Abstract
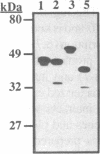
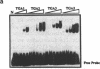
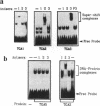
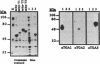
Activating sequence factor 1 (ASF-1) is a nuclear DNA-binding activity that is found in monocots and dicots. It interacts with several TGACG-containing elements that have been characterized from viral and T-DNA genes, the prototypes of which are the as-1 element of the CaMV 35S promoter and the ocs element from the octopine synthase promoter. This class of cis-acting elements can respond to auxin and salicylic acid treatments. Consistent with these observations, we have shown that ASF-1 can interact with promoter elements of an auxin-inducible tobacco gene GNT35, encoding a glutathione S-transferase. Characterization of the nuclear factors that make up ASF-1 activity in vivo will be an important step toward understanding this induction phenomenon. The TGA family of basic-leucine-zipper (bZIP) proteins are good candidates for the ASF-1 nuclear factor. However, there may be as many as seven distinct TGA genes in Arabidopsis, five of which have now been reported. In this study, we expressed the cDNAs that encode four of these five Arabidopsis TGA factors in vitro and compared their DNA-binding behavior using two types of TGACG-containing elements. With specific antisera prepared against three of the five known Arabidopsis TGA factors, we also investigated the relative abundance of these three proteins within the ASF-1 activities of root and leaf nuclear extracts. Our results indicate that these TGA factors bind to DNA with different degrees of cooperativity and their relative affinity toward as-1 also can differ significantly. The results of a supershift assay suggested that only one of the three TGA factors represented a significant component of nuclear ASF-1 activity. Arabidopsis TGA2 comprises approximately 33 and 50% of the ASF-1 activity detected in root and leaf nuclear extracts respectively. These results suggest that each member of the TGA factor family may be differentially regulated and that they may play different roles by virtue of their distinct DNA-binding characteristics. Furthermore, since transcripts for each of these factors can be detected in various plant tissues, post-transcriptional regulation may play an important part in determining their contribution to nuclear ASF-1 in a given cell type.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alliotte T., Tiré C., Engler G., Peleman J., Caplan A., Van Montagu M., Inzé D. An Auxin-Regulated Gene of Arabidopsis thaliana Encodes a DNA-Binding Protein. Plant Physiol. 1989 Mar;89(3):743–752. doi: 10.1104/pp.89.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfey P. N., Ren L., Chua N. H. The CaMV 35S enhancer contains at least two domains which can confer different developmental and tissue-specific expression patterns. EMBO J. 1989 Aug;8(8):2195–2202. doi: 10.1002/j.1460-2075.1989.tb08342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelle A. N., Chua N. H. Transcription regulatory proteins in higher plants. Curr Opin Genet Dev. 1993 Apr;3(2):254–258. doi: 10.1016/0959-437x(93)90031-j. [DOI] [PubMed] [Google Scholar]
- Chiu R., Angel P., Karin M. Jun-B differs in its biological properties from, and is a negative regulator of, c-Jun. Cell. 1989 Dec 22;59(6):979–986. doi: 10.1016/0092-8674(89)90754-x. [DOI] [PubMed] [Google Scholar]
- Coen E. S., Meyerowitz E. M. The war of the whorls: genetic interactions controlling flower development. Nature. 1991 Sep 5;353(6339):31–37. doi: 10.1038/353031a0. [DOI] [PubMed] [Google Scholar]
- Droog F. N., Hooykaas P. J., Libbenga K. R., van der Zaal E. J. Proteins encoded by an auxin-regulated gene family of tobacco share limited but significant homology with glutathione S-transferases and one member indeed shows in vitro GST activity. Plant Mol Biol. 1993 Mar;21(6):965–972. doi: 10.1007/BF00023595. [DOI] [PubMed] [Google Scholar]
- Ehrlich K. C., Cary J. W., Ehrlich M. A broad bean cDNA clone encoding a DNA-binding protein resembling mammalian CREB in its sequence specificity and DNA methylation sensitivity. Gene. 1992 Aug 15;117(2):169–178. doi: 10.1016/0378-1119(92)90726-6. [DOI] [PubMed] [Google Scholar]
- Feltkamp D., Masterson R., Starke J., Rosahl S. Analysis of the involvement of ocs-like bZip-binding elements in the differential strength of the bidirectional mas1'2' promoter. Plant Physiol. 1994 May;105(1):259–268. doi: 10.1104/pp.105.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley R. C., Grossman C., Ellis J. G., Llewellyn D. J., Dennis E. S., Peacock W. J., Singh K. B. Isolation of a maize bZIP protein subfamily: candidates for the ocs-element transcription factor. Plant J. 1993 May;3(5):669–679. [PubMed] [Google Scholar]
- Foster R., Izawa T., Chua N. H. Plant bZIP proteins gather at ACGT elements. FASEB J. 1994 Feb;8(2):192–200. doi: 10.1096/fasebj.8.2.8119490. [DOI] [PubMed] [Google Scholar]
- Fromm H., Katagiri F., Chua N. H. The tobacco transcription activator TGA1a binds to a sequence in the 5' upstream region of a gene encoding a TGA1a-related protein. Mol Gen Genet. 1991 Oct;229(2):181–188. doi: 10.1007/BF00272154. [DOI] [PubMed] [Google Scholar]
- Hai T. W., Liu F., Coukos W. J., Green M. R. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989 Dec;3(12B):2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- Harter K., Kircher S., Frohnmeyer H., Krenz M., Nagy F., Schäfer E. Light-regulated modification and nuclear translocation of cytosolic G-box binding factors in parsley. Plant Cell. 1994 Apr;6(4):545–559. doi: 10.1105/tpc.6.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri F., Lam E., Chua N. H. Two tobacco DNA-binding proteins with homology to the nuclear factor CREB. Nature. 1989 Aug 31;340(6236):727–730. doi: 10.1038/340727a0. [DOI] [PubMed] [Google Scholar]
- Katagiri F., Seipel K., Chua N. H. Identification of a novel dimer stabilization region in a plant bZIP transcription activator. Mol Cell Biol. 1992 Nov;12(11):4809–4816. doi: 10.1128/mcb.12.11.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawata T., Imada T., Shiraishi H., Okada K., Shimura Y., Iwabuchi M. A cDNA clone encoding HBP-1b homologue in Arabidopsis thaliana. Nucleic Acids Res. 1992 Mar 11;20(5):1141–1141. doi: 10.1093/nar/20.5.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter R., Vollbrecht E., Lowe B., Veit B., Yamaguchi J., Hake S. Sequence analysis and expression patterns divide the maize knotted1-like homeobox genes into two classes. Plant Cell. 1994 Dec;6(12):1877–1887. doi: 10.1105/tpc.6.12.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Buckley K., Costa M. A., An G. A 20 nucleotide upstream element is essential for the nopaline synthase (nos) promoter activity. Plant Mol Biol. 1994 Jan;24(1):105–117. doi: 10.1007/BF00040578. [DOI] [PubMed] [Google Scholar]
- Lam E. Analysis of tissue-specific elements in the CaMV 35S promoter. Results Probl Cell Differ. 1994;20:181–196. doi: 10.1007/978-3-540-48037-2_8. [DOI] [PubMed] [Google Scholar]
- Lam E., Benfey P. N., Gilmartin P. M., Fang R. X., Chua N. H. Site-specific mutations alter in vitro factor binding and change promoter expression pattern in transgenic plants. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7890–7894. doi: 10.1073/pnas.86.20.7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E., Chua N. H. ASF-2: a factor that binds to the cauliflower mosaic virus 35S promoter and a conserved GATA motif in Cab promoters. Plant Cell. 1989 Dec;1(12):1147–1156. doi: 10.1105/tpc.1.12.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E., Chua N. H. GT-1 binding site confers light responsive expression in transgenic tobacco. Science. 1990 Apr 27;248(4954):471–474. doi: 10.1126/science.2330508. [DOI] [PubMed] [Google Scholar]
- Lam E., Katagiri F., Chua N. H. Plant nuclear factor ASF-1 binds to an essential region of the nopaline synthase promoter. J Biol Chem. 1990 Jun 15;265(17):9909–9913. [PubMed] [Google Scholar]
- Lamb P., McKnight S. L. Diversity and specificity in transcriptional regulation: the benefits of heterotypic dimerization. Trends Biochem Sci. 1991 Nov;16(11):417–422. doi: 10.1016/0968-0004(91)90167-t. [DOI] [PubMed] [Google Scholar]
- Liu X., Lam E. Two binding sites for the plant transcription factor ASF-1 can respond to auxin treatments in transgenic tobacco. J Biol Chem. 1994 Jan 7;269(1):668–675. [PubMed] [Google Scholar]
- Lohmer S., Maddaloni M., Motto M., Salamini F., Thompson R. D. Translation of the mRNA of the maize transcriptional activator Opaque-2 is inhibited by upstream open reading frames present in the leader sequence. Plant Cell. 1993 Jan;5(1):65–73. doi: 10.1105/tpc.5.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkens A. E., Cashmore A. R. Isolation and characterization of a fourth Arabidopsis thaliana G-box-binding factor, which has similarities to Fos oncoprotein. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2522–2526. doi: 10.1073/pnas.91.7.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Z. H., Lam E. Construction of a trans-dominant inhibitor for members of the TGA family of transcription factors conserved in higher plants. Plant J. 1995 Jun;7(6):887–896. doi: 10.1046/j.1365-313x.1995.07060887.x. [DOI] [PubMed] [Google Scholar]
- Miao Z. H., Lam E. Targeted disruption of the TGA3 locus in Arabidopsis thaliana. Plant J. 1995 Feb;7(2):359–365. doi: 10.1046/j.1365-313x.1995.7020359.x. [DOI] [PubMed] [Google Scholar]
- Miao Z. H., Liu X., Lam E. TGA3 is a distinct member of the TGA family of bZIP transcription factors in Arabidopsis thaliana. Plant Mol Biol. 1994 Apr;25(1):1–11. doi: 10.1007/BF00024193. [DOI] [PubMed] [Google Scholar]
- Neuhaus G., Neuhaus-Url G., Katagiri F., Seipel K., Chua N. H. Tissue-Specific Expression of as-1 in Transgenic Tobacco. Plant Cell. 1994 Jun;6(6):827–834. doi: 10.1105/tpc.6.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X. F., Holuigue L., Horvath D. M., Chua N. H. Immediate early transcription activation by salicylic acid via the cauliflower mosaic virus as-1 element. Plant Cell. 1994 Jun;6(6):863–874. doi: 10.1105/tpc.6.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler U., Beckmann H., Cashmore A. R. TGA1 and G-box binding factors: two distinct classes of Arabidopsis leucine zipper proteins compete for the G-box-like element TGACGTGG. Plant Cell. 1992 Oct;4(10):1309–1319. doi: 10.1105/tpc.4.10.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T., Nakayama T., Mikami K., Iwabuchi M. HBP-1a and HBP-1b: leucine zipper-type transcription factors of wheat. EMBO J. 1991 Jun;10(6):1459–1467. doi: 10.1002/j.1460-2075.1991.tb07666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuhisa J. G., Singh K., Dennis E. S., Peacock W. J. A DNA-binding protein factor recognizes two binding domains within the octopine synthase enhancer element. Plant Cell. 1990 Mar;2(3):215–224. doi: 10.1105/tpc.2.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson C. R., Hai T., Boyd S. M. Dimerization specificity of the leucine zipper-containing bZIP motif on DNA binding: prediction and rational design. Genes Dev. 1993 Jun;7(6):1047–1058. doi: 10.1101/gad.7.6.1047. [DOI] [PubMed] [Google Scholar]
- Vinson C. R., Sigler P. B., McKnight S. L. Scissors-grip model for DNA recognition by a family of leucine zipper proteins. Science. 1989 Nov 17;246(4932):911–916. doi: 10.1126/science.2683088. [DOI] [PubMed] [Google Scholar]
- Vogt P. K., Bos T. J. The oncogene jun and nuclear signalling. Trends Biochem Sci. 1989 May;14(5):172–175. doi: 10.1016/0968-0004(89)90268-5. [DOI] [PubMed] [Google Scholar]
- Weigel D., Meyerowitz E. M. The ABCs of floral homeotic genes. Cell. 1994 Jul 29;78(2):203–209. doi: 10.1016/0092-8674(94)90291-7. [DOI] [PubMed] [Google Scholar]
- Zhang B., Foley R. C., Singh K. B. Isolation and characterization of two related Arabidopsis ocs-element bZIP binding proteins. Plant J. 1993 Oct;4(4):711–716. doi: 10.1046/j.1365-313x.1993.04040711.x. [DOI] [PubMed] [Google Scholar]
- Zhang B., Singh K. B. ocs element promoter sequences are activated by auxin and salicylic acid in Arabidopsis. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2507–2511. doi: 10.1073/pnas.91.7.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vetten N. C., Ferl R. J. Two genes encoding GF14 (14-3-3) proteins in Zea mays. Structure, expression, and potential regulation by the G-box binding complex. Plant Physiol. 1994 Dec;106(4):1593–1604. doi: 10.1104/pp.106.4.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Krol A. R., Chua N. H. The basic domain of plant B-ZIP proteins facilitates import of a reporter protein into plant nuclei. Plant Cell. 1991 Jul;3(7):667–675. doi: 10.1105/tpc.3.7.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Arnim A. G., Deng X. W. Light inactivation of Arabidopsis photomorphogenic repressor COP1 involves a cell-specific regulation of its nucleocytoplasmic partitioning. Cell. 1994 Dec 16;79(6):1035–1045. doi: 10.1016/0092-8674(94)90034-5. [DOI] [PubMed] [Google Scholar]