Abstract
Background
It is well known that enteric bacterial antigens drive the development of chronic colitis in a variety of different mouse models of the inflammatory bowel diseases (IBD). The objective of this study was to evaluate the role of gut-associated lymphoid tissue (GALT; Peyer’s patches, isolated lymphoid follicles), mesenteric lymph nodes (MLNs) and spleen in the pathogenesis of chronic colitis in mice.
Methods
Surgical as well as genetic approaches were used to generate lymphopenic mice devoid of one or more of these lymphoid tissues. For the first series of studies, we subjected recombinase activating gene-1-deficient mice (RAG−/−) to sham surgery (Sham), mesenteric lymphadenectomy (MLNx), splenectomy (Splx) or both (MLNx/Splx). In a second series of studies we intercrossed lymphotoxinβ-deficient (LTβ−/−) mice with RAG−/− animals to generate LTβ−/− × RAG−/− offspring that were anticipated to contain functional MLNs but be devoid of GALT and most peripheral lymph nodes. Flow purified naïve (CD4+CD45RBhigh) T-cells were adoptively transferred into the different groups of RAG−/− recipients to induce chronic colitis.
Results
We found that at 3–5 wks following T-cell transfer, all four of the surgically-manipulated RAG−/− groups (Sham, MLNx, Splx and MLNx/Splx) developed chronic colitis that was similar in onset and severity. Flow cytometric analysis revealed no differences among the different groups with respect to surface expression of different gut-homing markers nor were there any differences noted in IFN-γ and IL-17 generation by mononuclear cells isolated among these surgically-manipulated mice. Although we anticipated that LTβ−/− × RAG−/− mice would contain functional MLNs but be devoid of GALT and peripheral lymph nodes (PLNs), we found that LTβ−/− × RAG−/− mice were in fact devoid of MLNs as well as GALT and PLNs. Adoptive transfer of CD45RBhigh T-cells into LTβ−/− × RAG−/− mice or their littermate controls (LTβ+/+ × RAG−/−) induced rapid and severe colitis in both groups.
Conclusions
Taken together, our data demonstrate that: a) neither the GALT, MLNs nor PLNs are required for induction of chronic gut inflammation in this model of IBD and b) T-and/or B-cells may be required for the development of MLNs in LTβ−/− mice.
Keywords: inflammatory bowel disease, Crohn’s disease, gut-associated lymphoid tissue, T-cells, mesenteric lymph nodes (MLNs), lymphotoxin-beta
The intestinal tract is colonized by more than 100 trillion (1014) microorganisms, with the vast majority residing within the colonic lumen.1,2 The gut-associated lymphoid tissue (GALT; Peyer’s patches [PPs], isolated lymphoid follicles) as well as the gut-draining mesenteric lymph nodes (MLNs) play important roles in mounting mucosal immune responses to invading pathogenic and/or commensal microorganisms while minimizing the tissue inflammation and systemic immune activation that may arise from these protective responses.3–11 Studies from several different laboratories suggest that commensal bacterial antigens continuously gain access to the intestinal interstitium where they are endocytosed by intestinal dendritic cells (DCs) and then transported from the lamina propria and PPs to the draining MLNs.6–8,12 Using carefully regulated, low-level cellular immune responses to these nonpathogenic bacteria, the MLNs provide for both anatomical and functional compartmentalization of the adaptive immune responses, thereby generating a “firewall” to the systemic dissemination of these microorganisms.6,7 The mechanisms by which the cellular elements of the GALT and MLNs mediate these distinct and seemingly opposing functions are only now beginning to be revealed. Although there is good evidence demonstrating that MLNs play a critical role mounting and regulating mucosal immune responses to enteric bacteria, the evidence demonstrating a role for PPs as a major inductive site for adaptive immune responses is somewhat vague and ill-defined.6–8,13 Recent work by Worbs et al13 demonstrate that the MLNs (but not PPs) are required for the induction of systemic tolerance to orally administered luminal antigens. Those investigators convincingly showed that ovalbumin-loaded DCs are transported from the gut interstitium and PPs to the MLNs by the afferent lymphatics, where tolerance to these luminal antigens is induced. There is an accumulating literature suggesting that a break in tolerance to enteric antigens may be responsible for the induction of the chronic intestinal inflammation observed in patients who suffer from the inflammatory bowel diseases (IBDs; Crohn’s disease; ulcerative colitis).14–17 Indeed, the role of commensal bacteria in the pathogenesis of experimental IBD has been well characterized in number of different animal models of chronic gut inflammation.14,16,18 Although the etiology of these chronic inflammatory disorders has not been definitively elucidated, there is accumulating evidence to suggest that chronic gut inflammation arises from a complex interaction among genetic, immune, and environmental factors.16,18,19 A growing body of experimental and clinical data suggest that chronic gut inflammation arises from a dysregulated immune response to enteric bacterial antigens.
An immunologically important yet undefined aspect of disease pathogenesis in animal models of IBD is the anatomic location(s) where naïve T cells encounter enteric antigens to yield colitogenic effector cells. It has been assumed that naïve T cells migrate to the GALT/MLNs, where they are primed and polarized to yield T helper 1 (Th1) and/or Th17 effector cells. These colitogenic effector cells then exit the GALT and/or MLNs by way of the efferent lymphatic vessels, enter into the systemic circulation, and home to the gut where they initiate intestinal inflammation.15–18,20 In reality, there have been few studies that have assessed the role of the GALT and/or MLNs in the induction of chronic gut inflammation. Spahn et al21 demonstrated that dextran sulfate sodium (DSS)-induced colitis is more severe in mice devoid of MLNs, suggesting an overall suppressive function of these gut-draining lymph nodes, whereas Dohi et al22 showed an exacerbated inflammatory infiltrate and Th1 response in PP- and colonic patch-deficient mice exposed to intrarectal trinitrobenzene sulfonic acid (TNBS) dissolved in ethanol. In contrast to these studies, work by Makita et al23 suggests that neither the GALT, MLNs, spleen, nor peripheral lymph nodes were required for the induction (or suppression) of chronic colitis in mice. These investigators intercrossed lymphotoxin-α deficient (LTα−/−) mice with recombinase activating gene-2 deficient (RAG-2−/−) animals to generate LTα−/− × RAG−/− double-deficient progeny that are devoid of all organized lymphoid tissue.23 They found that adoptive transfer of naive or antigen-experienced (activated/memory) CD4+ T cells obtained from the lamina propria of mice with active colitis to LTα−/− × RAG-2−/− recipients induced colonic inflammation; however, the onset and severity of disease was delayed when compared to the disease induced by T-cell transfer to littermate controls (i.e., LTα+/+ × RAG-2−/− recipients). Although compelling, unanswered questions remain. For example, it is unclear how a global defect in LTα during development of lymphopenic RAG−/− mice affects lymphotoxin receptor-β expression and subsequent lymphotoxin signaling events associated with T-cell trafficking, priming, and polarization following adoptive transfer of naïve T-cells. Therefore, the objectives of this study were to evaluate, using both surgical as well as genetic approaches, the role of gut-associated and secondary lymphoid tissue in the conversion of naïve T-cells to colitogenic Th1/Th17 effector cells and their induction of chronic colitis. We present data demonstrating that neither the spleen, GALT, MLNs nor peripheral lymph nodes (PLNs) are required for the induction of chronic gut inflammation.
MATERIALS AND METHODS
Animals
Healthy wildtype (WT; C57BL/6) and recombinase activating gene-1 deficient (RAG−/−) mice were purchased from the Jackson Laboratories (Bar Harbor, ME), whereas lymphotoxin-β-deficient (LTβ−/−; C57Bl/6) mice were obtained from Dr. Nancy Ruddle (Yale University).24 LTβ−/− mice were intercrossed with RAG−/− animals to generate LTβ−/− × RAG−/− double-deficient offspring that were anticipated to possess functional MLNs but be devoid of GALT (isolated lymphoid follicles, PPs) and most secondary lymphoid tissue.4,9,10,21,24,25 Animals were maintained on 12/12-hour light/dark cycles in standard animal cages with filter tops under specific pathogen-free conditions in our animal care facility at Louisiana State University (LSU) Health Sciences Centers and given standard laboratory rodent chow and water ad libitum. All experimental procedures involving the use of animals were reviewed and approved by the Institutional Animal Care and Use Committee of LSU Health Sciences Center and performed according to the criteria outlined by the National Institutes of Health.
Surgery
For some experiments, the MLNs and/or spleen were surgically removed from RAG−/− mice by performing a mesenteric lymphadenectomy (MLNx) and/or splenectomy (Splx) using previously published protocols.13,23 Briefly, 6–8-week-old mice were anesthetized using isoflurane during surgery. A ventral incision was made on the abdomen and the intestinal loops were gently reflected to the right to expose the mesentery. A dissecting microscope was used to visualize the lymphatic vessels emerging from the rudimentary MLNs. The MLNs were excised by blunt dissection and incisions were closed with sutures. For splenectomy, a lateral incision was performed on the left abdomen. The spleen was removed after blood vessel ligation and incisions were closed with sutures. Sham-operated mice (Sham) were treated identically; however, neither the MLNs nor the spleen were removed. Following surgery mice were allowed to recover for 2 weeks. Confirmation that the MLNs and/or spleen had been removed was determined by stereomicroscopy at 8 weeks following T-cell transfer or when the animals lost >15% of their original body weight and were euthanized.
Antibodies
The following antibodies were purchased either from BD Pharmingen (San Diego, CA) or eBioscience (San Diego, CA) for cell isolation and flow cytometric analysis: CD45RB-FITC, CD4-APC, CD103-FITC, CD29-PE, lymphocyte Peyer’s patch adhesion molecule-1 (LPAM-1)-biotin + streptavidin-phycoerythrin (SA-PE)-Texas Red, CD25-FITC, CD49d-PE, CD4-PE-Cy5, IFN-γ-FITC, IL-17A-PE, and CD4-PerCP.
T-cell Transfer Model of Chronic Colitis
Chronic colitis was induced via adoptive transfer of naïve CD4+CD45RBhigh T cells derived from WT donors using our previously published method.26,27 Briefly, spleens were removed from donor WT mice and teased into single cell suspensions in phosphate-buffered saline (PBS) with 4% fetal bovine serum (FBS; FACS buffer) using the frosted sides of glass microslides. CD4+ T cells were enriched by negative selection using Dynal Mouse CD4 Negative Isolation Kit (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Enriched CD4+ T cells were labeled with anti-CD4 and anti-CD45RB antibodies and sorted into CD4+CD45RBhigh and CD4+CD45RBlow fractions by two-color sorting on a FACS Aria (Becton-Dickinson, San Jose, CA). The CD45RBhigh and CD45RBlow populations were defined as the 40% of cells exhibiting the brightest CD45RB staining and the 15% of cells with the dimmest CD45RB expression, respectively, and were found to be >98% pure on post-sort analysis. RAG−/− mice were intraperitoneally injected with 0.5 × 106 cells of either CD4+CD45RBhigh or CD4+CD45RBlow (as a healthy control group) T cells suspended in 500 µL PBS. Clinical evidence of disease (e.g., losing body weight and loose stool/diarrhea) was monitored and recorded weekly from the time of the injection.
Macroscopic and Histological Evaluation of Chronic Colitis
At 8 weeks following T-cell reconstitution or when animals lost >15% of their original body weight, RAG−/− mice were euthanized and the colons were removed, cleaned of fecal material, and scored for macroscopic evidence of inflammation using our previously published scoring criteria.26,27 Briefly, normal colonic morphology was assessed a score of 0; mild bowel wall thickening in the absence of visible hyperemia was assigned a score of 1; moderate bowel wall thickening and hyperemia was given a score of 2; severe bowel wall thickening with rigidity and marked hyperemia was assigned a score of 3; and severe bowel wall thickening with rigidity, hyperemia, and colonic adhesions was given a score of 4. Colonic length and weight were also measured to calculate weight-to-length ratios, which provide a quantitative index of inflammation.27 In addition to macroscopic inflammation, representative sections of distal colon were placed in 10% PBS-formalin, fixed overnight at 4°C, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). Blinded histopathological evaluation of the colons was performed by a pathologist unaware of the treatment groups using our published scoring criteria.27
Lymphocyte Isolation and Analysis
Lymphocytes were obtained from the spleen and/or MLNs (when present) as well as the colonic lamina propria and analyzed by flow cytometry as previously described.26–28 Briefly, spleens were removed from RAG−/− mice reconstituted with either CD4+CD45RBhigh T cells or CD4+CD45RBlow T cells and teased into a single cell suspension using the frosted ends of two glass slides in 4% FACS buffer on ice. Colonic lamina propria mononuclear cells (LPMCs) were prepared by the digestion of the finely minced intestinal pieces remaining after intestinal intraepithelial lymphocyte (IEL) isolation in RPMI-1640, 4% FBS, and collagenase type VIII (200 U/mL, Sigma, St. Louis, MO) for 40 minutes at 250 rpm in a 37°C shaker. Cells from the spleen and cLP were further enriched by centrifugation over a 44%/70% Percoll gradient. The cell pellet was washed and resuspended in FACS buffer and viable cells were counted using a solution of 0.4% Trypan blue in 1× PBS. For surface antigen staining, 1 × 106 cells were placed in individual wells of a 96-well plate, incubated first with Fc receptor (FcR) block (CD16/CD32), and then stained with the appropriate antibody cocktails. After staining, cells were fixed for 20 minutes on ice in freshly prepared 2% ultrapure formaldehyde (Polysciences, Warrington, PA) and surface expression levels of CD103, CD29, LPAM-1, CD25, and CD49d on CD4+ T cells were analyzed the next day on the FACS-Calibur or LSRII (BD Biosciences). Absolute numbers of CD4+ T cells in the spleen and colonic lamina propia of reconstituted animals were calculated by multiplying the total number of viable cells isolated from each tissue by the percentage of total cells positive for CD4 as determined by flow cytometric analysis. Flow cytometry data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Intracellular and Extracellular cytokine analyses
Colonic lamina propria mononuclear cells were prepared as described above and 1.5 × 105 cells were cultured for 16 hours in 200 µL of complete RPMI-1640 medium at 37°C and 5% CO2 in anti-CD3ε monoclonal antibody (mAb) (BD Pharmingen)-coated 96-well flat-bottom plates containing soluble anti-CD28 mAb (eBioscience, 1 µg/mL final concentration). BD GolgiStop (BD Pharmingen) was added for the last 6 hours. Cells were harvested, stained with anti-CD4, permeabilized using the BD cytofix/cytoperm kit (BD Pharmingen), stained with IFN-γ and IL-17A mAb, and analyzed for intracellular cytokine production by flow cytometry as previously described.27 To determine extracellular cytokine production, colonic LPMCs (1.0 × 105 cells) were cultured for 16 hours in 200 µL of complete RPMI-1640 medium at 37°C and 5% CO2 in an anti-CD3ε mAb-coated 96-well flat-bottom plate with 1 µg/mL soluble anti-CD28 mAb. The supernatants were collected and stored at −80°C until analyzed. IFN-γ and IL-17A levels in the supernatants were determined by sandwich enzyme-linked immunosorbent assay (ELISA) using the commercial kits Mouse IFN-γ or IL-17A ELISA (eBioscience) as described by the manufacturer.
Statistics
Data are presented as means ± standard error of the mean (SEM). Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by Dunnett’s post-hoc test. A probability value (P value) of P < 0.05 was considered significant.
RESULTS
Induction of Chronic Colitis in the Presence or Absence of MLNs and/or Spleen
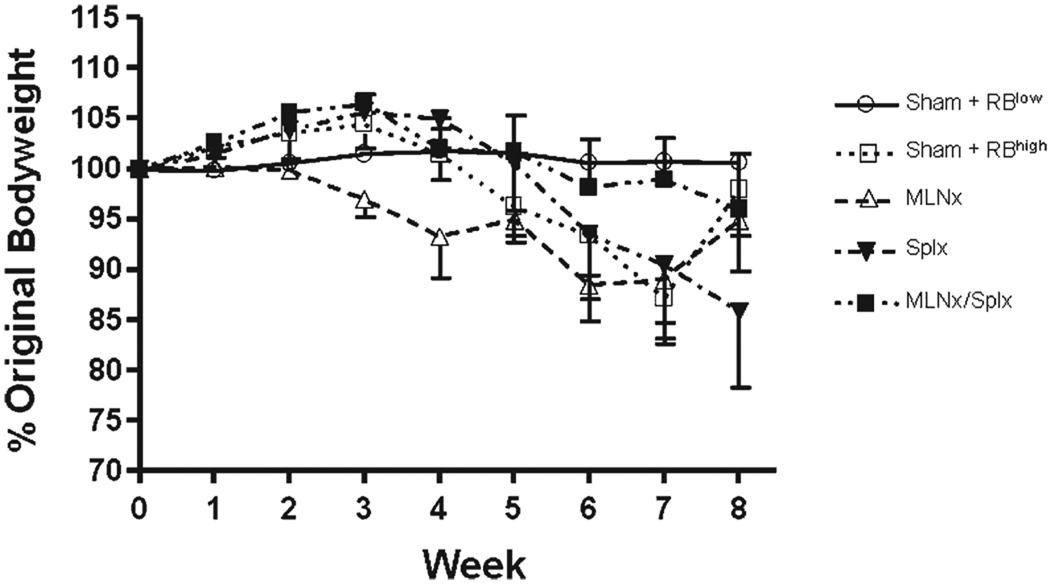
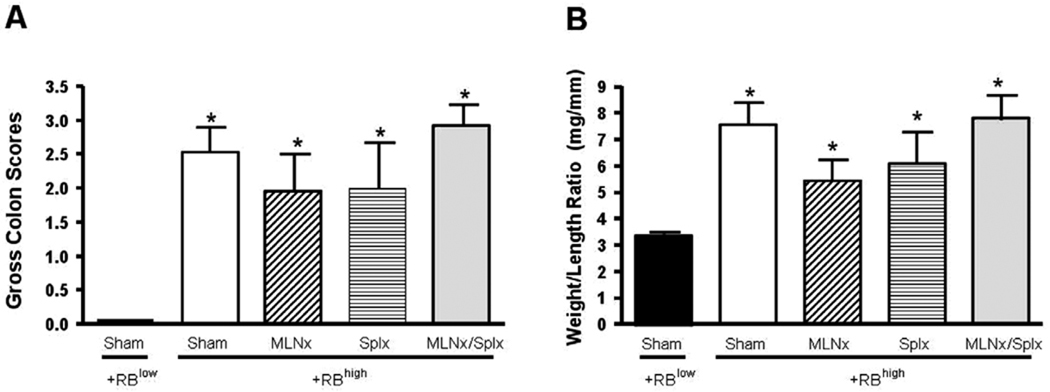
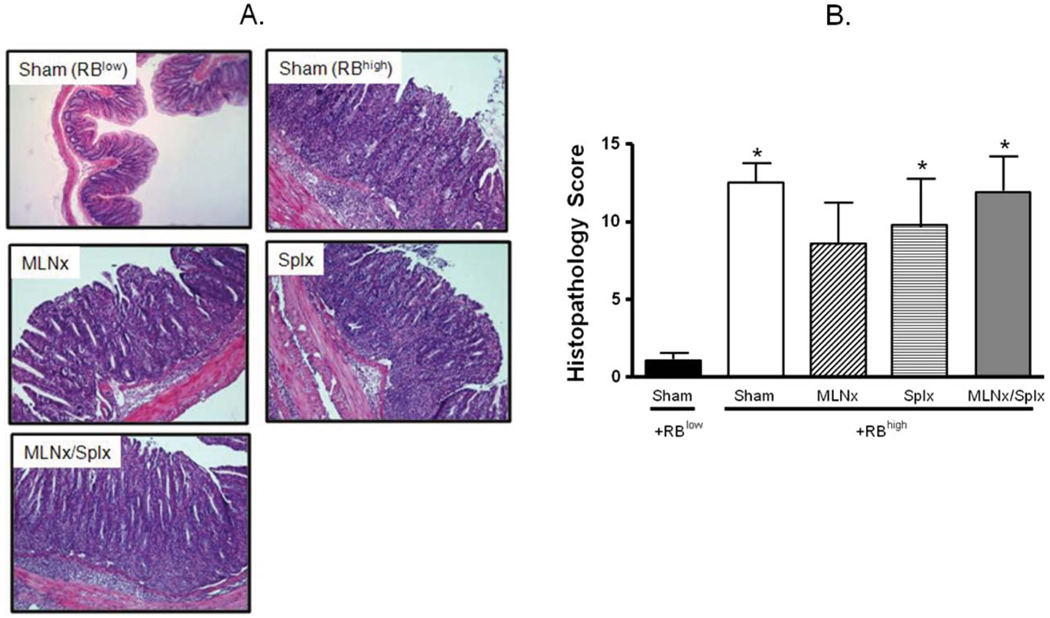
As a first step toward defining the roles of MLNs and/or spleen in enteric antigen-induced conversion of naïve T-cells to yield colitogenic effector cells, RAG−/− mice were subjected to sham, MLNx, and/or Splx surgeries prior to adoptive transfer of naïve CD4+CD45RBhigh T cells. For comparison, a group of sham-operated RAG−/− mice were reconstituted with CD4+CD45RBlow T cells. Beginning at 3–5 weeks following T-cell transfer, mice in the different groups reconstituted with CD4+CD45RBhigh T-cells began to lose body weight and developed visible signs of disease including hunched-over appearance, loose stools, and piloerection (Fig. 1). In contrast, RAG−/− mice that received CD4+CD45RBlow T cells appeared healthy and never lost weight throughout the 8-week observation period (Fig. 1). Macroscopic evidence of colonic inflammation (hyperemia, bowel wall thickening, and adhesions) at 8 weeks following T-cell transfer (or when mice lost >15% of their original body weight) was significantly increased in all four of the CD4+CD45RBhigh→RAG−/− groups compared to the healthy CD4+CD45RBlow→RAG−/− group (Fig. 2A). No statistically significant differences were noted among the different CD4+CD45RBhigh→RAG−/− groups. In addition to macroscopic colon scores, we also quantified the colon weight-to-length (W/L) ratio for each animal, as our previous studies have demonstrated this to be a quantitative index of colonic inflammation that correlates well with blinded histopathological analysis.27 We observed significant increases in the W/L ratios in the four CD4+CD45RBhigh→RAG−/− groups when compared to the CD4+CD45RBlow→RAG−/− controls; however, no statistical differences were identified among the four CD4+CD45RBhigh→RAG−/− groups (Fig. 2B). Blinded histopathological evaluation of the colons revealed that virtually all of the animals in the four CD4+CD45RBhigh→RAG−/− groups developed moderate-to-severe disease characterized by extensive transmural infiltration of polymorphonuclear leukocytes (PMNs), mononuclear leukocytes (monocytes, lymphocytes), bowel wall thickening, goblet cell loss, abnormal crypt architecture, and crypt abscesses when compared to their healthy CD4+CD45RBlow→RAG−/− controls (Fig. 3). Histopathology scores were found to be significantly increased in the Sham, Splx, and MLNx + Splx groups when compared to the CD4+CD45RBlow→RAG−/−group. Although the histopathology score for the MLNx group was 5-fold greater than that of the CD4+CD45RBlow→RAG−/−, this value did not achieve statistical significance. Nevertheless, no significant differences were observed among these four groups, suggesting that neither the onset nor the severity of chronic colitis is dependent on the presence of the MLNs and/or the spleen.
FIGURE 1.
Body weights of recombinase activating gene-1-deficient (RAG−/−) mice reconstituted with naïve T-cells. C57BL/6 RAG−/− mice underwent sham surgery (Sham), mesenteric lymphadenectomy (MLNx), splenectomy (Splnx), or both (MLNx/Splx), allowed to recover for 2 weeks, and then reconstituted with 5 × 105 CD4+CD45RBhigh (naïve) T-cells derived from wildtype C57BL/6 donors. For comparison, 5 × 105 CD4+CD45RBlow T cells were transferred to sham-operated RAG−/− mice. Animal body weights were recorded weekly and are shown as percentages of their original weights. Data represent means ± SEM for n = 6–8 mice in each group. Comparisons between groups were determined by one-way ANOVA.
FIGURE 2.
Development of chronic colitis at 8 weeks following T-cell transfer. (A) Macroscopic colon scores. Colon scores were obtained using our previously published criteria as described in Materials and Methods. (B) Colon weight-to-length ratios. Data represent means ± SEM for n = 6–8 mice in each group. Comparisons between means were determined by one-way ANOVA followed by Dunnett’s post-hoc test; *P < 0.05 versus RBlow group.
FIGURE 3.
Histopathology at 8 weeks following T-cell transfer. (A) Representative histopathology. (B) Blinded histopathological scores of colons were assigned by a pathologist unaware of the treatment groups using our previously published scoring criteria.27 Data represent means ± SEM for n = 6–8 mice in each group. Comparisons between groups were determined by one-way ANOVA followed by Dunnett’s post-hoc test; *P < 0.05 versus RBlow group.
Colonic T-cell Accumulation and Integrin Expression in the Presence or Absence of MLNs and/or Spleen
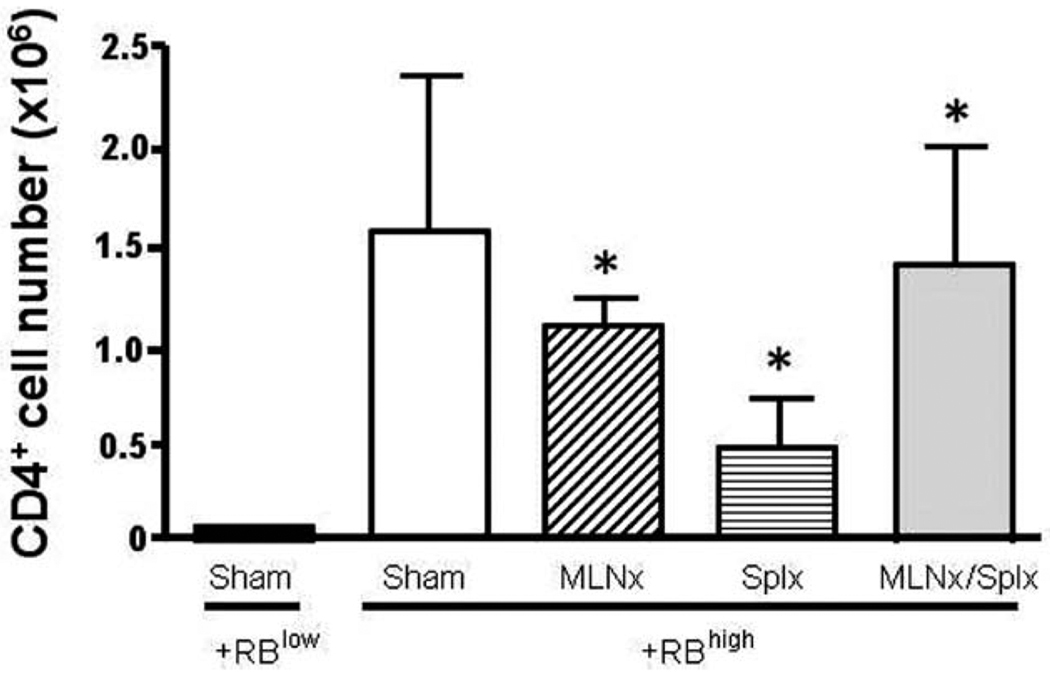
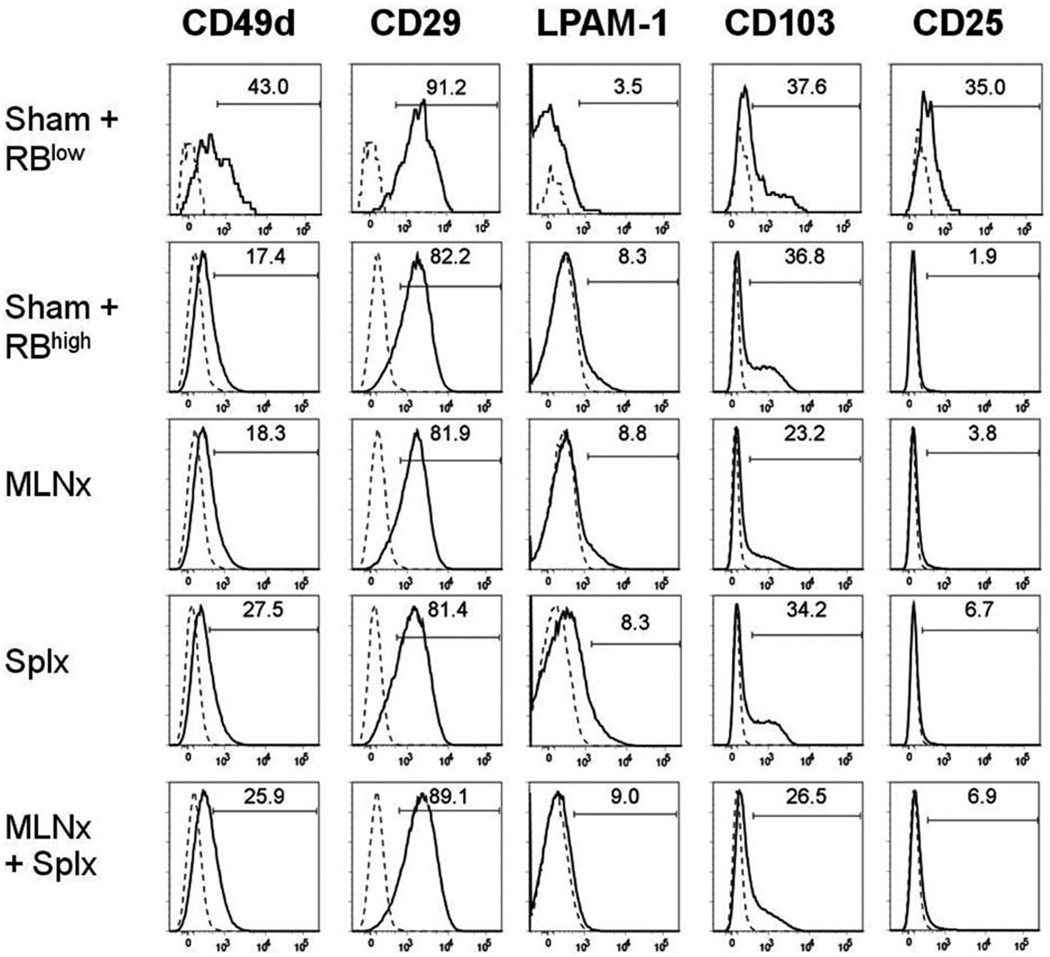
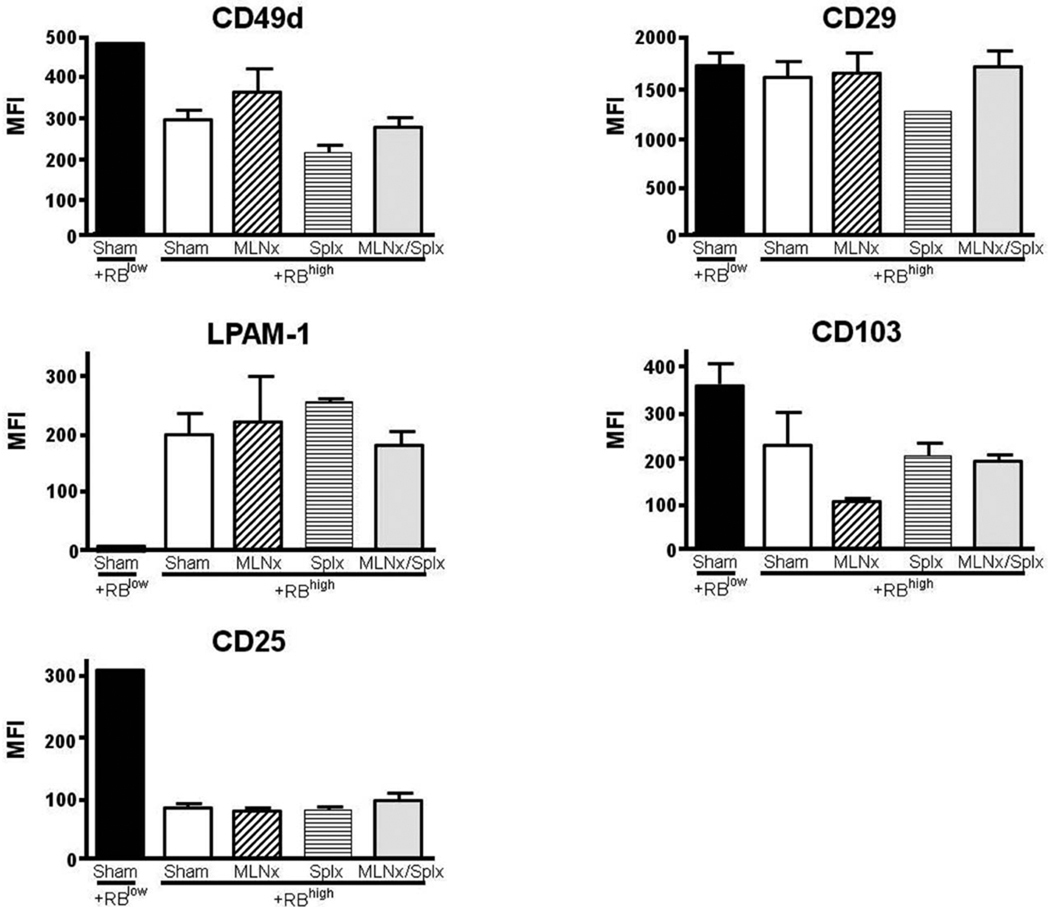
Having established that neither the MLNs nor the spleen are required for induction of chronic colitis, we wished to determine how the absence of MLNs and/or the spleen affected T-cell accumulation and induction of gut-homing marker expression on T cells obtained from the colonic lamina propria of the different mice. We found that the numbers of CD4+ T cells within the colonic lamina propria of all four CD4+CD45RBhigh→RAG−/− groups were significantly greater when compared to their CD4+ CD45RBlow→RAG−/− controls (Fig. 4). Although we noted a trend for fewer T cells in the Splnx group, this difference was not statistically different from the other three groups that received CD4+CD45RBhigh T cells. We next examined how the absence of MLNs and/or spleen affected the surface expression of CD49d (α4 integrin), CD29 (β1 integrin), LPAM-1 (α4β7 integrin), CD103 (αE integrin), and CD25 on CD4+ T cells obtained from colonic lamina propria of reconstituted RAG−/− mice. We observed that CD49d expression decreased from ≈43% in the CD4+CD45RBlow→RAG−/− to ≈17, 18, 27, and 26% in the sham, MLNx, Splx, and MLNx+Splx groups, respectively (Fig. 5). This same trend was also observed for CD49d expression when expressed as median fluorescence intensity (MFI; Fig. 6). A similar trend was observed for CD103 expression in that the MFI was decreased by 30–40% in the four CD4+CD45RBhigh→RAG−/− groups when compared to the CD4+CD45RBlow→RAG−/− controls (Fig. 6). A large decrease (>80%) in CD25 surface expression was observed in all four of the CD4+CD45RBhigh→RAG−/− groups when compared to the healthy CD4+CD45Rlow→RAG−/− controls (Figs. 5, 6). Conversely, although the expression of the gut-homing integrin α4β7 (LPAM-1) was relatively low compared to freshly isolated WT T cells (data not shown), it was increased significantly in the four CD4+CD45RBhigh→RAG−/− groups when compared to the noncolitic CD4+CD45RBlow→RAG−/− group (Figs. 5, 6). Finally, we observed little or no alteration in CD29 expression among the five different groups (Figs. 5, 6).
FIGURE 4.
CD4+ T-cell numbers in the colonic lamina propria of RAG−/− mice reconstituted with naïve T-cells. CD4+-cell numbers were obtained by multiplying the total number of viable cells isolated from the colonic lamina propria by the percentage of total cells positive for CD4 as determined by flow cytometry. Data represent means ± SEM for n = 3–4 mice in each group. Comparisons between groups were determined by one-way ANOVA.
FIGURE 5.
Expression of different surface markers on CD4+ T cells isolated from the colonic lamina propria of RAG−/− mice reconstituted with naïve T-cells. Surface expression of CD49d (α4 integrin), CD29 (β1 integrin), LPAM-1 (α4β7 integrin), CD103 (αE integrin), and CD25 on CD4+ T cells were analyzed using flow cytometry. Solid lines represent staining with mAb specific for the indicated molecules, whereas the broken line represents the isotype control staining. Numbers represent the percentage of positive cells for each surface molecule among CD4+ T cells. Data shown are representative of at least two separate experiments with 2–4 mice/group.
FIGURE 6.
Median fluorescence intensity (MFI) of different surface molecules on CD4+ T cells obtained from the colonic lamina propria of RAG−/− mice reconstituted with naïve T-cells. The MFIs of (A) CD49d (α4 integrin), (B) CD29 (β1 integrin), (C) LPAM-1 (α4β7 integrin), (D) CD103 (αE integrin), and (E) CD25 expression on CD4+ T cells were obtained using FlowJo software. Data represent means ± SEM. Comparisons between groups were determined by one-way ANOVA.
T-cell Polarization and Cytokine Production in the Presence or Absence of MLNs and/or Spleen
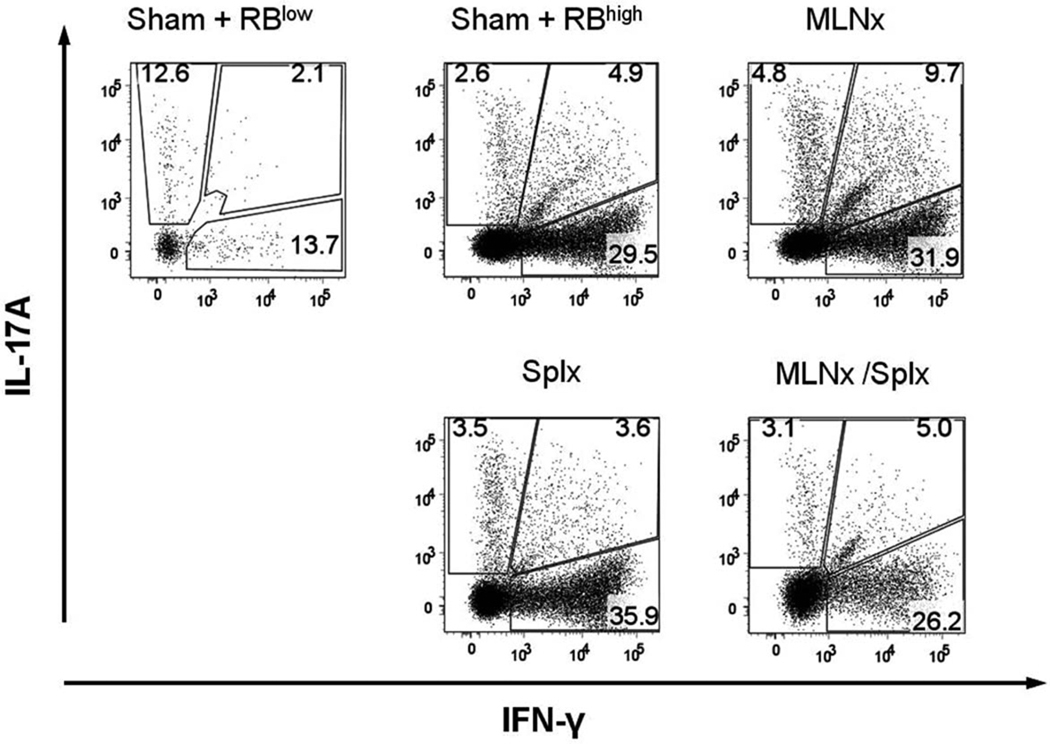
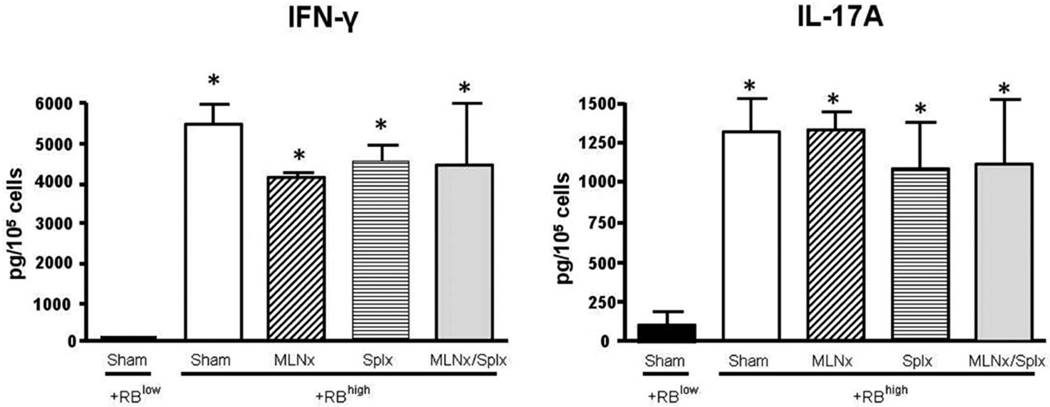
Because MLNs and/or spleen is/are thought to be important for enteric antigen-dependent priming and polarization of naïve T cells to yield disease-producing effector cells, we investigated how the absence of MLNs and/or the spleen affected this process. To do this we stimulated LPMCs obtained from the colonic lamina propria of reconstituted RAG−/− mice with plate-bound CD3ε and soluble CD28 mAbs ex vivo, and quantified the intracellular production of IFN-γ and IL-17A in CD4+ T cells using flow cytometry. We observed that the percent of CD4+ T cells expressing IFN-γ increased from 16% in the CD4+CD45RBlow→RAG−/− animals to ≈35, 42, 40, and 31% in the sham, MLNx, Splx, and MLNx+Splx groups, respectively (Fig. 7). In addition, we found that the percent of CD4+ T-cells expressing IL-17A ranged from 15% in T cells isolated from CD4+CD45RBlow→RAG−/− mice to ≈8, 15, 7, and 8% in the sham, MLNx, Splx, and MLNx+Splx groups, respectively (Fig. 7). Finally, we observed that the percent of T cells producing both IFN-γ and IL-17A increased from 2% in the CD4+CD45RBlow→RAG−/− animals to ≈5, 10, 4, and 5% in the sham, MLNx, Splx, and MLNx+Splx mice, respectively (Fig. 7). In addition to intracellular cytokine production, we also quantified extracellular release of the two cytokines following ex vivo activation of LPMCs obtained from the colons of the different mice. We found that the production of IFN-γ increased from very low levels in LPMCs obtained from CD4+CD45RBlow→RAG−/− mice (57 pg/105 cells) to levels ranging from 4220 to >5500 pg/105 cells for the sham, MLNx, Splx, and MLNx+Splx CD4+CD45RBhigh→RAG−/− animals (Fig. 8). Although the percentage of CD4+ expressing IL-17A was not increased in colitic mice, the secreted levels of this cytokine were significantly increased from 133 pg/105 cells for cells obtained from CD4+CD45RBlow→RAG−/− to >1100 pg/105 cells for cells taken from all four CD4+ CD45RBhigh→RAG−/− groups (Fig. 8). No significant differences were observed in the levels of the cytokines among these four groups (Fig. 8).
FIGURE 7.
Intracellular expression of IFN-γ and IL-17A by CD4+ T cells obtained from the colonic lamina propria of RAG−/− mice reconstituted with naïve T-cells. Intracellular IFN-γ and IL-17A levels were determined using flow cytometry following ex vivo stimulation of LPMCs with CD3ε and CD28 mAbs for 16 hours. Numbers represent the percentages of CD4+ T cells expressing the different cytokines. Data shown are representative from at least two separate experiments with 2–4 mice/group.
FIGURE 8.
Secreted cytokine levels by colonic LPMCs obtained from colitic mice. LPMCs were stimulated ex vivo with CD3ε and CD28 mAbs for 16 hours. (A) IFN-γ and (B) IL-17A levels in the supernatants were quantified by sandwich ELISA. Data represent means ± SEM for n = 3 mice in each group. Comparisons between groups were determined by one-way ANOVA followed by Dunnett’s post-hoc test; *P < 0.05 versus RBlow group.
Induction of Chronic Colitis in RAG−/− Mice Lacking GALT, MLNs, and Peripheral Lymph Nodes
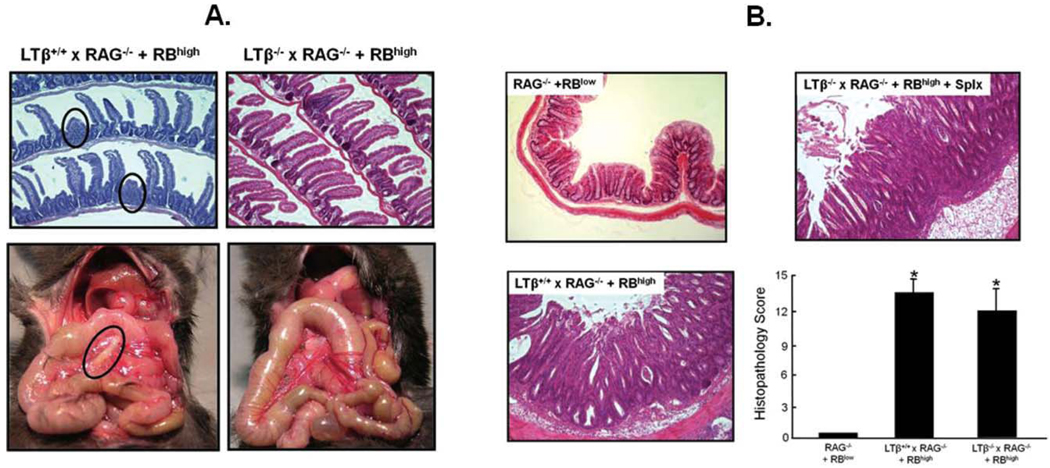
Data obtained from the studies presented above suggested that surgical removal of both the MLNs and spleen had little effect on enteric antigen-dependent priming and polarization of naïve T-cells to yield colitogenic effector (Th1/Th17) cells. Indeed, adoptive transfer of naïve T-cells into MLNx and/or Splx RAG−/− recipients induced chronic colitis with an onset and severity very similar to sham-operated controls. It could be argued that other lymphoid tissues such as the GALT and/or PLNs could substitute for the MLNs and spleen in promoting the conversion of naïve T cells to disease-producing effector cells. Therefore, we next determined whether adoptive transfer of naïve T cells into LTβ−/− × RAG−/− recipients developed disease, as these mice would be predicted to possess functional MLNs but lack PPs, isolated lymphoid follicles (ILFs) and most PLNs.4,9,10,21,24,25 Surprisingly, and in contrast to what has been described for LTβ−/− mice, LTβ−/− × RAG−/− lacked GALT (ILFs, PPs), PLNs, and MLNs (Fig. 9A). Furthermore, we found that adoptive transfer of naïve T-cells into LTβ−/− × RAG−/− mice induced moderate to severe colitis that began to develop within 2–3 weeks following T-cell transfer (Fig. 9B). This accelerated disease was also observed in their littermate LTβ+/+ × RAG−/− controls as well. The reason for this accelerated disease does not appear to be classical graft versus host disease since we transferred syngeneic WT T-cells into LTβ−/− × RAG−/− and LTβ+/+ × RAG−/− recipients.
FIGURE 9.
(A) LTβ−/− × RAG−/− double-deficient mice are devoid of PPs, MLNs, and most peripheral lymphoid tissue. LTβ−/− × RAG−/− double-deficient mice were generated by interbreeding RAG−/− mice with LTβ−/− animals (both on the C57Bl/6 background) to generate LTβ−/− × RAG−/− or their LTβ +/+ × RAG−/− littermate controls. Mice were injected (ip) with 5 × 105 CD45RBhigh T cells (RBhigh) to induce colitis. Inspection of mice at 2–3 weeks post-T-cell transfer revealed that LTβ−/− × RAG−/− mice were devoid of PPs and MLNs (right panels) as well as lymphoid follicles and most peripheral lymph nodes (not shown), whereas their littermate controls contained developing/residual PPs (red circles) and MLNs similar to RAG−/− (black circle). (B) Adoptive transfer of naive T cells into LTβ−/− × RAG−/− or LTβ+/+ × RAG−/− induces chronic colitis. At 2–3 weeks following T-cell transfer, mice were euthanized and their colons scored in a blinded fashion using our published scoring criteria27; n = 3 mice for each group.
DISCUSSION
The literature contains numerous studies demonstrating that the GALT and/or MLNs are the major inductive sites where naïve T-cells encounter enteric bacterial antigens such that the appropriate immune response may be initiated. Thus, it has been assumed that the GALT and/or MLNs play critical roles in enteric antigen-induced chronic gut inflammation.20,29 Previous studies have suggested that MLNs may function to limit or suppress the induction of the erosive, self-limiting colitis induced by toxic chemicals,21 whereas another study suggested that neither the GALT, MLNs, nor the peripheral lymph nodes are required for the induction of chronic colitis in RAG−/− mice reconstituted with naive or antigen-experienced (activated) CD4+ T cells.23 In the latter study, investigators used a model very similar to our own.23 Although a provacative study, Makita et al23 did not assess how a systemic defect in LTα expression during the development of the lymphopenic RAG−/− mice might affect LT receptor-β expression and LT signaling events associated with T-cell trafficking, priming, and polarization following adoptive transfer of T cells, especially if the MLN environment is important for regulating immune responses to commensal bacterial antigens. Therefore, we surgically removed the MLNs and/or spleen in healthy RAG−/− to evaluate the roles of these gut-draining and peripheral lymphoid tissues in the pathogenesis of T-cell-induced colitis. Although our initial focus was the MLNs, we also wished to determine how the absence of the spleen might affect the onset and severity of chronic gut inflammation in this mouse model of IBD. It is quite possible that commensal enteric antigens may gain access to the systemic circulation where splenic DCs would endocytose, process and present these gut-derived antigens to naïve T cells, thereby promoting their conversion to colitogenic effector cells within the spleen.8 Indeed, Eksteen et al30 have recently demonstrated that splenic DCs can enhance expression of gut-homing surface proteins CCR9 and α4β7 on T-cells. Data obtained in the current study clearly demonstrate that neither the MLNs nor the spleen are required for induction of chronic gut inflammation. In addition, we show that the absence of MLNs and/or spleen did not affect expression of the different T-cell-associated integrins nor did their absence alter cytokine production of T-cells obtained from the colonic lamina propria of colitic mice. Of note however, was the significant decrease in CD25 expression on T-cells obtained from the four CD45RBhigh→RAG−/− groups compared to their healthy CD45RBlow→RAG−/− counter-parts (Figs. 5, 6). The reasons for the decreased expression of the IL-2 receptor are not clear at the present time but may be related to the fact that the large majority of transferred CD45RBlow T-cells express CD25 whereas CD45RBhigh T-cells do not. Another interesting observation noted in the current study was that both intracellular as well as secreted levels of IFN-γ from LPMCs obtained from the colons of all four CD45RBhigh→RAG−/− groups were ≈3-fold greater than for IL-17A (Figs. 7, 8), suggesting a skewing toward a more Th1-like inflammation in this model. Taken together, these data suggest that neither the MLNs nor spleen are required for the conversion of naïve T cells to their disease-producing phenotype. These data are somewhat surprising given the large body of work demonstrating that enteric antigen-loaded DCs migrate from the intestinal lamina propria and PPs to the MLNs by way of the afferent lymphatics in order to induce immune responses to commensal bacteria.5–8,12,13,31,32 However, it could be argued that other gut-associated or secondary lymphoid tissues may substitute as inductive sites for naïve T-cell priming and polarization. Therefore, we generated RAG−/− mice that we assumed would possess functional MLNs but lack PPs, ILFs, and most PLNs.4,9,10,21,24,25 Surprisingly, and in contrast to what has been described for LTβ−/− mice, LTβ−/− × RAG−/− lacked GALT (ILFs, PPs), PLNs, and MLNs (Fig. 9A). The mechanisms responsible for this novel finding are not clear; however, it may be that T- and/or B-cells are required for MLN development in the absence of LTβ. Indeed, one report demonstrated that deletion of the T-cell-associated LTβ receptor ligand (LIGHT) in LTβ−/− mice resulted in a dramatic decrease in the size and numbers of MLNs.33 Nevertheless, we found that adoptive transfer of naïve T cells into LTβ−/− × RAG−/− induced rapid and severe colitis that was virtually identical to the colitis induced in their littermate RAG−/− controls (i.e., LTβ+/+ × RAG−/−). Unlike Makita et al, we did not observe a delay in onset in the LTβ−/− × RAG−/− mice compared to their RAG−/− littermates, but rather, observed an acceleration of disease in both groups of mice. The reasons for the accelerated disease in both groups of mice are not clear at the present time but do not appear to be some form of graft versus host disease (GvHD) because: 1) recipients were reconstituted with syngeneic C57Bl/6 T-cells, and 2) the mice never exhibited the classic signs of GvHD such as loss of body hair, facial swelling, and dermatitis. It may be that intercrossing these two mutant mice produced offspring with enhanced expression of one or more sensitivity factors or generated progeny with reduced expression of certain protective genes. Studies to identify the mechanisms responsible for this accelerated disease are currently under way. Taken together, these data demonstrate that neither the GALT, MLNs nor PLNs are required for the induction of chronic colitis induced by adoptive transfer of naïve T-cells into lymphopenic recipients. The anatomic locations where naïve T-cells are converted to colitogenic effector cells by enteric antigens remain to be defined.
Acknowledgments
Supported by PO1-DK43785 (Project 1, Animal Models Core and Histopathology Core).
REFERENCES
- 1.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehrchen JM, Roth J, Roebrock K, et al. The absence of cutaneous lymph nodes results in a Th2 response and increased susceptibility to Leishmania major infection in mice. Infect Immun. 2008;76:4241–4250. doi: 10.1128/IAI.01714-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ivanov II, Diehl GE, Littman DR. Lymphoid tissue inducer cells in intestinal immunity. Curr Top Microbiol Immunol. 2006;308:59–82. doi: 10.1007/3-540-30657-9_3. [DOI] [PubMed] [Google Scholar]
- 5.Kwa SF, Beverley P, Smith AL. Peyer’s patches are required for the induction of rapid Th1 responses in the gut and mesenteric lymph nodes during an enteric infection. J Immunol. 2006;176:7533–7541. doi: 10.4049/jimmunol.176.12.7533. [DOI] [PubMed] [Google Scholar]
- 6.Macpherson AJ, Uhr T. Compartmentalization of the mucosal immune responses to commensal intestinal bacteria. Ann N Y Acad Sci. 2004;1029:36–43. doi: 10.1196/annals.1309.005. [DOI] [PubMed] [Google Scholar]
- 7.Macpherson AJ, Smith K. Mesenteric lymph nodes at the center of immune anatomy. J Exp Med. 2006;203:497–500. doi: 10.1084/jem.20060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 9.Spahn TW, Weiner HL, Rennert PD, et al. Mesenteric lymph nodes are critical for the induction of high-dose oral tolerance in the absence of Peyer’s patches. Eur J Immunol. 2002;32:1109–1113. doi: 10.1002/1521-4141(200204)32:4<1109::AID-IMMU1109>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 10.Spahn TW, Kucharzik T. Modulating the intestinal immune system: the role of lymphotoxin and GALT organs. Gut. 2004;53:456–465. doi: 10.1136/gut.2003.023671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spahn TW, Muller MK, Domschke W, et al. Role of lymphotoxins in the development of Peyer’s patches and mesenteric lymph nodes: relevance to intestinal inflammation and treatment. Ann N Y Acad Sci. 2006;1072:187–193. doi: 10.1196/annals.1326.029. [DOI] [PubMed] [Google Scholar]
- 12.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Worbs T, Bode U, Yan S, et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–527. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elson CO, Cong Y, McCracken VJ, et al. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 15.Powrie F, Read S, Mottet C, et al. Control of immune pathology by regulatory T cells. Novartis Found Symp. 2003;252:92–98. [PubMed] [Google Scholar]
- 16.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uhlig HH, Powrie F. The role of mucosal T lymphocytes in regulating intestinal inflammation. Springer Semin Immunopathol. 2005;27:167–180. doi: 10.1007/s00281-005-0206-6. [DOI] [PubMed] [Google Scholar]
- 18.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bamias G, Nyce MR, De La Rue SA, et al. New concepts in the pathophysiology of inflammatory bowel disease. Ann Intern Med. 2005;143:895–904. doi: 10.7326/0003-4819-143-12-200512200-00007. [DOI] [PubMed] [Google Scholar]
- 20.Sakuraba A, Sato T, Kamada N, et al. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn’s disease. Gastroenterology. 2009;137:1736–1745. doi: 10.1053/j.gastro.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 21.Spahn TW, Herbst H, Rennert PD, et al. Induction of colitis in mice deficient of Peyer’s patches and mesenteric lymph nodes is associated with increased disease severity and formation of colonic lymphoid patches. Am J Pathol. 2002;161:2273–2282. doi: 10.1016/S0002-9440(10)64503-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dohi T, Rennert PD, Fujihashi K, et al. Elimination of colonic patches with lymphotoxin beta receptor-Ig prevents Th2 cell-type colitis. J Immunol. 2001;167:2781–2790. doi: 10.4049/jimmunol.167.5.2781. [DOI] [PubMed] [Google Scholar]
- 23.Makita S, Kanai T, Nemoto Y, et al. Intestinal lamina propria retaining CD4+CD25+ regulatory T cells is a suppressive site of intestinal inflammation. J Immunol. 2007;178:4937–4946. doi: 10.4049/jimmunol.178.8.4937. [DOI] [PubMed] [Google Scholar]
- 24.Koni PA, Sacca R, Lawton P, et al. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- 25.Alimzhanov MB, Kuprash DV, Kosco-Vilbois MH, et al. Abnormal development of secondary lymphoid tissues in lymphotoxin beta-deficient mice. Proc Natl Acad Sci U S A. 1997;94:9302–9307. doi: 10.1073/pnas.94.17.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ostanin DV, Furr KL, Pavlick KP, et al. T cell-associated CD18 but not CD62L, ICAM-1, or PSGL-1 is required for the induction of chronic colitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1706–G1714. doi: 10.1152/ajpgi.00573.2006. [DOI] [PubMed] [Google Scholar]
- 27.Ostanin DV, Bao J, Koboziev I, et al. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am J Physiol Gastrointest Liver Physiol. 2009;296:G135–G146. doi: 10.1152/ajpgi.90462.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ostanin DV, Pavlick KP, Bharwani S, et al. T cell-induced inflammation of the small and large intestine in immunodeficient mice. Am J Physiol Gastrointest Liver Physiol. 2006;290:G109–G119. doi: 10.1152/ajpgi.00214.2005. [DOI] [PubMed] [Google Scholar]
- 29.Gullberg E, Soderholm JD. Peyer’s patches and M cells as potential sites of the inflammatory onset in Crohn’s disease. Ann N Y Acad Sci. 2006;1072:218–232. doi: 10.1196/annals.1326.028. [DOI] [PubMed] [Google Scholar]
- 30.Eksteen B, Mora JR, Haughton EL, et al. Gut homing receptors on CD8 T cells are retinoic acid dependent and not maintained by liver dendritic or stellate cells. Gastroenterology. 2009;137:320–329. doi: 10.1053/j.gastro.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agace WW. T-cell recruitment to the intestinal mucosa. Trends Immunol. 2008;29:514–522. doi: 10.1016/j.it.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Mueller DL. Mechanisms maintaining peripheral tolerance. Nat Immunol. 2010;11:21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 33.Scheu S, Alferink J, Potzel T, et al. Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin beta in mesenteric lymph node genesis. J Exp Med. 2002;195:1613–1624. doi: 10.1084/jem.20020215. [DOI] [PMC free article] [PubMed] [Google Scholar]