Abstract
Freshwater crayfish have three known photoreceptive systems: the compound eyes, extraretinal brain photoreceptors, and caudal photoreceptors. The primary goal of the work described here was to explore the contribution of the brain photoreceptors to circadian locomotory activity and define some of the underlying neural pathways. Immunocytochemical studies of the brain photoreceptors in the parastacid (southern hemisphere) crayfish Cherax destructor reveal their expression of the blue light-sensitive photopigment cryptochrome and the neurotransmitter histamine. The brain photo-receptors project to two small protocerebral neuropils, the brain photoreceptor neuropils (BPNs), where they terminate among fibers expressing the neuropeptide pigment-dispersing hormone (PDH), a signaling molecule in arthropod circadian systems. Comparable pathways are also described in the astacid (northern hemisphere) crayfish Procambarus clarkii. Despite exhibiting markedly different diurnal locomotor activity rhythms, removal of the compound eyes and caudal photoreceptors in both C. destructor and P. clarkii (leaving the brain photoreceptors intact) does not abolish the normal light/dark activity cycle in either species, nor prevent the entrainment of their activity cycles to phase shifts of the light/dark period. These results suggest, therefore, that crayfish brain photoreceptors are sufficient for the entrainment of loco-motor activity rhythms to photic stimuli, and that they can act in the absence of the compound eyes and caudal photoreceptors. We also demonstrate that the intensity of PDH expression in the BPNs varies in phase with the locomotor activity rhythm of both crayfish species. Together, these findings suggest that the brain photoreceptor cells can function as extraretinal circadian photoreceptors and that the BPN represents part of an entrainment pathway synchronizing locomotor activity to environmental light/dark cycles, and implicating the neuropeptide PDH in these functions. (Author correspondence: bbeltz@wellesley.edu)
Keywords: Behavior, Circadian rhythm, Crayfish, Cryptochrome, Photosensitivity, Pigment dispersing hormone
INTRODUCTION
The day/night cycle exerts a powerful influence on crustaceans. Photo-periodic rhythmicity can be found in locomotory activity, size of the response of compound eye receptor potentials (ERGs), movement of pigment in the eyes and on the body (Aréchiga et al., 1993; Fanjul-Moles & Pietro-Sagredo, 2003), levels of serotonin in the brain (Benton et al., 2008; Wildt et al., 2003), and rate of neurogenesis in adult animals (Goergen et al., 2002). Many of these rhythms persist in conditions of constant darkness and are therefore under the control of endogenous circa-dian oscillators (Aréchiga et al., 1993).
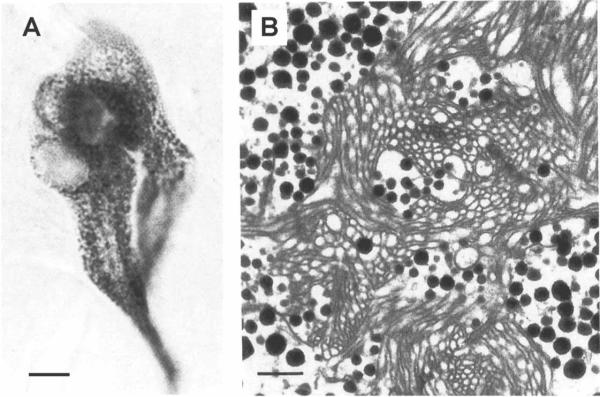
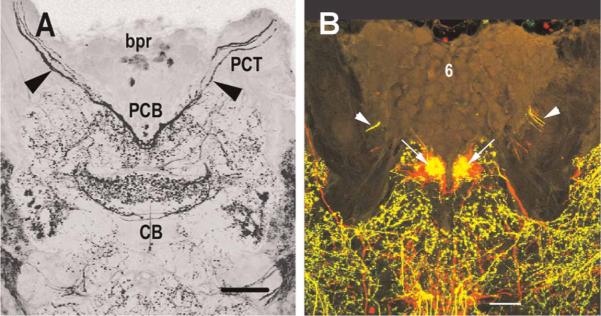
Photoperiodic rhythms require photosensitive detectors for entrainment to environmental light/dark (LD) cycles. Two photoreceptive systems, in addition to the compound eyes, have been identified in crayfish: caudal photoreceptors in the terminal abdominal ganglion (Prosser, 1934; Wilkens & Larimer, 1972) and brain photoreceptors in the supraesophageal ganglion (Bobkova et al., 2003; Sandeman et al., 1990). The brain photoreceptors of the parastacid (southern hemisphere) crayfish Cherax destructor, located in an anterior median brain soma Cluster (Cluster 6; nomenclature according to Sandeman et al., 1992; see Figure 1), can be visualized without staining as they contain dark screening pigment granules (see Figure 2A; Sandeman et al., 1990). This natural pigmentation has enabled the anatomical and physiological characterization of these cells. Individual photoreceptor cells form Clusters around a central rhabdom-like structure in which the cell membranes of the contributing cells are convoluted into interdigitating microvillae identical to those of the retinula cells in the compound eyes (see Figure 2B; Sandeman et al., 1990). Electrical recordings from these cells confirm that they depolarize when exposed to light, and that they respond best to green and blue light but are relatively insensitive to red light (Sandeman et al., 1990). The axons of the brain photoreceptors terminate in and are restricted to two round, finefibered neuropils that lie within the “V” of the protocerebral bridge (see Figure 3A; Sandeman et al., 1990). We refer to these regions as the brain photoreceptor neuropils (BPNs) in this paper. Electron microscope studies have also shown the presence of rhabdomeric structures in Cluster 6 cells in the brains of the astacid (northern hemisphere) crayfish Orconectes limosus and Pacifastacus leniusculus (Bobkova et al., 2003). These cells, however, do not contain the dark screening pigment granules found in C. destructor. While the structure of these cells is entirely consistent with that of the brain photoreceptors of C. destructor (Bobkova et al., 2003), physiological recordings have not yet been made of their responses to light.
FIGURE 1.
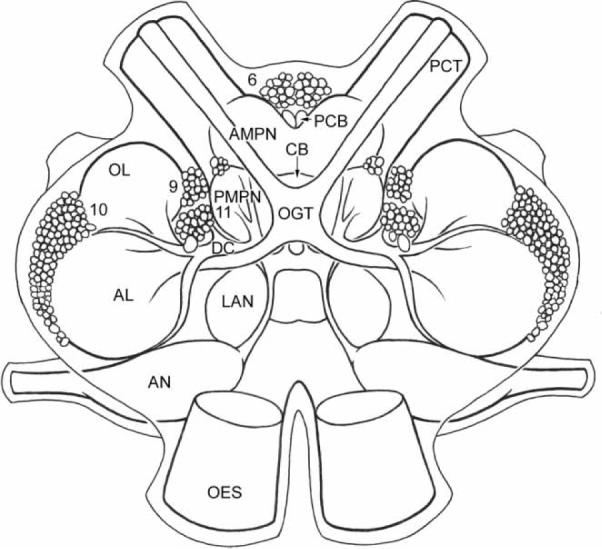
Diagram of the crayfish brain showing the cell body Clusters and neuropil areas relevant to the results presented in this paper. The protocerebral tract connects the median protocerebrum to the lateral protocerebral neuropils (terminal medulla, hemiellipsoid bodies) located in the eyestalk proximal to the optic neuropils (internal medulla, external medulla, and lamina ganglionaris; see Figure 11). Abbreviations: 6, 9, 10, 11 = cell body Clusters; AL = accessory lobe; AMPN = anterior median protocerebral neuropil; AN = antenna 2 neuropil; CB = central body; DC = deutocerebral = commis-sure; LAN = lateral antennular neuropil; OES = esophageal connective; OGT = olfactory globular tract; OL = olfactory lobe; PCB = protocerebral bridge; PCT = protocerebral tract; PMPN = posterior median protocerebral neuropil.
FIGURE 2.
Brain photoreceptors of C.destructor. (A) Light micrograph of an unstained Cluster of photoreceptor cells in Cluster 6. The somata of the three cells contain many dark granules and are fused around a solid central core. (B) Low power electron micrograph shows dense pigment granules in the cytoplasm of the cells and reveals the solid core seen in light micrographs (A) to consist of intertwined microtubules from the individual cells. Scale bars: A, 10 μm. B, 1μm. (Modified from Sandeman et al., 1990).
FIGURE 3.
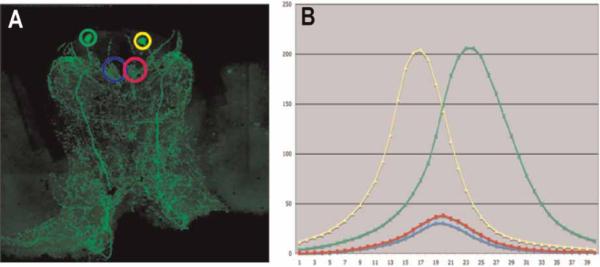
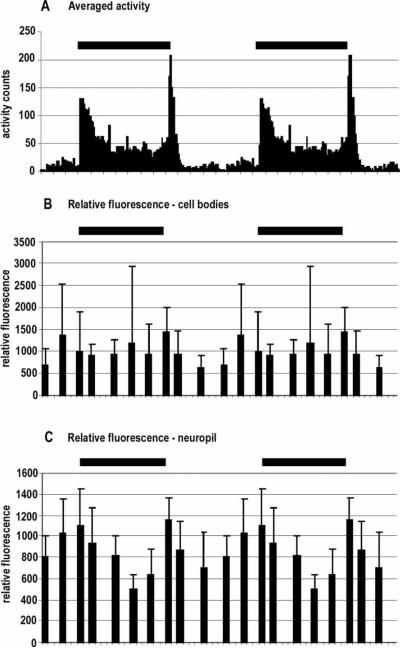
Analysis of the intensity of PDH immunoreactivity. (A) Scan of a whole mount of a C. destructor brain in which large immunoreactive cells in Cluster 6 and the brain photoreceptor neuropils are encircled to show regions of interest (ROI) chosen for analysis. (B) Curves from scanning through the entire depth of each ROI (x-axis) for which the total fluorescence intensity is indicated (y-axis). Colors of the circles around the ROIs are matched with the colors of graphs for that region. Amplitude of the fluorescence intensities for each ROI was calculated from the area beneath the individual curves.
Under standard LD conditions (12 h light phase followed by 12 h dark phase; 12 : 12 LD), astacid crayfish exhibit circadian rhythms of locomotor activity in which activity is largely confined to the D phase (Fernández de Miguel & Aréchiga, 1994; Fuentes-Pardo et al., 2003; Miranda-Anaya, 2004; Page & Larimer, 1972, 1975; Styrishave et al., 2007; Viccon-Pale & Fuentes-Pardo, 1994). This rhythm has been most extensively studied in Procambarus clarkii, a species that exhibits a bimodal locomotory rhythm with peaks of activity around the onset of both the D and L phases (Fernández de Miguel & Aréchiga, 1994; Fuentes-Pardo et al., 2003; Miranda-Anaya, 2004; Page & Larimer, 1972, 1975; Viccon-Pale & Fuentes-Pardo, 1994). Surgical ablation studies in which inputs from the retina and the caudal photoreceptors are removed suggest both that the endogenous oscillator generating circadian locomotor activity rhythms is located within the brain and that extraretinal photoreception plays a role in the entrainment of this oscillator to environmental LD cycles (Page & Larimer, 1972, 1975). Apart from that of Paranephrops zealandicus (Quilter & Williams, 1977), the loco-motor activity patterns of most parastacid crayfish, including C. destructor, remain uncharacterized.
Cryptochrome (CRY), a blue light-absorbing photopigment, is associated with circadian oscillators synchronized to environmental LD cycles in both invertebrates and vertebrates (Hall, 2000; Yu & Hardin, 2006). In Drosophila, this conserved pterin/flavin-containing protein serves dual roles, functioning both as a circadian photoreceptor and transcriptional repressor in the molecular feedback loop of the circadian clock (Dubruille & Emery, 2008). Immunocytochemical studies in P. clarkii have demonstrated the presence of CRY in cells of the terminal medulla in the eyestalk and median protocerebrum in the brain (Escamilla-Chimal & Fanjul-Moles, 2008; Fanjul-Moles et al., 2004), and behavioral studies indicate that locomotory rhythms in these animals can entrain to monochromatic blue light (Miranda-Anaya & Fanjul-Moles, 1997). The abundance of CRY in the median protocerebrum, but not eyestalk, has also been shown to vary in a circadian fashion (Escamilla-Chimal & Fanjul-Moles, 2008; Fanjul-Moles et al., 2004). Together, these results suggest that CRY may represent an important component of crayfish circadian systems.
In addition to the expression of CRY, the median protocerebum of astacid crayfish also contains an extensive network of neurons immuno-reactive to the neuropeptide pigment-dispersing hormone, or PDH (Mangerich & Keller, 1988; Mangerich et al., 1987). PDH is a homologue of pigment-dispersing factor, or PDF, a critical component in the generation and synchronization of circadian rhythmicity in Drosophila (Helfrich-Förster, 1997; Helfrich-Förster & Homberg 1993; Helfrich-Förster et al. 1998; Yoshii et al., 2009) that acts as a modulator between pacemaker neurons and neural circuits controlling behavior and other rhythmic outputs (Helfrich-Förster et al., 2000). In P. clarkii, PDH application in vitro has been shown to induce phase changes in the circadian rhythm of retinal photoreceptor photosensitivity, suggesting that it may play a comparable role in crayfish circadian systems (Verde et al., 2007). Neither the expression of PDH nor the localization of CRY has yet been examined in the brains of parastacid crayfish.
In the present study, we took advantage of the natural pigmentation of the brain photoreceptors of C. destructor to examine the expression of CRY and PDH in these cells and their target neuropils in the median protocerebrum, the BPNs. We also undertook behavioral and surgical ablation studies to examine the influence of extraretinal brain photoreception on locomotor activity rhythms. Immunohistochemical and intracellular labeling revealed that the brain photoreceptors of C. destructor express CRY and that their axons arborize among PDH-immunoreactive neurons in the BPNs. These anatomical studies also identify histamine as a presumptive neurotransmitter of the brain photoreceptors and provide a description of the BPN in an astacid crayfish, P. clarkii. Behavioral studies reveal that the locomotor activity rhythms of C. destructor and P. clarkii differ markedly in several important aspects. Both species, however, are able to entrain their locomotory rhythms to phase shifts in the LD cycle following ablation of both retinal and caudal photoreceptors, leaving only the brain photoreceptors intact. Finally, we provide evidence that expression of PDH in the BPNs of both C. destructor and P. clarkii varies in phase with the animals' locomotor activity rhythm. These studies contribute to our understanding of the control systems underlying rhythmic behaviors in crayfish, in terms of both the photoreceptive systems entraining endogenous clocks to environmental changes in light intensity and the neurotransmitter systems contributing to these pathways.
MATERIAL AND METHODS
Animals
Adult C. destructor (Malacostraca, Decapoda, Parastacidae) were obtained from suppliers in northern New South Wales, Australia, and P. clarkii (Malacostraca, Decapoda, Astacidae) from commercial vendors (Carolina Biological Supply Company, Burlington, North Carolina, USA; Niles Biological, Inc., Sacramento, California, USA). All crayfish were maintained at 20°C in recirculating artificial pond water in the Animal Care Facility at Wellesley College. Light cycles were varied as described below. The experimental protocols conformed to the international ethical standards set out in Portaluppi et al. (2008).
Immunohistochemistry
Target tissues (brain, compound eyes, ventral nerve cords) were dissected from the animals in cold crayfish saline (mM: 205 NaCl; 5.4 KCl; 34.4 CaCl2; 1.2 MgCl2; 2.4 NaHCO3; pH 7.4) and then fixed overnight in 4% paraformaldehyde in 0.1M phosphate buffer (PB) at 4°C for PDH, CRY, and synapsin labeling or 4% N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (Sigma) in PB for histamine (HA) labeling. The tissues were then rinsed with several changes of PB containing 0.3% Triton X-100 (PBTx) for a minimum of 1.5 h. This was followed by the procedures detailed below either on whole mounts or brains sectioned at 100 μm with a vibratome after suspension of the tissue in 7% Noble agar. Primary and secondary antibodies were diluted in PBTx, and all antibody incubations were conducted overnight at 4°C. Primary antibody incubations were followed by a 4 h rinse in PBTx. Secondary antibody incubations were followed by final washing in PB, after which the preparations were mounted on slides in Gelmount (Biømeda, Foster City, California, USA).
The following antibodies were used in these studies:
Mouse anti-synapsin (1 : 50, gift from Dr. E. Buchner, Würzburg, Germany) followed by goat anti-mouse Alexa 488 (1 : 50; Invitrogen, USA) or goat anti-mouse Cy5 (1 : 50, Jackson ImmunoResearch, USA);
Rabbit anti-histamine (1 : 400, Progen Immuno-Diagnostika, Heidelberg, Germany) followed by goat anti-rabbit Alexa 594 (1 : 50, Invitrogen);
Rabbit anti-Drosophila cryptochrome (1 : 100, Alpha Diagnostic International Inc, USA; for characterization, see Escamilla-Chimal & Fanjul-Moles, 2008; Fanjul-Moles et al., 2004), followed by goat anti-rabbit Alexa 594 (1 : 50, Invitrogen); and
Rabbit anti-PDH (1 : 1000, gift of Dr. H. Dircksen, Stockholm, Sweden; for production and characterization, see Dircksen et al., 1987) followed by goat anti-rabbit Alexa 488 (1 : 50, Invitrogen).
Experiments involving double labeling with primary antibodies raised in the same species (HA and PDH) were performed using a modification of the method of Jensen and Norrild (1999), in which 4% paraformaldehyde was substituted for 3% paraformaldehyde and 2% glutaraldehyde. This technique involves fixation of the sections between the staining cycles of the two primary antibodies to inactivate residual binding sites in the tissue-bound antibodies of the first cycle and to fix these antibodies in place, thus preventing cross-labeling during the second cycle.
Intracellular Staining of Individual Brain Photoreceptors
Brains were dissected free in cold crayfish saline and desheathed in the regions surrounding the ventral surface of the median protocerebrum and Cluster 6. Preparations were then viewed using a fixed-stage Nikon compound microscope equipped with Nomarski optics. The morphology of individual brain photoreceptors was examined by intracellular labeling of the cells with Lucifer yellow CH (Sigma). Brain photoreceptors were identified by their dark screening pigment granules and then penetrated in the soma and stained by iontophoretic injection of Lucifer yellow using hyperpolarizing current pulses of up to 6 nA (500 msec in duration, 1 Hz in frequency) for 30–60 mins. After the injection of Lucifer yellow, preparations were fixed overnight in 4% paraformaldehyde and processed for PDH immunolabeling as detailed above.
Confocal Microscopy and Image Processing
Following immunohistochemical processing or intracellular staining, preparations were viewed with a Leica TCS SP laser-scanning confocal microscope equipped with argon, krypton, and helium-neon lasers. Serial optical sections were taken at intervals of 1 μm and saved as both three-dimensional stacks and two-dimensional projections. Subsequently, the specimens were examined with a Nikon Microphot FXA microscope at a series of focal planes with both epifluorescence and transmitted light to confirm the coincidence of fluorescent immunolabeling with the dark screening pigment granules characteristic of the brain photoreceptors.
Activity Measurements
Locomotory activity of individual animals was measured using a radio-telemetry system designed for small mammals (Series ER-4000 Mini-Mitter; Respironics Company, Bend, Oregon, USA) and adapted for use with crayfish. Small transponders (15.5 × 6 mm; 0.52 g in water) were attached to the dorsal medial surface of the cephalothorax of each crayfish with superglue. Each individual crayfish was placed in a tank (30 cm × 60 cm) containing washed gravel and a short length of black plastic pipe as a shelter and filled to a depth of 20 cm with artificial pond water. The Mini-Mitter transducers did not impede the animals in their movement or from entering and leaving their shelters. Damage to, or removal of, the compound eyes will induce molting in crustaceans and hence, in our animals, the shedding of the transponders. In such cases, we waited several days before re-attaching the devices. Therefore, uninterrupted recordings over periods >60 days were seldom achieved.
The eight individual monitoring systems were mounted in a rack in which illumination, controlled by a time switch, was provided by wide spectrum fluorescent lamps (Sylvania 40 watt GRO-Lux), arranged so that all tanks received the same light intensity (135 lux). The rack was contained in a light- and sound-proof room maintained at 18–20°C. The Mini-Mitter transducers provided a continuous, quantitative measure of the animals' activity, summed into 10 min bins, over the duration of each experiment. The telemetry system does not provide information about positional or directional changes so that no distinction is made, for example, between an animal that moves in a tight circle to one moving at the same speed in a straight line, but will discriminate between these if the animals are moving at different speeds, so covering greater distances per unit time. We found that movements <1.5 mm/s were not recorded.
The raw locomotory activity data were first displayed as actograms and then analyzed, following visual inspection, using the software supplied with the Mini-Mitter device (Actiview™ 1.3 Mini-Mitter Co., Bend, Oregon, USA). This provided us with information on the average levels of dark and light activity and dark/light activity ratios. The periods of the activity rhythms in LD and DD were determined using both chi-square periodograms and fast Fourier transform (FFT) analysis. FFT analyses were found useful for determining the presence of diurnal rhythmicity in relatively brief “windows” of activity in experiments where the different experimental conditions followed one another at short intervals. Nevertheless, cognizant of the shortcomings of this method for the accurate determination of diurnal (instead of ultradian) rhythms (Refinetti, 2006), all runs were also analyzed with chi-square periodograms. The results of these analyses—namely, period (τ), average activity in the light (ρ), activity in the dark (α), light/dark activity ratios (ρ /α), and phase shift from the zeitgeber time (T) in constant conditions (Δφ)—are provided in the figure legends.
Photoreceptor Ablations
Compound Eye Retinal Cells
Disablement of the compound eyes can be achieved by complete eyestalk ablation, but this destroys the optic and lateral protocerebral neuropils, and also the X organ-sinus gland complex, an important neuroendocrine system. In order to limit the ablation to the photoreceptors alone, we applied a 1 mm broad thermal nichrome probe to the entire surface of the cornea. The probe was heated to a point where the corneal surface beneath it became opaque without breaking the cuticle.
Caudal Photoreceptors
The ventral nerve cord of crayfish can be visualized through the transparent arthrodial membrane on the ventral side of the abdomen. The caudal photoreceptors were disabled by first making a small transverse incision in this membrane just anterior to the terminal abdominal ganglion. The ventral nerve cord was then severed at this point with a pair of small scissors.
All ablations were carried out rapidly on animals cooled to immobility in ice. None of the ablated animals died as a direct result of the operations. The completeness of the ablations was tested both behaviorally and anatomically. After the ablations, but before locomotor activity recordings, the animals were tested for their response to sudden changes in light intensity by rapidly covering or uncovering them with a black card. Animals with only a few square millimeters of intact retina will respond to such stimuli with abrupt movements of the chelipeds, limbs, or antennae. Complete section of the ventral nerve cord anterior to the terminal abdominal ganglion resulted in the animals no longer responding to touching the telson with a soft paintbrush, although local reflexive retractions of the uropods often occurred. Touching the dorsal surface of the abdomen of these animals produced an immediate and vigorous avoidance response. At the end of each experiment, the behavioral responses of the animals were again tested, after which they were sacrificed and the ventral nerve cord examined to ensure that reconnection to the terminal abdominal ganglion had not occurred. Sections of the compound eyes and optic ganglia were processed with an antibody to synapsin, as described above. Individuals that responded positively to behavioral tests, or that showed some evidence of retinal regeneration, were excluded from the study.
Measurement of PDH Expression
To test the effects of diurnal light cues on PDH levels, animals were kept in 12:12 LD conditions for at least five days and then sacrificed at 3:00, 5:30, 6:30, 9:30, 12:30, 15:00, 17:30, 18:30, 21:30, and 24:00 h over the course of one 24 h period. Lights-on occurred at 06:00 h and lights-off at 18:00 h. The fixed brains were immunolabeled for PDH, as described above, and the level of PDH expression in a pair of cell somata in Cluster 6 and the brain photoreceptor neuropils assessed using a semi-quantitative method described by Benton et al. (2007). This method relies on the probability that the intensity of fluorescence emitted from a defined region of interest (ROI), labeled with an antibody carrying fluorophores and recorded by a confocal microscope, will be proportional to the amount of epitope present in that region. While this does not allow quantification, it does provide a measure of the relative amounts of labeling for an epitope present in different ROIs. Comparison of different ROIs required that the output of the irradiating laser be calibrated with a power meter designed for this purpose (Fieldmaster™, Coherent, California, USA) and that the laser output and the sensitivity of the confocal recording system remained at the same levels during the imaging for all preparations.
Each brain was scanned without averaging in 1 μm increments with a confocal microscope (Leica TCS SP), through the depth of the Cluster 6 cell somata and brain photoreceptor neuropils, and the individual images of the stacks saved. Using the Leica confocal analysis software (Leica Systems™), a ROI was defined for the Cluster 6 cell bodies and the BPNs, based on the standard size necessary to encompass these regions (see Figure 3A). These ROIs remained the same size within each brain region for all preparations. The analysis software provided a measure of the level of brightness, and hence PDH immunoreactivity, for each ROI (see Figure 3B). These values were used to compare PDH expression between the brains of different individuals. Differences in PDH immunolabeling were analyzed for statistical significance with one-way ANOVA and Tukey multiple comparisons analyses using SPSS software.
RESULTS
The primary goal of this study was to examine the role of the crustacean brain photoreceptors in the control of circadian locomotor activity rhythms. In pursuing this aim, we have ablated the other known photoreceptive systems of the crayfish (i.e., compound eyes and caudal photoreceptors) and examined the effects on photoentrainment of their locomotory rhythms. In addition, we have also examined the distribution of immunoreactivity to CRY, HA, and PDH in the brain photoreceptors, BPN, and other brain regions, because these molecules have all been closely linked to the control of circadian activity rhythms in both crustaceans and insects (Fanjul-Moles et al., 2004; Hardin, 2005; Helfrich-Förster, 2005; Shafer et al., 2006).
Distribution of Cryptochrome (CRY), Histamine (HA), and Pigment Dispersing Hormone (PDH) in the Protocerebrum
The brain photoreceptors in C. destructor can be visualized without labeling, because they contain dark screening pigment granules (see Figure 2) and are located in an anterior median Cluster of cell somata (Cluster 6; see Figure 1). In the present study, we used this feature to unequivocally identify these photoreceptors to further characterize the cells and the neuropil region to which they project (the BPN), and to explore their relationship to presumptive circadian systems. Clusters of darkly pigmented cells are also occasionally found in Cluster 6 of juvenile P. clarkii brains, and these have, in the light microscope, a similar anatomy to those found in C. destructor, leading to the conclusion that functional brain photoreceptors are also present in this species. As the brain photoreceptors of adult P. clarkii seldom express dark screening pigments, we were unable to perform anatomical studies on these cells comparable to those for C. destructor. We were able, however, to provide a description of the brain photoreceptor neuropil in this species.
Brain Photoreceptors and the BPN
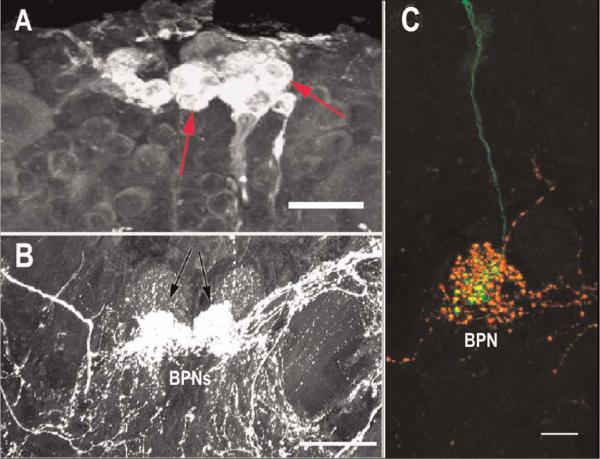
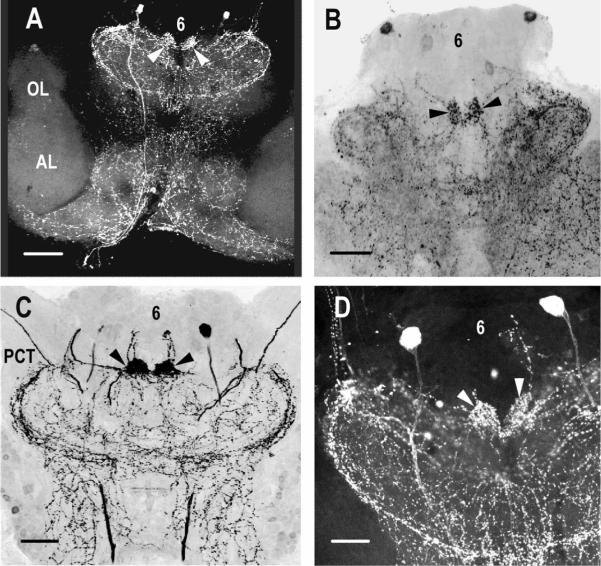
Immunohistochemical labeling in C. destructor revealed the expression of CRY in brain photoreceptor somata in Cluster 6 and in their axons projecting to the BPNs (see Figure 4A). The brain photoreceptor neuropil is also extensively innervated by neurons intensely immunoreactive to PDH (see Figure 4b); intracellular labeling of individual brain photoreceptors indicated that they arborize within the brain photoreceptor neuropil in close proximity to the PDH-immunoreactive neurons (see Figure 4C). Immunolabeling of the brain of C. destructor for HA demonstrated that the brain photoreceptors are also immunoreactive for this biogenic amine (see Figure 5A). Double labeling for HA and PDH revealed extensive HA immunoreactivity in the BPNs, much of which could be traced to the brain photoreceptors (see Figure 5B). Histamine has been identified as a transmitter in insects and crustaceans (Beltz, 1999; Nässel, 1999) and specifically as a transmitter in many photoreceptor systems (Stuart, 1999; Stuart et al., 2007); it appears that the brain photoreceptors of C. destructor also employ this amine for signal transmission with central brain pathways. An attempt to double label for HA and CRY was not undertaken, but given the positive identification in each case of the brain photoreceptors (with dark pigment granules), we are confident that HA and CRY are co-localized in these cells.
FIGURE 4.
Immunoreactivity to CRY and PDH in the protocerebrum of C. destructor. (A) Photo-receptor cells along the anterior edge of Cluster 6 that label intensely with antibodies to CRY (red arrows), project to and terminate in the brain photoreceptor neuropils (BPNs), and (B) that lie close to the protocerebral bridge and are immunoreactive to PDH (black arrows). (C) Intracellular fill of a brain photoreceptor with Lucifer yellow (green fiber) showing that the cell arborises within the BPN in close proximity to PDH-immunoreactive neurons (red). Scale bars: A, 50 μm; B, 100 μm; C, 20 μm.
FIGURE 5.
Histamine (HA) immunoreactivity in the brain of C. destructor. (A) Brain photoreceptors (bpr) along the anterior edge of the median protocerebrum labeled with antibodies to HA. HA immunoreactivity is also intense in axons (arrowheads) in the protocerebral tract (PCT) that cross the brain close to the protocerebral bridge (PCB). Broad and narrow transverse bands in the central body (CB) label strongly for HA. (B) C. destructor brain labeled with antibodies to PDH (red) and HA (green). Preparation shows histamine is concentrated within the brain photoreceptor neuropils (BPN, arrows) and that both PDH- and HA-immunoreactive fibers mingle over a large area of the anterior median protocerebrum. Cluster 6 cell bodies in this image were artificially brightened and not labeled with either antibody. Short lengths of the red and yellow labeled primary neurites (arrowheads) extend from labeled Cluster 6 cell bodies, which, like those of the brain photoreceptors, are not in the plane of this section. Scale bars: A, 80 μm; B, 100 μm.
PDH-Immunoreactive Neurons in the Protocerebrum and Optic Neuropils
PDH-immunoreactive neurons exhibited a similar distribution in the anterior median protocerebrum of C. destructor and P. clarkii (see Figure 6). An overview of PDH labeling in a whole mount of the brain of C. destructor shows labeled fiber tracts encircling the anterior median protocerebrum that form part of a complex network associated with broad areas of this region (see Figure 6A). Fibers that extend into the median protocerebrum from the protocerebral tract arborize in the center of this encircled region. The most intense PDH labeling is found in the brain photoreceptor neuropils (see Figure 6, arrowheads). Some PDH-labeled fibers extend rostrally into Cluster 6 from this small neuropil and also laterally and caudally into areas of the anterior median protocerebral neuropil. Labeling is scattered over the lateral antennular neuropils and the antenna 2 neuropils of the tritocerebrum (see Figure 6A).
FIGURE 6.
PDH immunoreactivity in the brains of C. destructor (A,C,D) and P. clarkii (B). (A & B) Low power images of whole mounts of C. destructor (A) and P. clarkii (B) brains show the characteristic distribution of PDH-labeled fibers over the anterior regions of the median protocerebrum but not into the olfactory (OL) or accessory (AL) lobes. The large paired, labeled cell bodies in Cluster 6 (6) analyzed for the intensity of PDH labeling can be seen in both C. destructor (A) and P. clarkii (B), with those of P. clarkii lying closer to the anterior margin of the cell Cluster than those of C. destructor. The fiber extending from one of these cell bodies, posteriorly through the brain and into the circumesophageal connectives, can be seen on the left side in the C. destructor preparation (A). (C) In C. destructor, large axons extending along the protocerebral tract (PCT) are also labeled with antibodies to PDH. The brain photoreceptor neuropils (BPNs), in which the brain photoreceptors terminate, label intensely in both species (arrowheads in (A–D)). (D) Large PDH-immunoreactive cells in C. destructor branch extensively through the anterior regions of the median protocerebrum. Scale bars: A, 100 μm; B & C, 150 μm; D, 200 μm.
Two large cell bodies in Cluster 6 in both C. destructor and P. clarkii label consistently with the PDH antibody, revealing details of their morphology. These cells branch extensively in lateral regions of the anterior median protocerebrum, and some fibers may extend to the brain photoreceptor neuropils (see Figure 6). Their large axons, one on each side of the brain, extend caudally and leave the brain through the circumesophageal connectives (see Figure 6A). Their final destinations have not been determined in C. destructor, but neurons with very similar morphology have been described in the astacid crayfish Orconectes limosus (Mangerich & Keller, 1988), and axons of these neurons pass down the circumesophageal connectives, branching in each segmental ganglion in the thorax and continuing into the abdominal nerve cord. Apart from these two large and intensely labeled cell bodies, labeling in cell somata was rarely seen in the brain, though faint labeling of an additional four neuronal somata was occasionally observed in Cluster 6 of P. clarkii (data not shown). This would suggest that many of the PDH-immunoreactive fibers innervating the neuropils of the proto-, deuto-, and tritocerebrum are from cell somata located in the lateral protocerebrum or optic ganglia in the eyestalks, or from ascending pathways from the ventral nerve cord.
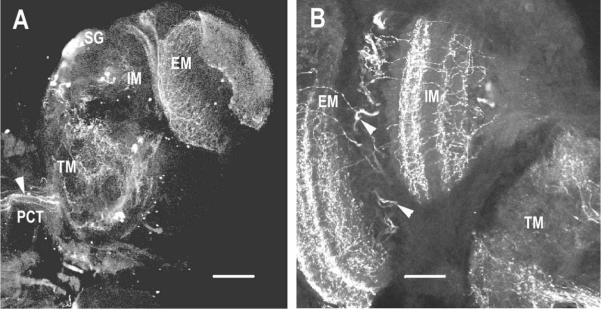
Immunolabeling for PDH in the lateral protocerebral and optic neuropils in the eyestalks of C. destructor reveals all elements described previously in the eyestalks of O. limosus (Mangerich & Keller, 1988), the crabs Carcinus maenas and Cancer productus (Hsu et al., 2008; Mangerich et al., 1987), and 80% embryonic lobsters (Homarus americanus; Harzsch et al., 2009). In whole mounts of the eyestalk neuropils of C. destructor, large neurites projecting from the terminal medulla extend up over the internal medulla to end in the external medulla, close to where tangential fibers running between the internal medulla and external medulla end in a glomerular-like complex in the sinus gland (see Figure 7A). Clusters of labeled cell bodies associated with the terminal medulla are located between the terminal medulla and internal medulla (see Figure 7A). Sections of eyestalk neuropils show that PDH-immunoreactive fibers are distributed throughout the terminal medulla and in two tangential layers in the internal medulla (see Figure 7B). Fibers cross the region between the internal and external medulla. The external medulla contains three tangential layers (see Figure 7B).
FIGURE 7.
PDH immunoreactivity in sections through the optic neuropils and lateral protocerebral neuropils in C. destructor. (A) Large axons (arrowhead) in the protocerebral tract (PCT) innervate the terminal medulla (TM), internal medulla (IM), and external medulla (EM). Tangential fibers cross the internal edge of the external medulla to end in the region of the sinus gland (SG). (B) Section through the eyestalk shows the tangential layers of labeled fibers in the IM and EM and fibers that cross between these two neuropils (arrowheads). Scale bars: A, 250 μm; B, 100 μm.
Locomotory Activity Rhythms of C. Destructor and P. Clarkii
Locomotory activity is a measure commonly used to detect the presence of circadian rhythms entrained by external signals and to test if these rhythms are maintained by endogenous oscillators in the absence of external zeitgebers. While the circadian locomotor rhythms of P. clarkii and other astacid crayfish have been examined in several studies, circadian rhythmicity has not yet been studied in detail in parastacid crayfish, such as C. destructor.
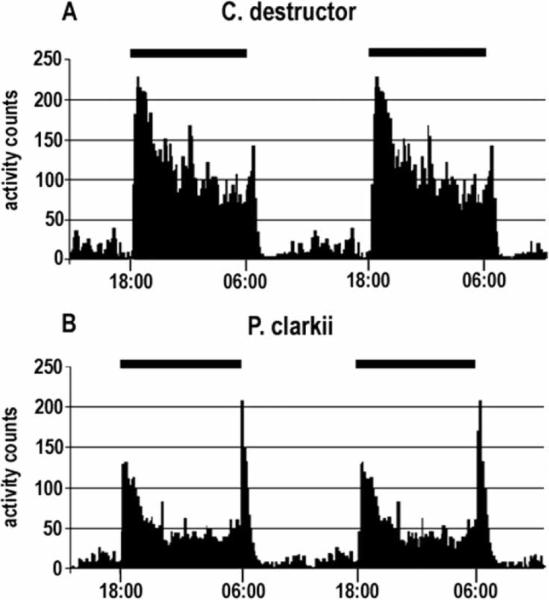
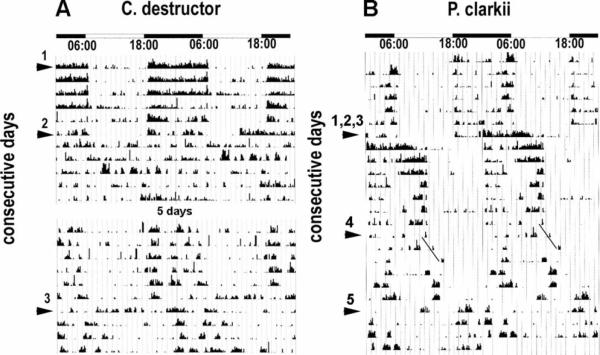
Locomotor activity rhythms of C. destructor and P. clarkii were found to differ significantly in several respects (see Figures 8 and 9). Under normal LD conditions, C. destructor responds, after a delay of up to 30 min, with a burst of activity at lights-off, after which activity is reduced but often continuous throughout the dark period. At lights-on, a small increase in locomotory activity over a short period is then reduced over the next 30 min to almost complete rest, after which activity increases again but to a level that is significantly lower than that maintained in the dark (see Figure 8A). Individuals vary considerably in terms of their level of activity, but all conform to the same general pattern. There is no evidence that C. destructor exhibits any anticipation of the approaching lights-off or lights-on, even after several weeks of stable LD conditions. The activity of most animals placed in DD became arrhythmic (see Figure 9A). In some animals, an initial resumption of activity at dark levels was observed after about 4 h, which was then maintained for up to 12 h; during the next three to five days, however, activity became sporadic and not related to the previous LD cycle. Animals exposed to LL conditions also became arrhythmic. Animals returned to LD after constant conditions, or subjected to a 6 h phase shift of the LD cycle, immediately adjusted their activity to the prevailing conditions, with tight synchrony between activity and periods of light and dark (data not shown).
FIGURE 8.
Comparison of the LD activity patterns in individual crayfish (C. destructor and P. clarkii), averaged over a period of 11 days. (The daily activity of these animals is shown in Figure 9). The average activity over a single 24 h period is shown twice here so the complete LD periods can be more easily seen. The delayed and abrupt onset and termination of C. destructor activity, and the absence of any anticipation of the onset of change in the illumination (A), contrast with that of P. clarkii, in which activity increases immediately at lights-off, decreases to a plateau overnight, and then clearly increases again several hours before lights-on. Lights-off: 18 : 00 h; lights-on: 06 : 00 h.
FIGURE 9.
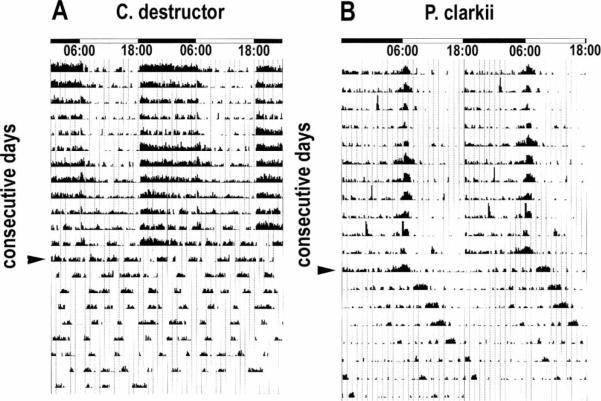
Comparison between LD and DD locomotory rhythms of intact C. destructor and P. clarkii. Actograms in this and Figure 10 show the recorded locomotory activity of individual animals. Each horizontal line corresponds to two consecutive LD cycles (indicated by bars at top of figure) in which the second LD period is repeated and appears first in the next line (i.e., line 1: day 1, day 2; line 2: day 2, day 3, and so on). Each vertical line on the recordings represents a measure of the animals' activity over a 10 min period. The counts have been normalized for each individual, and the highest count represents the full scale on the vertical axis. (A) Actogram of C. destructor over a period of 21 days. Animals were subjected to 12 : 12 LD for 12 days and then to DD (arrowhead) for nine days. Activity in LD increases abruptly ~20 min after the onset of darkness and stops soon after the onset of light. Activity during the day is significantly less. Robust locomotor activity rhythms were not observed in any animals (n>32) in DD. (B) Subjecting P. clarkii to the same regime shows this animal is also largely night active but has a very different activity profile, with less activity at the onset of darkness and a peak of activity that begins several hours before dawn that is maintained for about 1 h after the light onset. The entire rhythm is maintained in DD (arrowhead) but with the onset of activity delayed each day. In contrast to C. destructor, therefore, P. clarkii exhibits a robust, endogenously driven locomotory rhythm, with a 26 h cycle time. ((A) C. destructor LD: τ = 24.03 h; ρ = 58.1; α = 168, ρ/α = 0.35. DD: τ>30 h; ρ = 37.1; α = 35.3, ρ/α = 1.05; (B) P. clarkii LD: τ = 24.0 h; ρ = 43.5; α = 86.1, ρ/α = 0.50 DD: τ = 26.25 h; Δφ = −22.25 h; ρ = 39.3; α = 77.2, ρ/α = 0.51)
The C. destructor animals used in these experiments all originated in eastern Australia. The initial measurements were made on animals that had been in the laboratory and subjected to American Eastern Standard Time (EST; UTC-5) for several months. With a new shipment of animals that arrived in the United States during this study, we were able to explore the possibility that animals being flown from summer in the southern hemisphere to winter in the northern hemisphere may show some residual effects of photoperiod/or jet lag. In transit, the animals were housed in lightproof containers for two days. Immediately upon arrival, eight animals were equipped with transponders, placed in the Mini-Mitter setup, and provided with a 12 : 12 LD cycle equivalent to Australian EST (UTC + 10) from which they had come. After ten days in this LD cycle, they were submitted to DD (six days), then back to LD (six days), and finally into LD but shifted to American EST. The animals changed their activity patterns immediately in each of these situations, with no sign of a slow adjustment to the new light conditions (data not shown).
P. clarkii exposed to LD conditions are, like C. destructor, more active in the dark than light but exhibit two distinct peaks of activity. There is an abrupt increase in activity at lights-off, and the level of activity slowly declines overnight. Activity then increases in intensity before lights-on, providing an indication that the animals are anticipating the approaching lights-on. Lights-on is accompanied by a further increase in activity, which gradually decreases over the next 1–3 h (see Figure 8B). Placing P. clarkii in DD conditions results in a continuation of the lights-off and lights-on activity peaks, but with an increasing delay and with a period of 26 h (see Figure 9B). Under LL conditions, these animals become arrhythmic (data not shown). The endogenous oscillator in P. clarkii appears, therefore, to signal both the lights-off and lights-on activity peaks, although activity never anticipates the onset of darkness in LD conditions. In DD conditions, activity during the original dawn period persists and at almost the same level as in LD, suggesting light, itself, is not the dominant factor maintaining the activity.
Contribution of Photoreceptive Systems to Entrainment of Activity Rhythms
Experiments designed to test the influence of the three photoreceptive systems (i.e., caudal photoreceptors, compound eyes, and brain photo-receptors) on locomotory activity in both C. destructor and P. clarkii were conducted on animals that were initially subjected to a standard 12 : 12 LD cycle. The inputs from the caudal photoreceptors were then interrupted by sectioning the ventral nerve cord just anterior to the terminal abdominal ganglion, and the animals' responses monitored. The cornea and retina of both compound eyes were then ablated, leaving only the brain photoreceptors intact; animals were again placed in LD conditions and their responses monitored. The LD cycle was then advanced or delayed by 6 h and the locomotory activity monitored over several days to determine if the brain photoreceptors alone were able to entrain the locomotory rhythm. Finally, the animals were subjected to DD for several days and then returned to the original LD cycle.
Interruption of the input from the caudal photoreceptors had little quantifiable effect on the locomotory rhythms of either species in a 12 : 12 LD cycle (see Figures 10A and 10B). Subsequent ablation of the cornea and underlying retinal cells, after disabling the caudal photo-receptors, was followed by an increase in activity in both the light and dark spans, but a resumption of the normal LD response after two to three days in P. clarkii or up to ten days in C. destructor. Thereafter, both species adjusted immediately to a 6 h phase shift in the LD cycle, suggesting the brain photoreceptors are fully capable of responding to changes in light and entraining the locomotory rhythm. Interestingly, the characteristic forms of the activity cycles seen in intact individuals of each species were also retained. Subjecting the animals to DD produced the same result as in the intact animals: C. destructor became arrhythmic, whereas P. clarkii maintained a weak, but bimodal, locomotory rhythm with a free-running period of 25–26 h (see Figure 10B).
FIGURE 10.
Actograms from C. destructor and P. clarkii showing the effects of ablations of the caudal and compound eye photoreceptors in animals in which the brain photoreceptors remained intact. (A) C. destructor without caudal photoreceptors (1) responded to LD with distinct locomotory rhythms, but after retinal ablation (2), they became arrhythmic for a period of up to ten days, after which they again responded to LD and followed a phase shift (3) in which the onset of the dark period was delayed by 6 h. (B) P. clarkii in which both caudal and retinal photoreceptors were removed and the animals simultaneously subjected to a phase shift in which the dark period was delayed by 6 h (1–3). These animals immediately shifted their activity rhythm to accommodate the phase delay. After seven days in the new LD rhythm, the animals were placed in DD (4) and continued to exhibit rhythmic activity, although weaker than in LD. The burst of activity at lights-on persisted for three days, and a line joining these common events was calculated to represent a period of 25.7 h. Returning the animals to an LD regime with the original timing (5) restored their original activity. ((A) C. destructor LD1: τ = 24.01 h; ρ = 14.6; α = 95.5, ρ/α = 0.15. LD2, after ten days: τ 24.02 h; ρ = 14.5; α = 117.8, ρ/α = LD3, τ = 24.09 h; ρ = 19.8; α = 66.7, ρ/α = 0.3; (B) P. clarkii: LD: τ = 24.02 h; ρ = 8.3; α = 43.3, ρ/α = 0.19. LD1,2,3: τ = 24.11 h; ρ = 10.6; α = 65.2; ρ/α = 0.16. LD4: τ = 25.7 h; Δφ = −1.7 h; ρ = 7.3; α = 12.3, ρ/α = 0.59. LD5: τ = 24.19 h; ρ = 13.4; α = 51.7, ρ/α = 0.26).
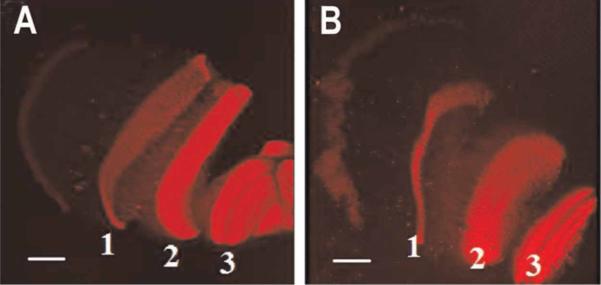
At the termination of the experiments, the animals that had been subjected to corneal and retinal ablation were first tested behaviorally for their responses to a shadow, then sacrificed, and the eyestalks sectioned and stained. The degree of damage to the retina and integrity of the optic neuropils were then examined (see Figure 11). Antibodies to the synaptic protein synapsin labeled the lamina ganglionaris (the site of the first synaptic exchange between the photoreceptors and second-order neurons), the internal medulla, and external medulla, indicating that these areas were not damaged by the ablation of the cornea and retina (see Figure 11B). Touching the telson of animals with their ventral nerve cords sectioned did not produce the normal startle response of the intact animals, and examination of the ventral nerve cords revealed that no reconnection had taken place between the terminal abdominal ganglion and the intact portion of the ventral nerve cord.
FIGURE 11.
Histology of optic neuropils following retinal cautery. Immunoreactive labeling for synapsin in a normal intact eye (A) and one that had been subjected to retinal destruction (B) shows synapsin labeling typically found in the lamina ganglionaris (1), external medulla (2), and internal medulla (3) of the untreated eye is also found in the treated eye, suggesting that despite the disruption of the retina, the downstream neuropil layers remain intact. Scale bars: 100 μm.
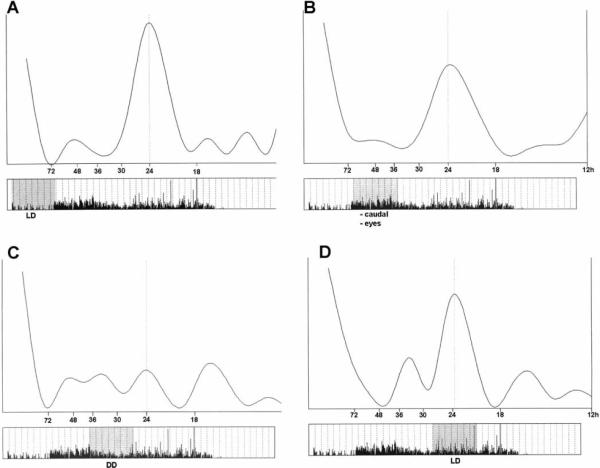
In a final experiment, the caudal photoreceptors and entire eyestalks of P. clarkii were ablated after the initial LD entrainment, and the animals' responses to LD, DD, and again LD were recorded. Those animals that had exhibited a robust response before the ablations resumed their responses to lights-off and lights-on after two to three days of increased and sometimes continuous activity. Exposure to DD, however, no longer produced a convincing free-running locomotory rhythm, and instead the activity became arrhythmic and continuous. When restored to LD, the activity was again strongly modulated (see Figure 12).
FIGURE 12.
Locomotory activity in P. clarkii following ablation of the caudal photoreceptors and entire removal of the eyestalks. Graphs show fast Fourier transform (FFT) analysis of sections of the activity (shaded portions of the actograms). The FFT analyses provide a measure of the relative amplitudes of periodic rhythms between 12 and 100 h. (A) In LD before ablation. (B) In LD after ablation of the caudal photoreceptors and eyestalks. (C) In DD. (D) After return to LD. The strong 24 h rhythm in A is attenuated but still dominant after ablations (B), virtually absent in constant darkness (C), restored with return to LD (D). ((A) τ = 24.01 h; ρ = 11.4; α 32; ρ/α 0.35. (B) τ 23.51 h; ρ 79.9; α = 139.7, ρ/α = 0.57. (C) τ>30 h; ρ=53.6; α = 76.7, ρ/α = 0.7. (D) τ = 23.57 h; ρ = 45.6; α = 62.2, ρ/α = 0.73).
We conclude from these ablation experiments that the brain photo-receptors of both P. clarkii and C. destructor are able to synchronize locomo-tory rhythms to environmental LD cycles. Nevertheless, it is apparent that some eyestalk neuropils in the eyestalks of P. clarkii, possibly including the X organ-sinus gland complex, need to be intact for the endogenously driven locomotory rhythm of P. clarkii to be manifested.
PDH Cycling
In P. clarkii that have only brain photoreceptors intact, the maintenance and entrainment of photoperiodic locomotory rhythms suggests that these cells contribute to the synchronization of behavioral rhythms and that the BPNs represent integral neuropils in the circadian systems of these animals. Given the intense PDH immunolabeling in the BPN and the close association of its homologue PDF with circadian locomotory activity in Drosophila (Hardin, 2005; Helfrich-Förster, 2005; Yoshii et al., 2009), we asked whether the expression of PDH in the BPN and the somata of two large interneurons that may innervate this neuropil bear any relationship to the locomotor activity rhythms (see Figures 13 and 14).
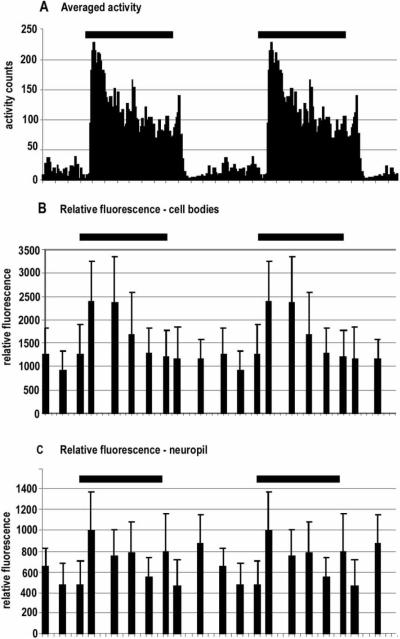
FIGURE 13.
PDH expression and locomotion in C. destructor. (A) Locomotory activity averaged over 11 days (from Figure 8A), compared with the expression of PDH, measured as relative fluorescence intensity, in Cluster 6 cell bodies (B) and brain photoreceptor neuropils (C) in the brains of C. destructor during a single LD period. PDH expression in the cell bodies follows a profile that closely reflects the locomotory behavior, while measurement of PDH immunoreactivity in the brain photoreceptor neuropils is less regular, although it does increase within the first third of the dark period. Changes in the levels of PDH expression do not anticipate the onset of either the dark or light periods in C. destructor. The data in A, B, and C are from a single LD cycle, but shown twice. Error bars in B and C represent standard deviations of the mean. (one-way ANOVA: between groups, cell bodies p = 0.004, F = 3.246; neuropils p = 0.001, F = 3.838. Tukey multiple comparisons: peak to trough, cell p = 0.002; neuropils p = 0.02).
FIGURE 14.
PDH expression and locomotion in P. clarkii. (A) Locomotory activity averaged over 11 days (from Figure 8B), compared with the expression of PDH, measured as relative fluorescence intensity, in Cluster 6 cell bodies (B) and brain photoreceptor neuropils (C) in brains of P. clarkii during a single LD period. Levels of PDH immunoreactivity in brain photoreceptor neuropils follow a profile that approximates activity, including an apparent anticipatory increase in expression before the onset of both the dark and light periods. No significant differences are seen in levels of PDH in the cell bodies at different times of the day. The data in A, B, and C are from a single LD cycle, but shown twice. Error bars in B and C represent standard deviations of the mean. (one-way ANOVA: between groups, cell bodies p = 0.474, F = 0.986; neuropils p 0.001, F = 11.422. Tukey multiple comparisons: peak to trough, cell bodies, no significant differences; neuropils p<0.001).
C. destructor
The intensity of PDH immunoreactivity in the large cell somata in Cluster 6 is relatively constant during the light period and then increases almost two-fold after lights-off (see Figure 13B). PDH expression in these cells decreases gradually over the next 7–8 h to a level that is not significantly different from that of the daytime level. There is no increase in PDH expression at lights-on. The profile of expression closely matches dynamic changes in the locomotory pattern but not the static, basal activity levels in the light and dark: lights-off is followed by a burst of locomotory activity as well as a sudden increase in PDH expression; at lights-on, locomotory activity decreases after a small transient increase, whereas there is no change in the intensity of PDH immunoreactivity (see Figures 13A and 13B). Expression of PDH in the brain photoreceptor neuropils also increases at lights-off and declines over the dark period, and it is highly variable during the light period. Although this pattern shares some elements with that of the large interneurons in Cluster 6, rhythmic changes in the intensity of PDH labeling in these neuropils are not as pronounced (see Figure 13C).
P. clarkii
PDH expression in P. clarkii also follows a cycle that matches the locomotory activity rhythm. Unlike in C. destructor, however, PDH levels in the brain photoreceptor neuropils more closely resemble the locomotor activity pattern than do levels in the Cluster 6 interneurons (see Figure 14), which do not show a statistically significant rhythm (see below). It is of particular interest that changes in the intensity of PDH immunoreactivity in P. clarkii precede both lights-off and lights-on. A large increase in PDH expression in the brain photoreceptor neuropils precedes dawn and decreases over the lights-on period, matching the temporal pattern of locomotory behavior.
Estimation of the abundance of PDH by measuring relative levels of fluorescence after immunocytochemical labeling is semi-quantitative, and the potential for variability is high; in addition, there is inherent and characteristic variability in locomotory activity and behavior between individual crayfish. To address this problem, we used a relatively large number of animals for the PDH studies (C. destructor, n = 45; P. clarkii, n = 45), each of which gave us two measurements (left and right hemibrains). In spite of the large variability, one-way ANOVA revealed differences between timepoints in C. destructor for both the BPN (p < 0.001, F = 3.838) and Cluster 6 cell bodies (p < 0.004, F = 3.246). Tukey multiple comparisons showed statistically significant differences in C. destructor between peaks and troughs for both the BPN (p < 0.02) and Cluster 6 cell bodies (p < 0.01). One-way ANOVA treatment of the P. clarkii data revealed significant differences between timepoints for the BPN (p < 0.001) but not for the Cluster 6 cell bodies (p = 0.474, F = 0.986). Tukey multiple comparisons showed statistically significant differences in P. clarkii between peak and trough values for the BPN (p < 0.001, F = 11.422), but no significant differences between any of the Cluster 6 cell body measurements.
DISCUSSION
Extraretinal photoreceptors are found in the brains and optic lobes of a variety of invertebrate taxa and are thought to have a number of functions, including entrainment of circadian rhythms (Numata et al., 1997; Page, 1982). In decapod crustaceans, extraretinal brain photoreceptors have only been described in freshwater crayfish (Bobkova et al., 2003; Sandeman et al., 1990). Among those crayfish species thus far examined, the brain photoreceptors of Cherax sp. are unique in their expression of dark screening pigment granules. In the present study, we utilized this endogenous pigmentation to examine the neurochemistry of the brain photoreceptors of C. destructor and that of their target neuropil, the BPN. Immunohistochemical and intracellular labeling revealed that the brain photoreceptors express the neurotransmitter histamine and the circadian photopigment CRY, and also that they arborize in the BPN in close proximity to PDH-immunoreactive interneurons. PDH levels in the BPN, in both C. destructor and P. clarkii, were found to vary in phase with the diurnal locomotory rhythms characteristic of each species. Surgical ablation studies suggest the brain photoreceptors in both species play a role in the synchronization of these locomotory rhythms to the day/night cycle.
The intense histamine immunoreactivity observed within the axons and terminals of the brain photoreceptors in C. destructor suggests that, like most retinal and ocellar photoreceptors in adult arthropods (Nässel, 1999; Stuart, 1999; Stuart et al., 2007), these cells utilize histamine for signaling at their central synapses. Similarly, the extraretinal eyelets of insects, small Clusters of rhodopsin-expressing photoreceptors located in the optic lobes, are histaminergic (Hamasaka & Nässel, 2006; Nässel et al., 1988; Pollack & Hofbauer, 1991). Photoreceptors in the most extensively characterized of the insect eyelets, the Hofbauer-Buchner eyelet (H-B eyelet) of Drosophila, have also been shown to express choline acetyltransferase (Yasuyama & Meinertzhagen, 1999), suggesting they also use acetylcholine as a neurotransmitter. Further studies of the brain photoreceptors of C. destructor will therefore be required to determine whether histamine represents the principal neurotransmitter of these cells or whether they employ multiple neuroactive substances.
Unlike the H-B eyelet photoreceptors, which do not appear to express CRY (Yoshii et al., 2008), the brain photoreceptors of C. destructor exhibit strong CRY immunoreactivity. CRY proteins are core components of the circadian oscillators of both plants and animals (Cashmore, 2003; Dardente & Cermakian, 2007). Molecular evolutionary studies in insects indicate that cry gene expression varies between taxa (Rubin et al., 2006; Zhu et al., 2005). Drosophila has one CRY (dCRY) with dual circadian functions. In the brain, dCRY is expressed in circadian pacemaker neurons (Benito et al., 2008; Yoshii et al., 2008) and plays a role in photoentrainment (Emery et al., 1998, 2000; Stanewsky et al., 1998). In some peripheral tissues, however, dCRY also functions as a transcriptional repressor within the circadian oscillator (Collins et al., 2006). In contrast, the butterfly (Danaus plexippus) and mosquito (Anopheles gambiae) express two CRY proteins: the photosensitive CRY1 and the light insensitive CRY2 that exhibits transcriptional repressive activity (Zhu et al., 2005). A third mode of cry gene expression occurs in bees (Apis mellifera, Bombus impatiens) and the red flour beetle (Tribolium castaneum) that express only CRY2 (Rubin et al., 2006; Yuan et al., 2007). Little is known about the structure or function of the CRYs of freshwater crayfish (Escamilla-Chimal & Fanjul-Moles, 2008; Fanjul-Moles et al., 2004). The conserved circadian function of CRY proteins across the metazoa, however, suggests brain photoreceptors may have multiple, overlapping functions as both input and core-pacemaker components in the central circadian system.
Recent behavioral studies in Drosophila mutants have provided evidence that, in addition to its roles in circadian control circuits, CRY can also mediate light-dependent magnetoreception (Gegear et al., 2008). While there is no evidence that freshwater crayfish are able to sense magnetic fields, it has been proposed that spiny lobsters (Panulirus argus) navigate using cues from the Earth's magnetic field (Boles & Lohmann, 2003). It is possible, therefore, that CRY-expressing brain photoreceptor cells in C. destructor could have functions beyond the photic regulation of locomotory behavior.
Intracellular dye fills of the brain photoreceptors have demonstrated that the axons of these cells terminate in and are restricted to a single protocerebral neuropil, the BPN (see Figure 4C; Sandeman et al., 1990). Within the BPN, the photoreceptors arborize among the neurites of neurons exhibiting intense immunoreactivity to PDH. PDH and the PDF of insects belong to the same family of neuropeptides (Rao, 2001), and both peptides appear to act as signaling molecules in circadian systems (PDH: Verde et al., 2007; PDF: Lin et al., 2004; Renn et al., 1999; Saifullah & Tomioka, 2003; Schneider & Stengl, 2005; Wülbeck et al., 2008). Like the brain photoreceptors of C. destructor, the eyelet photoreceptors of insects terminate in a single neuropil, the accessory medulla (Hofbauer & Buchner, 1989; Nässel et al., 1988; Yasuyama et al., 2006), in close proximity to PDF-expressing interneurons (Hamasaka & Nässel, 2006; Helfrich-Förster et al., 2002; Malpel et al., 2002). Ultrastructural studies in the blowfly (Protophormia terraenovae) have demonstrated that the eyelet photoreceptors are, in fact, presynaptic to PDF-immunoreactive neurons in the accessory medulla (Yasuyama et al., 2006). In Drosophila, PDF-expressing neurons innervating the accessory medulla have been identified as core circadian pacemaker neurons (Helfrich-Förster, 2003; Nitabach & Taghert, 2008). Owing to the intense PDH immunoreactivity in the BPN of C. destructor, it was difficult to accurately determine which PDH-expressing neurons innervate this neuropil. An important challenge for future studies, therefore, will be to identify which neurons innervate the BPN, in order to elucidate the central pathways to which the brain photoreceptors provide inputs.
Under standard 12 : 12 LD conditions, most C. destructor exhibited a robust diurnal rhythm in locomotor activity characterized by a pronounced peak in activity following lights-off. This rhythm differs from the bimodal activity pattern characteristic of the astacid crayfish P. clarkii (Fernández de Miguel & Aréchiga, 1994; Fuentes-Pardo et al., 2003; Miranda-Anaya, 2004; Page & Larimer, 1972, 1975; Viccon-Pale & Fuentes-Pardo, 1994). Similarly, Quilter and Williams (1977) observed in the parastacid crayfish Paranephrops zealandicus that most animals (64%; n = 47) exhibit a unimodal diurnal locomotory rhythm with a peak at lights-off. Some P. zealandicus also exhibited a bimodal activity pattern (23%) with peaks at lights-off and lights-on, while others were arrhythmic (13%). Such inter-animal (and intra-animal) variability in diurnal locomotor activity patterns appears to be characteristic of freshwater crayfish and does not seem to be correlated with age, sex, or molt stage (Fanjul-Moles et al., 1996; Page & Larimer, 1972; Quilter & Williams, 1977; as well as the present study). Surprisingly, unlike P. zealandicus, in which locomotory rhythms continue for up to 60 days in DD (Quilter & Williams, 1977), placing C. destructor in DD conditions resulted in arrhythmic locomotor activity. The locomotory rhythm in P. clarkii, on the other hand, did not cease in DD, suggesting that the rhythm is governed by an endogenous oscillator. This oscillator appears to be compromised, however, by the entire removal of the compound eyes and the associated protocerebral neuropils, because the rhythmicity under these conditions was not maintained in DD.
Another surprising finding of the present study was that while P. clarkii exhibited robust locomotory rhythms in both LD and DD conditions, elements of these rhythms differed markedly from those observed in previous studies of this species. In our experimental setting, P. clarkii exhibited a bimodal locomotory rhythm in LD, with an activity peak following (but never anticipating) lights-off and another anticipating and spanning the lights-on transition. In contrast, several previous studies of P. clarkii have observed a bimodal rhythm dominated by a large activity peak centered around (and often anticipating) lights-off and a second, smaller peak following (and never anticipating) lights-on (Fernández de Miguel &Aréchiga, 1994; Fuentes-Pardo et al., 2003; Miranda-Anaya, 2004; Page & Larimer, 1972). While both the lights-off and lights-on activity peaks continued in DD conditions in the present study, the lights-on peak has been observed in some previous studies to disappear immediately upon the transition to DD (Fernández de Miguel & Aréchiga, 1994; Page & Larimer, 1972) or following surgical ablation of the retina (Page & Larimer, 1972). Furthermore, in most previous studies in which the largest activity peak occurred at lights-off, the free-running period of the locomotory rhythm in DD has had a period <24 h, conforming to Aschoff's rule for a nocturnally-active species (Aschoff, 1960; Hoffmann, 1965). In the present study, however, the locomotory activity rhythm of P. clarkii had a free-running period of 26 h in DD conditions, consistent with a diurnally-active species (Aschoff, 1960; Hoffmann, 1965). Interestingly, Miranda-Anaya (2004) described an intermediate behavioral rhythm in P. clarkii in which the locomotory rhythm was dominated by a large peak in activity at lights-off but exhibited a free-running period >24 h in DD conditions.
Together, these results provide evidence for a pronounced plasticity in the circadian control of locomotor activity in P. clarkii. Several studies of insects and non-decapod crustaceans have shown that locomotor activity rhythms can vary between adult individuals of the same species, depending on the experimental light conditions in which they are raised (Barrett & Page, 1989; Sheeba et al., 2002; Tomioka & Chiba, 1989), their geographic location (Rossano et al., 2008; Shinkawa et al., 1994), or social environment (Bloch & Robinson, 2001; Shemesh et al., 2007). As the individuals used in the present study were obtained from wild populations, it is difficult to determine precisely how their previous environments may have influenced their circadian control systems. Future experiments with laboratory-reared animals will be important, therefore, in elucidating factors modulating this plasticity in the circadian locomotory rhythms of P. clarkii.
The locomotor activity rhythms of both C. destructor and P. clarkii readily adjust to 6 h phase shifts in the LD cycle. In order to examine possible roles of the brain photoreceptors in photoentrainment of circadian rhythms, we investigated the ability of these crayfish to perform this resynchronization following surgical ablation of the two other known photoreceptor organs in these animals: the compound eye photoreceptors and caudal photoreceptors. The results of these experiments suggest that the brain photoreceptors themselves are able to entrain locomotor activity rhythms to photic stimuli, as are the extraretinal eyelets of insects (Rieger et al., 2003; Veleri et al., 2007). Behavioral experiments with Drosophila mutants have shown that CRY-expressing circadian pacemaker neurons transduce light signals cell-autonomously into circadian phase information that can entrain locomotory rhythms (Emery et al., 1998; Helfrich-Förster et al., 2001; Klarsfeld et al., 2004; Rieger et al., 2003; Stanewsky et al., 1998). Additionally, clock gene-expressing dorsal neurons have been shown to be photosensitive through unknown mechanisms (Rieger et al., 2003, 2006; Veleri et al., 2003). Photoentrainment in freshwater crayfish, therefore, may also be influenced by CRY-expressing neurons in the brain (Fanjul-Moles et al., 2004) or by other undescribed photoreceptive neurons (Edwards, 1984).
To further examine possible contributions of the brain photoreceptor pathway to circadian rhythmicity, we examined variations in the levels of PDH expression in the BPN across the day/night cycle. In both C. destructor and P. clarkii, PDH levels in the BPN varied with the time of day in a manner mirroring the locomotory rhythm characteristic of each species. As the BPN is likely to be innervated by several PDH-expressing neurons, these results suggest that PDH levels in this population of neurons may cycle synchronously. In C. destructor, a significant diurnal rhythm in PDH levels was also observed within the somata of a pair of intensely PDH-immunoreactive Cluster 6 interneurons. These neurons appear to be homologues of neurons, described in detail previously in O. limosus (Mangerich & Keller, 1988), that descend from the brain via the circumesophageal connectives and branch within the subesophageal and thoracic ganglia. As these ganglia contain the motor centers for locomotion, these interneurons represent a candidate neuronal pathway for the translation of the brain photoreceptor outputs into behavioral rhythms.
In summary, the brain photoreceptors of C. destructor exhibit strong immunoreactivity to the circadian photopigment CRY and arborize within the median protocerebrum among neurons expressing PDH. Surgical ablation studies suggest that inputs from the brain photoreceptors contribute to the synchronization of the locomotory rhythms of C. destructor and P. clarkii to the day/night cycle. Histaminergic neurotransmission appears to mediate the synaptic transfer of light entrainment signals from the photoreceptors to central neurons. Together, these results provide several lines of evidence that the brain photoreceptors of freshwater crayfish can control rhythmic locomotory behaviors, and that in some species they can entrain the endogenous oscillator.
ACKNOWLEDGMENTS
We thank P. Carey and V. LePage for animal care, and H. Dircksen and E. Buchner for kindly providing antibodies. This work was supported by NIH R01 MH67157, NSF-IBN 0344448, NSF-IOS 0818259, and The Maren Foundation, Mount Desert Island Biological Laboratory.
Footnotes
Publisher's Disclaimer: The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
REFERENCES
- Aréchiga H, Fernández-Quiróz F, Fernández de Miguel F, Rodríguez-Sosa L. The circadian system of crustaceans. Chronobiol. Int. 1993;10:1–19. doi: 10.3109/07420529309064477. [DOI] [PubMed] [Google Scholar]
- Aschoff J. Exogenous and endogenous components in circadian rhythms. Cold Spring Harb. Symp. Quant. Biol. 1960;25:11–28. doi: 10.1101/sqb.1960.025.01.004. [DOI] [PubMed] [Google Scholar]
- Barrett RK, Page TL. Effects of light on circadian pacemaker development. I. The free running period. J. Comp. Physiol. 1989;A 165:41–49. doi: 10.1007/BF00613798. [DOI] [PubMed] [Google Scholar]
- Beltz BS. Distribution and functional anatomy of amine-containing neurons in decapod crustaceans. Microsc. Res. Tech. 1999;44:105–120. doi: 10.1002/(SICI)1097-0029(19990115/01)44:2/3<105::AID-JEMT5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Benito J, Houl JH, Roman GW, Hardin PE. The blue-light photoreceptor CRYPTOCHROME is expressed in a subset of circadian oscillator neurons in the Drosophila CNS. J. Biol. Rhythms. 2008;23:296–307. doi: 10.1177/0748730408318588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton JL, Sandeman DC, Beltz BS. Nitric oxide in the crustacean brain: Regulation of neurogenesis and morphogenesis in the developing olfactory pathway. Dev. Dyn. 2007;236:3047–3060. doi: 10.1002/dvdy.21340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton JL, Goergen EM, Rogan SC, Beltz BS. Hormonal and synaptic influences of serotonin on adult neurogenesis. Gen. Comp. Endocrinol. 2008;158:183–190. doi: 10.1016/j.ygcen.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch G, Robinson GE. Reversal of honeybee behavioural rhythms. Nature. 2001;410:1048. doi: 10.1038/35074183. [DOI] [PubMed] [Google Scholar]
- Bobkova M, Grève P, Meyer-Rochow VB, Martin G. Description of intracerebral ocelli in two species of North American crayfish: Orconectes limosus (Cambaridae) and Pacifastacus leniusculus (Astacidae) Invert. Biol. 2003;122:158–165. [Google Scholar]
- Boles LC, Lohmann KJ. True navigation and magnetic maps in spiny lobsters. Nature. 2003;421:60–63. doi: 10.1038/nature01226. [DOI] [PubMed] [Google Scholar]
- Cashmore AR. Cryptochromes: Enabling plants and animals to determine circadian time. Cell. 2003;114:537–543. [PubMed] [Google Scholar]
- Collins B, Mazzoni EO, Stanewsky R, Blau J. Drosophila CRYPTOCHROME is a circadian transcriptional repressor. Curr. Biol. 2006;16:441–449. doi: 10.1016/j.cub.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Dardente H, Cermakian N. Review: Molecular circadian rhythms and peripheral clocks in mammals. Chronobiol. Int. 2007;24:195–213. doi: 10.1080/07420520701283693. [DOI] [PubMed] [Google Scholar]
- Dircksen H, Zahnow CA, Gaus G, Keller R, Rao KR, Riehm JP. The ultrastructure of nerve endings containing pigment-dispersing hormone (PDH) in crustacean sinus glands: Identification by an antiserum against a synthetic PDH. Cell Tissue Res. 1987;250:377–387. [Google Scholar]
- Dubruille R, Emery P. A plastic clock: How circadian rhythms respond to environmental cues in Drosophila. Mol. Neurobiol. 2008;38:129–145. doi: 10.1007/s12035-008-8035-y. [DOI] [PubMed] [Google Scholar]
- Edwards DH. Crayfish extraretinal photoreception I. Behavioural and motoneuronal response to abdominal illumination. J. Exp. Biol. 1984;109:291–306. doi: 10.1242/jeb.109.1.291. [DOI] [PubMed] [Google Scholar]
- Emery P, So WV, Kaneko M, Hall JC, Rosbash M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell. 1998;95:669–679. doi: 10.1016/s0092-8674(00)81637-2. [DOI] [PubMed] [Google Scholar]
- Emery P, Stanewsky R, Helfrich-Förster C, Emery-Le M, Hall JC, Rosbash M. Drosophila CRY is a deep brain circadian photoreceptor. Neuron. 2000;26:493–504. doi: 10.1016/s0896-6273(00)81181-2. [DOI] [PubMed] [Google Scholar]
- Escamilla-Chimal EG, Fanjul-Moles ML. Daily and circadian expression of cryptochrome during the ontogeny of crayfish. Comp. Biochem. Physiol. A. 2008;151:461–470. doi: 10.1016/j.cbpa.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Fanjul-Moles ML, Pietro-Sagredo J. The circadian system of crayfish: A developmental approach. Microsc. Res. Tech. 2003;60:291–301. doi: 10.1002/jemt.10268. [DOI] [PubMed] [Google Scholar]
- Fanjul-Moles ML, Miranda-Anaya M, Prieto J. Circadian locomotor activity rhythm during ontogeny in crayfish Procambarus clarkii. Chronobiol. Int. 1996;13:15–26. doi: 10.3109/07420529609040838. [DOI] [PubMed] [Google Scholar]
- Fanjul-Moles ML, Escamilla-Chimal EG, Gloria-Osorio A, Hernández-Herrera G. The crayfish Procambarus clarkii CRY shows daily and circadian variation. J. Exp. Biol. 2004;207:1453–1460. doi: 10.1242/jeb.00900. [DOI] [PubMed] [Google Scholar]
- Fernández de Miguel F, Aréchiga H. Circadian locomotor activity and its entrainment by food in the crayfish Procambarus clarkii. J. Exp. Biol. 1994;190:9–21. doi: 10.1242/jeb.190.1.9. [DOI] [PubMed] [Google Scholar]
- Fuentes-Pardo B, Guzmán-Gómez AM, Lara-Aparicio M, López de Medrano S. A qualitative model of a motor circadian rhythm. BioSystems. 2003;71:61–69. doi: 10.1016/s0303-2647(03)00110-2. [DOI] [PubMed] [Google Scholar]
- Gegear RJ, Casselman A, Waddell S, Reppert SM. Cryptochrome mediates light-dependent magnetosensitivity in Drosophila. Nature. 2008;454:1014–1019. doi: 10.1038/nature07183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goergen EM, Bagay LA, Rehm K, Benton JL, Beltz BS. Circadian control of neurogenesis. J. Neurobiol. 2002;53:90–95. doi: 10.1002/neu.10095. [DOI] [PubMed] [Google Scholar]
- Hall JC. Cryptochromes: Sensory reception, transduction, and clock functions subserving circadian systems. Curr. Opin. Neurobiol. 2000;10:456–466. doi: 10.1016/s0959-4388(00)00117-3. [DOI] [PubMed] [Google Scholar]
- Hamasaka Y, Nässel DR. Mapping of serotonin, dopamine, and histamine in relation to different clock neurons in the brain of Drosophila. J. Comp. Neurol. 2006;494:314–330. doi: 10.1002/cne.20807. [DOI] [PubMed] [Google Scholar]
- Hardin PE. The circadian timekeeping system of Drosophila. Curr. Biol. 2005;15:R714–R722. doi: 10.1016/j.cub.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Harzsch S, Dircksen H, Beltz BS. Development of pigment-dispersing hormone-immunoreactive neurons in the American lobster: Homology to the insect circadian pacemaker system? Cell Tissue Res. 2009;335:417–429. doi: 10.1007/s00441-008-0728-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Förster C. Development of pigment-dispersing hormone immunoreactive neurons in the central nervous system of Drosophila melanogaster. J. Comp. Neurol. 1997;380:355–354. doi: 10.1002/(sici)1096-9861(19970414)380:3<335::aid-cne4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C. The neuroarchitecture of the circadian clock in the brain of Drosophila melanogaster. Microsc. Res. Tech. 2003;62:94–102. doi: 10.1002/jemt.10357. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C. Neurobiology of the fruit fly's circadian clock. Genes Brain Behav. 2005;4:65–76. doi: 10.1111/j.1601-183X.2004.00092.x. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C, Homberg U. Pigment-dispersing hormone-immunoreactive neurons in the nervous system of wild-type Drosophila melanogaster and of several mutants with altered circadian rhythmicity. J. Comp. Neurol. 1993;337:177–190. doi: 10.1002/cne.903370202. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C, Stengl M, Homberg U. Organization of the circadian system in insects. Chronobiol. Int. 1998;15:567–594. doi: 10.3109/07420529808993195. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C, Täuber M, Park JH, Mühlig-Versen M, Schneuwly S, Hofbauer A. Ectopic expression of the neuropeptide pigment-dispersing factor alters behavioral rhythms in Drosophila melanogaster. J. Neurosci. 2000;20:3339–3353. doi: 10.1523/JNEUROSCI.20-09-03339.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Förster C, Winter C, Hofbauer A, Hall JC, Stanewsky R. The circadian clock of fruit flies is blind after elimination of all known photoreceptors. Neuron. 2001;30:249–261. doi: 10.1016/s0896-6273(01)00277-x. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C, Edwards T, Yasuyama K, Wisotzki B, Schneuwly S, Stanewsky R, Meinertzhagen IA, Hofbauer A. The extraretinal eyelet of Drosophila: Development, ultrastructure, and putative circadian function. J. Neurosci. 2002;22(21):9255–9266. doi: 10.1523/JNEUROSCI.22-21-09255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer A, Buchner E. Does Drosophila have seven eyes? Naturwissenschaften. 1989;76:335–336. [Google Scholar]
- Hoffmann K. Overt circadian frequencies and circadian rule. In: Aschoff J, editor. Circadian clocks. North Holland; Amsterdam: 1965. pp. 87–94. [Google Scholar]
- Hsu YA, Stemmler EA, Messinger DI, Dickinson PS, Christie AE, de la Iglesia HO. Cloning and differential expression of two β-pigment-dispersing hormone (β-PDH) isoforms in the crab Cancer productus: Evidence for authentic β-PDH as a local neurotransmitter and β-PDH II as a humoral factor. J. Comp. Neurol. 2008;508:197–211. doi: 10.1002/cne.21659. [DOI] [PubMed] [Google Scholar]
- Jensen HL, Norrild B. Easy and reliable double-immunogold labelling of herpes simplex virus type-1 infected cells using primary monoclonal antibodies and studied by cryosection electron microscopy. Histochem. J. 1999;31:525–533. doi: 10.1023/a:1003840006848. [DOI] [PubMed] [Google Scholar]
- Klarsfeld A, Malpel S, Michard-Vanhee C, Picot M, Chelot E, Rouyer F. Novel features of cryptochrome-mediated photoreception in the brain circadian clock of Drosophila. J. Neurosci. 2004;24:1468–1477. doi: 10.1523/JNEUROSCI.3661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Stormo GD, Taghert M. The neuropeptide pigment-dispersing factor coordinates pacemaker interactions in the Drosophila circadian system. J. Neurosci. 2004;24:7951–7957. doi: 10.1523/JNEUROSCI.2370-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpel S, Klarsfeld A, Rouyer F. Larval optic nerve and adult extra-retinal photoreceptors sequentially associate with clock neurons during Drosophila brain development. Development. 2002;129:1443–1453. doi: 10.1242/dev.129.6.1443. [DOI] [PubMed] [Google Scholar]
- Mangerich S, Keller R. Localization of pigment-dispersing hormone (PDH) immunoreactivity in the central nervous system of Carcinus maenas and Orconectes limosus (Crustacea), with reference to FMRFamide immunoreactivity in O. limosus. Cell Tissue Res. 1988;253:199–208. doi: 10.1007/BF00221755. [DOI] [PubMed] [Google Scholar]
- Mangerich S, Keller R, Dircksen H, Rao KR, Riehm JP. Immunocytochemical localization of pigment-dispersing hormone (PDH) and its coexistence with FMRFamide-immunoreactive material in the eyestalks of the decapod crustaceans Carcinus maenas and Orconectes limosus. Cell Tissue Res. 1987;250:365–375. [Google Scholar]
- Miranda-Anaya M. Circadian locomotor activity in freshwater decapods: An ecological approach. Biol. Rhythm Res. 2004;35:69–78. [Google Scholar]
- Miranda-Anaya M, Fanjul-Moles ML. Nonparametric effects of monochromatic light on the activity rhythm of juvenile crayfish. Chronobiol. Int. 1997;14:25–34. doi: 10.3109/07420529709040539. [DOI] [PubMed] [Google Scholar]
- Nässel DR. Histamine in the brain of insects: A review. Microsc. Res. Tech. 1999;44(2–3):121–136. doi: 10.1002/(SICI)1097-0029(19990115/01)44:2/3<121::AID-JEMT6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Nässel DR, Holmqvist MH, Hardie RC, Håkanson R, Sundler F. Histamine-like immunoreactivity in photoreceptors of the compound eyes and ocelli of flies. Cell Tissue Res. 1988;253:639–646. doi: 10.1007/BF00219755. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Taghert PH. Organization of the Drosophila circadian control circuit. Curr. Biol. 2008;18:R84–R93. doi: 10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- Numata H, Shiga S, Morita A. Photoperiodic receptors in arthropods. Zool. Sci. 1997;14:187–197. [Google Scholar]
- Page TL. Extraretinal photoreception in entrainment and photoperiodism in invertebrates. Experientia. 1982;38:1007–1013. [Google Scholar]
- Page TL, Larimer JL. Entrainment of the circadian locomotor activity rhythm in crayfish. J. Comp. Physiol. 1972;78:107–120. [Google Scholar]
- Page TL, Larimer JL. Neural control of circadian rhythmicity in the crayfish. I. The locomotor rhythm. J. Comp. Physiol. 1975;97:59–80. [Google Scholar]
- Pollack I, Hofbauer A. Histamine-like immunoreactivity in the visual system and brain of Drosophila melanogaster. Cell Tissue Res. 1991;266:391–398. doi: 10.1007/BF00318195. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Touitou Y, Smolensky H. Ethical and methodological standards for laboratory and medical biological rhythm research. Chronobiol. Int. 2008;25:999–1016. doi: 10.1080/07420520802544530. [DOI] [PubMed] [Google Scholar]
- Prosser CL. Action potentials in the nervous system of the crayfish II. Responses to illumination of the eye and the caudal ganglion. J. Cell Comp. Physiol. 1934;4:363–377. [Google Scholar]
- Quilter CG, Williams BG. Circadian activity rhythms in crayfish Paranephrops zealandicus (Crustacea) J. Zool. (Lond.) 1977;182:559–571. [Google Scholar]
- Rao KR. Crustacean pigmentary-effector hormones: Chemistry and functions of RPCH, PDH and related peptides. Am. Zool. 2001;41:364–379. [Google Scholar]
- Refinetti R. Circadian physiology. Taylor and Francis; Boca Raton, Fla.: 2006. p. 688. [Google Scholar]
- Renn SCP, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each causes severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- Rieger D, Stanewsky R, Helfrich-Förster C. Cryptochrome, compound eyes, Hofbauer-Buchner eyelets, and ocelli play different roles in the entrainment and masking pathway of the locomotor activity rhythm in the fruit fly Drosophila melanogaster. J. Biol. Rhythms. 2003;18:377–391. doi: 10.1177/0748730403256997. [DOI] [PubMed] [Google Scholar]
- Rieger D, Shafer OT, Tomioka K, Helfrich-Förster C. Functional analysis of circadian pacemaker neurons in Drosophila melanogaster. J. Neurosci. 2006;26:2531–2543. doi: 10.1523/JNEUROSCI.1234-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossano C, Morgan E, Scapini F. Variation of the locomotor activity rhythms in three species of Talitrid amphipods, Talitrus saltator, Orchestria montagui, and O. gammarellus, from various habitats. Chronobiol. Int. 2008;25:511–532. doi: 10.1080/07420520802257869. [DOI] [PubMed] [Google Scholar]
- Rubin EB, Shemesh Y, Cohen M, Elgavish S, Roberston HM, Bloch G. Molecular and phylogenetic analyses reveal mammalian-like clockwork in the honey bee (Apis mellifera) and shed new light on the molecular evolution of the circadian clock. Genome Res. 2006;16:1352–1365. doi: 10.1101/gr.5094806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saifullah AS, Tomioka K. Pigment-dispersing factors sets the night state of the medulla bilateral neurons in the optic lobe of the cricket, Gryllus bimaculatus. J. Insect Physiol. 2003;41:231–239. doi: 10.1016/s0022-1910(02)00270-6. [DOI] [PubMed] [Google Scholar]
- Sandeman DC, Sandeman RE, de Couet HG. Extraretinal photoreceptors in the brain of the crayfish Cherax destructor. J. Neurobiol. 1990;21:619–629. doi: 10.1002/neu.480210409. [DOI] [PubMed] [Google Scholar]
- Sandeman DC, Sandeman R, Derby C, Schmidt M. Morphology of the brain of crayfish, crabs and spiny lobsters: A common nomenclature for homologous structures. Biol. Bull. 1992;183:304–326. doi: 10.2307/1542217. [DOI] [PubMed] [Google Scholar]
- Schneider NL, Stengl M. Pigment-dispersing factor and GABA synchronize cells of the isolated circadian clock of the cockroach Leucophaea maderae. J. Neurosci. 2005;25:5138–5147. doi: 10.1523/JNEUROSCI.5138-A-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer OT, Helfrich-Förster C, Renn SC, Taghert PH. Reevaluation of Drosophila melanogaster's neuronal circadian pacemakers reveals new neuronal classes. J. Comp. Neurol. 2006;498:180–193. doi: 10.1002/cne.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeba V, Chandrashekaran MK, Joshi A, Sharma VK. Developmental plasticity of the locomotor activity rhythm of Drosophila melanogaster. J. Insect Physiol. 2002;48:25–32. doi: 10.1016/s0022-1910(01)00139-1. [DOI] [PubMed] [Google Scholar]
- Shemesh Y, Cohen M, Bloch G. Natural plasticity in circadian rhythms is mediated by reorganization in the molecular clockwork in honeybees. FASEB J. 2007;21:2304–2311. doi: 10.1096/fj.06-8032com. [DOI] [PubMed] [Google Scholar]
- Shinkawa Y, Takeda S, Tomioka K, Matsumoto A, Oda T, Chiba Y. Variability in circadian activity patterns within the Culex pipiens complex (Diptera: Culicidae) J. Med. Entomol. 1994;31:49–56. doi: 10.1093/jmedent/31.1.49. [DOI] [PubMed] [Google Scholar]
- Stanewsky R, Kaneko M, Emery P, Beretta B, Wager-Smith K, Kay SA, Rosbash M, Hall JC. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell. 1998;95:681–692. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- Stuart AE. From fruit flies to barnacles, histamine is the neurotransmitter of arthropod photoreceptors. Neuron. 1999;22:431–433. doi: 10.1016/s0896-6273(00)80699-6. [DOI] [PubMed] [Google Scholar]
- Stuart AE, Borycz J, Meinertzhagen IA. The dynamics of signaling at the histaminergic photoreceptor synapse of arthropods. Prog. Neurobiol. 2007;82:202–227. doi: 10.1016/j.pneurobio.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Styrishave B, Bojsen BH, Witthøfft H, Andersen O. Diurnal variations in physiology and behavior of the noble crayfish Astacus astacus and the signal crayfish Pacifastacus leniusculus. Mar. Fresh Behav. Physiol. 2007;40:63–77. [Google Scholar]
- Tomioka K, Chiba Y. Photoperiod during post-embryonic development affects some parameters of adult circadian rhythm in the cricket Gryllus bimaculatus. Zool. Sci. 1989;6:565–571. [Google Scholar]
- Veleri S, Rieger D, Helfrich-Förster C, Stanewsky R. Hofbauer-Buchner eyelet affects circadian photosensitivity and coordinates TIM and PER expression in Drosophila clock neurons. J. Biol. Rhythms. 2007;22:29–42. doi: 10.1177/0748730406295754. [DOI] [PubMed] [Google Scholar]
- Verde MA, Barriga-Montoya C, Fuentes-Pardo B. Pigment dispersing hormone generates a circadian response to light in the crayfish, Procambarus clarkii. Comp. Biochem. Physiol. 2007;147:983–992. doi: 10.1016/j.cbpa.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Viccon-Pale JA, Fuentes-Pardo B. Synchronization by light of the circadian rhythm of motor activity in the crayfish. Biol. Rhythm Res. 1994;25:267–276. [Google Scholar]
- Wildt M, Goergen EM, Benton JL, Sandeman DC, Beltz BS. Regulation of serotonin levels by multiple light-entrainable endogenous rhythms. J. Exp. Biol. 2003;207:3765–3774. doi: 10.1242/jeb.01205. [DOI] [PubMed] [Google Scholar]
- Wilkens LA, Larimer JL. The CNS photoreceptor of crayfish: Morphology and synaptic activity. J. Comp. Physiol. 1972;80:389–407. [Google Scholar]
- Wülbeck C, Grieshaber E, Helfrich-Förster C. Pigment-dispersing factor (PDF) has different effects on Drosophila's circadian clocks in the accessory medulla and in the dorsal brain. J. Biol. Rhythms. 2008;23:409–424. doi: 10.1177/0748730408322699. [DOI] [PubMed] [Google Scholar]
- Yasuyama K, Meinertzhagen IA. Extraretinal photoreceptors at the compound eye's posterior margin in Drosophila melanogaster. J. Comp. Neurol. 1999;412:193–202. doi: 10.1002/(sici)1096-9861(19990920)412:2<193::aid-cne1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Yasuyama K, Okada Y, Hamanaka Y, Shiga S. Synaptic connections between eyelet photoreceptors and pigment dispersing-immunoreactive neurons of the blowfly Protophormia terranovae. J. Comp. Neurol. 2006;494:331–344. doi: 10.1002/cne.20812. [DOI] [PubMed] [Google Scholar]
- Yoshii T, Todo T, Wülbeck C, Stanewsky R, Helfrich-Förster C. Cryptochrome is present in the compound eyes and a subset of Drosophila's clock neurons. J. Comp. Neurol. 2008;508:952–966. doi: 10.1002/cne.21702. [DOI] [PubMed] [Google Scholar]
- Yoshii T, Wülbeck C, Sehadova H, Veleri S, Bichler D, Stanewsky R, Helfrich-Förster C. The neuropeptide pigment-dispersing factor adjusts period and phase of Drosophila's clock. J. Neurosci. 2009;29:2597–2610. doi: 10.1523/JNEUROSCI.5439-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Hardin PE. Circadian oscillators of Drosophila and mammals. J. Cell Sci. 2006;119:4793–4795. doi: 10.1242/jcs.03174. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Metterville D, Briscoe AD, Reppert SM. Insect cryptochromes: Gene duplication and loss define diverse ways to construct insect circadian clocks. Mol. Biol. Evol. 2007;24:948–955. doi: 10.1093/molbev/msm011. [DOI] [PubMed] [Google Scholar]
- Zhu H, Yuan Q, Briscoe AD, Froy O, Casselman A, Reppert SM. The two CRYs of the butterfly. Curr. Biol. 2005;15:R953–R954. doi: 10.1016/j.cub.2005.11.030. [DOI] [PubMed] [Google Scholar]