Abstract
Background
We have demonstrated previously that in vivo supplementation of tetrahydrobiopterin (BH4); a co-factor for neuronal nitric oxide synthase (nNOS) significantly restored delayed gastric emptying and attenuated nitrergic relaxation in diabetic rat. In this study, we have investigated whether supplementation of sepiapterin (SEP), a precursor for BH4 biosynthesis via salvage pathway restores gastric emptying and nitrergic system in female diabetic rats.
Methods
Diabetic rats (streptozotocin-induced) were supplemented with BH4 or SEP (20 mg kg−1 body weight). Gastric nitrergic relaxation in the presence or absence of high glucose and SEP were measured by electric field stimulation. Gastric muscular strips from healthy or diabetic female rats were incubated in the presence or absence of high glucose, SEP and/or methotrexate (MTX). Nitric oxide release was measured colorimetrically by NO assay kit. The expression of nNOSα and dimerization was detected by Western blot.
Key Results
In vitro studies on gastric muscular tissues showed that MTX, an inhibitor of BH4 synthesis via salvage pathway, significantly decreased NO release. In vivo treatment with MTX reduced both gastric nitrergic relaxation and nNOSα dimerization. Supplementation of SEP significantly attenuated delayed gastric emptying in diabetic rats. In addition, SEP supplementation restored impaired nitrergic relaxation, gastric nNOSα protein expression and dimerization in diabetic rats.
Conclusions & Inferences
The above data suggests that supplementation of SEP accelerated gastric emptying and attenuated reduced gastric nNOSα expression, and dimerization. Therefore, SEP supplementation is a potential therapeutic option for female patients of diabetic gastroparesis.
Keywords: diabetes, female rat, gastroparesis, nitrergic relaxation, neuronal nitric oxide synthase dimerization, sepiapterin, tetrahydrobiopterin
INTRODUCTION
Gastroparesis is a clinical condition associated with abnormal gastric motility which is characterized by delayed gastric emptying. The most common etiologies include diabetes, postsurgical and idiopathic and the symptoms are nausea, vomiting and epigastric pain.1 Our laboratory previously demonstrated that differences in nitrergic regulation of gastric motility cause the reduction in gastric emptying in female rats after diabetes induction and female gastric motility being more influenced by nitrergic neurons.2
Gastric motility is largely regulated by synchronized interplay of excitatory (mainly cholinergic) and inhibitory (mainly nitrergic) neurons innervating smooth muscle directly or through interstitial cells of Cajal. In the stomach, nitrergic signaling in the form of the gaseous neurotransmitter nitric oxide (NO) plays a critical role in the control of gastric accommodation and pyloric relaxation in response to a meal. In disorders such as diabetic gastroparesis, loss of NO signaling is postulated to be an important contributor to the disordered motility and delay in emptying.3–5
Nitric oxide is synthesized in cells by a class of L-arginine dependent nitric oxide synthases (NOS) that catalyze the transformation of L-arginine to L-citrulline with the formation of NO.6–8 The NOS enzymes are dimeric in their active form and are associated with two molecules of calmodulin (CaM) and several cofactors.9 An essential cofactor of all NOS isoforms [endothelial (e), neuronal (n), inducible (i)] is tetrahydrobiopterin (BH4), which is intracellularly produced from GTP (guanosine tri phosphate) via GTP-cyclohydrolase I or, alternatively, from sepiapterin (SEP) via salvage pathway.10 Although the exact mechanism whereby BH4 regulates NOS activity is not known, several different hypotheses have been demonstrated such as, BH4 exerts an allosteric action to stabilize the active dimeric state of NOS, it plays a redox active role in stimulating NOS, it increases the binding of L-arginine to NOS and scavenges reactive free radicals.11–13 Importance of BH4 as NOS regulation and alteration in this system in various pathophysiological conditions became a focus of recent studies.
In keeping with these physiological roles, beneficial effect of BH4 supplementation has been documented in many pathological conditions associate with BH4 deficiency, including diabetes. Treatment of diabetic vascular endothelial cells with SEP (the BH4 precursor in the salvage pathway), significantly improves NO synthesis.14 In addition, dietary supplementation of SEP increases acetylcholine (Ach)-induced vascular relaxation in diabetic mice.15
However, there have been no studies on the effects of SEP on nitrergic mediated gastric motility dysfunction in diabetic rodents. The aim of the present study was, therefore, to investigate whether supplementation of SEP attenuates impaired nitrergic signaling and gastric dysmotility in female diabetic rats. The objective of the present study was also to investigate the effects of the oral supplementation of SEP on gastric nNOS function from diabetic rats. We chose female rats because gastroparesis predominantly affects women, and we have previously shown that female rats are more vulnerable to changes in nitrergic signaling induced by diabetes.2
MATERIALS AND METHODS
Experimental rats and induction of diabetes
Adult female Sprague Dawley rats (9 week old) were procured from Harlan (Houston, TX, USA) and Harlan Sprague Dawley Inc. (Indianapolis, IN, USA) and maintained in the institutional animal care facility under controlled temperature, humidity and light-dark cycle (12 : 12-h), with free access to rodent chow and water. All experiments in this study were approved by the Institutional Animal Care and Use Committees at the University of Texas Medical Branch, Galveston, Texas and Meharry Medical College, Nashville, Tennessee, in accordance with the recommendations of National Institutes of Health, Guide for the Care and Use of Laboratory Animals.
Diabetes was induced in overnight fasted animals by a single intraperitoneal injection of streptozotocin (STZ, 55 mg kg−1) (Sigma Chemical, St. Louis, MO, USA) prepared in 9 mmol citrate buffer, pH 4.0. Control animals were injected with the vehicle (9 mmol citrate buffer, pH 4.0).2 Blood glucose levels were examined in overnight fasted animals, 48 h post STZ injection. Animals exhibiting blood glucose levels more than 250 mg dL−1 were considered diabetic and included in the study. Blood glucose levels in vehicle-treated overnight fasting rats ranged between 80–95 mg dL−1. Both control and diabetic female rats were selected during the diestrus phase of the estrous cycle using vaginal smear testing method [by measuring vaginal cytology (90–100% of leucocytes) under microscope] for further experiments. As reported earlier, we noticed that 60–70% of diabetic rats show a persistent diestrus stage of the estrous cycle (data not shown).16,17
Experimental design
At the end of 7th week of diabetes induction, animals were divided into four groups, i.e. control female rats (C), diabetic female rats (DB), BH4 supplemented diabetic female rats (DB + BH4) and SEP supplemented diabetic female rats (DB + SEP). DB + BH4 were provided with BH4 pellets (20 mg kg−1 body wt day−1) for next 2 weeks. DB + SEP were provided with SEP tablets (20 mg kg−1 body wt day−1) for 10 days. BH4 or SEP pellets (one gram size flavored with chocolate, TestDiet, Land O’Lakes Purina Feed, LLC, Richmond, IN) were fed to each animal housed in a separate cage before they were fed with normal rat chow. We selected 20 mg kg−1 BH4/SEP dose based on the published studies.18,19 In addition, in our recent report we used two doses for BH4 (5 and 20 mg kg−1) for gastric motility studies and we didn’t find any significant difference between the two doses.20 Therefore we have chosen 20 mg kg−1 dose for BH4/SEP in the current study. On the last day of BH4 and SEP supplementation, animals were sacrificed to collect gastric muscular tissue for future analysis. Tissue samples were snap frozen in liquid nitrogen and stored at −80°C until analyzed. BH4 or SEP-pellets used in this study were prepared by compressing (Schircks Laboratories, Switzerland) with rodent chow and stored at −20°C until used. To avoid oxidation of BH4 or SEP, heat and water was not employed in pellet preparation.
Solid gastric emptying studies
At the end of 10 days of SEP supplementation solid gastric emptying studies were performed according to the method of Martinez et al. with slight modification.2,21 Our recent published data using this protocol demonstrate that gender differences exist in solid gastric emptying and BH4 treatment restores delayed gastric emptying only in female but not in males in the onset of diabetes.2,20 In addition, using the similar gastric emptying protocol, we have noticed accelerated gastric emptying instead of delayed gastric emptying in spontaneous diabetic female but not in male diabetic animals.22 We have noticed that 60–70% diabetic rats displayed delayed gastric emptying as reported in humans in the onset of diabetes.
According to the protocol, animals were fasted over night (provide water). On the next day, known amount of food was fed to the animals for 3 h. At the end of 3 h collected the remaining food from the cage and calculated the amount of food intake. Fast the animals for 4 h without food and water. At the end of fast, animals were euthanized, collected the gastric tissue and measured the weight of the whole stomach. The food contents was removed by opening the stomach and measured the empty stomach weight. The rate of gastric emptying was calculated according to the following equation: gastric emptying (%in 4 h) = (1 − gastric content food intake−1) × 100.
Organ bath studies
Electric field stimulation (EFS)-induced Non adrenergic Non cholinergic NANC relaxation was studied in circular gastric antrum muscle strips. Muscle strips were tied with silk thread at both ends and were mounted in 10-ml water-jacketed organ baths containing Krebs buffer (11 mmol L−1 glucose) at 37°C and continuously bubbled with 95% O2–5% CO2 (Radnoti Glass Technology, Monrovia, CA, USA). Tension for each muscle strip was monitored with an isometric force transducer and analyzed by a digital recording system (Biopac Systems, Santa Barbara, CA, USA). A passive tension equal to 2 g was applied on each strip in the 1 h equilibration period through an incremental increase (0.5 g, four times, at 15 min interval). Gastric antrum muscle strips were exposed to atropine, phentolamine and propranolol (10 μmol each) in bath solution for 1 h to block cholinergic and adrenergic responses. 5-hydroxytryptamine (5-HT; 100 μmol L−1) pre-contracted strips were exposed to EFS (90 V, 2 Hz, 1-ms pulse for duration of 1 min) to elicit NANC relaxation.
Relaxation response elicited by low frequency (EFS; 2 Hz) stimulus under NANC conditions, as used in this study, was demonstrated as predominantly nitrergic in origin.2,7
To investigate the in vivo effect of methotrexate (MTX) on EFS induced nitrergic relaxation; a group of animals were supplemented with MTX (inhibitor of dihydro folate reductase, DHFR) 3.75 mg kg−1 body wt. per twice a day for 4 days. Gastric strips from control animals and MTX treated animals were incubated in organ bath and nitrergic relaxation was measured by EFS.
At the end of each experiment, the muscle strip was blotted dry with filter paper and weighed. Comparisons between groups were performed by measuring the area tinder the curve (AUC mg−1 tissue) of the EFS-induced relaxation (AUCR) for 1 min and the baseline for 1 min (AUCB) according to the formula (AUCR)−(AUCB) weight of tissue (mg)−1 = AUC mg of tissue−1.
In vitro NO release
Animals from control groups were killed by CO2 asphyxiation, the abdominal cavity opened, and the stomach dissected and transferred in chilled oxygenated Krebs bicarbonate solution of the following composition (in mmol): 118.0 NaCl, 4.7 KCl, 25.0 NaHCO3, 1.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, and 11.5 glucose (pH 7.4). Antrum tissue was harvested and cut into mucosa-free strips and were cultured for 48 h (37°C, 5% CO2) in 500 μL of phenol red-free DMEM supplemented with NB27 (2%) and antibiotics (1%) in the presence of normal glucose (control), control + MTX (100 μmol L−1), control + MTX + SEP (100 μmol L−1, 100 μmol L−1). On completion of incubation, DMEM (500 μL) was collected and stored at −80°C for analysis of NO released in medium during incubation period. NO released in the medium was analyzed as total nitrite (metabolic byproduct of NO) following the protocol supplied with a commercially available kit (EMD Chemicals, Gibbstown, NJ, USA).
Western blot analysis
nNOSα protein was quantified in gastric antrum homogenates from all groups using standard western blot analysis, as described in our previous study.2 Proteins were measured by Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA) and 30 μg protein was separated by 6% SDS polyacrylamide gel electrophoresis (SDS-PAGE). The membrane was immunoblotted with polyclonal nNOSα primary antibody (Zymed Laboratories Inc., CA, USA) and anti-rabbit IgG conjugated with horseradish peroxidase (Sigma Chemical, St. Louis, MO) as secondary antibody. Binding of antibodies to the blots was detected with enhanced chemiluminescence system (ECL, Amersham Pharmacia Biotech, Piscataway, NJ, USA) following manufacturer’s instructions. Stripped blots were reprobed with γ-tubulin specific polyclonal antibodies (Sigma Chemical, St. Louis, MO, USA) to enable normalization of signals between samples. Band intensities were analyzed using Bio-Rad Gel Doc (Bio-Rad, Hercules, CA, USA).
nNOSα dimerization in rat gastric antrum
Levels of nNOSα monomer and dimer were quantified by western blotting via low temperature (LT)-PAGE in gastric antrum homogenates as described previously.2 LT-SDS-PAGE was performed on ice. The low-temperature process was used to identify nNOS dimers and monomers in the native state as low temperature is known to prevent monomerization of nNOS dimers. For the low-temperature processing, 30 μg of protein in standard Laemmli buffer at 4°C was used for SDS-PAGE. The mixture was incubated at 0°C for 30 min before LT-SDS-PAGE using a 6% separating gel. All gels and buffers were pre-equilibrated to 4°C prior to electrophoresis and the buffer tank placed in an ice-bath during electrophoresis to maintain the gel temperature below 15°C. A polyclonal antibody specific to nNOSα (Zymed Laboratories) and anti-rabbit IgG conjugated with horseradish peroxidase (Sigma Chemical, St. Louis, MO, USA) were used as the primary and secondary antibodies, respectively.
Statistics
Data were presented as mean ± standard error (SE). Statistical comparisons between groups were determined by Student’s t-test or the Tukey test after one-way analysis of variance (ANOVA), using GraphPad prism Version 5.0 (GraphPad software, San Diego, CA, USA). A P value of less than 0.05 was considered statistically significant.
RESULTS
Effect of supplementation of SEP on blood glucose and body weight in female diabetic rats
Table 1 demonstrate whether supplementation of SEP attenuated the elevated blood glucose and reduced body weights in female rats after diabetes induction. A significant weight loss (174.3 ± 4.8) was noted in the diabetic rats compared to age-matched control group (255.9 ± 2.3). Supplementation of SEP had no significant effect (188 ± 4.3) on the diabetes-induced body weight loss. Fasting blood glucose levels were significantly elevated in female rats (521.3 ± 35.01 mg dL−1) after diabetes induction (Table 1). Blood glucose levels were unchanged upon supplementation of SEP in both control and in diabetic female rats.
Table 1.
Blood glucose level and body weight in the control and diabetic female rats
| C | C + SEP | DB | DB + SEP | |
|---|---|---|---|---|
| Body Weight, g | 255.9 ± 2.3 | 242 ± 7.7 | 174.3 ±4.8* | 188 ± 4.3* |
| Blood Glucose, mg dL−1 | 102 ± 3.2 | 112 ± 1.0 | 521.3 ±35.01* | 494.5 ± 13.96* |
C, Control group; DB, Diabetes; SEP, Sepiapterin. Results are expressed as mean ± SEM.
P < 0.05 compared to control.
Attenuation of diabetes-induced solid gastric emptying by SEP in vivo
Fig. 1 shows the effect of SEP supplementation on solid gastric emptying (% in 4 h) in female diabetic rats. A significant reduction (30.0 ± 8.0) in the solid gastric emptying was observed in diabetic rats compared to control (76.0 ± 5.6). According to the Fig. 1, SEP supplementation did not affect the solid gastric emptying in control rats (74 ± 4), whereas, a significant induction (67.0 ± 8.2) in the solid gastric emptying was observed when diabetic animals were supplemented with SEP.
Figure 1.
Effect of sepiapterin (SEP) treatment on diabetes-induced solid gastric emptying in female rats. Groups (4–6) of diabetic rats received dietary SEP (20 mg kg−1 body wt) daily for 10 days after diabetic induction with single injection of streptozotocin (STZ 55 mg kg−1 body wt; ip). Control group was injected with vehicle (9 mmol citrate buffer) only. The values are mean ± SE for 4–6 animals. Statistical significance was determined by Tukey test after one-way ANOVA. *P < 0.05 compared with control group; #P < compared with diabetic |DB) group.
We next explored the underlying mechanisms for this effect, beginning with changes in gastric antrum nitrergic relaxation and NO release.
Effect of SEP on nitrergic relaxation in vivo
The effects of SEP on diabetic-induced induction of nitrergic relaxation in gastric antrum muscle strips from female rats are presented in Fig. 2 following EFS (2 Hz). Induction of diabetes caused a 3.8-fold decrease (−0.13 ± 0.04) in the nitrergic relaxation compared with control rats (−0.49 ± 0.09). Supplementation of SEP resulted in almost complete reversal (−0.35 ± 0.03) of diabetes-induced alteration of nitrergic relaxation. However, no change in nitrergic relaxation was noticed in control rats treated with SEP (−0.51 ± 0.06).
Figure 2.
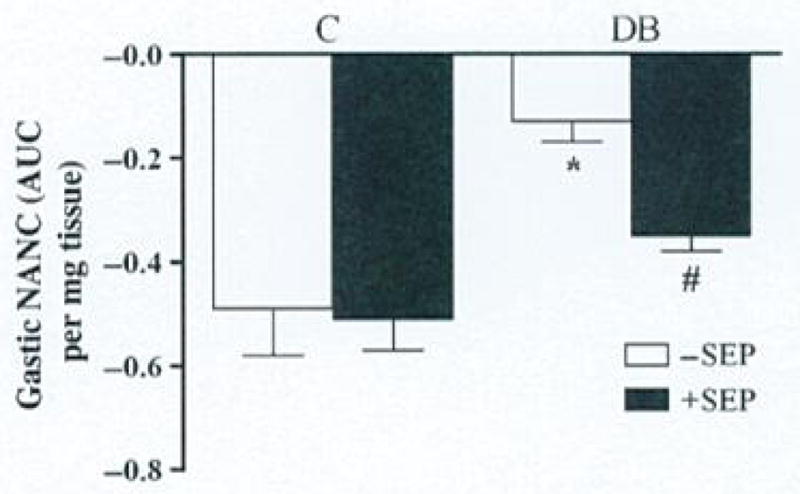
Effect of sepiapterin (SEP) on nitrergic relaxation in diabetic rat gastric muscular tissues in vivo. Nitrergic relaxation was measured following daily exposure to dietary SEP (20 mg kg−1 body wt) for 10 days after diabetic induction with single injection of streptozotocin (STZ 55 mg kg−1 body wt ip). Control group was injected with vehicle (9 mmol citrate buffer) only. Values are mean ± SE (n = 4–6). Statistical significance was determined by Tukey test after one-way ANOVA.
*P < 0.05 compared with control group; #P < compared with diabetic [DB] group.
We next explored the underlying mechanisms for this effect, beginning with changes in nNOSα structure and function which we have previously shown to be profoundly affected in diabetes. To show that these changes in nNOSα expression and structure were of functional significance we performed further experiments in vitro and in vivo.
Effects of in vivo MTX treatment on nitrergic relaxation and nNOSα dimer expression
We used inhibitor of BH4 biosynthesis, MTX, to analyze the role of BH4 or SEP on NO production in gastric tissue. MTX inhibits the enzyme DHFR and decreases the availability of BH4 via salvage pathway.23 To demonstrate the role of SEP in stomach function, we measured the effect of MTX on the nitrergic relaxation in vivo. According to Fig. 3A, gastric tissue from control healthy female rats exhibited substantial relaxation following EFS (2 Hz) (fundus: −1.1 ± 0.24; antrum: −0.5 ± 0.14; pylorus: −0.33 ± 0.05). MTX treatment significantly decreased the nitrergic relaxation in all areas of gastric muscular tissues (fundus: −0.17 ± 0.076; antrum: −0.08 ± 0.04; pylorus: −0.13 ± 0.05).
Figure 3.
(A) Effect of methotrexate (MTX)-induced nitrergic relaxation in female diabetic rats in vivo. Nitrergic relaxation was measured following daily exposure to intraperitoneal injection (i.p) of MTX (3.75 mg kg−1 body wt, two times a day), for 4 days. The values are mean ± SE of 4–6 animals. Statistical significance was determined by Student t-test. *P < 0.05 compared with control group. (B) Effect of MTX-induced neuronal nitric oxide synthaseα (nNOSα) dimcr expression in female diabetic rats in vivo. Western blot was done following daily exposure to MTX (3.75 mg kg−1 body wt, two times a day), for 4 days. Representative immunoblot and densitometric analysis data for nNOSα protein dimerization in female rat gastric antrum. Values are mean ± SE (n = 4). Statistical significance was determined by Tukey test after one-way ANOVA. *P < 0.05 compared with control group #P < compared with MTX group. (C) Effect of MTX and sepiapterin (SEP) supplementation on nitric oxide (NO) release in female diabetic rats in vitro. Nitric oxide levels were measured using the NO assay kit following 48 h incubation with MTX (100 μmol L−1), MTX + SEP (100 μmol L−1, 100 μmol L−1). Values are mean ± SE (n = 4–6). Statistical significance was determined by Tukey test after one-way ANOVA. *P < 0.05 compared with control group; #P < compared with MTX group.
To measure nNOSα dimer/monomer levels in MTX treated rats, we performed the dimerization study by LT-PAGE gel. Fig. 3B shows a significant decrease in the dimer/monomer ratio in MTX treated group (0.2 ± 0.04) when compared to control group (0.45 ± 0.2).
Effect of MTX and SEP supplementation on gastric NO release in female diabetic rats in vitro
To examine whether the NO production is dependent on SEP pathway, we demonstrated the NO release in MTX treated gastric antrum muscle strip. MTX exposure caused a significant decrease (P < 0.05) in NO release in vitro (Fig. 3C). Inhibition of DHFR, the critical enzyme for the production of BH4 in salvage pathway, significantly reduced nitrergic relaxation in healthy rats (1.28 ± 0.08 vs 0.73 ± 0.15). MTX-induced decrease in NO release was attenuated by SEP treatment (1.02 ± 0.19).
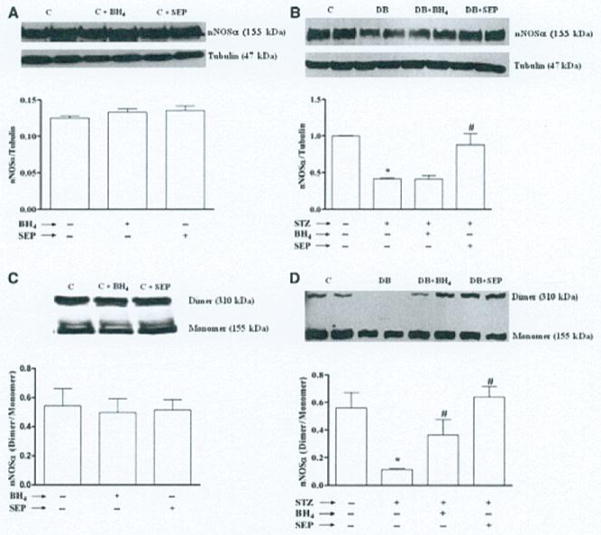
Effect of SEP on nNOSα protein expression and dimerization
According to data presented in Fig. 4B, the protein level of nNOSα, the only functional isoform of nNOS in gastric antrum tissue was significantly decreased (0.42 ± 0.01) following 9 weeks of diabetes. Supplementation of SEP to diabetic female rats results in significant restoration of nNOSα protein level (0.88 ± 0.15), whereas supplementation of BH4 for two weeks did not alter the nNOSα protein level (0.42 ± 0.05). However, no change in nNOSα protein expression was observed in control rats treated with either BH4 or SEP (Fig. 4A).
Figure 4.
Effect of sepiapterin (SEP) on neuronal nitric oxide synthaseα (nNOSα) protein expression and nNOSα dimerization of diabetic rat gastric tissues. nNOSα protein expression and nNOSα dimerization was measured following either daily exposure to SEP (20 mg kg−1 body wt) for 10 days or tetrahydrobiopterin (BH4) supplementation (20 mg kg−1 body wt per day) for 2 weeks after diabetic induction with single injection of STZ (55 mg kg−1 body wt ip). Control group was injected with vehicle (9 mmol citrate buffer) only. (A) Representative immunoblot and densitometric analysis data for nNOSα protein expression in female control rat gastric antrum supplemented with either BH4 or SEP. (B) Representative immunoblot and densitometric analysis data for nNOSα protein expression in female diabetic rat gastric antrum. (C) Representative immunoblot and densitometric analysis data for nNOSα protein dimerization in female control rat gastric antrum supplemented with either BH4 or SEP. (D) Representative immunoblot and densitometric analysis data for nNOSα protein dimerization in female diabetic rat gastric antrum. Values are mean ± SE (n = 4). Statistical significance was determined by Tukey test after one-way ANOVA. *P < 0.05 compared with control group; #P < compared with diabetic (DB) group.
To measure whether decrease in the gastric antrum nNOSα was the result of altered nNOSα dimer levels in diabetic rats, we performed the dimerization study by LT-PAGE gel. As depicted in Fig. 4D, a significant decrease in the gastric antrum nNOSα dimer/monomer ratio was seen in diabetic female rats compared to control (0.11 ± 0.01 vs 0.56 ± 0.11). Supplementation of SEP resulted in complete reversal of diabetes-induced alteration of nNOSα dimer/monomer level (0.64 ± 0.07). Though two weeks of BH4 supplementation did not change the nNOSα protein level (Fig. 4B), it significantly increased the nNOSα dimer/monomer level (0.36 ± 0.11) compared with diabetes-induced female rats (Fig. 4D).
DISCUSSION
The present study established that SEP treatment attenuated delayed solid gastric emptying in diabetic female rats. In this study, we also demonstrated that supplementation of SEP prevented the down regulation of both nNOSα protein level and dimer/monomer level in diabetic female rats. Our result suggests that increased gastric dysfunction in diabetic rats restores by SEP or BH4 treatment.
In diabetic gastroparesis, delayed gastric emptying is generally resulted from impaired phasic antral contractions, tonic motor defects, and increased liquid retention in the fundus. Another reason of delayed emptying is increased outflow resistance in the pylorus and abnormal pyloric contraction.24 It has been reported earlier that diabetic induction causes differences in nitrergic regulation of gastric motility associated with the reduction in gastric emptying.2,25–27
SEP can serve as a BH4 precursor and is metabolized in mammalian cells by sepiapterin reductase to BH2. Though SEP is not considered a physiologic metabolite in humans or animals, it has been used as an exogenous source.28 BH4 deficiencies have been associated with diabetic complications.29 BH4 is essential for NOS to synthesize NO.30 Low BH4 levels impair the production of NO, and leads to increased superoxide radical production, due to nNOS uncoupling. The superoxide radical then reacts with NO resulting in the production of peroxynitrite. This further reduces biological availability of NO.31
This information provides a rational basis for the use of supplemental SEP in diabetic conditions. We demonstrate that supplementation of SEP can normalize the delayed gastric emptying associated with diabetes in female rats (Fig. 1). This is in good agreement with our previous study, where we showed that supplementation of BH4 restored the delayed gastric emptying associated with diabetes in female rats.20 This results are further supported by attenuation of diabetes induced altered nitrergic relaxation in vivo (Fig. 2).
nNOS, which produces NO, is an important neuronal enzyme. NO can serve as a neuromodulator in a second messenger system for neuron-to-neuron communications.32,33 It has been well known that BH4, a critical cofactor for NOS activity, acts as a redox switch in the oxygenase domain of NOS.34 In this study, we examined the effects of inhibitors of BH4 biosynthesis MTX to find out the involvement of salvage pathway. Nitrergic relaxation and nNOSα dimerization was affected significantly by MTX in healthy female rats (Fig. 3A and 3B); SEP restored the NO production (Fig. 3C). These results revealed that BH4 biosynthesis is regulated not only by the main de novo pathway but also by the salvage pathway. This result are in good agreement with Robbins et al.35, who showed that MTX inhibits NO production in murine lung epithelial cells in vitro. These findings suggest a potential strategy for reducing NO production in vivo.
In our previous study, we showed that induction of diabetes reduced the expression of nNOSα protein in pylorus.20 This notion was further evident by the reductions in the dimer level of nNOSα and supplementation of dietary BH4 for 3 weeks stabilizes the functionally active, dimeric form of nNOSα in pylorus.20 We and others showed that BH4 also inhibits monomerization of nNOS, and the inactivation of the enzyme.20,36 The present results demonstrate that induction of diabetes causes decrease in both nNOSα monomer and dimer levels, which has been restored by BH4 or SEP treatment in gastric antrum (Fig. 4). It is possible that SEP or BH4 may protect degradation of enzyme and to improve the stabilization of nNOS dimer and activity of the enzyme.
Recently, we have reported that induction of diabetes caused intracellular BH4 depletion that was accompanied by a decrease in nNOS activity followed by reduced gastric motility in pylorus.34 In addition to the de novo biosynthesis of BH4, mammalian cells can also generate BH4 by an alternate pathway where SEP is converted to BH4 by sepiapterin reductase and DHFR.37 Cellular BH4 levels have been increased both in vitro and in vivo by exogenous supply of BH4 via salvage pathway.38 SEP treatment may selectively reverse the effect of diabetes by enhancing the intracellular BH4 that helped preserve the nNOS dimer level. Although our current study did not report that SEP treatment increase BH4, we speculate that either increase in the intracellular BH4 level and/or because of the change in the ratio of BH2/BH4 by SEP may prevent NOS uncoupling, resulting in restoration of appropriate NOS activity, nitrergic relaxation and gastric emptying.
Thus, SEP or BH4 offer a protection against diabetes-induced activation of gastric motility. SEP can move across the cell membrane in both an inward and outward direction.39 BH4, however, is virtually unable to cross the cell membrane in either direction.39 The two successive reactions, SEP to BH2, and BH2 to BH4, favor production of BH4, due to cellular redox-homeostasis. SEP is enforcedly taken up by the cell and BH4 is accumulated in the cytosol in a continuous fashion.39 This is in agreement with our current findings such that 10 day treatment with SEP restored nitrergic function. Whereas, our recent studies demonstrated that 3 week supplementation of dietary BH4 restored nNOS function.24 Taken together, both BH4 and SEP may be more effective therapeutic reagents in the treatment of diabetes induced gastric dysfunction.
In summary, the present data suggests that impaired bioavailability of NO may be associated with decreased BH4 biosynthesis via salvage pathway. Supplementation of SEP accelerated gastric emptying and gastric nNOSα expression and nNOSα dimerization.
Acknowledgments
We thank Dr. Veera Rajaratnam, Director of Scientific Publications at the Center for Women’s Health Research, Meharry Medical College, for her excellent scientific editing of the manuscript. We also thank Pravcen Gupta for technical help.
Financial Support for the original research was provided by the N1H-NIDDK R21DKO76704, 3R1DKO76704-03S1 awarded to Pandu R. Gangula and from RCM1 G12RR03032 provided to Pandu R. Gangula as start-up funds at Meharry Medical College, Nashville, TN, USA.
Footnotes
DISCLOSURES
There are no conflict of interest to disclose for the authors except Pandu R.R. Gangula and Pankaj J Pasricha (the University of Texas Medical Branch, Galveston, TX has filed a patent application in their name). Pandu R Gangula and Pankaj J Pasricha were involved in the design of experiment, and the interpretation of results as well as writing the manuscript. Sutapa Mukhopadhyay and Kalpana Ravella were involved in conducting experiments as well as writing the manuscript.
References
- 1.Waseem S, Moshiree B, Draganov PV. Gastroparesis: symptoms, evaluation, and treatment. World J Gastroenterol. 2009;15:25–37. doi: 10.3748/wjg.15.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gangula PR, Maner WL, Micci MA, Garfield RE, Pasricha PJ. Diabetes induces sex-dependent changes in neuronal nitric oxide synthase dimerization and function in the rat gastric antrum. Am J Physiol Gastrointest Liver Physiol. 2007;292:G725–33. doi: 10.1152/ajpgi.00406.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vijay SV, Lyford G, Gores G, Farrugia G. Nitric oxide in gastrointestinal health and disease. Gastroenterology. 2004;126:903–13. doi: 10.1053/j.gastro.2003.11.046. [DOI] [PubMed] [Google Scholar]
- 4.Toda N, Herman AG. Gastrointestinal function regulation by nitrergic efferent nerves. Pharmacol Rev. 2005;57:315–38. doi: 10.1124/pr.57.3.4. [DOI] [PubMed] [Google Scholar]
- 5.Holzer P, Schicho R, Holzer-Petsche U, Lippe IT. The gut as a neurological organ. Wien Klin Wochenschr. 2001;113:647–60. [PubMed] [Google Scholar]
- 6.Marletta MA. Nitric oxide synthase: aspects concerning structure and catalysis. Cell. 1994;78:927–30. doi: 10.1016/0092-8674(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 7.Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–8. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 8.Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298:249–58. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun J, Druhan LJ, Zwcier JL. Dose dependent effects of reactive oxygen and nitrogen species on the function of neuronal nitric oxide synthase. Arch Biochem Biophys. 2008;471:126–33. doi: 10.1016/j.abb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiefenbacher CP. Tetrahydrobiopterin: a critical cofactor for eNOS and a strategy in the treatment of endothelial dysfunction? Am J Physiol Heart Circ Physiol. 2001;280:H2484–8. doi: 10.1152/ajpheart.2001.280.6.H2484. [DOI] [PubMed] [Google Scholar]
- 11.Klatt P, Schmid M, Leopold E, Schmidt K, Werner ER, Mayer B. The pteridine binding site of brain nitric oxide synthase Tetrahydrobiopterin binding kinetics, specificity, and allosteric interaction with the substrate domain. J Biol Chem. 1994;269:13861–6. [PubMed] [Google Scholar]
- 12.Kojima S, Ona S, Iizuka I, Arai T, Mori H, Kubota K. Antioxidative activity of 5,6,7,8-tetrahydrobiopterin and its inhibitory effect on paraquat-induced cell toxicity in cultured rat hepatocytes. Free Radic Res. 1995;23:419–30. doi: 10.3109/10715769509065263. [DOI] [PubMed] [Google Scholar]
- 13.Mayer B, Werner ER. In search of a function for tetrahydrobiopterin in the biosynthesis of nitric oxide. Naunyn Schmiedebergs Arch Pharmacol. 1995;351:453–63. doi: 10.1007/BF00171035. [DOI] [PubMed] [Google Scholar]
- 14.Ishii M, Shimizu S, Momose K, Yamamoto T. SIN-1-induced cytotoxicity in cultured endothelial cells involves reactive oxygen species and nitric oxide: protective effect of sepiapterin. J Cardiovasc Pharmacol. 1999;33:295–300. doi: 10.1097/00005344-199902000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Pannirselvam M, Simon V, Verma S, Anderson T, Triggle CR. Chronic oral supplementation with sepiapterin prevents endothelial dysfunction and oxidative stress in small mesenteric arteries from diabetic (db/db) mice. Br J Pharmacol. 2003;140:701–6. doi: 10.1038/sj.bjp.0705476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blades RA, Bryant KR, Whitehead SA. Feedback effects of steroids and gonadotrophin control in adult rats with streptozotocin-induced diabetes mellitus. Diabetologia. 1985;28:348–54. doi: 10.1007/BF00283142. [DOI] [PubMed] [Google Scholar]
- 17.Kirchick HJ, Keyes PL, Frye BE. An explanation for anovulation in immature alloxan-diabetic rats treated with pregnant mare’s serum gonadotropin: reduced pituitary response to gonadotropin-releasing hormone. Endocrinology. 1979;105:1343–9. doi: 10.1210/endo-105-6-1343. [DOI] [PubMed] [Google Scholar]
- 18.Fiege B, Ballhausen D, Kierat L, et al. Plasma tetrahydrobiopterin and its pharmacokinetic following oral administration. Mol Genetics and Metabolism. 2004;81:45–51. doi: 10.1016/j.ymgme.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Levy HL, Milanowski A, Chakrapani A, et al. Efficacy of sapropterin dihydrochloride (tetrahydrobiopterin, 6R-BH4) for reduction of phenylalanine concentration in patients with phenylketonuria: a phase III randomised placebo-controlled study. Lancet. 2007;370:504–10. doi: 10.1016/S0140-6736(07)61234-3. [DOI] [PubMed] [Google Scholar]
- 20.Gangula PR, Mukhopadhyay S, Ravella K, et al. Tetrahydrobiopterin (BH4), a cofactor for nNOS, restores gastric emptying and nNOS expression in female diabetic rats. Am J Physiol Gastrointest Liver Physiol. 2010;298:G692–9. doi: 10.1152/ajpgi.00450.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez V, Barquist E, Rivier J, Tache Y. Central CRF inhibits gastric emptying of a nutrient solid meal in rats: the role of CRF2 receptors. Am J Physiol Gastrointest Liver Physiol. 1998;274:G965–70. doi: 10.1152/ajpgi.1998.274.5.G965. [DOI] [PubMed] [Google Scholar]
- 22.Gangula PR, Ravella K, Navarro MJ, Pasricha PJ. Gender dependent changes in gastric nitrergic expression and function in health and spontaneous diabetic rats. Gastroenterology. 2010;138(Supplement 1):S–457. [Google Scholar]
- 23.Nichol CA, Lee CL, Edelstein MP, Chao JY, Duch DS. Biosynthesis of tetrahydrobiopterin by de novo and salvage pathways in adrenal medulla extracts, mammalian cell cultures, and rat brain in vivo. Proc Natl Acad Sci USA. 1983;80:1546–50. doi: 10.1073/pnas.80.6.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacy B, Weiser K. Gastric motility, gastroparesis, and gastric stimulation. Surg Clin North Am. 2005;85:967–87. doi: 10.1016/j.suc.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Zandecki M, Vanden Berghe P, Depoortere I, et al. Characterization of myenteric neuropathy in the jejunum of spontaneously diabetic BB-rats. Neurogastroenterol Motil. 2008;20:818–28. doi: 10.1111/j.1365-2982.2008.01091.x. [DOI] [PubMed] [Google Scholar]
- 26.Cellek S, Foxwell NA, Moncada S. Two phases of nitrergic neuropathy in streptozotocin-induced diabetic rats. Diabetes. 2003;52:2353–62. doi: 10.2337/diabetes.52.9.2353. [DOI] [PubMed] [Google Scholar]
- 27.Watkins CC, Sawa A, Jaffrey S, et al. Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy. J Clin Invest. 2000;106:373–84. doi: 10.1172/JCI8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moens AL, Kass DA. Therapeutic potential of tetrahydrobiopterin for treating vascular and cardiac disease. J Cardiovasc Pharmacol. 2007;50:238–46. doi: 10.1097/FJC.0b013e318123f854. [DOI] [PubMed] [Google Scholar]
- 29.Shinozaki K, Kashiwagi A, Nishio Y, et al. Abnormal biopterin metabolism is a major cause of impaired endothelium-dependent relaxation through nitric oxide/O2- imbalance in insulin-resistant rat aorta. Diabetes. 1999;48:2437–15. doi: 10.2337/diabetes.48.12.2437. [DOI] [PubMed] [Google Scholar]
- 30.Delgado-Esteban M, Almeida A, Medina JM. Tetrahydrobiopterin deficiency increases neuronal vulnerability to hypoxia. J Neurochem. 2002;82:1148–59. doi: 10.1046/j.1471-4159.2002.01055.x. [DOI] [PubMed] [Google Scholar]
- 31.Pall ML. Nitric oxide synthase partial uncoupling as a key switching mechanism for the NO/ONOO-cycle. Med Hypotheses. 2007;69:821–5. doi: 10.1016/j.mehy.2007.01.070. [DOI] [PubMed] [Google Scholar]
- 32.Collier J, Valiance P. Second messenger role for NO widens to nervous and immune systems. Trends Pharmacol Sci. 1989;10:427–31. doi: 10.1016/s0165-6147(89)80001-x. [DOI] [PubMed] [Google Scholar]
- 33.Ross CA, Bredt D, Snyder SH. Messenger molecules in the cerebellum. Trends Neurosci. 1990;13:216–22. doi: 10.1016/0166-2236(90)90163-5. [DOI] [PubMed] [Google Scholar]
- 34.Tayeh MA, Marletta MA. Macrophage oxidation of 1-arginine to nitric oxide, nitrite, and nitrate: tetrahydrobiopterin is required as a cofactor. J Biol Chem. 1989;264:19654–8. [PubMed] [Google Scholar]
- 35.Robbins RA, Jinkins PA, Bryan TW, Prado SC, Milligan SA. Methotrexate inhibition of inducible nitric oxide synthase in murine lung epithelial cells in vitro. Am J Respir Cell Mol Biol. 1998;18:853–9. doi: 10.1165/ajrcmb.18.6.3070. [DOI] [PubMed] [Google Scholar]
- 36.Reif A, Frohlich LG, Kotsonos P, et al. Tetrahydrobiopterin inhibits monomerization and is consumed during catalysis in neuronal NO synthase. J Biol Chem. 1999;274:24921–9. doi: 10.1074/jbc.274.35.24921. [DOI] [PubMed] [Google Scholar]
- 37.Thony B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J. 2000;347:1–16. [PMC free article] [PubMed] [Google Scholar]
- 38.Hasegawa H, Sawabe K, Nakanishi N, Wakasugi OK. Delivery of exogenous tetrahydrobioptcrin (BH4) to cells of target organs: Role of salvage pathway and uptake of its precursor in effective elevation of tissue BR4. Molec Genetics and Metabolism. 2005;86:2–10. doi: 10.1016/j.ymgme.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Sawabe K, Yamamoto K, Harada Y, et al. Cellular uptake of sepiapterin and push-pull accumulation of tetrahydrobioptcrin. Mol Genet Metab. 2008;94:410–6. doi: 10.1016/j.ymgme.2008.04.007. [DOI] [PubMed] [Google Scholar]