman AW, Dembroski TM, Crump D. Speech rate, loudness, and cardiovascular reactivity. J Behav Med. 1992;15:519–539. doi: 10.1007/BF00844945. [DOI] [PubMed] [Google Scholar]
- 24.Strawn WB, Bondjers G, Kaplan JR, Manuck SB, Schwenke DC, Hansson GK, Shively CA, Clarkson TB. Endothelial dysfunction in response to psychosocial stress in monkeys. Circ Res. 1991;68:1270–1279. doi: 10.1161/01.res.68.5.1270. [DOI] [PubMed] [Google Scholar]
- 25.Erami C, Zhang H, Tanoue A, Tsujimoto G, Thomas SA, Faber JE. Adrenergic catecholamine trophic activity contributes to flow-mediated arterial remodeling. Am J Physiol Heart Circ Physiol. 2005;289:H744–H753. doi: 10.1152/ajpheart.00129.2005. [DOI] [PubMed] [Google Scholar]
- 26.Head RJ. Hypernoradrenergic innervation and vascular smooth muscle hyperplastic change. Blood Vessels. 1991;28:173–178. doi: 10.1159/000158858. [DOI] [PubMed] [Google Scholar]
- 27.von Kanel R, Mills PJ, Ziegler MG, Dimsdale JE. Effect of beta2-adrenergic receptor functioning and increased norepinephrine on the hypercoagulable state with mental stress. Am Heart J. 2002;144:68–72. doi: 10.1067/mhj.2002.123146. [DOI] [PubMed] [Google Scholar]
- 28.Fuligni AJ, Telzer EH, Bower J, Cole SW, Kiang L, Irwin MR. A preliminary study of daily interpersonal stress and C-reactive protein levels among adolescents from Latin American and European backgrounds. Psychosom Med. 2009;71:329–333. doi: 10.1097/PSY.0b013e3181921b1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lande MB, Carson NL, Roy J, Meagher CC. Effects of childhood primary hypertension on carotid intima media thickness: a matched controlled study. Hypertension. 2006;48:40–44. doi: 10.1161/01.HYP.0000227029.10536.e8. [DOI] [PubMed] [Google Scholar]
- 30.Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, Berenson GS. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA. 2003;290:2271–2276. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]