Abstract
Two decades have passed since the first attempts were made to establish systematic ethical review of human research in the Baltic States. Legally and institutionally much has changed. In this paper we provide an historical and structural overview of ethical review of human research and identify some problems related to the role of ethical review in establishing quality research environment in these countries. Problems connected to (a) public availability of information, (b) management of conflicts of interest, (c) REC composition and motivation of REC members, and (d) differing levels of stringency of ethical review for different types of studies, are identified. Recommendations are made to strengthen cooperation among the Baltic RECs.
Keywords: Research ethics committees, transparency, ethical review, Baltic States
INTRODUCTION
In the Baltic States of Estonia, Latvia and Lithuania, as in most countries of Central and Eastern Europe, systematic ethical review of biomedical research started in the late 1980s. Two decades have passed, but information on the day-to-day functioning of Research Ethics Committees (RECs) is still rather scarce and sporadic.1 In this paper we attempt to close this information gap.
Our goal is twofold: (1) to present a general overview of ethical review of human research in the Baltic States and (2) to identify some of the problems related to the role of ethical review in establishing a high quality research environment. We believe that this overview may provide some important insights into the processes of ethical review and the protection of research participants not only in the Baltic States but also in other transition societies.
HISTORICAL AND STRUCTURAL OVERVIEW
The emergence of ethical review of human research in the Baltics, which began in late 1980s, can be traced back to certain individuals who were knowledgeable about the workings of ethical review in the West and who were eagerly anticipating the first chance to join international research.2 However, even though the Baltic States share many socio-economic characteristics, all three have developed rather different institutional models of ethical review, despite their common motivation to facilitate local researcher involvement in international research.
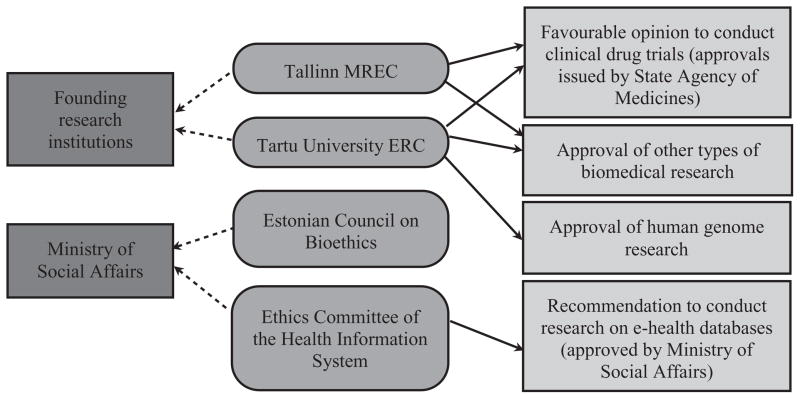
In Estonia, ad hoc committees for clinical trials were created as early as 1989.3 The first permanent RECs were established in 1990 by the University of Tartu and in 1992 by the Institute of Experimental and Clinical Medicine (now National Institute for Health Development) in Tallinn. These two RECs, affiliated with and dependent upon mother institutions, also perform functions of regional RECs since they also review research proposals from outside their own institutions. A national bioethics board, the Estonian Council on Bioethics, was established in 1998. One of its tasks is to coordinate activities of regional RECs. A special REC to review research conducted within the Estonian Genome Project was established in 2001, but its functions were later passed to the Ethics Review Committee on Human Research of the University of Tartu. In 2008, a special kind of REC, reviewing only matters related to research with medical data from Estonian e-health databases, was established. It is called the Ethics Committee of the Health Information System.
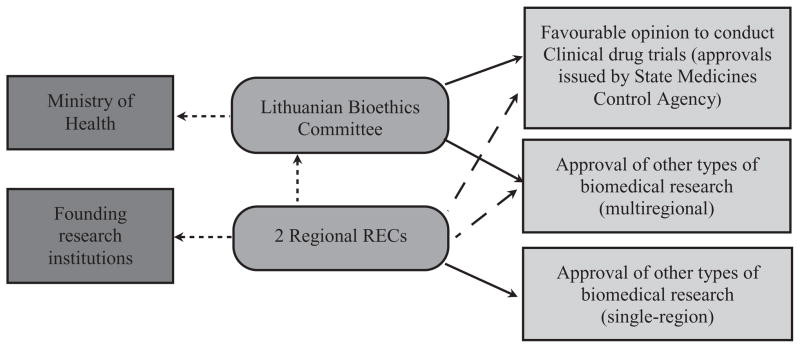
The emergence of the Lithuanian ethical review system was similar to that of Estonia. Two RECs were created in the late 1980s as a consequence of local research institution initiative, and not as a realization of some national policy. In 1994, the Law on the Health System4 was adopted, thus setting the conditions for the official establishment of the national REC, the Lithuanian Medical Ethics Committee (now Lithuanian Bioethics Committee) a year later. The Committee functions as a national bioethics council and also has a subcommittee for ethical review of biomedical research. In 2001, the Law on Ethics of Biomedical Research5 introduced a two-tier system with regional RECs. The Kaunas Regional Biomedical Research Ethics Committee was thus established at Kaunas University of Medicine in 2001. The second regional REC was established at Vilnius University in 2008.
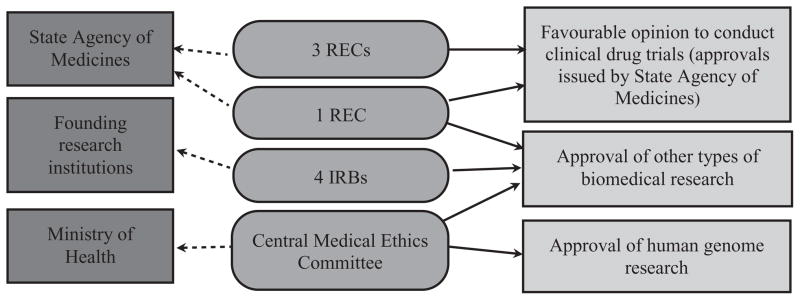
Latvia was the last of the Baltic States to start this process. The first committee was founded at the Latvian Institute of Cardiology in 1992 and was then called the Ethics Committee of Clinical and Experimental Research. The second REC was created by Riga Stradins University in 1996, prior to any legal provisions being enacted. In 1997, the Parliament adopted the Medical Treatment Law6 creating a legal platform for establishing medical ethics committees. In 1998, the Statutes of Central Medical Ethics Committee7 were approved.
The situation in Latvia is more complex than in the other two countries. There are several kinds of RECs with different jurisdictions and scopes of operation. There is a national body, the Central Medical Ethics Committee, and there are other RECs of two kinds: institutional ones and those which are sometimes referred to as ‘regional’.8 However, these so-called ‘regional’ committees are not assigned to any particular region, all are located in Riga, Latvia’s capital, and the law does not require that applications for ethical review be submitted according to region of origin. It would be more appropriate, therefore, to distinguish between RECs with a narrow scope of review and RECs with a wide scope of review. Three committees review only clinical drug trials, one reviews drug trials and other types of human biomedical research, while four review all types of biomedical research, except clinical drug trials.
In all three Baltic States, ethical review of human research developed in bottom-up fashion. Initially RECs were established in particular university hospitals or medical faculties and only then were legal regulations put in place. Consequently the institutional structure of ethical review was reshaped by legal developments.
Currently all three systems of ethical review can be seen as structures that include a national body and local or regional committees established at various institutions that engage in biomedical research. It is only in Lithuania and Estonia where such local or regional committees are assigned geographically defined jurisdictions although some Latvian committees could be regarded as ‘regional committees’ in that they review research proposals from outside their founding institution even though there is no defined geographic region.
The relationship between national and local or regional RECs differs a lot across the Baltic States. For example, Estonian RECs simply provide to the national body annual reports about main facts and trends of their operation. The RECs themselves have taken the initiative. While in Lithuania, the division of responsibilities and accountability between national and local bodies is clearly defined in different normative documents.
All three national bodies (the Estonian Council on Bioethics, the Latvian Central Medical Ethics Committee and the Lithuanian Bioethics Committee) are state institutions. For example, the Lithuanian Bioethics Committee is an institution subordinate to the Ministry of Health. However, national bodies differ in their functions. For example, it is only the Lithuanian committee that supervises the rest of the committees and reviews their decisions upon appeal. The Lithuanian and Latvian committees differ from their Estonian counterpart in that they review research protocols. Before the introduction of the two-tiered system, the Lithuanian committee was the sole reviewer in the country. It is still the only body that issues decisions on clinical drug trials and on biomedical research studies that take place in more than one region. The Latvian committee reviews: (a) research proposals that are related to the National Genome Project and (b) non-drug trials. The Estonian Council on Bioethics does not review research.
In all three countries approvals of clinical trials of medicinal products cannot be granted by RECs alone. Each country has a State Agency of Medicines (called State Medicines Control Agency in Lithuania) that also participates in approval process. REC approval is a necessary but not sufficient condition of issuing an approval for a trial of this type. This is a direct outcome of the adoption of the Directive 2001/20/EC.9
REC workload, summarized in Table 1, lists the number of approvals by country (the figures exclude student research).
Table 1.
Workload of RECs in the Baltic States (excluding student research)
| Lithuania |
Latvia |
Estonia |
|||||
|---|---|---|---|---|---|---|---|
| Population (millions) |
3.56 |
2.23 |
1.34 |
||||
| Year | 2007 | 2008 | 2007 | 2008 | 2007 | 2008 | |
| Clinical drug trials | Number of approvals | 112 | 108 | 85 | 88 | 93 | 103 |
| Number of committees | 1 | 1 | 4 | 4 | 2 | 2 | |
| Other human research | Number of approvals | 111 | 119 | N/A | N/A | 114 | 127 |
| Number of committees | 2 | 2 | 6 | 6 | 2 | 2 | |
Figures 1–3 depict the organizational structure of ethical review in the Baltic States. Solid lines show which institutions issue approvals (or recommendations to issue approvals) for particular types of research. Punctuated lines show accountability of institutions. Dashed lines (in Lithuanian figure only) show recommendations to other committee.
Figure 1.
Ethical review system in Lithuania.
Figure 3.
Ethical review system in Estonia.
ETHICAL REVIEW AND QUALITY RESEARCH ENVIRONMENT
As reflected in Figures 1–3, the Baltic States have developed the legal and institutional structure for the ethical review of human research. However, the fact that a basic legal and institutional structure exists, does not automatically guarantee high quality ethical review. Although there can be no guarantees, there could be more confidence in a review process that at a minimum required: transparency in reporting REC activities and findings; clear, precise and justifiable rules and principles governing committee processes; specification of the composition and required qualifications for committee members, requirements for reporting of research process and findings; reliable funding and facilities for the committee; and legal protection of intellectual property. In this section we will briefly address several problematic areas connected to the quality of ethical review. These include: (a) availability of information, (b) management of conflicts of interest, (c) committee composition and motivation of REC members, and (d) differing stringency of ethical review for different types of studies.
Availability of information
Very few RECs in the region have websites that provide information about their procedures of functioning. Information on statutes, de facto composition, basic statistics on the number of reviewed research protocols (not including the list of approved and rejected research projects), in many cases, is not publicly available.10 Sometimes this information is not available even if relevant institutions are contacted directly. The limited availability of information adds to low public and professional awareness of RECs.
In general, there is growing international support for the dissemination of information on clinical trials via publicly available clinical trial registries and databases.11 However, publicly accessible registries and databases are typically lacking in smaller countries. In their absence, RECs might become vehicles for improving the publicity and transparency of biomedical research by providing information on approved research projects on their own websites.12
Conflicts of interest
The management of conflicts of interest has received only limited attention in laws and regulations. The Baltic systems of ethical review of human research rely solely on voluntarily disclosure of potential conflicts of interest.13 This is not very troubling by itself – voluntarily disclosure is a rather widespread model of managing conflicts of interest in a number of different countries. However, most of the RECs in the Baltic States are units within universities or medical schools, and many or even most of their members are representatives of the same institution that conducts research projects. In addition, biomedical communities are rather small and compact and subject to subtle peer pressure. Therefore it is very difficult to determine the extent to which decisions of RECs are made independently of the interests of researchers and sponsors, how often conflicts of interest occur, how serious they are, and how are they avoided in practice. Open for discussion is whether RECs that are affiliated with research institutions and draw most of their members from that institution (similar to IRBs in the USA) can avoid conflicts of interest.
Another potential problem is that funding of RECs in Estonia and Latvia is directly dependent on the number of reviewed protocols.14 The Lithuanian system follows a redistributive model, where the fees are first paid to the budget of a third party (i.e. the state), from which the committees are financed. Arguably, the former system may be less likely to issue negative decisions, especially in cases where researchers are free to choose the REC. In Lithuania and Estonia, if approval is denied by a REC it is not possible to seek approval for the same protocol from another REC. For example, Estonian RECs, on a quarterly basis, exchange lists of applications to help eliminate ‘shopping for RECs’. However, in Latvia, where different RECs are not assigned defined geographical regions, there is no system to exchange information on refused applications. Therefore a protocol refused by one REC can, at least in theory, be submitted to another.
Committee composition and motivation of REC members
RECs in the Baltic States usually include 7 to 15 members, most of whom have medical qualifications and affiliations. Selection and composition criteria vary significantly among committees in regard to their specificity in defining the qualifications of individual members and in the requirements for representation from various bodies and organizations. For example, although composition criteria for Lithuanian regional RECs are defined in detail by special ministerial decree,15 most committees rely on general principles. For example, the only requirement for the composition of the subcommittee for ethical review of biomedical research of the Lithuanian Bioethics Committee is to keep the balance between biomedical and non-biomedical representation.16 Also, although the membership of the Latvian Central Medical Ethics Committee must represent 11 organizations (Ministry of Health, Council of Science, University of Latvia Institute for Experimental and Clinical Medicine, Latvian Nurses Association, Latvian Pensioners Federation, etc.),17 the rationale for their membership is not clear. Furthermore, except for being delegated by one of these organizations, no formal individual qualifications are defined. In most cases, however, criteria are stated relatively vaguely. For example, the statutes of Ethics Review Committee on Human Research of the University of Tartu state that it should be composed of,
persons representing various different fields of life with the preparation in the specialties of biomedicine as well as in other specialties. Each member of the Ethics Review Committee shall be a recognized specialist in his or her field with the necessary expertise to perform the duties of a member of the Ethics Review Committee and shall have an impeccable reputation.18
The definition of ‘necessary expertise’ is not further specified.
The RECs themselves have identified the problem of insufficient motivation of members. Reading the protocols and attending meetings is time consuming and usually members are not adequately paid for the work, causing difficulty in recruiting highly qualified professionals.19 The lack of financial incentives is not outweighed by other types of motivation, such as professional development, prestige or social status. For example, there are no systematic training programs for the members in the Baltic States. Most of the education is acquired through practical work on the committee. Some members participate in conferences or workshops, but this is not done in any systematic or coordinated way. Lack of respect by academic researchers for the requirement of ethical review can also prevent qualified scientists from working on RECs. Many perceive the process as slowing down and hindering scientific research. In general, the lack of motivators can result in both poorer quality of ethical reviews and a reduction in the number of interested potential committee candidates.20
Ethical review by types of human research
Procedural clarity and the scope of ethical review may differ for different types of human research. Some types of human research may even escape ethical review altogether. Although, as previously mentioned, clinical drug trials enjoy special status in all three countries, due to the provisions of Directive 2001/20/EC,21 the situation is less uniform in other cases. It is only in Lithuania where a legal definition of biomedical research, and the requirement to subject such research to review by REC, exists. The Law on Ethics of Biomedical Research defines biomedical research as ‘verification of hypotheses of biomedicine by methods of scientific investigation and development of knowledge about characteristics of human health’.22 This broad definition includes research ‘carried out in individuals or their groups, a foetus, tissues, organs, cells and genetic material, cadavers and medical documents’.23 Neither Latvian nor Estonian national law includes a definition of biomedical research or clarifies why, in practice, some kinds of research involving human subjects require ethical review by RECs and others do not. Sometimes specifications can be found in the statutes of particular RECs established at academic institutions, but sanctions for non-compliance with these regulations are limited to the jurisdiction of that institution. For example, it seems that no sanctions could be applied to researchers who are affiliated with institutions that do not have a REC (e.g. any Estonian research institution except the University of Tartu and the National Institute for Health Development) for failing to seek ethical review.24
At the moment, according to law in the Baltic States, non-biomedical human studies do not fall within the scope of REC approval. Ethical review is not required for conducting sociological, anthropological or psychological research outside of healthcare context. When there are no legally binding requirements, ethical review may be enforced by what might be called ‘softer’ social regulations, such as policies of RECs established at the universities or research institutes, requirements for authors wishing to publish in scientific journals and guidelines promulgated by different funding bodies sponsoring human research. At least in one case, provisions to cover non-biomedical human research are included in REC policies. According to the statutes of Ethics Review Committee on Human Research of the University of Tartu, the REC shall also assess the ethical aspects of human research if a danger to the physical or mental health of human(s) may occur while conducting the aforementioned research.25 Despite differences in REC systems and existing practices, we suggest that closer networking and cooperation of RECs in the Baltic region would help to reduce problems related to the quality of the research environment and open up possibilities for the advancement of research ethics both on the regional and national levels, including the development of common guidelines for ethical review and the joint training of REC members. Baltic RECs have already experienced positive results of such networking over the past few years.26
CONCLUDING REMARKS
The experience of the Baltic States, as transition societies, may well be relevant to other transition societies. Over the past two decades, the ethical review of human research in the Baltic States has undergone significant legal and institutional development, with each country evolving in a separate direction. In many ways, the resulting systems adhere to international standards. However, problems remain. For example, the limited transparency and procedural clarity of ethical review hinder the creation of a quality research environment. RECs should be encouraged to increase the availability of data on reviewed protocols on their websites. New methods to manage conflicts of interest need to be created taking into account the influence that the structure of RECs has on those conflicts. More effort should be made to establish the optimal REC model in terms of composition of committees and motivation of their members. The legal environment should also be improved. Networking among RECs could be a potent tool to encourage the above-mentioned processes.
Figure 2.
Ethical review system in Latvia.
Acknowledgments
The project was supported by Research Grant # R25TW007085 from the Fogarty International Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Fogarty International Center or the National Institutes of Health.
We would also like to thank the RECs which kindly agreed to present empirical material via completed questionnaires and an anonymous reviewer for valuable suggestions on how to make this paper clearer.
Biography
Vilius Dranseika (MPhil) is a researcher at the Department of Medical History and Ethics (DMHE) at the Vilnius University, Lithuania. Eugenijus Gefenas (MD, PhD) is the head of DMHE, Vilnius University and is a member of the Lithuanian Bioethics Committee. Asta Cekanauskaite (MPhil) is a researcher at DMHE, Vilnius University and is a member of the Lithuanian Bioethics Committee. Kristina Hug (MA) is a doctoral researcher at the Department of Medical Ethics, Lund University, Sweden. Signe Mezinska (MPhil) is a doctoral candidate in the Riga Stradins University (RSU), Latvia. Eimantas Peicius (PhD) is a lecturer at the Department of Philosophy and Social Sciences, Kaunas University of Medicine, Lithuania and is also a member of the Kaunas Regional Biomedical Research Ethics Committee. Vents Silis (MPhil) is a researcher and lecturer at the Department of Humanities at RSU. Andres Soosaar (MD, PhD) is a lecturer at the Department of Public Health, University of Tartu, Estonia and a member of the University of Tartu Human Research Ethics Committee. Martin Strosberg (PhD) is a professor at the Union Graduate College – Mount Sinai School of Medicine Bioethics Program, Schenectady, New York, USA.
Footnotes
Conflict of interest statement: No conflicts declared
References
- 1.One of the first descriptions of RECs in Baltic States can be found in Glasa J, editor. Ethics Committees in Central and Eastern Europe. Strasbourg: Council of Europe; 2000. However, recently there have been some Europe-wide initiatives to collect information on ethical review of human research that also covered the Baltic States. One important source is the PRIVIREAL project, started in 2002. However, the information provided by the project’s website ( Privireal. Research Ethics Committees – Countries. Sheffield, UK: Privireal; 2005. [Accessed 31 Mar 2010]. Available at: http://www.privireal.org/content/rec/countries.php..) is in some respects already outdated. Results were published as Beyleveld D, Townend D, Wright J, editors. Research Ethics Committees, Data Protection and Medical Research in European Countries. Hants: Ashgate; 2005. . Another initiative is the European Forum for Good Clinical Practice (EFGCP) ongoing survey of ethical review procedures and practices related to clinical drug trials. Even though information provided by this survey is very recent (report was published in 2007 ( EFGCP Ethics Working Party on the Structure and Function of Research Ethics Committees in the European Union. The Procedure for the Ethical Review of Protocols for Clinical Research Projects in the European Union. Int J Pharm Med. 2007;21:1–113..); updates on the website are correct as of 2009 ( EFGCP. Update of the Report, as of 2009. Brussels: EFGCP; 2009. [Accessed 31 Mar 2010]. Available at: http://www.efgcp.be/html.asp?what=efgcpreport.htm&L1=5&L2=1..)) and comprehensive, it covers only a limited area of human research because drug-unrelated clinical trials as well as other types of biomedical research are not covered by the EU Clinical Trials Directive ( European Commission. Directive 2001/20/EC of 4 April 2001, of the European Parliament and of the Council on the approximation of the laws, Regulations and administrative provisions of the Member States relating to implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. OJ L. 2001 Jan 05;121:34..) that the EFGCP activities are based on.
- 2.As is indicated by Lithuanian bioethicists, ‘[t]he process of development of ethical review in Lithuania (and probably many other Central and Eastern European countries) […] was strongly facilitated by foreign pharmaceutical companies.’ Cekanauskaite A, Gefenas E. Research Ethics Committees in Lithuania. In: Beyleveld, et al., editors. op cit. 2005. pp. 140–147.pp. 141 note 1.. Similar views were expressed by Estonian and Latvian experts as well. In the questionnaire received from one of the Estonian RECs, it was indicated that the motive for the establishment was to embrace ‘new possibilities to take part in international medical research. […] The international requirements demanded independent body for the overview of research projects.’ One of the Latvian RECs also reported that the main motive was ‘the necessity to review the research involving human participants, as well as rapid development of drugs clinical research’.
- 3.Veidebaum T. Research Ethics in Estonia. In: Beyleveld, et al., editors. op cit. 2005. pp. 41–43.pp. 41 note 1. [Google Scholar]
- 4.Seimas of the Republic of Lithuania. Republic of Lithuania Law on the Health System. [Accessed 31 Mar 2010];Parliamentary Record. 1995 Dec 01;:12. Available at: http://www3.lrs.lt/pls/inter3/dokpaieska.showdoc_e?p_id=23358.
- 5.Seimas of the Republic of Lithuania. [Accessed 31 Mar 2010];Republic of Lithuania Law on Ethics of Biomedical Research. 2000 Available at: http://www3.lrs.lt/pls/inter3/dokpaieska.showdoc_e?p_id=148740.
- 6.Latvijas Republikas Saeima (Saeima of the Republic of Latvia) Ārstniecı̄bas likums (Medical Treatment Law) [Accessed 31 Mar 2010];Latvijas vēstnesis. 1998 Jan 07;(Nr. 167) Available at: http://www.likumi.lv/doc.php?id=44108.
- 7.Latvijas Republikas Ministru kabinets (Cabinet of Ministers of the Republic of Latvia) Centrālās medicı̄nas ētikas komitejas nolikums (Statutes of Central Medical Ethics Committee) [Accessed 31 Mar 2010];Latvijas vēstnesis. 1998 15 01;(Nr. 10) Available at: http://www.likumi.lv/doc.php?id=46597.
- 8.Eg. Privireal. Latvia – RECs and Medical Research. Sheffield, UK: Privireal; 2005. [Accessed 31 Mar 2010]. Available at: http://www.privireal.org/content/rec/latvia.php. [Google Scholar]
- 9.European Commission. op cit. note 1. [Google Scholar]
- 10.An exception is the website of the Lithuanian Bioethics Committee ( [Accessed 31 Mar 2010]; Available at: http://bioetika.sam.lt.). This institution also has an official template for information requests and is obliged to answer inquiries. However, even here information sometimes is not regularly updated. For example, at the time of this research, statistics on the number of reviewed protocols had not been updated since mid-2005.
- 11.An example of one such database is ClinicalTrials.gov, a service of the US National Institutes of Health. [Accessed 31 Mar 2010]; Available at: http://clinicaltrials.gov.). Also, Article 19 of Helsinki Declaration ( World Medical Association. Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects. Helsinki: 1964. ; amended Tokyo: 1975; Venice: 1983; Hong Kong: 1989; Republic of South Africa: 1996; Edinburgh: 2000; Washington: 2002; Tokyo 2004; Seoul 2008). [Accessed 31 Mar 2010]; Available at: http://www.wma.net/en/30publications/10policies/b3/index.html..) states that: ‘Every clinical trial must be registered in a publicly accessible database before recruitment of the first subject.’ This idea was also supported by the International Committee of Editors of Medical Journals which stipulates that member journals require authors to register their trials (including methodology) in a registry that is accessible to the public free of charge ( International Committee of Medical Journal Editors. [Accessed 31 Mar 2010];Obligation to Register Clinical Trials. 2009 Available at: http://www.icmje.org/publishing_10register.html..). The European Commission also issued guidelines on the list of fields contained in the EudraCT clinical trials database that should be made publicly available ( European Commission. List of fields contained in the ‘EudraCT’ clinical trials database to be made public, in accordance with Article 57(2) of Regulation (EC) No 726/2004 and its implementing guideline 2008/C168/02. [Accessed 31 Mar 2010];2009 Available at: http://ec.europa.eu/enterprise/pharmaceuticals/eudralex/vol-10/2009_02_04_guideline.pdf..).
- 12.The Ethics Review Committee on Human Research of the University of Tartu has published an annual list of all approvals since 2008; see Tartu Ülikooli inimuuringute eetika komitee (Ethics Review Committee on Human Research of the University of Tartu). 2009. Tartu Ülikooli inimuuringute eetika komitee menetlus 2008 (Approvals given by the Ethics Review Committee on Human Research of the University of Tartu in 2008). [Accessed 31 Mar 2010]; Available at: http://www.ut.ee/orb.aw/class=file/action=preview/id=595718/Ec_2008_kokkuv6te.pdf.
- 13.For example, the Statutes of Tallinn MREC [provided by the REC] simply state that members who are not ‘independent from the performers of the research’ may not vote.
- 14.In Latvia, it is true only of those RECs that review clinical drug trials.
- 15.Lietuvos Respublikos Sveikatos apsaugos ministerija (Ministry of Health of the Republic of Lithuania). 2008. Lietuvos Respublikos sveikatos apsaugos ministro įsakymas dėl regioninių biomedicininių tyrimų etikos komitetų narių skyrimo tvarkos aprašo patvirtinimo (Decree of the Minister of Health of the Republic of Lithuania on the Procedure of Nomination of the Members of Regional Biomedical Research Ethics Committees). Valstybės žinios, 2008-06-17, Nr. 69-2635. [Accessed 31 Mar 2010]; Available at: http://www3.lrs.lt/pls/inter3/dokpaieska.showdoc_l?p_id=322379&p_query=&p_tr2=
- 16.Lietuvos bioetikos komitetas (Lithuanian Bioethics Committee). 2007. Lietuvos bioetikos komiteto pirmininko įsakymas V-8 Dėl Lietuvos bioetikos komiteto biomedicininių tyrimų ekspertų grupės darbo reglamento patvirtinimo (Decree of the Lithuanian Bioethics Committee no. V-8 on the Working Procedures of the Biomedical Research Experts’ Group). Valstybės žinios, 2008-02-28, Nr. 24-893. [Accessed 31 Mar 2010]; Available at: http://www3.lrs.lt/pls/inter3/dokpaieska.showdoc_l?p_id=315164&p_query=&p_tr2=
- 17.Latvijas Republikas Ministru kabinets (Cabinet of Ministers of the Republic of Latvia), op. cit. note 7. Art. 3.
- 18.University of Tartu. [Accessed 31 Mar 2010];Statute of the Ethics Review Committee on Human Research of the University of Tartu. 2007 Available at: http://www.ut.ee/orb.aw/class=file/action=preview/id=276573/TY+Inimuuringute_et-en_070828_9015_statuut_en_ok-ED.pdf.
- 19.For a general argument that REC members should be adequately remunerated, see Druml Ch, et al. Research Ethics Committees in Europe: Trials and Tribulations. Intensive Care Med. 2009;35:1636–1640. doi: 10.1007/s00134-009-1544-y.
- 20.This also leads to the issue of rotation of REC members. Lack of potential candidates may be dealt with by having no formal term limits for an REC member. For example, in Estonia, term limits were introduced only in 2007.
- 21.European Commission. op cit. note 1. [Google Scholar]
- 22.Seimas of the Republic of Lithuania. op cit. note 5, Art 2.1. [Google Scholar]
- 23.Ibid: Art 3.1.
- 24.For Estonia, some guidance can be found in the Oviedo Convention (Council of Europe. 1997. Convention for the Protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine. [Accessed 31 Mar 2010]; Available at: http://conventions.coe.int/Treaty/en/Treaties/Html/164.htm..). This treaty has been ratified by Estonia and Lithuania but not Latvia. The Convention states that human scientific research in the field of biology and biomedicine cannot be conducted without ‘multidisciplinary review of its ethical acceptability’ (Art 16.3).
- 25.Human research conducted by students in educational setting may also involve significant risks to research subjects and therefore should receive ethical scrutiny. However, it may be impracticable to apply stringent procedures to student research. There is no definition of student research in Lithuanian, Latvian or Estonian legislation. Most medical schools in the Baltic States have internal regulations concerning the need for ethical review of student research. These internal regulations are based on local initiative not on overarching national policies.
- 26.Information on the Baltic research ethics network activities can be found on the website of Lithuanian Bioethics Committee. Available at: [Accessed 31 Mar 2010]; http://bioetika.sam.lt/index.php?-809972162.