Abstract
Vaginal gels may act as physical barriers to HIV following semen deposition. However, the extent and significance of this effect are not well understood. During male-to-female sexual transmission of HIV, semen containing infectious HIV is present within the lower female reproductive tract. In cases where a topical gel has previously been applied to the vaginal epithelium, virions must move through gel layers before reaching vulnerable tissue. This additional barrier could affect the functioning of anti-HIV microbicide gels and placebos. To better understand HIV transport in gels, we: (1) quantified diffusion coefficients of HIV virions within semi-solid delivery vehicles; and (2) tested the barrier functioning of thin gel layers in a Transwell system. Two gels used as placebos in microbicides clinical trials, hydroxyethyl cellulose (HEC) and methylcellulose (MC), were found to hinder HIV transport in vitro. The diffusion coefficients for HIV virions in undiluted HEC and MC were 4 ± 2 × 10−12 cm2/s and 7 ± 1 × 10−12 cm2/s respectively. These are almost 10,000 times lower than the diffusion coefficient for HIV in water. Substantial gel dilution (80%: diluent/gel, v/v) was required before diffusion coefficients rose to even two orders of magnitude lower than those in water. In the Transwell system, gel layers of approximately 150-μm thickness reduced HIV transport. There was a log reduction in the amount of HIV that had breached the Transwell membrane after 0-, 4-, and 8- hour incubations. The ability of a gel to function as a physical barrier to HIV transport from semen to tissue will also depend on its distribution over the epithelium and effects of dilution by vaginal fluids or semen. Results here can serve as a baseline for future design of products that act as barriers to HIV transmission. The potential barrier function of placebo gels should be considered in the design and interpretation of microbicides clinical trials.
Keywords: HIV prevention, microbicides, gels, diffusion, drug delivery, placebos
1. Introduction
Vaginal gels may act as physical barriers to Human Immunodeficiency Virus (HIV) following semen deposition, but the extent and significance of this effect are poorly understood. During male-to-female sexual transmission of HIV, semen containing infectious HIV is distributed in the lower female reproductive tract. HIV must penetrate epithelial tissues to establish local infection of target immune cells. Systemic infection occurs after viral dissemination from the mucosal site of infection to lymphatic tissue (Haase, 2005).
In cases where a topical gel has been applied to the vaginal epithelium, HIV must additionally move through gel layers before reaching vulnerable tissue. Gels are used commonly for vaginal drug delivery (das Neves and Bahia, 2006; Justin-Temu et al., 2004) and have been used to formulate microbicides, agents applied topically to prevent transmission of sexually transmitted infections (Buckheit et al., 2010; Cutler and Justman, 2008; Ndesendo et al., 2008; Rohan and Sassi, 2009). Previous studies in our lab have found that vaginal gels are deployed in vivo in layers approximately 100-500 μm thick (Henderson et al., 2007; Henderson et al., 2005; Mauck et al., 2008).
Several researchers have pointed to the importance of intervening at early events in mucosal HIV transmission to prevent infection (Johnston and Fauci, 2007; Miller et al., 2005; Trapp et al., 2006), especially since viral reservoirs may be established within 10 days of infection (McMichael et al., 2010). In this regard, microbicides would be a valuable additional tool in comprehensive HIV prevention programs. The biological significance of hindering virion transport at mucosal surfaces, as it relates to increasing microbicide effectiveness, is poorly understood.
There are several mechanisms by which vaginal gels, by hindering virion transport, could contribute to HIV prevention. Clinical studies have shown that likelihood of infection in male-to-female sexual HIV transmission is related to blood viral load (Gray et al., 2001; Quinn et al., 2000; Wawer et al., 2005), which is likely also related to the viral inoculum in semen (Kalichman et al., 2008). Hindering virion transport at mucosal surfaces could reduce the effective viral inoculum that reaches target cells by trapping virions. Trapped virions could then be cleared from the lower reproductive tract with other vaginal fluids. Furthermore, because HIV infectivity decreases over time in vitro, delaying viral contact may reduce the potential of target cell infection. Hindering virion transport could also allow microbicide active agents or innate defense factors a greater opportunity to neutralize virus. Further studies are needed to elucidate the viability of HIV within the lower female reproductive tract and the time required for HIV to traverse mucosal barriers to reach targets for infection.
Transport of HIV virions from semen to vaginal epithelial surfaces is due, in general, to two mechanisms – convection and diffusion. During coitus, initial gel coating of vaginal surfaces is smoothed (Barnhart et al., 2004; Lai et al., 2009a) and semen is distributed over that coating. These are both processes in which convection dominates diffusion. Following coitus, convective motions in the vagina still exist (e.g. due to changes in posture and leakage of vaginal fluid from the introitus) but diffusion likely becomes the persistent mechanism of sustained HIV migration to vaginal epithelium. It is this latter scenario that is addressed in the present study.
In the present study, we evaluated the physical barrier functioning of two vaginal gels to HIV in vitro by: (1) quantifying the diffusion coefficients of HIV virions within these gels; and (2) directly testing the barrier functioning of thin gel layers in a Transwell system. The diffusion coefficient provides an objective means of comparing HIV transport in different materials. In Fickian diffusion, the diffusion coefficient relates diffusive flux and concentration gradient for particles within a given medium (Truskey et al., 2009). The purpose of measuring the diffusion coefficient here is to help quantify the transport of HIV virions in scenarios relevant to HIV prevention. We hypothesized that the diffusion coefficients of HIV virions in these semi-solid gels would be lower than those in water. To investigate the effect of dilution on HIV transport, we also measured the diffusion coefficients of HIV virions in biologically-relevant dilutions of these gels in PBS. We hypothesized that diffusion coefficients of HIV would increase with level of dilution.
We also evaluated the barrier functioning of vaginal gels in a Transwell system that simulates the spatial geometry of HIV transmission in vivo. Transwell systems are used commonly in drug delivery research to assess the permeability of polarized cell monolayers to drugs or nanoparticulate drug carriers (Balimane et al., 2000; Behrens et al., 2002; Behrens et al., 2001; Cecchelli et al., 1999; Forbes and Ehrhardt, 2005; Mathias et al., 1996; Pontier et al., 2001). Transwell systems have also been used in microbicide development to create models of HIV infection (Dezzutti et al., 2004; Guenthner et al., 2005; Van Herrewege et al., 2007). Transwell plates have an insert that forms upper and lower chambers separated by a porous membrane (Figure 1). In each Transwell, we applied a gel layer of characterized thickness (100-300 μm) to the membrane or no gel for the control condition. A solution of HIV was then added to the top chamber. The insert was placed in the bottom plate, which contained cell culture medium. The plate was incubated for a given time, and then samples from the top and bottom chambers were assayed for infectious HIV using the TZM-bl assay. We hypothesized that gel layers would reduce levels of HIV in the bottom compartment compared to controls where no gel had been applied. A reduction in the number of infectious virions in the bottom compartment of the Transwell was taken as an indicator of viral restriction.
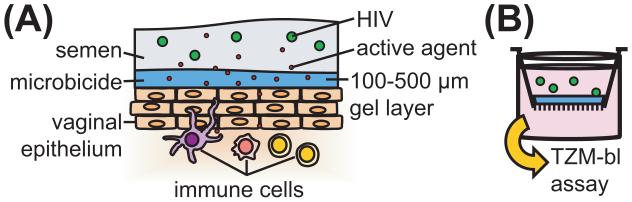
Figure 1.
Biological context of Transwell system used to evaluate barrier functioning of thin gel layers. (A) Schematic of microbicide functioning in vivo. Microbicide gels are applied topically prior to challenge by HIV. Gels form layers of approximately 100-500 μm thick on epithelial surfaces. HIV must traverse these gel layers to reach vulnerable tissue. (B) Transwell system simulates HIV transmission in the presence of vaginal gels. A thin gel layer is applied to the Transwell membrane. A suspension of HIV is added to the top compartment. After incubation, levels of HIV in the bottom compartment are quantified using the TZM-bl assay.
We tested two gels that are commonly used in vaginal drug delivery and have been used as placebos in microbicides clinical trials, hydroxyethyl cellulose (HEC) and methylcellulose (MC). As placebos, these materials are presumed to have minimal effect on virion transport. However, HIV transport through these gels has not been previously quantified. Our results here contribute to the quantitative understanding of the baseline barrier functioning of typical vaginal gels. Furthermore, the results of this study may help to understand the barrier functioning of placebo gels used in microbicides clinical trials.
2. Materials and Methods
2.1. Gels Tested
We tested two gels used as placebos in clinical trials, 2.7% (w/w) hydroxyethyl cellulose (HEC) (Study No. C03-090, Batch 03724326, CONRAD, Arlington, VA) (Tien et al., 2005) and 2.5% methylcellulose (MC) (Batch 100306, Population Council, New York, NY) (Maguire et al., 1998). Both HEC and MC are commonly used in the formulation of vaginal gels (das Neves and Bahia, 2006; Justin-Temu et al., 2004). HEC is the “universal placebo” for microbicide clinical trials (Tien et al., 2005). The batch of HEC used in this study was created for the Phase III clinical trial of Ushercell, or cellulose sulfate (Halpern et al., 2008; Tao et al., 2008; Van Damme et al., 2008). Variations of HEC gels have also been used in other clinical trials (Abdool Karim et al., 2009; Feldblum et al., 2008; Microbicide Trials Network, 2009; Peterson et al., 2007). HEC is an uncharged linear polymer that has been shown to lack anti-HIV activity in in vitro assays and macaque models (Tien et al., 2005). HEC has also been shown to be safe and acceptable to humans (Schwartz et al., 2007). HEC has been presumed not to provide “the physical barrier protection of high-yield strength gelling agents” (Tien et al., 2005). However, the actual barrier functioning of HEC has not been previously characterized. The formulation of MC used in this study was used as the placebo in clinical trials of the microbicide candidate Carraguard (Skoler-Karpoff et al., 2008). MC has been shown to lack anti-HIV activity in vitro (Phillips, 1996) and does not protect macaques from SHIV infection (Turville et al., 2008; Veazey et al., 2003).
2.2. Particle tracking of fluorescently-labeled HIV virions
2.2.1. HIV-1 virions
HIV-1 virions fluorescently-labeled with Alexa Fluor 488 C5 maleimide were provided by Dr. Jeffrey D. Lifson (HIV-1 BAL/SUPT1-CCR5 CL.30 [Alexa 488 labeled /NEM Tx], Lot SP1551A, NIH, Frederick, MD). Treatment with the fluorophore, which is an N-Ethylmaleimide (NEM) analog, eliminates infectivity of virions by cross-linking zinc finger structures needed for viral replication (Morcock et al., 2005). Envelope glycoproteins are unaffected by NEM treatment, retaining their structure and function. Virions prepared using these methods have been used previously in fluorescence correlation spectroscopy and microscopy studies of HIV transport in human cervical mucus (Boukari et al., 2009). Virions were stored at −80°C and thawed immediately before use. To validate that measured diffusion coefficients were reasonable, we compared diffusion coefficients for HIV virions with those of similarly sized fluorescently-labeled latex beads (F-8801, red fluorescent (580/605) 0.1 μm carboxylate-modified FluoSpheres® beads, Invitrogen, Carlsbad, CA). We found that diffusion coefficients were not statistically significantly different (t-test, p < 0.05) for all media tested except for 20% HEC (results not shown).
2.2.2. Sample preparation and microscopy
Gels were evaluated undiluted (100%), diluted to 50% (gel/diluent, v/v) in 1× phosphate-buffered saline (PBS) (from OmniPur 10× PBS concentrate, EMD chemicals, Darmstadt, Germany), or diluted to 20% (v/v) in PBS. Samples of virions and gels were prepared by combining 1 μl solution of HIV-1 virions (32.8 μg/ml p24, 0.239 mg/ml estimated total protein concentration) and 100 μl gel. Samples were mixed by pipetting. Slides were prepared by placing a Secure Seal Spacer (Electron Microscopy Sciences, Hatfield, PA) on a glass slide, applying 11.1 μl of sample, and sealing with a coverslip. The thickness of the sample was determined by the spacer, which is 120 μm thick.
Fluorescence microscopy (Zeiss Axio Observer with 100x/1.46 oil Plan Apochromat DIC objective, QuantEM backthinned EM-CCD camera) was used to image the position of diffusing particles over time. Images were acquired using MetaMorph 7.5 Stream Acquisition. Images of HIV virions were acquired with exposure times of 5 ms and 0.041 s between frames for 1000 frames. Typically, approximately 2-10 particles were observed in each stack. For each sample, 10 image stacks were acquired from different regions. Care was taken to ensure that the z-position during imaging was sufficiently above the coverslip, where particles may stick to the glass. For each condition, independent experiments were performed on 3 different days using separate samples to obtain n = 3. All experiments were performed at 37°C.
2.2.3. Analysis of particle tracking data to obtain diffusion coefficients
We analyzed images to calculate the effective diffusion coefficients. We quantified the x and y positions over time for a given particle using the software Video Spot Tracker developed by the Center for Computer Integrated Systems for Microscopy and Manipulation at the University of North Carolina, Chapel Hill. Image stacks where directional drift of all particles was observed were excluded from analysis. This was determined visually by watching the time-lapse images and noting when all particles in the field moved in the same direction. Directional drift was likely caused by convective flow in the sample or movement of the slide or microscope.
Coordinates for x and y positions were used to calculate the mean-square displacement (MSD), 〈Δr2(τ)〉, as follows (Qian et al., 1991; Saxton, 1997; Suh et al., 2005):
| (1) |
Here τ is the time interval, or time lag, over which the displacement was calculated. See Supplementary Materials for detailed methods of calculating MSD for different time lags. For two-dimensional diffusion in a Newtonian fluid, the effective diffusion coefficient (Deff) is related to the MSD as follows:
| (2) |
Thus, we obtained the diffusion coefficients by plotting MSD with respect to τ and using a linear regression to find the slope, which is equivalent to 4Deff. We fitted MSD values to a linear regression in MATLAB using the linear least squares method (using MATLAB function “fit” with method “LinearLeastSquares”). Values were weighted using the inverse of the variance (Saxton, 1997). The best-fit line was defined as the one that minimized the sum of the square of the weighted residuals. We used the function for calculating the variance for successive determinations of MSD developed by Qian et al. (Qian et al., 1991; Saxton, 1997).
Statistical analyses were performed using JMP 8 software (SAS, Cary, NC). ANOVA was used to compare diffusion coefficients for all conditions; post-hoc t-tests were then used to make comparisons between different groups. Values for p < 0.05 were considered statistically significant.
2.3. Transwell experiments for testing barrier functioning of thin gel layers
2.3.1. Virus
We evaluated two CCR5-tropic, well-characterized, reference strains of HIV-1 isolated from acute, sexually transmitted infections: HIV-1 DU156 (Clade C, 500 TCID50) and HIV-1 TRO (Clade B, 1000 TCID50). Viruses were grown in peripheral blood mononuclear cells (PBMCs) so that virions had surface properties relevant to sexually transmitted virus.
2.3.2. Transwell experiments
Thin gel layers (approximately 100-300 μm thick) were applied to the membranes of 96-well Transwell plates (HTS Transwell®-96 Well Plate, polycarbonate membrane, pore size = 5 μm Cat. No. 3388, Corning Incorporated, Corning, NY) using a custom applicator (see Supplementary Materials for detailed methods). Each gel layer was challenged by a solution of 75 μl of HIV-1 virions, added to the top chamber. The bottom compartment of each Transwell contained 235 μl of cell culture medium. Cell culture medium consisted of D-MEM with 10% heat-inactivated fetal bovine serum (Cat. No. SH30071.03, Hyclone, Logan, UT), 25 mM HEPES (Cat. No. 15630, Gibco, Grand Island, NY), and 50 μg/ml gentamicin (Cat. No. G1272, Sigma, St. Louis, MO). Transwell plates were incubated in a 37°C, 5% CO2 incubator for 0, 4, 8, or 12 hours.
After the specified incubation time, the Transwell plate was removed from the incubator and samples of solutions from the top and bottom chambers were removed for quantification of infectious HIV using the TZM-bl assay.
2.3.3. TZM-bl assay
The TZM-bl cell line is a CXCR4-postive HeLa cell clone that has been engineered to express CD4 and CCR5 as well as integrated reporter genes for firefly luciferase and E. coli β-galactosidase under control of an HIV long-terminal repeat sequence (Hammonds et al., 2005; Mascola et al., 2005; Montefiori, 2010). Luciferase reporter gene expression is induced by the viral Tat protein soon after single round infection. The level of infection is quantified by incubating the cells with the luciferin substrate. Luciferase, generated by infected cells, cleaves the luciferin substrate to generate luminescence. Luminescence is quantified using a luminometer. There is a linear range in which luminescence is directly proportional to levels of HIV infection.
Samples from the top and bottom chambers of the Transwell plate were incubated with TZM-bl cells to quantify levels of HIV. For the top compartment, 50-μl samples were transferred to the TZM-bl assay plate and mixed with 100 μl additional cell culture media. For the bottom compartment, 150-μl samples were transferred to the TZM-bl assay plate. Then, 100 μl of TZM-bl cell suspension (105 cells/ml in culture medium with 15 μg/ml DEAE dextran) was added to the assay plate. TZM-bl cells were suspended using treatment with trypsin-EDTA (Cat. No. 25200-056, Invitrogen): Cells were incubated at room temperature with 2.5 ml 0.25% trypsin-EDTA for 30 s. The trypsin-EDTA solution was then removed and cells were incubated for an additional 4 minutes at 37° C. Cells were then suspended in 10 ml growth medium for counting. Cells were further diluted with growth medium to 105 cells/ml. Plates were incubated for 48 hours at 37°C, 5% CO2.
To read plates, we removed 150 μl medium from each well. The remaining 100 μl was incubated with 100 μl luciferin substrate for 2 minutes (Britelite Reagent, PerkinElmer, Waltham, MA). Samples were mixed by pipetting and transferred to 96-well black plates for reading (Corning 3915, Corning, NY). Luminescence was quantified in Relative Luminescence Units (RLUs) using a plate reader (Wallac 1420 Victor3, PerkinElmer, Waltham, MA).
Controls were used to ensure the proper functioning of the TZM-bl assay for quantifying levels of infectious virus. Negative controls consisted of cells without virus: 150 μl cell culture medium and 100 μl of TZM-bl cells solution were mixed in the assay plate at the time of sampling from the Transwell. Positive controls consisted of cells and virus solution applied to the top compartment of the Transwell: Upon initiation of the Transwell assay, 50 μl of virus solution was mixed with 100 μl cell culture medium in the TZM-bl assay plate. These positive controls were incubated for the length of incubation of the Transwell plate, and 100 μl of TZM-bl cells solution was added at the time of sampling from the Transwell.
We characterized the linear range of the assay for the two strains of HIV-1 used, in which the total number of infectious virions is directly proportional to the luminescence of the sample (results not shown). In performing our experiments, all RLU values were within the linear range of the TZM-bl assay (results not shown). We also used the TZM-bl assay to confirm that the placebo gels tested, HEC and MC, do not neutralize virus at the concentrations used within the Transwell, and thus, reductions in the levels of HIV in the bottom compartment were due to hindrance of transport and not viral neutralization (results not shown).
2.3.4. Analysis of results
RLU levels varied from experiment to experiment and over time due to the finite lifetime of HIV, so results were normalized to the control, in which no gel was applied to the Transwell membrane:
| (3) |
Statistical analyses were performed using JMP 8 software (SAS, Cary, NC). ANOVA was used to compare results for all conditions; post-hoc Student’s t-tests were then used to make individual comparisons of means. Values for p < 0.05 were considered statistically significant.
2.3.5. Characterization of thin gel layers
Gel layer thickness was characterized in independent experiments from the Transwell experiments of HIV transport. Gels were labeled with a fluorescent dye, fluorescein (Invitrogen, Carlsbad, CA). A standard curve relating fluorescence to gel thickness was generated by finding the fluorescence intensity of gel layers of defined thickness, created by placing fluorescent gel in chambers of defined height. Those chambers were created by stacking Secure Seal Spacers (Electron Microscopy Sciences). The thicknesses of gel layers applied to Transwell membranes were then quantified: A fluorescent plate reader to was used to determine the fluorescence intensity of gels applied to Transwell membranes, and the standard curve to was used to relate intensity to thickness.
3. Results
3.1. Particle tracking of fluorescently-labeled HIV virions
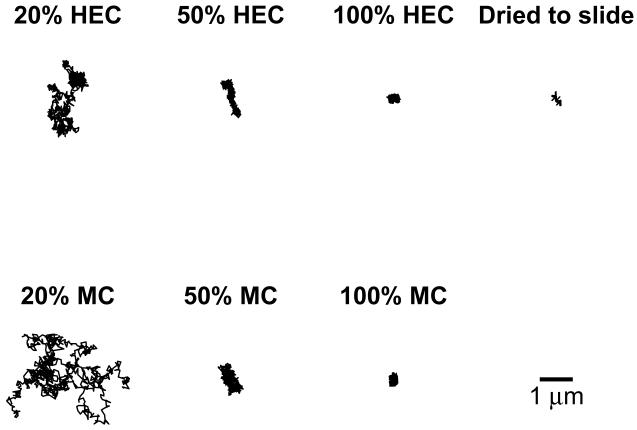
Particle-tracking experiments measured the diffusion coefficients of fluorescently-labeled HIV virions in HEC and MC. To examine effects of dilution on diffusion coefficients, we also performed particle-tracking experiments using HEC and MC diluted to 50% (v/v) gel in PBS, and diluted to 20% (v/v) gel in PBS. Figure 2 shows examples of particle tracks obtained over the time of observation, approximately 41 s. Qualitatively, we observed that particle motion increased with increased dilution by PBS. Undiluted HEC and MC appeared to trap HIV virions. Tracks for undiluted HEC and MC appeared similar to tracks for 100-nm beads dried to the glass slide. These beads were completely immobilized, and any apparent motion represented noise, or the lower limit of detection of the method.
Figure 2.
Examples of tracks obtained for fluorescently-labeled HIV virions in HEC and MC, and for 100-nm bead dried to glass slide. Particles were tracked for 41s.
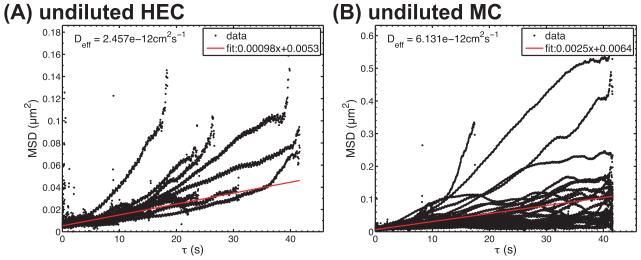
Tracks were processed to calculate mean squared displacements (MSD). Figure 3 shows representative plots of MSD vs. timescale (τ) from single experiments. These data were weighted by the inverse of their variance and fitted to a linear regression. The diffusion coefficient was computed as one-fourth of the slope (cf. Equation 2).
Figure 3.
Examples of plots for MSD vs. τ for HIV in (A) HEC and (B) MC. Data represent different virions within single experiments.
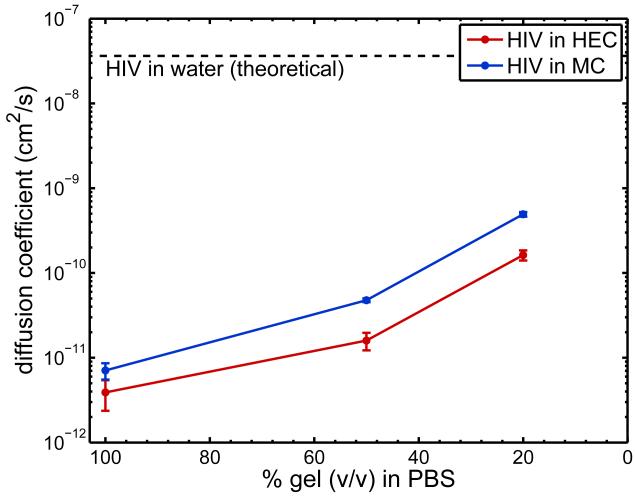
Figure 4 summarizes diffusion coefficients obtained from three independent experiments for particle tracking of HIV virions in HEC and MC, with and without dilution by PBS. Diffusion coefficients for HIV virions in undiluted HEC and MC were 4 ± 2 × 10−12 cm2/s and 7 ± 1 × 10−12 cm2/s, respectively. These are approximately 10,000 times lower than the diffusion coefficient for HIV in water predicted by the Stokes-Einstein equation, 3.6 × 10−8 cm2/s at 37° C (Lai et al, 2009a). Diffusion coefficients in HEC and MC were not significantly different (t-test, p > 0.05).
Figure 4.
Diffusion coefficients (mean ± SE, n = 3 independent experiments) obtained using particle tracking of fluorescently-labeled HIV in HEC and MC undiluted, diluted to 50% (v/v) in PBS, and diluted to 20% (v/v) in PBS.
Diffusion coefficients increased with increasing level of dilution by PBS. However, HIV-virion diffusion was still hindered in diluted gels when compared to water. Diffusion coefficients in 50% (v/v) dilutions of HEC and MC were approximately 3 orders of magnitude lower than those in water, whereas diffusion coefficients in 20% (v/v) dilutions of gels were approximately 2 orders of magnitude lower than those in water.
3.2. Transwell experiments for testing barrier functioning of thin gel layers
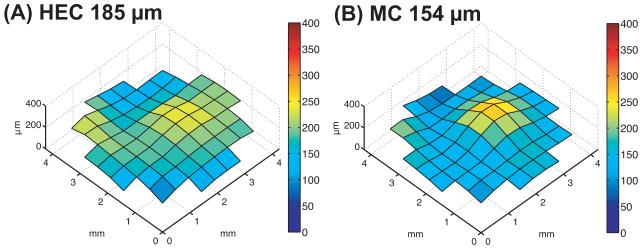
HIV barrier functioning was evaluated for gel layers of thicknesses comparable to those measured for human vaginal coating in vivo, in experiments that applied thin gel layers to the membranes of Transwell inserts. Figure 5 shows examples of distributions of gels on membranes. We measured the thickness of gel layers by labeling gels with fluorescent dye and relating fluorescence intensity to thickness. For each gel, we generated a standard curve relating fluorescence intensity to thickness. Levels of fluorescence were linearly related to thickness for the range of thicknesses tested. For comparing HEC and MC, we attempted to form gel layers of approximately 150-μm thickness. Actual thicknesses were 185 ± 5 (n = 12) and 153 ± 4 μm (n = 6) for HEC and MC, respectively. These gel layers were formed by applying either 6.3 μl of HEC or 8.4 μl of MC to the applicator tip. Although gel layers were not entirely uniform, the entire surface of the membrane was always covered with gel.
Figure 5.
Examples of (A) HEC and (B) MC gel distributions applied to membranes in Transwell system.
We tested the barrier functioning of these thin gel layers to HIV by applying a solution of the virions to the top compartment of the Transwell. After incubation for 0, 4, 8, or 12 hours, samples from the bottom compartment of the Transwell were tested for levels of infectious HIV using the TZM-bl assay. RLU levels varied from experiment to experiment and over time due to time dependent differences in infectivity and variations in target cell number between experiments, so results were normalized to the control, in which no gel was applied to the Transwell membrane.
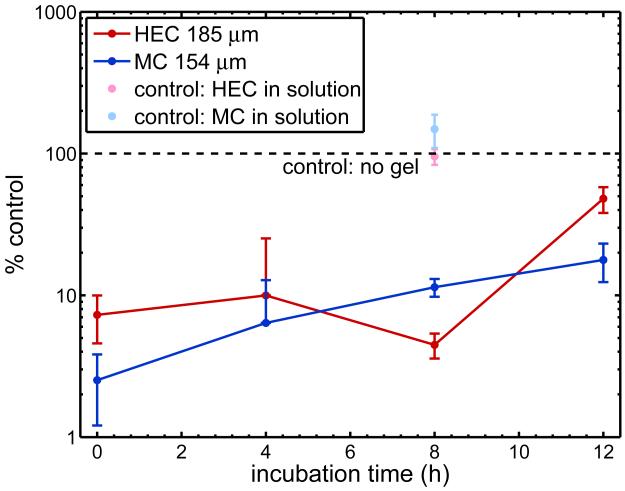
Results show that both HEC and MC reduced the amount of infectious virus in the bottom compartment of the Transwell for all of the incubation times tested (Figure 6). There was no statistically significant difference (p > 0.05) between the two strains of HIV-1, so we grouped them for analysis. Levels of HIV in the bottom compartment were significantly different between control (no gel) and gel experiments (p < 0.05). Notably, there was a log reduction the amount of HIV in the bottom compartment for HEC and MC at 0, 4, and 8 hours. In neutralization assays, log reductions in HIV are considered biologically significant. When data were grouped by gel (over all incubation times), MC appeared to be a slightly superior barrier to HEC, yielding approximately 20% lower levels of HIV in the bottom compartment (p < 0.05).
Figure 6.
Levels of HIV-1 in bottom compartment of Transwell for two semi-solid gels, HEC and MC (n ≥ 4 independent experiments). Experimental results are normalized to controls where no gel was applied to the membrane, such that % control = RLUbottom,experiment/RLUbottom,control. Additional control experiments were performed in which an equivalent amount of gel was mixed in to solutions. For 0-, 4-, and 8- hour incubations, there was a log reduction in levels of HIV-1 in the bottom compartment.
We also performed control experiments in which gel was mixed into solution and incubated in the Transwell for 8 hours. The volume of gel mixed into solution was equivalent to the volume of gel applied to the Transwell membrane. We found that there was no significant difference in HIV levels in the bottom compartment for this “mixed” condition compared control (p > 0.05). However, there was a significant difference between the “mixed” control and the experiments with gel layers (p < 0.05). This indicated that reduction of HIV in the bottom compartment was due to reduction of transport, not neutralization by the placebo gels.
Levels of HIV in the bottom compartment for t = 0 h were significantly different from control. If transport from the top to the bottom compartment occurred due to diffusion alone, we would have observed no HIV in the bottom compartment for all conditions at t = 0 h. The observed log reduction suggests that there was some convective transport from the top to bottom compartment, which probably occurred due to hydrostatic pressure differences introduced when the top insert was separated from the bottom plate at the end of the incubation period. This effect may have lessened for longer incubation times, as the gel became more dilute at the time of separation of the top and bottom compartments. Levels of HIV in the bottom compartment appeared to approach the no-gel control as time increased.
4. Discussion
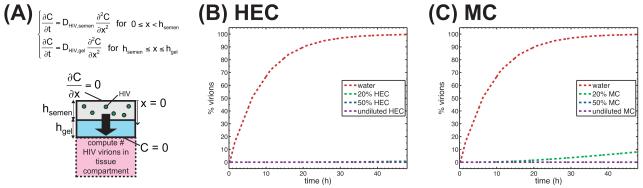
Following ejaculation, HIV is believed to remain infectious in semen for a period of hours, although the precise range of the interval is not known. During this time, diffusion is likely to be a principal mechanism of HIV transport from semen to vaginal epithelial surfaces. Our in vitro experiments found that common vaginal gels, if undiluted, can hinder transport of HIV, reducing diffusion coefficients by a factor of almost 104 vs. diffusion in water. Such a phenomenon may, therefore, also occur in vivo. Within the vagina, gels contact ambient fluids (vaginal transudate, mucus) as well as semen. Typical microbicide gel applied volumes are around 3 – 4 ml, the volume of the human ejaculate ranges 2 – 5 ml (Owen and Katz, 2005), and the volume of ambient fluid in the vagina is about 0.5 – 0.75 ml (Owen and Katz, 1999). Thus on average, a gel could be diluted with vaginal fluid to about 75% gel-to-fluid (v:v), but dilution could be higher in localized regions where gel coating thickness is small (Henderson et al, 2007). Dilution with semen would triple this typical dilution. Our results indicate that only when gel dilution is extremely high (e.g. 20% gel:PBS, v:v) do virion diffusion coefficients increase to a level even two orders of magnitude lower than those in water. This suggests that where there is local gel coating of the vaginal epithelium, HIV transport is significantly slowed. Such retardation of viral transport was analyzed quantitatively in our recent compartmental model of HIV diffusion to vaginal epithelial surfaces (Lai et al, 2009a): The model examined tradeoffs amongst extent and thickness of vaginal coating, and HIV diffusion coefficient, on HIV transport rates to the vaginal epithelium. We have input the present results for HIV diffusion coefficients into that modeling framework (Figure 7). Here, there was uniform 150-μm thick epithelial coating by gel. As seen in Figure 7, undiluted HEC and MC presented a robust barrier to HIV -- over 48 hours were required for virions to reach the tissue surface. Even with 50% (v/v) dilution of the gel, fewer than 1% of virions reached the tissue surface within 48 hours. In contrast, nearly all of the virions reached tissue when migrating with the diffusion coefficient for HIV in water, as predicted by the Stokes-Einstein equation. Diffusion coefficients for 20% (v/v) gel in PBS produced intermediate results: For 20% (v/v) HEC, 0.7% of virions reached tissue within 48 hours, and for 20% (v/v) MC, 8% of virions reached tissue within 48 hours. According to this mathematical model, log reductions in diffusion coefficients are necessary to achieve a significant effect on HIV diffusion through a gel layer of typical thickness found in vivo. Diffusion coefficients 1000-10,000 times lower than diffusion coefficients in water are needed for a gel layer to function as a barrier to HIV diffusion over the time period of 48 hours.
Figure 7.
Interpretation of experimentally-measured diffusion coefficients using mathematical model of diffusion of HIV virions from semen, through microbicide layer, to tissue compartment. (A) Schematic of mathematical model. Measured diffusion coefficients were input to the model. Plots show percent of virions in the tissue compartment, as a function of time, for various concentrations of (B) HEC and (C) MC. In (B), curves for 20% HEC, 50% HEC, and undiluted HEC appear superimposed. In (C), curves for 50% MC and undiluted MC appear superimposed.
The diffusion coefficients measured here for HIV in placebo gels are similar to prior results for HIV virions in human cervicovaginal mucus. Cervical mucus likely evolved to function, in part, as a physicochemical barrier to pathogens (Cone, 2009). A recent study using particle tracking of HIV virus-like particles (VLPs) found that diffusion coefficients of HIV VLPs in fresh, human cervicovaginal mucus (from six different donors) were less than 10−11 cm2/s (Lai et al., 2009b). Another study, using time-resolved confocal microscopic particle tracking and fluorescence correlation spectroscopy to examine movement of fluorescently-labeled HIV virions, found a more modest effect: Virions were slowed by approximately 200 times in mucus compared to water (Boukari et al., 2009). Differences in results of the two studies may be attributed to differences in mucus collection methods and sample preparation. Thus, the diffusion coefficients measured here for HEC and MC were similar or lower than those previously measured for human cervicovaginal mucus, a natural physical barrier to HIV.
Diffusion coefficients measured here were also similar to those for HIV virions in a reversibly crosslinked hydrogel engineered to hinder HIV transport (Jay et al., 2009). That gel was designed using phenylboronic acid (PBA) and salicylhydroxamic acid (SHA) crosslinks that respond to changes in pH, hindering virion diffusion at vaginal pH. Diffusion coefficients from particle tracking experiments of fluorescently-labeled HIV-1 virions in the hydrogel ranged from 6 × 10−10 cm2/s at pH 4.3 to below 2 × 10−12 cm2/s at pH 4.8. These diffusion coefficients roughly correspond with the diffusion coefficients measured in this study for HIV in 20% (v/v) HEC in PBS and undiluted HEC, respectively.
Results from our Transwell system also indicated that the vaginal gels presented physical barriers HIV transport. There was a log reduction in levels of HIV after 0-, 4-, and 8- hour incubation where gel layers of approximately 150 μm had been applied to the Transwell membrane. In HIV neutralization assays, log reductions are typically considered biologically significant. Transwell results suggest that MC is a slightly superior barrier to HEC. This was unexpected given the higher diffusion coefficients measured for HIV virions in MC compared to HEC. Further, the thicknesses of MC layers were less than those of HEC layers. The result may be explained by different reactions of the gels to the dilution process. Perhaps MC is less prone to dilution when placed in contact with tissue culture medium. In HIV neutralization assays, log reductions are typically considered biologically significant, so the small differences between HEC and MC observed here are probably not biologically significant.
Strictly speaking, levels of HIV in the bottom compartment of the Transwell were higher than those predicted using the mathematical model of HIV diffusion with diffusion coefficients measured for HEC and MC. This can be attributed to: (1) convection from the top compartment to the bottom compartment during separation of the Transwell insert and bottom plate; and (2) dilution of gel layers reducing the level of viral hindrance by the gel. The former was likely created, in part, by hydrostatic pressure differences between the top and bottom fluid compartments of the small Transwells. In addition, dilution of the gel within the Tranwell system likely increased the effective diffusion coefficient of HIV within the gel. The volume ratio of gel to culture medium in the Transwell was approximately 2%. Levels of dilution of the thin gel layer within the Transwell system were greater than levels of dilution observed in vivo. However, even with these high levels, our results do indicate that there was a barrier effect of the thin gel layers compared to Transwells where no gel had been applied.
Our findings for HEC and MC, combined with our recent compartmental modeling (Lai et al, 2009a) suggest that gels used as placebos in microbicide clinical trials may function as physical barriers to HIV. Retarded HIV transport and actual trapping could provide increased time for lumenally active factors – microbicide ingredients, host defense molecules – to contact and neutralize virus. However, to the extent that those factors do not succeed in viral neutralization, viral trapping in a gel coating layer might prolong the infectious lifetime of the virions.
The success of the recent CAPRISA 004 study has buoyed the microbicides field, providing the first proof of principle that vaginal microbicide gels can successfully function, in a clinical trial setting, to reduce the rate of HIV transmission. This was a gel placebo-controlled trial. The possible prophylactic activity of placebo gels in clinical trials is recognized as a deficiency of blinded, placebo-controlled clinical trial designs for microbicides, since it could reduce the estimated effectiveness of the test gels from its actual value (Kilmarx and Paxton, 2003; Lagakos et al., 2008). However this effect, while unknown, is likely low. For example, while it is difficult to accurately quantify the efficacy of the HEC placebo in the HPTN trial, it is likely to be less than 10% (Masse et al., 2009). Results of the present study suggest that such a low placebo efficacy could be due, at least in part, to incomplete deployment of the gel in vivo. That is, vaginal coating may have been incomplete, leaving bare spots of epithelium exposed to the viral inoculum in semen.
Gels remain primary vehicles, together with intravaginal rings, for delivery of microbicides against HIV and other sexually transmitted pathogens. Their active pharmaceutical ingredients may act against HIV within the fluids of the lumen (e.g. entry inhibitors), or within the vaginal epithelium and stroma (e.g. reverse transcriptase inhibitors, as in the CAPRISA 004 trial). The potential barrier functioning of a gel could influence its biological functioning in both cases. For the former, there is a clear benefit to slowing viral transit to epithelial surfaces. An initial compartmental model developed in our lab illustrated this for the candidate microbicide Cyanovirin-N (Geonnotti and Katz, 2006). At present there are a number of antiretroviral drugs being tested as microbicides; these would act within the tissue. The mission of their vehicles would be to create and sustain a reservoir of prophylactic concentrations of drug throughout the epithelium exposed to invading HIV virions. Hindrance of virion diffusion would extend the safe time, after product insertion, for delivery of agents prior to the arrival of virions at the tissue surface.
The quantitative methods presented here can contribute to our understanding of the functioning of microbicide gels. Their results can help in the interpretation of information on microbicide pharmacokinetics. The time to gel effectiveness after application is governed by how rapidly microbicide ingredients are delivered by gel in relation to the time course of sexual activity, deposition of semen, HIV transport, and the initial interactions of virions with host cells. Thus, the methods here are also informative with respect to the pharmacodynamics of HIV neutralization itself.
Supplementary Material
Acknowledgements
We thank Dr. Jeffrey Lifson and Julian Bess of NCI Frederick for generously providing the fluorescently-labeled HIV virions used in particle tracking experiments; Dr. Sam Johnson and Dr. Yasheng Gao of the Duke University Light Microscopy Core Facility for assistance with imaging; the Center for Computer Integrated Systems for Microscopy and Manipulation (CISMM) at the University of North Carolina, Chapel Hill supported by the NIH NIBIB (NIH 5-P41-RR02170) for providing the Video Spot Tracker software, particularly Dr. Jeremy Cribb, Dr. Benjamin Evans, and Dr. Richard Superfine; and Barbara Sokolik-Wolak for assistance with the TZM-bl assay.
This study was supported from National Institutes of Health grant U19 A1077289, developmental grants from Duke University CFAR (NIH P30 AI 64518), and a National Science Foundation Graduate Research Fellowship for BEL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdool Karim S, Coletti A, Richardson B, Ramjee G, Hoffman I, Chirenje M, Taha T, Kapina M, Maslankowski L, Soto-Torres L. Safety and effectiveness of vaginal microbicides BufferGel and 0.5% PRO 2000/5 gel for the prevention of HIV infection in women: results of the HPTN 035 Trial; 16th Conference on Retroviruses and Opportunistics Infections; Montreal, QC, CANADA. 2009. [Google Scholar]
- Balimane PV, Chong S, Morrison RA. Current methodologies used for evaluation of intestinal permeability and absorption. Journal of Pharmacological and Toxicological Methods. 2000;44:301–312. doi: 10.1016/s1056-8719(00)00113-1. [DOI] [PubMed] [Google Scholar]
- Barnhart KT, Pretorius ES, Timbers K, Shera D, Shabbout M, Malamud D. In vivo distribution of a vaginal gel: MRI evaluation of the effects of gel volume, time and simulated intercourse. Contraception. 2004;70:498–505. doi: 10.1016/j.contraception.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Behrens I, Pena AIV, Alonso MJ, Kissel T. Nanoparticles in Human Intestinal Cell Lines and Rats: The Effect of Mucus on Particle Adsorption. Pharmaceutical Research. 2002 doi: 10.1023/a:1019854327540. [DOI] [PubMed] [Google Scholar]
- Behrens I, Stenberg P, Artursson P, Kissel T. Transport of Lipophilic Drug Molecules in a New Mucus-Secreting Cell Culture Model Based on HT29-MTX …. Pharmaceutical Research. 2001 doi: 10.1023/a:1010974909998. [DOI] [PubMed] [Google Scholar]
- Boukari H, Brichacek B, Margolis L, Nossal R. HIV-virions Appear To Be Trapped By Human Cervical Mucus. Biophysical Journal. 2009;96:35a. doi: 10.1021/bm900344q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckheit RW, Watson KM, Morrow KM, Ham AS. Development of topical microbicides to prevent the sexual transmission of HIV. Antiviral Research. 2010;85:142–158. doi: 10.1016/j.antiviral.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchelli R, Dehouck B, Descamps L, Fenart L, BuÈe-Scherrer V, Duhem C, Lundquist S, Rentfel M, Torpier G, Dehouck MP. In vitro model for evaluating drug transport across the blood-brain barrier. Advanced Drug Delivery Reviews. 1999;36:165–178. doi: 10.1016/s0169-409x(98)00083-0. [DOI] [PubMed] [Google Scholar]
- Cone R. Barrier properties of mucus. Advanced Drug Delivery Reviews. 2009;61:75–85. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Cutler B, Justman J. Vaginal microbicides and the prevention of HIV transmission. The Lancet Infectious Diseases. 2008;8:685–697. doi: 10.1016/S1473-3099(08)70254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- das Neves JJ, Bahia MMF. Gels as vaginal drug delivery systems. 2006;318:14. doi: 10.1016/j.ijpharm.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Dezzutti CS, James VN, Ramos A, Sullivan ST, Siddig A, Bush TJ, Grohskopf LA, Paxton L, Subbarao S, Hart CE. In vitro comparison of topical microbicides for prevention of human immunodeficiency virus type 1 transmission. Antimicrobial Agents And Chemotherapy. 2004;48:3834–3844. doi: 10.1128/AAC.48.10.3834-3844.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldblum PJ, Adeiga A, Bakare R, Wevill S, Lendvay A, Obadaki F, Olayemi MO, Wang L, Nanda K, Rountree W. SAVVY Vaginal Gel (C31G) for Prevention of HIV Infection: A Randomized Controlled Trial in Nigeria. PLoS ONE. 2008;3:e1474. doi: 10.1371/journal.pone.0001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes B, Ehrhardt C. Human respiratory epithelial cell culture for drug delivery applications. European Journal of Pharmaceutics and Biopharmaceutics. 2005;60:193–205. doi: 10.1016/j.ejpb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Geonnotti AR, Katz DF. Dynamics of HIV neutralization by a microbicide formulation layer: Biophysical fundamentals and transport theory. Biophysical Journal. 2006;91:2121–2130. doi: 10.1529/biophysj.106.086322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RH, Wawer MJ, Brookmeyer R, Sewankambo NK. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. The Lancet. 2001;357:1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- Guenthner PC, Secor WE, Dezzutti CS. Trichomonas vaginalis-induced epithelial monolayer disruption and human immunodeficiency virus type 1 (HIV-1) replication: implications for the sexual transmission of HIV-1. Infection and immunity. 2005;73:4155–4160. doi: 10.1128/IAI.73.7.4155-4160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nature Reviews Immunology. 2005;5:783–792. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- Halpern V, Ogunsola F, Obunge O, Wang C, Onyejepu N, Oduyebo O, Taylor D, McNeil L, Mehta N, Umo-Otong J, Otusanya S, Crucitti T, Abdellati S. Effectiveness of cellulose sulfate vaginal gel for the prevention of HIV infection: results of a Phase III trial in Nigeria. PLoS ONE. 2008;3:e3784. doi: 10.1371/journal.pone.0003784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammonds J, Chen X, Fouts T, DeVico A, Montefiori D, Spearman P. Induction of Neutralizing Antibodies against Human Immunodeficiency Virus Type 1 Primary Isolates by Gag-Env Pseudovirion Immunization. J. Virol. 2005;79:14804–14814. doi: 10.1128/JVI.79.23.14804-14814.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, Couchman, Walmer, Peters, Owen, Brown, Lavine, Katz Optical imaging and analysis of human vaginal coating by drug delivery gels. Contraception. 2007;75:142–151. doi: 10.1016/j.contraception.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson MH, Peters JJ, Walmer DK, Couchman GM, Katz DF. Optical instrument for measurement of vaginal coating thickness by drug delivery formulations. Review of Scientific Instruments. 2005;76 [Google Scholar]
- Jay JI, Shukair S, Langheinrich K, Hanson MC, Cianci GC, Johnson TJ, Clark MR, Hope TJ, Kiser PF. Modulation of Viscoelasticity and HIV Transport as a Function of pH in a Reversibly Crosslinked Hydrogel. Advanced Functional Materials. 2009;19:2969–2977. doi: 10.1002/adfm.200900757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MI, Fauci AS. An HIV Vaccine -- Evolving Concepts. N Engl J Med. 2007;356:2073–2081. doi: 10.1056/NEJMra066267. [DOI] [PubMed] [Google Scholar]
- Justin-Temu M, Damian F, Kinget R, Van Den Mooter G. Intravaginal gels as drug delivery systems. J Womens Health (Larchmt) 2004;13:834–844. doi: 10.1089/jwh.2004.13.834. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Di Berto G, Eaton L. Human immunodeficiency virus viral load in blood plasma and semen: review and implications of empirical findings. Sexually Transmitted Diseases. 2008;35:55–60. doi: 10.1097/olq.0b013e318141fe9b. [DOI] [PubMed] [Google Scholar]
- Kilmarx PH, Paxton L. Need for a true placebo for vaginal microbicide efficacy trials. The Lancet. 2003;361:785–786. doi: 10.1016/S0140-6736(03)12645-1. [DOI] [PubMed] [Google Scholar]
- Klasse PJ, Shattock R, Moore JP. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annual Review of Medicine. 2008;59:455–471. doi: 10.1146/annurev.med.59.061206.112737. [DOI] [PubMed] [Google Scholar]
- Lagakos SW, Gable A, Institute of Medicine. Committee on the Methodological Challenges in, H.I.V.P.T. Methodological challenges in biomedical HIV prevention trials. National Academies Press; Washington, D.C.: 2008. [Google Scholar]
- Lai BE, Henderson MH, Peters JJ, Walmer DK, Katz DF. Transport Theory for HIV Diffusion through In Vivo Distributions of Topical Microbicide Gels. Biophysical Journal. 2009a;97:2379–2387. doi: 10.1016/j.bpj.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SK, Hida K, Shukair S, Wang Y-Y, Figueiredo A, Cone R, Hope TJ, Hanes J. Human Immunodeficiency Virus Type 1 Is Trapped by Acidic but Not by Neutralized Human Cervicovaginal Mucus. J. Virol. 2009b;83:11196–11200. doi: 10.1128/JVI.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire RA, Zacharopoulos VR, Phillips DM. Carrageenan-based nonoxynol-9 spermicides for prevention of sexually transmitted infections. Sexually Transmitted Diseases. 1998;25:494–500. doi: 10.1097/00007435-199810000-00010. [DOI] [PubMed] [Google Scholar]
- Mascola JR, D’Souza P, Gilbert P, Hahn BH, Haigwood NL, Morris L, Petropoulos CJ, Polonis VR, Sarzotti M, Montefiori DC. Recommendations for the Design and Use of Standard Virus Panels To Assess Neutralizing Antibody Responses Elicited by Candidate Human Immunodeficiency Virus Type 1 Vaccines. J. Virol. 2005;79:10103–10107. doi: 10.1128/JVI.79.16.10103-10107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masse B, Boily M-C, Dimitrov D, Desai K. Efficacy dilution in randomized placebo-controlled vaginal microbicide trials. Emerging Themes in Epidemiology. 2009;6:5. doi: 10.1186/1742-7622-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias NR, Yamashita F, Lee VHL. Respiratory epithelial cell culture models for evaluation of ion and drug transport. Advanced Drug Delivery Reviews. 1996;22:215–249. [Google Scholar]
- Mauck CK, Katz DF, Sandefer EP, Nasution MD, Henderson M, Digenis GA, Su I, Page R, Barnhart K. Vaginal distribution of Replens and K-Y Jelly using three imaging techniques. Contraception. 2008;77:195–204. doi: 10.1016/j.contraception.2007.11.016. [DOI] [PubMed] [Google Scholar]
- McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol. 2010;10:11–3. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Microbicide Trials Network . Fact Sheet: HPTN 035 at a glance. Microbicide Trials Network; Pittsburgh, PA: 2009. [Google Scholar]
- Miller CJ, Li Q, Abel K, Kim EY, Ma ZM, Wietgrefe S, La Franco-Scheuch L, Compton L, Duan L, Shore MD, Zupancic M, Busch M, Carlis J, Wolinsky S, Wolinksy S, Haase AT. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. Journal of Virology. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefiori DC. Standardized Assessments of Neutralizing Antibodies for HIV/AIDS Vaccine Development. Durham: 2010. [Google Scholar]
- Morcock DR, Thomas JA, Gagliardi TD, Gorelick RJ, Roser JD, Chertova EN, Bess JW, Jr., Ott DE, Sattentau QJ, Frank I, Pope M, Lifson JD, Henderson LE, Crise BJ. Elimination of Retroviral Infectivity by N-Ethylmaleimide with Preservation of Functional Envelope Glycoproteins. J. Virol. 2005;79:1533–1542. doi: 10.1128/JVI.79.3.1533-1542.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndesendo VM, Pillay V, Choonara Y, Buchmann E, Bayever D, Meyer LC. A review of current intravaginal drug delivery approaches employed for the prophylaxis of HIV/AIDS and prevention of sexually transmitted infections. Aaps Pharmscitech. 2008;9:505–520. doi: 10.1208/s12249-008-9073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999;59:91–95. doi: 10.1016/s0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- Owen DH, Katz DF. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J Androl. 2005;26:459–469. doi: 10.2164/jandrol.04104. [DOI] [PubMed] [Google Scholar]
- Peterson L, Nanda K, Opoku B, Ampofo W, Owusu-Amoako M, Boakye A, Rountree W, Troxler A, Dominik R, Roddy R, Dorflinger L. SAVVY (C31G) gel for prevention of HIV infection in women: a Phase 3, double-blind, randomized, placebo-controlled trial in Ghana. PLoS ONE. 2007;2:e1312. doi: 10.1371/journal.pone.0001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. Intravaginal formulations to prevent HIV infection. Perspectives in Drug Discovery and Design. 1996;5:213–224. [Google Scholar]
- Pontier C, Pachot J, Botham R, Lenfant B, Arnaud P. HT29-MTX and Caco-2/TC7 monolayers as predictive models for human intestinal absorption: Role of the mucus layer. Journal of Pharmaceutical Sciences. 2001;90:1608–1619. doi: 10.1002/jps.1111. [DOI] [PubMed] [Google Scholar]
- Qian H, Sheetz MP, Elson EL. Single particle tracking. Analysis of diffusion and flow in two-dimensional systems. Biophysical Journal. 1991;60:910–921. doi: 10.1016/S0006-3495(91)82125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, Meehan MO, Lutalo T, Gray RH, The Rakai Project Study Group Viral Load and Heterosexual Transmission of Human Immunodeficiency Virus Type 1. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- Rohan LC, Sassi AB. Vaginal Drug Delivery Systems for HIV Prevention. The AAPS Journal. 2009;11:78–87. doi: 10.1208/s12248-009-9082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton MJ. Single-particle tracking: the distribution of diffusion coefficients. Biophysical Journal. 1997;72:1744–1753. doi: 10.1016/S0006-3495(97)78820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JL, Ballagh SA, Kwok C, Mauck CK, Weiner DH, Rencher WF, Callahan MM. Fourteen-day safety and acceptability study of the universal placebo gel. Contraception. 2007;75:136–141. doi: 10.1016/j.contraception.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Skoler-Karpoff S, Ramjee G, Ahmed K, Altini L, Plagianos MG, Friedland B, Govender S, De Kock A, Cassim N, Palanee T, Dozier G, Maguire R, Lahteenmaki P. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1977–1987. doi: 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]
- Suh J, Dawson M, Hanes J. Real-time multiple-particle tracking: applications to drug and gene delivery. Advanced Drug Delivery Reviews. 2005;57:63–78. doi: 10.1016/j.addr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Tao W, Richards C, Hamer D. Short Communication: Enhancement of HIV Infection by Cellulose Sulfate. AIDS Research and Human Retroviruses. 2008;24:925–929. doi: 10.1089/aid.2008.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien D, Schnaare RL, Kang FR, Cohl G, McCormick TJ, Moench TR, Doncel G, Watson K, Buckheit RW, Lewis MG, Schwartz J, Douville K, Romano JW. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. Aids Research and Human Retroviruses. 2005;21:845–853. doi: 10.1089/aid.2005.21.845. [DOI] [PubMed] [Google Scholar]
- Trapp S, Turville SG, Robbiani M. Slamming the door on unwanted guests: why preemptive strikes at the mucosa may be the best strategy against HIV. J Leukoc Biol. 2006;80:1076–1083. doi: 10.1189/jlb.0206121. [DOI] [PubMed] [Google Scholar]
- Truskey GA, Yuan F, Katz DF. Transport Phenomena in Biological Systems. 2nd ed. Pearson Education, Inc.; Upper Saddle River, New Jersey: 2009. [Google Scholar]
- Turville S, Aravantinou M, Miller T, Kenney J, Teitelbaum A, Hu L, Chudolij A, Zydowsky T, Piatak M, Bess J, Lifson J, Blanchard J, Gettie A, Robbiani M. Efficacy of Carraguard-based microbicides in vivo despite variable in vitro activity. PLoS ONE. 2008;3:e3162. doi: 10.1371/journal.pone.0003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme L, Govinden R, Mirembe FM, Gu√©dou F, Solomon S, Becker ML, Pradeep BS, Krishnan AK, Alary M, Pande B, Ramjee G, Deese J, Crucitti T, Taylor D, Group, C.S.S. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. The New England Journal of Medicine. 2008;359:463–472. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- Van Herrewege Y, Michiels J, Waeytens A, De Boeck G, Salden E, Heyndrickx L, van den Mooter G, de Bethune M-P, Andries K, Lewi P, Praet M, Vanham G. A dual chamber model of female cervical mucosa for the study of HIV transmission and for the evaluation of candidate HIV microbicides. Antiviral Research. 2007;74:111–124. doi: 10.1016/j.antiviral.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, Ketas T, Marx PA, Klasse PJ, Burton DR, Moore JP. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, Kiwanuka N, Kigozi G, Kiddugavu M, Lutalo T, Nalugoda F, Wabwire-Mangen F, Meehan MP, Quinn TC. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.