Abstract
Diabetes mellitus is one of the leading causes of death, and the majority of these deaths are associated with cardiovascular diseases. Development and progression of myocardial infarction leading to heart failure is much more complex and multifactorial in diabetics compared with non-diabetics. Despite significant advances in pharmacological interventions and surgical techniques, the disease progression leading to diabetic end-stage heart failure remains very high. Recently, cell therapy has gained much attention as an alternative approach to treat various heart diseases. However, transplanted stem cell studies in diabetic animal models are very limited. In this review, we discuss the pathogenesis of the diabetic infarcted heart and the potential of stem cell therapy to repair and regenerate.
Keywords: Diabetes, Embryonic stem cells, Myocardial infarction, Oxidative stress, Hyperglycemia
Introduction
Diabetes mellitus is responsible for a host of conditions such as heart disease and stroke, nephropathy, retinopathy, blindness, neuropathy, gastroparesis, and periodontal disease. Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in patients with diabetes, accounting for an estimated 80% of all diabetic deaths in North America [1, 2]. Three quarters of these deaths are attributable to coronary artery disease (CAD) leading to myocardial infarction (MI) and heart failure. Acute MI, in the condition of diabetes, results in coagulative necrosis of the myocardium, myocyte cell loss, and infiltration of neutrophils. Cardiac myocyte cell loss in the infarcted region occurs via necrosis or apoptosis and has long been thought to be irreversible. Moreover, cardiac hypertrophy is considered to be a major mechanism to meet increased demands under pathophysiological conditions. Other means to restore cardiac function of the diabetic MI heart may have a great therapeutic potential. Current treatment strategies for diabetic infarcted heart and subsequent heart failure include drugs (aspirin, angiotensin converting enzyme inhibitors, and β-blockers), angioplasty, thrombolytic therapy, ventricular assist devices, and, ultimately, heart transplantation [1, 3]. However, recent advances in cell transplantation in the injured myocardium have enhanced the reality of improved cardiac function in the diseased myocardium [4–6].
Cell transplant studies in the infarcted hearts have reported differentiated heart cell types from transplanted adult and embryonic stem (ES) cells [4, 7]. Thus, cell transplantation may be applicable in the regeneration of diabetic infarcted myocardium. In this review, we first highlight differences in the pathophysiology of diabetic versus non-diabetic infarcted hearts. This is followed by a discussion of the mechanisms in the development of cardiac pathogenesis in the diabetic infarcted heart. Finally, the review focuses on the potential of stem cells as a future therapeutic intervention.
Incidence of myocardial infarction in diabetic versus non-diabetic patients
At present, the rate of hospitalized MI is about 180 per 100,000, and ~25% of these MI patients have also been diagnosed with diabetes [8, 9]. Furthermore, patients with diabetes without prior history of MI have as high a risk of MI as non-diabetic patients with history of MI [10, 11]. Following MI, a greater risk of morbidity and recurrent ischemic events is observed in the diabetic population compared with non-diabetic equivalents [12, 13]. The GUSTO-1 angiographic study revealed that diabetes is an independent determinant of 30-day acute MI mortality with a rate of 11.3% in diabetic versus 5.9% in non-diabetic subjects [3]. The MONICA study, comparing diabetics to non-diabetics, determined that the overall mortality following MI was 4 and 7 times higher in diabetic men and women, respectively [12]. Additionally, patients with diabetes following non-fatal MI encounter more severe complications than their non-diabetic counterparts including larger infarcts, increased frequency of post-infarction angina, and enhanced susceptibility to congestive heart failure [3, 14, 15]. Although the source of these differences in outcomes has yet to be fully understood and delineated, the current evidence suggests diabetic MI pathogenesis is more complex and multi-factorial.
Animal models
Appropriate animal models are powerful tools for understanding the pathogenesis and preventative strategies in diabetes research. There are several chemicals (streptozotocin (STZ) and alloxan) and genetically induced mouse and rat animal models as listed in Table 1. MI can be generated in these diabetic animal models using a coronary artery ligation technique, which closely mimics several features of the diabetic infarcted human heart (Table 1). Moreover, Table 1 lists various type 2 diabetic (T2D) animal models and their use in MI studies [16–34].
Table 1.
| T2D models | Description | Type 2 diabetic characteristics | Used in MI/IR studies? | References |
|---|---|---|---|---|
| ob/ob mouse | Leptin deficient | Obesity, dyslipidemia, insulin resistance, hyperinsulinemia, hyperglycemia, impaired glucose tolerance | Yes/Yes | 16, 17, 18, 19 |
| db/db mouse | Leptin resistant | Obesity, dyslipidemia, insulin resistance, hyperinsulinemia, hyperglycemia | Yes/Yes | 17, 19, 20, 21 |
| KK/Ay mouse | Genetically derived (Ay mutation) | Obesity, insulin resistance, hyperinsulinemia, islet cell hyperplasia, hyperglycemia, impaired glucose tolerance | No/No | 16, 20, 22 |
| Zucker (fa/fa) rat | Leptin resistant | Obesity, dyslipidemia, insulin resistance, hyperinsulinemia, hyperglycemia, impaired glucose tolerance, hypertension | Yes/Yes | 20, 31, 32, 34 |
| Goto Kakizaki rat | Spontaneous diabetes by selective breeding | Insulin resistance, hyperinsulinemia, hyperglycemia, impaired glucose tolerance | Yes/Yes | 16, 20, 23, 32, 33 |
| NZO mouse | Polygenic diabetes model by selective inbreeding | Obesity, insulin resistance, hyperinsulinemia, hyperglycemia, impaired glucose tolerance | No/No | 20 |
| NSY mouse | Spontaneous diabetes by selective inbreeding | Insulin resistance, hyperglycemia | No/No | 16 |
| OLETF rat | Spontaneous diabetes by selective inbreeding | Mild obesity, dyslipidemia, hyperinsulinemia, hyperglycemia, impaired glucose tolerance | No/Yes | 16, 20, 30 |
| Cohen diabetic rat | Diet-induced diabetes | Hyperinsulinemia, hyperglycemia, hypertension | No/No | 20, 24, 25 |
| Low dose ALX- or STZ-treated rodents | Chemical ablation of β cells | Dyslipidemia, hyperglycemia, hyperinsulinemia | Yes/Yes | 20, 28, 29 |
| C57BL/6J | High fat diet-induced | Obesity, dyslipidemia, insulin resistance, hyperglycemia, impaired glucose tolerance | Yes/Yes | 20, 26, 27 |
NZO New Zealand obese, NYS Nagoya-Shibata-Yasuda, OLETF Otsuka Long-Evans Tokushima fatty, ALX alloxan, and STZ streptozotocin, IR ischemia/reperfusion
Factors responsible for pathophysiologic mechanisms
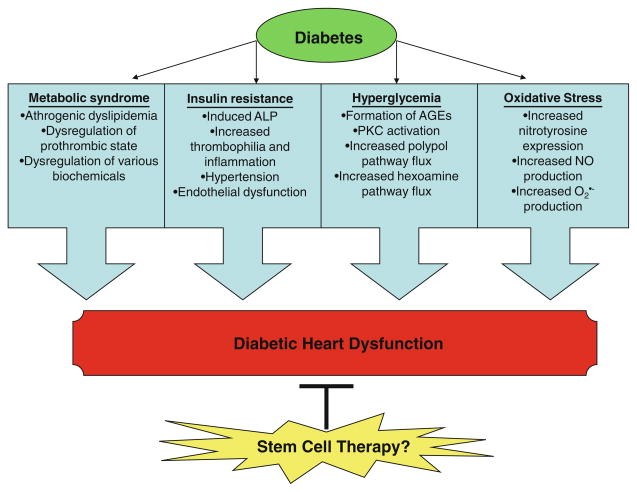
The development and progression of MI in diabetes is multifactorial including metabolic syndrome, insulin resistance, hyperglycemia, and oxidative stress (Fig. 1). However, due to multifaceted factors, there is no clear understanding of the precise pathophysiologic mechanisms of the infarcted diabetic heart.
Fig. 1.
List of various factors involved in the development and progression of heart failure in diabetes
Metabolic syndrome
The metabolic syndrome, a complex disorder that encompasses several risk factors including impaired glucose regulation, insulin resistance, hypertension, obesity, dysli-pidemia, and microalbuminuria, is associated with the infarcted diabetic heart [35]. In diabetic MI patients, the metabolic syndrome is associated with the prothrombotic state, which develops by decreased endothelial thrombo-resistance, platelet hyperactivity, increased plasmatic coagulation, and hypofibrinolysis [36, 37]. Moreover, atherogenic dyslipidemia is also closely associated with the metabolic syndrome and T2D. Atherogenic dyslipidemia is comprised of 3 constituents including elevated low-density-lipoproteins (LDL), reduced high-density-lipoproteins (HDL), and increased triglyceride levels [38]. Importantly, it has been reported that accumulated LDL particles are associated with oxidation, impairment of endothelial cell function, inflammation and adhesion, and promotion of vascular smooth muscle cell anomalies that may lead to MI. High levels of LDL are associated with an increased risk for the development of MI as much as observed in post-MI [2, 38].
Insulin resistance
Insulin, an anabolic hormone, plays an essential role in carbohydrate and lipid homeostasis and protein metabolism. Additionally, insulin has pertinent non-metabolic functions including thrombosis, homeostasis, and modulation of vascular tone and blood flow [39–41]. Insulin’s cellular actions are impaired in insulin-resistant conditions including obesity, T2D, stress, metabolic syndrome, hypertension, and MI. Hyperinsulinemia and insulin resistance give rise to hyperglycemia, decreased lipolysis, increased adipose mass, dysfunctional energy balance and appetite, and an imbalance in the signaling pathway downstream of the insulin receptor. In turn, these pathogenic components induce hypertension, increased thrombophilia and inflammation, endothelial dysfunction, and, ultimately, atherosclerotic cardiovascular disease [40, 42]. Furthermore, previous investigations have shown that the extent of insulin resistance is directly related to the incidence of MI and stroke in diabetes [2].
Hyperglycemia
Elevated plasma blood glucose level, hyperglycemia, is now established as a major contributor in the pathogenesis of cardiovascular diseases including MI leading to heart failure and death [2, 43, 44]. Hyperglycemia has been shown to be associated with increased oxidative stress, enhanced inflammatory processes, non-enzymatic and enzymatic glycosylation of proteins, delayed endothelial cell replication, increased cellular apoptosis, and accelerated atherosclerosis [2, 43]. Chronic elevated glucose induces vasculature and cardiac myocyte cell death through several processes including formation of advanced glycation end-products (AGE) via non-enzymatic glycosylation of proteins and lipids, activation of protein kinase C (PKC), increased polyol pathway flux, and enhancement of flow through the hexosamine pathway [2, 43, 45, 46].
One of the most deleterious effects of chronic hyperglycemia is glycation of proteins and lipids. AGEs are formed as a result of non-enzymatic reactions of reducing sugars with free amino groups of proteins, lipids, and nucleic acids. AGEs contribute to the development of some pathological modifications associated with various CVDs through multiple mechanisms: interrupting normal function of proteins and lipids, diminishing ligand/receptor recognition of proteins and lipids, releasing inflammatory cytokines and growth factors from macrophages, and altering enzymatic activity, extracellular matrix (ECM) permeability, and circulating lipoproteins [2, 43, 47]. These mechanisms initiate a continuous cycle of cellular insult and vascular complications, which potentiate and contribute to the development of diabetic vasculopathy and CVDs [2, 43, 47].
Mechanistic activation of PKC pathway contributes to a myriad of pathogenic consequences by altering expression of angiotensinogen II (AT-II), activator protein-1 (AP-1), endothelial nitric oxide synthetase (eNOS), endothelin-1 (ET-1), vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), and production of increased reactive oxygen species (ROS). These alterations in signaling pathways lead to vasoconstriction, capillary occlusion, pro-inflammatory gene expression, hypertension, vascular smooth muscle cell growth, and protein functions [45, 46].
Moreover, hyperglycemia further causes vascular/cardiac complications by inducing oxidative stress via increased polyol pathway flux. Generation of reactive oxygen species (ROS) is attributable to two enzymes within the pathway. The first, aldose reductase, utilizes NADPH to reduce glucose to sorbitol. Under non-pathological conditions, this reaction is a minute percentage (<3%) of total glucose use, whereas in a hyperglycemic environment, glucose metabolism by aldose reductase is increased by 30–35% [48]. This leads to a decrease in NADPH availability, reducing glutathione regeneration and NO synthase activity, and ultimately augments oxidative stress [46, 49]. Sorbitol dehydrogenase (SDH), the second enzyme, oxidizes sorbitol to fructose with simultaneous production of NADPH, which may be used to produce superoxide [46, 49]. Taken together, ROS generated within the polyol pathway in the presence of elevated glucose concentrations has been shown to play an important role in the development of diabetic vascular complications [50].
Additionally, previous studies suggest hyperglycemia-dependent activation of the hexosamine pathway impairs various nuclear and cytoplasmic protein activity through O-linked glycosylation modification (O-GlcNAcylation) leading to endothelial and cardiomyocyte dysfunction [46, 51, 52].
Oxidative stress in the diabetic heart
Oxidative stress is responsible for a myriad of diabetic complications including cardiomyopathy [53], impaired wound healing [54], retinopathy [55, 56], bone disorder [57], endothelial dysfunction [58], nephropathy [59], neuropathy [60], and MI [61]. Oxidative stress is an imbalance between production of reactive oxygen species (ROS) and its breakdown by enzymes including superoxide dismutase (SOD), glutathione peroxidase, catalase, and vitamins C and E. ROS include a variety of oxygen and nitrogen derivatives including superoxide ( ), hydroxyl (OH•), hydrogen peroxide (H2O2), and peroxynitrite (ONOO−). Detailed reviews of ROS generation in the cardiovascular system have been previously published [62–64]. However, potential sources of ROS generation include dysfunctional mitochondrial electron transport chain, arachidonic acid metabolism, neutrophil infiltration and activation, xanthine-xanthine oxidase, and catecholamines [65, 66]. A perturbance in the balance of ROS activates mitochondrial pathways, caspases, and mitogen-activated protein kinases (MAPKs), which ultimately have been implicated in the onset and progression of many cardiovascular diseases such as diabetic MI [65, 66].
Apoptosis in the diabetic heart
Apoptosis is programmed cell death mediated through a succession of biochemical events including cell blebbing and shrinkage, chromatin condensation, and DNA fragmentation. Collected evidence suggests apoptosis occurs in cardiovascular diseases and plays a significant role in the development of heart failure [5, 67, 68]. Despite intense investigation into apoptosis in cardiovascular diseases, the exact stimulus for apoptosis remains controversial. The balance between endogenous apoptotic stimuli and inhibitors decide the fate of the cell, i.e. death versus survival.
Evidence of apoptosis has been reported in the pathogenesis of various complications including nephropathy, cardiomyopathy, retinopathy, and neuropathy [69–72]. Several studies have identified hyperglycemia as an independent risk factor for cardiac cell insult, damage, and, ultimately, cell death leading to many cardiovascular diseases including MI [67, 69, 73]. Additionally, diabetes has been shown to be associated with increased myocardial cell death in the months following the acute MI phase [67, 74]. Adverse cardiac remodeling was observed in the diabetic MI that included cardiomyocyte-apoptotic cell loss, hypertrophy, and fibrosis [75]. Moreover, several mechanisms of hyperglycemia-induced myocardial apoptosis have been identified. However, future studies are necessary to understand the enormity and complexity of the initiation and progression of diabetic cardiomyocyte loss.
Stem cell therapy
Engineered cells and tissues have gained much attention as an alternate method to repair or regenerate injured myocardium following MI. A number of candidate cell types have been transplanted into MI animal models demonstrating their ability to improve the structural and functional capacity of the heart; for example, skeletal myoblasts, bone marrow-derived hematopoietic stem cells, mesenchymal stem cells, endogenous cardiac stem cells, induced pluripotent stem (iPS) cells, embryonic stem (ES) cells [5, 6, 76–78]. Clinical trials have also been ongoing to study the effects of cell transplantation in patients with MI. The Reinfusion of Enriched Progenitor cells And Infarct Remodeling in Acute Heart Failure (REPAIR-AMI) clinical trial investigated the effects of infused bone marrow stem cells in patients day 4 post-MI and reported improvement in left ventricular ejection fraction (LVEF) [79]. However, the Autologous Stem cell Transplantation in Acute Myocardial Infarction (ASTAMI) clinical trial reported no ventricular function improvement in stem cell transplant–treated group compared to the control group [79]. Overall, the clinical data have reached no conclusive decision regarding the efficacy of adult stem cells for transplantation in MI. However, we are still in search of the optimal cell type for enhanced cardiac repair and regeneration.
Moreover, cell transplantation has also been examined in diabetic cardiomyopathy and infarcted hearts [80–83]. Transplanted MSCs improved cardiac function through increased angiogenesis and matrix metalloproteinase-2 (MMP-2) expression and decreased collagen content and transcription of MMP-9 in diabetic cardiomyopathy hearts [83]. Bone marrow stem cells were also tested in the diabetic cardiomyopathy setting in which improved cardiac function was observed [82]. Govaert et al. transplanted diabetic bone marrow mononuclear cells (BMMCs) and healthy BMMCs into db/db mice with ischemic myocardium. They demonstrated diabetic BMMCs were unable to improve cardiac function post-MI, whereas healthy BMMCs were able to preserve fractional shortening [80]. Additionally, transplanted MSCs initiated increased heart rate, left ventricular developed pressure, and contractility index as well as decreased systolic blood pressure in the diabetic animal model [81].
Current adult stem cell transplantation studies in the diabetic heart are very limited and require further investigation. Moreover, as per the best of our knowledge, there is no study performed on either a diabetic infarcted or cardiomyopathy heart using ES or iPS cells. ES and iPS cells possess many desirable traits, making them a more promising approach to attenuate the damaged myocardium. ES cells, derived from the inner cell mass of a blastocyst, are pluripotent, undifferentiated cells. They are capable of self-renewal and are able to differentiate into multiple cell types in the body including functional cardiomyocytes, endothelial cells, and vascular smooth muscle cells [4]. Previous studies have demonstrated the ability of ES cells transplanted into the infarcted heart to engraft, differentiate into cardiomyocytes, contribute to heart regeneration, and improve heart function [4–6]. Although the molecular mechanism of myocardial repair by transplanted ES cells has yet to be elucidated, it remains an active area of continued research. Nevertheless, an optimized ES cell therapy holds great promise for the treatment of diabetic injured myocardium.
Another emerging approach of cell transplantation therapy is the creation of iPS cells. iPS cells are reprogrammed adult cells exhibiting pluripotent cell characteristics through forced gene expression of Oct 3/4, Sox2, Klf4, and c-myc. These cells can then be directed to differentiate into specific cell types though mechanisms similar to ES cell differentiation. Fibroblast-derived iPS cells have recently been tested in a MI model and demonstrated the ability to engraft into the host myocardium, differentiate into all three major heart cells such as cardiac myocytes, smooth muscle and endothelial cells, repair the ventricular wall, and restore contractile function [78]. Although still in infancy, iPS cell transplantation holds tremendous potential for use in the repair of diabetic MI damaged myocardium.
Future perspectives
Patients with diabetes have improved their lifestyle with strict pharmacological interventions and non-pharmacological management (exercise, weight, smoking, etc.). However, the relative frequency and death occurring from MI remain drastically increased in the T2D patients compared to their non-diabetic counterparts. Thus, there is an eminent need to develop new therapeutic options. Recent studies suggest that stem cells transplanted in the infarcted heart have significantly improved cardiac function along with differentiation into all three major heart cell types. Moreover, transplanted adult stem cells in STZ-induced diabetic cardiomyopathy show improved cardiac function. However, there are no studies that define the role of ES cells for the treatment of infarcted diabetic hearts. More recently, generation of iPS cells and their applications to treat MI with improved heart function has raised new hope to bring stem cell therapy in the clinic. Overall, we propose that ES or iPS cells could have additional beneficial effects for the treatment of diabetic infarcted hearts.
Acknowledgments
We acknowledge support provided by 1R21 HL085795-01A1 and 1R01HL090646-01 (to DKS). Dr. Singal is the holder of the Naranjan Dhalla Chair in Cardiovascular Research, supported by the St. Boniface Hospital & Research Foundation.
Contributor Information
Carley E. Glass, Biomolecular Science Center, Burnett School of Biomedical Sciences, College of Medicine, University of Central Florida, 4000 Central Florida BLVD, Room 224, Orlando, FL 32816, USA
Pawan K. Singal, Institute of Cardiovascular Sciences, St. Boniface General Hospital Research Centre, University of Manitoba, Winnipeg, MB, Canada
Dinender K. Singla, Email: dsingla@mail.ucf.edu, Biomolecular Science Center, Burnett School of Biomedical Sciences, College of Medicine, University of Central Florida, 4000 Central Florida BLVD, Room 224, Orlando, FL 32816, USA
References
- 1.Aronson D, Rayfield EJ, Chesebro JH. Mechanisms determining course and outcome of diabetic patients who have had acute myocardial infarction. Ann Intern Med. 1997;126:296–306. doi: 10.7326/0003-4819-126-4-199702150-00006. [DOI] [PubMed] [Google Scholar]
- 2.Rahman S, Rahman T, Ismail AA, Rashid AR. Diabetes-associated macrovasculopathy: pathophysiology and pathogenesis. Diabetes Obes Metab. 2007;9:767–780. doi: 10.1111/j.1463-1326.2006.00655.x. [DOI] [PubMed] [Google Scholar]
- 3.Woodfield SL, Lundergan CF, Reiner JS, Greenhouse SW, Thompson MA, Rohrbeck SC, Deychak Y, Simoons ML, Califf RM, Topol EJ, Ross AM. Angiographic findings and outcome in diabetic patients treated with thrombolytic therapy for acute myocardial infarction: the GUSTO-I experience. J Am Coll Cardiol. 1996;28:1661–1669. doi: 10.1016/s0735-1097(96)00397-x. [DOI] [PubMed] [Google Scholar]
- 4.Singla DK, Hacker TA, Ma L, Douglas PS, Sullivan R, Lyons GE, Kamp TJ. Transplantation of embryonic stem cells into the infarcted mouse heart: formation of multiple cell types. J Mol Cell Cardiol. 2006;40:195–200. doi: 10.1016/j.yjmcc.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Singla DK, Lyons GE, Kamp TJ. Transplanted embryonic stem cells following mouse myocardial infarction inhibit apoptosis and cardiac remodeling. Am J Physiol Heart Circ Physiol. 2007;293:H1308–H1314. doi: 10.1152/ajpheart.01277.2006. [DOI] [PubMed] [Google Scholar]
- 6.Min JY, Yang Y, Converso KL, Liu L, Huang Q, Morgan JP, Xiao YF. Transplantation of embryonic stem cells improves cardiac function in postinfarcted rats. J Appl Physiol. 2002;92:288–296. doi: 10.1152/jappl.2002.92.1.288. [DOI] [PubMed] [Google Scholar]
- 7.Jing D, Parikh A, Canty JM, Jr, Tzanakakis ES. Stem cells for heart cell therapies. Tissue Eng Part B Rev. 2008;14:393–406. doi: 10.1089/ten.teb.2008.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tenerz A, Lonnberg I, Berne C, Nilsson G, Leppert J. Myocardial infarction and prevalence of diabetes mellitus. Is increased casual blood glucose at admission a reliable criterion for the diagnosis of diabetes? Eur Heart J. 2001;22:1102–1110. doi: 10.1053/euhj.2000.2445. [DOI] [PubMed] [Google Scholar]
- 9.Roger VL, Weston SA, Gerber Y, Killian JM, Dunlay SM, Jaffe AS, Bell MR, Kors J, Yawn BP, Jacobsen SJ. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation. 2010;121:863–869. doi: 10.1161/CIRCULATIONAHA.109.897249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 11.Hu G, Jousilahti P, Qiao Q, Peltonen M, Katoh S, Tuomilehto J. The gender-specific impact of diabetes and myocardial infarction at baseline and during follow-up on mortality from all causes and coronary heart disease. J Am Coll Cardiol. 2005;45:1413–1418. doi: 10.1016/j.jacc.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 12.Lundberg V, Stegmayr B, Asplund K, Eliasson M, Huhtasaari F. Diabetes as a risk factor for myocardial infarction: population and gender perspectives. J Intern Med. 1997;241:485–492. doi: 10.1111/j.1365-2796.1997.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee CD, Folsom AR, Pankow JS, Brancati FL. Cardiovascular events in diabetic and nondiabetic adults with or without history of myocardial infarction. Circulation. 2004;109:855–860. doi: 10.1161/01.CIR.0000116389.61864.DE. [DOI] [PubMed] [Google Scholar]
- 14.Mak KH, Topol EJ. Emerging concepts in the management of acute myocardial infarction in patients with diabetes mellitus. J Am Coll Cardiol. 2000;35:563–568. doi: 10.1016/s0735-1097(99)00628-2. [DOI] [PubMed] [Google Scholar]
- 15.Lomuscio A, Castagnone M, Vergani D, Verzoni A, Beltrami A, Ravaglia R, Pozzoni L. Clinical correlation between diabetic and non diabetic patients with myocardial infarction. Acta Cardiol. 1991;46:543–554. [PubMed] [Google Scholar]
- 16.Rees DA, Alcolado JC. Animal models of diabetes mellitus. Diabet Med. 2005;22:359–370. doi: 10.1111/j.1464-5491.2005.01499.x. [DOI] [PubMed] [Google Scholar]
- 17.Nishina PM, Lowe S, Wang J, Paigen B. Characterization of plasma lipids in genetically obese mice: the mutants obese, diabetes, fat, tubby, and lethal yellow. Metabolism. 1994;43:549–553. doi: 10.1016/0026-0495(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 18.McGaffin KR, Zou B, McTiernan CF, O’Donnell CP. Leptin attenuates cardiac apoptosis after chronic ischaemic injury. Cardiovasc Res. 2009;83:313–324. doi: 10.1093/cvr/cvp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gundewar S, Calvert JW, Elrod JW, Lefer DJ. Cytoprotective effects of N, N, N-trimethylsphingosine during ischemia-reperfusion injury are lost in the setting of obesity and diabetes. Am J Physiol Heart Circ Physiol. 2007;293:H2462–H2471. doi: 10.1152/ajpheart.00392.2007. [DOI] [PubMed] [Google Scholar]
- 20.Srinivasan K, Ramarao P. Animal models in type 2 diabetes research: an overview. Indian J Med Res. 2007;125:451–472. [PubMed] [Google Scholar]
- 21.Greer JJ, Ware DP, Lefer DJ. Myocardial infarction and heart failure in the db/db diabetic mouse. Am J Physiol Heart Circ Physiol. 2006;290:146–153. doi: 10.1152/ajpheart.00583.2005. [DOI] [PubMed] [Google Scholar]
- 22.Iwatsuka H, Shino A, Suzuoki Z. General survey of diabetic features of yellow KK mice. Endocrinol Jpn. 1970;17:23–35. doi: 10.1507/endocrj1954.17.23. [DOI] [PubMed] [Google Scholar]
- 23.Chandler MP, Morgan EE, McElfresh TA, Kung TA, Rennison JH, Hoit BD, Young ME. Heart failure progression is accelerated following myocardial infarction in type 2 diabetic rats. Am J Physiol Heart Circ Physiol. 2007;293:H1609–H1616. doi: 10.1152/ajpheart.01338.2006. [DOI] [PubMed] [Google Scholar]
- 24.Cohen AM, Rosenmann E, Rosenthal T. The Cohen diabetic (non-insulin-dependent) hypertensive rat model. Description of the model and pathologic findings. Am J Hypertens. 1993;6:989–995. doi: 10.1093/ajh/6.12.989. [DOI] [PubMed] [Google Scholar]
- 25.Weksler-Zangen S, Yagil C, Zangen DH, Ornoy A, Jacob HJ, Yagil Y. The newly inbred cohen diabetic rat: a nonobese normolipidemic genetic model of diet-induced type 2 diabetes expressing sex differences. Diabetes. 2001;50:2521–2529. doi: 10.2337/diabetes.50.11.2521. [DOI] [PubMed] [Google Scholar]
- 26.Matsushima S, Kinugawa S, Yokota T, Inoue N, Ohta Y, Hamaguchi S, Tsutsui H. Increased myocardial NAD(P)H oxidase-derived superoxide causes the exacerbation of postinfarct heart failure in type 2 diabetes. Am J Physiol Heart Circ Physiol. 2009;297:H409–H416. doi: 10.1152/ajpheart.01332.2008. [DOI] [PubMed] [Google Scholar]
- 27.Thakker GD, Frangogiannis NG, Bujak M, Zymek P, Gaubatz JW, Reddy AK, Taffet G, Michael LH, Entman ML, Ballantyne CM. Effects of diet-induced obesity on inflammation and remodeling after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;291:H2504–H2514. doi: 10.1152/ajpheart.00322.2006. [DOI] [PubMed] [Google Scholar]
- 28.Huang JP, Huang SS, Deng JY, Hung LM. Impairment of insulin-stimulated Akt/GLUT4 signaling is associated with cardiac contractile dysfunction and aggravates I/R injury in STZ-diabetic rats. J Biomed Sci. 2009;16:77. doi: 10.1186/1423-0127-16-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song GY, Wu YJ, Yang YJ, Li JJ, Zhang HL, Pei HJ, Zhao ZY, Zeng ZH, Hui RT. The accelerated post-infarction progression of cardiac remodelling is associated with genetic changes in an untreated streptozotocin-induced diabetic rat model. Eur J Heart Fail. 2009;11:911–921. doi: 10.1093/eurjhf/hfp117. [DOI] [PubMed] [Google Scholar]
- 30.Miki T, Miura T, Hotta H, Tanno M, Yano T, Sato T, Terashima Y, Takada A, Ishikawa S, Shimamoto K. Endoplasmic reticulum stress in diabetic hearts abolishes erythropoietin-induced myocardial protection by impairment of phospho-glycogen synthase kinase-3beta-mediated suppression of mitochondrial permeability transition. Diabetes. 2009;58:2863–2872. doi: 10.2337/db09-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dixon RA, Davidson SM, Wynne AM, Yellon DM, Smith CC. The cardioprotective actions of leptin are lost in the Zucker obese (fa/fa) rat. J Cardiovasc Pharmacol. 2009;53:311–317. doi: 10.1097/FJC.0b013e31819d6152. [DOI] [PubMed] [Google Scholar]
- 32.Kristiansen SB, Lofgren B, Stottrup NB, Khatir D, Nielsen-Kudsk JE, Nielsen TT, Botker HE, Flyvbjerg A. Ischaemic preconditioning does not protect the heart in obese and lean animal models of type 2 diabetes. Diabetologia. 2004;47:1716–1721. doi: 10.1007/s00125-004-1514-4. [DOI] [PubMed] [Google Scholar]
- 33.Vahtola E, Louhelainen M, Forsten H, Merasto S, Raivio J, Kaheinen P, Kyto V, Tikkanen I, Levijoki J, Mervaala E. Sirtuin1–p53, forkhead box O3a, p38 and post-infarct cardiac remodeling in the spontaneously diabetic Goto-Kakizaki rat. Cardiovasc Diabetol. 2010;9:5. doi: 10.1186/1475-2840-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li TS, Takahashi M, Suzuki R, Kobayashi T, Ito H, Mikamo A, Hamano K. Pravastatin improves remodeling and cardiac function after myocardial infarction by an antiinflammatory mechanism rather than by the induction of angiogenesis. Ann Thorac Surg. 2006;81:2217–2225. doi: 10.1016/j.athoracsur.2005.12.065. [DOI] [PubMed] [Google Scholar]
- 35.Bonora E. The metabolic syndrome and cardiovascular disease. Ann Med. 2006;38:64–80. doi: 10.1080/07853890500401234. [DOI] [PubMed] [Google Scholar]
- 36.Durina J, Remkova A. Prothrombotic state in metabolic syndrome. Bratisl Lek Listy. 2007;108:279–280. [PubMed] [Google Scholar]
- 37.Tschoepe D, Roesen P. Heart disease in diabetes mellitus: a challenge for early diagnosis and intervention. Exp Clin Endocrinol Diabetes. 1998;106:16–24. doi: 10.1055/s-0029-1211944. [DOI] [PubMed] [Google Scholar]
- 38.Rizzo M, Berneis K. Small dense low-density-lipoproteins and the metabolic syndrome. Diabetes Metab Res Rev. 2007;23:14–20. doi: 10.1002/dmrr.694. [DOI] [PubMed] [Google Scholar]
- 39.Anfossi G, Russo I, Doronzo G, Trovati M. Relevance of the vascular effects of insulin in the rationale of its therapeutical use. Cardiovasc Hematol Disord Drug Targets. 2007;7:228–249. doi: 10.2174/187152907782793581. [DOI] [PubMed] [Google Scholar]
- 40.Cersosimo E, Defronzo RA. Insulin resistance and endothelial dysfunction: the road map to cardiovascular diseases. Diabetes Metab Res Rev. 2006;22:423–436. doi: 10.1002/dmrr.634. [DOI] [PubMed] [Google Scholar]
- 41.Steinberg HO, Baron AD. Vascular function insulin resistance and fatty acids. Diabetologia. 2002;45:623–634. doi: 10.1007/s00125-002-0800-2. [DOI] [PubMed] [Google Scholar]
- 42.Zinn A, Felson S, Fisher E, Schwartzbard A. Reassessing the cardiovascular risks and benefits of thiazolidinediones. Clin Cardiol. 2008;31:397–403. doi: 10.1002/clc.20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palumbo F, Bianchi C, Miccoli R, Del PS. Hyperglycaemia and cardiovascular risk. Acta Diabetol. 2003;40(Suppl 2):S362–S369. doi: 10.1007/s00592-003-0121-z. [DOI] [PubMed] [Google Scholar]
- 44.Chyun DA, Young LH. Diabetes mellitus and cardiovascular disease. Nurs Clin North Am. 2006;41:681–685. doi: 10.1016/j.cnur.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Bartnik M, Norhammar A, Ryden L. Hyperglycaemia and cardiovascular disease. J Intern Med. 2007;262:145–156. doi: 10.1111/j.1365-2796.2007.01831.x. [DOI] [PubMed] [Google Scholar]
- 46.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 47.Basta G, Schmidt AM, De CR. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63:582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Ramana KV, Chandra D, Srivastava S, Bhatnagar A, Srivastava SK. Nitric oxide regulates the polyol pathway of glucose metabolism in vascular smooth muscle cells. FASEB J. 2003;17:417–425. doi: 10.1096/fj.02-0722com. [DOI] [PubMed] [Google Scholar]
- 49.Bonnefont-Rousselot D. Glucose and reactive oxygen species. Curr Opin Clin Nutr Metab Care. 2002;5:561–568. doi: 10.1097/00075197-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 50.Jay D, Hitomi H, Griendling KK. Oxidative stress and diabetic cardiovascular complications. Free Radic Biol Med. 2006;40:183–192. doi: 10.1016/j.freeradbiomed.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 51.Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J Biol Chem. 2003;278:44230–44237. doi: 10.1074/jbc.M303810200. [DOI] [PubMed] [Google Scholar]
- 52.Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, Sbraccia P, Spagnoli LG, Sesti G, Lauro R. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002;106:466–472. doi: 10.1161/01.cir.0000023043.02648.51. [DOI] [PubMed] [Google Scholar]
- 53.Watanabe K, Thandavarayan RA, Gurusamy N, Zhang S, Muslin AJ, Suzuki K, Tachikawa H, Kodama M, Aizawa Y. Role of 14–3-3 protein and oxidative stress in diabetic cardiomyopathy. Acta Physiol Hung. 2009;96:277–287. doi: 10.1556/APhysiol.96.2009.3.3. [DOI] [PubMed] [Google Scholar]
- 54.Peppa M, Stavroulakis P, Raptis SA. Advanced glycoxidation products and impaired diabetic wound healing. Wound Repair Regen. 2009;17:461–472. doi: 10.1111/j.1524-475X.2009.00518.x. [DOI] [PubMed] [Google Scholar]
- 55.Zheng L, Kern TS. Role of nitric oxide, superoxide, peroxynitrite and PARP in diabetic retinopathy. Front Biosci. 2009;14:3974–3987. doi: 10.2741/3505. [DOI] [PubMed] [Google Scholar]
- 56.Madsen-Bouterse SA, Kowluru RA. Oxidative stress and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Rev Endocr Metab Disord. 2008;9:315–327. doi: 10.1007/s11154-008-9090-4. [DOI] [PubMed] [Google Scholar]
- 57.Hamada Y, Fujii H, Fukagawa M. Role of oxidative stress in diabetic bone disorder. Bone. 2009;45(Suppl 1):S35–S38. doi: 10.1016/j.bone.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 58.Potenza MA, Gagliardi S, Nacci C, Carratu’ MR, Montagnani M. Endothelial dysfunction in diabetes: from mechanisms to therapeutic targets. Curr Med Chem. 2009;16:94–112. doi: 10.2174/092986709787002853. [DOI] [PubMed] [Google Scholar]
- 59.Ha H, Hwang IA, Park JH, Lee HB. Role of reactive oxygen species in the pathogenesis of diabetic nephropathy. Diabetes Res Clin Pract. 2008;82(Suppl 1):S42–S45. doi: 10.1016/j.diabres.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 60.Figueroa-Romero C, Sadidi M, Feldman EL. Mechanisms of disease: the oxidative stress theory of diabetic neuropathy. Rev Endocr Metab Disord. 2008;9:301–314. doi: 10.1007/s11154-008-9104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di FC, Cuzzocrea S, Rossi F, Marfella R, D’Amico M. Oxidative stress as the leading cause of acute myocardial infarction in diabetics. Cardiovasc Drug Rev. 2006;24:77–87. doi: 10.1111/j.1527-3466.2006.00077.x. [DOI] [PubMed] [Google Scholar]
- 62.Misra MK, Sarwat M, Bhakuni P, Tuteja R, Tuteja N. Oxidative stress and ischemic myocardial syndromes. Med Sci Monit. 2009;15:RA209–RA219. [PubMed] [Google Scholar]
- 63.Hori M, Nishida K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovasc Res. 2009;81:457–464. doi: 10.1093/cvr/cvn335. [DOI] [PubMed] [Google Scholar]
- 64.Lopez FA, Casado S. Heart failure, redox alterations and endothelial dysfunction. Hypertension. 2001;38:1400–1405. doi: 10.1161/hy1201.099612. [DOI] [PubMed] [Google Scholar]
- 65.Kumar D, Jugdutt BI. Apoptosis and oxidants in the heart. J Lab Clin Med. 2003;142:288–297. doi: 10.1016/S0022-2143(03)00148-3. [DOI] [PubMed] [Google Scholar]
- 66.Kumar D, Lou H, Singal PK. Oxidative stress and apoptosis in heart dysfunction. Herz. 2002;27:662–668. doi: 10.1007/s00059-002-2430-3. [DOI] [PubMed] [Google Scholar]
- 67.Backlund T, Palojoki E, Saraste A, Eriksson A, Finckenberg P, Kyto V, Lakkisto P, Mervaala E, Voipio-Pulkki LM, Laine M, Tikkanen I. Sustained cardiomyocyte apoptosis and left ventricular remodelling after myocardial infarction in experimental diabetes. Diabetologia. 2004;47:325–330. doi: 10.1007/s00125-003-1311-5. [DOI] [PubMed] [Google Scholar]
- 68.Melo LG, Pachori AS, Kong D, Gnecchi M, Wang K, Pratt RE, Dzau VJ. Molecular and cell-based therapies for protection, rescue and repair of ischemic myocardium: reasons for cautious optimism. Circulation. 2004;109:2386–2393. doi: 10.1161/01.CIR.0000128597.37025.00. [DOI] [PubMed] [Google Scholar]
- 69.Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes. 2002;51:1938–1948. doi: 10.2337/diabetes.51.6.1938. [DOI] [PubMed] [Google Scholar]
- 70.Kumar D, Robertson S, Burns KD. Evidence of apoptosis in human diabetic kidney. Mol Cell Biochem. 2004;259:67–70. doi: 10.1023/b:mcbi.0000021346.03260.7e. [DOI] [PubMed] [Google Scholar]
- 71.Ejaz S, Chekarova I, Ejaz A, Sohail A, Lim CW. Importance of pericytes and mechanisms of pericyte loss during diabetes retinopathy. Diabetes Obes Metab. 2008;10:53–63. doi: 10.1111/j.1463-1326.2007.00795.x. [DOI] [PubMed] [Google Scholar]
- 72.Kamboj SS, Vasishta RK, Sandhir R. N-acetylcysteine inhibits hyperglycemia-induced oxidative stress and apoptosis markers in diabetic neuropathy. J Neurochem. 2010;112:77–91. doi: 10.1111/j.1471-4159.2009.06435.x. [DOI] [PubMed] [Google Scholar]
- 73.Tuo QH, Zeng H, Stinnett A, Yu H, Aschner JL, Liao DF, Chen JX. Critical role of angiopoietins/Tie-2 in hyperglycemic exacerbation of myocardial infarction and impaired angiogenesis. Am J Physiol Heart Circ Physiol. 2008;294:H2547–H2557. doi: 10.1152/ajpheart.01250.2007. [DOI] [PubMed] [Google Scholar]
- 74.Backlund T, Lakkisto P, Palojoki E, Gronholm T, Saraste A, Finckenberg P, Mervaala E, Tikkanen I, Laine M. Activation of protective and damaging components of the cardiac renin-angiotensin system after myocardial infarction in experimental diabetes. J Renin Angiotensin Aldosterone Syst. 2007;8:66–73. doi: 10.3317/jraas.2007.018. [DOI] [PubMed] [Google Scholar]
- 75.Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999;79:215–262. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- 76.Menasche P. Skeletal myoblasts and cardiac repair. J Mol Cell Cardiol. 2008;45:545–553. doi: 10.1016/j.yjmcc.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 77.Kim H, Kim SW, Nam D, Kim S, Yoon YS. Cell therapy with bone marrow cells for myocardial regeneration. Antioxid Redox Signal. 2009;11:1897–1911. doi: 10.1089/ars.2009.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120:408–416. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cleland JG, Freemantle N, Coletta AP, Clark AL. Clinical trials update from the American Heart Association: REPAIR-AMI, ASTAMI, JELIS, MEGA, REVIVE-II, SURVIVE and PROACTIVE. Eur J Heart Fail. 2006;8:105–110. doi: 10.1016/j.ejheart.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 80.Govaert JA, Swijnenburg RJ, Schrepfer S, Xie X, van der Bogt KE, Hoyt G, Stein W, Ransohoff KJ, Robbins RC, Wu JC. Poor functional recovery after transplantation of diabetic bone marrow stem cells in ischemic myocardium. J Heart Lung Transplant. 2009;28:1158–1165. doi: 10.1016/j.healun.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bdel Aziz MT, El-Asmar MF, Haidara M, Atta HM, Roshdy NK, Rashed LA, Sabry D, Youssef MA, Bdel Aziz AT, Moustafa M. Effect of bone marrow-derived mesenchymal stem cells on cardiovascular complications in diabetic rats. Med Sci Monit. 2008;14:BR249–BR255. [PubMed] [Google Scholar]
- 82.Li JH, Zhang N, Wang JA. Improved anti-apoptotic and anti-remodeling potency of bone marrow mesenchymal stem cells by anoxic pre-conditioning in diabetic cardiomyopathy. J Endocrinol Invest. 2008;31:103–110. doi: 10.1007/BF03345575. [DOI] [PubMed] [Google Scholar]
- 83.Zhang N, Li J, Luo R, Jiang J, Wang JA. Bone marrow mesenchymal stem cells induce angiogenesis and attenuate the remodeling of diabetic cardiomyopathy. Exp Clin Endocrinol Diabetes. 2008;116:104–111. doi: 10.1055/s-2007-985154. [DOI] [PubMed] [Google Scholar]