Abstract
There are few pharmaceuticals superior to opiates for the treatment of pain. However, with concerns of addiction, withdrawal and questionable efficacy for all types of pain, these compounds are far from a magical panacea for pain-relief. As it is unlikely that other classes of compounds will supersede the opioids in the very near future, it is important to both optimize current opioid therapies and curb the astounding diversion of opioids from their intended analgesic use to non-medical abuse. In optimizing opioid therapeutics it is necessary to enhance the clinical awareness of the benefits of treating pain and combine this with aggressive strategies to reduce diversion for non-medical use. At the heart of the issue of opioid misuse is the role of opioid systems in the reward circuitry, and the adaptive processes associated with repetitive opioid use that manifest during withdrawal. Emerging pharmacological insights of opioid receptors will be reviewed that provide future hope for developing opioid-based analgesics with reduced addictive properties and perhaps, reduced opponent processes. In addition, with the increased understanding of nociceptive circuitry and the molecules involved in transmitting pain, new therapeutic targets have become evident that may result in effective analgesics either alone or in combination with current opioid therapies.
1. Controversial issues surrounding opioid therapeutics
1.1. The importance of opioid pain medications
Opioid therapeutics are the most effective analgesics available for certain types of pain. Opioid-based treatment of pain has been considered for several centuries. Thomas Sydenham, a 17th-century English medical pioneer, wrote: “Among the remedies which pleased almighty God to give to man to relieve his sufferings, none is so universal and as efficacious as opium.” For many decades the opium derivatives morphine and codeine have been used to relieve the pain associated with a range of ailments such as diarrhea, coughing, post-operative pain and cancer (Hamilton and Baskett, 2000). In spite of this long history of the clinical benefits of opioids and a valuable selection of available natural alkaloids, semi-synthetic, and synthetic opioid medications, pain has traditionally been under-treated for a number of reasons. The most significant reason being that pain is considered a symptom of the primary illness, and the medical focus has been on treating the illness without addressing the associated pain. Pain has been considered either as an endurable consequence or as an indicator of the underlying disease. Another common misconception has been that chronic pain should not be treated by opioids due to their side effects (Rosenblum et al., 2008).
There are serious consequences that may arise by ignoring the treatment of pain associated with certain illnesses. Dr. John Liebeskind’s research has brought awareness to these consequences, establishing that pain is not just a byproduct of illness, but that it also adversely impacts recovery. In 1993, he studied the effects of surgery-related pain in rats with lung cancer and determined that tumors metastasized faster in rats that did not receive analgesics, compared to rats that were given morphine. This demonstrated that the stress, resulting from pain, inhibited immunological defenses and that in rodents, as well as in humans, “pain can kill” (Liebeskind, 1991). Throughout his career Dr. Liebeskind argued that doctors and medical students should be better trained in pain management to ensure that patients do not suffer the debilitating consequences of untreated pain.
There are several consequences of untreated pain. Immunosupression can be induced by both untreated perioperative pain and severe thermal injury (Daniel et al., 2007). Untreated pain can exacerbate underlying medical conditions, decrease activity and conditioning, decrease productivity, delay rehabilitation, and increase emotional distress causing psychological symptoms, sleep deprivation, and inability to manage daily activities (Pasero, 2007). Prolonged suffering from acute pain can lead to intractable pain through peripheral and central sensitization and result in neurohumoral changes and neuronal remodeling (Dunwoody et al., 2008).
Dr. John Liebeskind’s efforts to rectify the under-treatment of pain were subsequently echoed by the educational initiatives of academic programs, accreditation organizations, professional pain societies, and the pharmaceutical industry (Carr and Reuben, 2005). These initiatives focused on hospitalized patients and succeeded in increasing the use of opioids for acute pain due to cancer, AIDS, or other life-threatening illnesses. However, medical educators and patient advocates soon argued that individuals should not have to be on the verge of death to merit aggressive pain management. As a result, the clinical success of opioid-based treatment of chronic non-malignant pain appeared in the medical literature (Jackman et al., 2008). Pain specialists unable to keep up with the demands of those suffering from debilitating ailments such as back, neck and joint pain, advocated that primary care physicians should prescribe opioids for better pain management (Portenoy and Russell, 1996).
Opioid analgesics provide more than just reprieve from physical and psychological pain; they can also relieve stress, negative emotional states, insomnia, and induce a sense of well-being. Some individuals are uniquely susceptible to the rewarding effects of opioids. In addition, taking an opioid-based analgesic offers expedient relief and is far more convenient than any lifestyle changes that could reduce pain such as a weight loss or physical therapy programs aimed at reducing musculoskeletal pain. These factors contribute to an overtreatment of pain often accompanied by a tacit reluctance on the part of some pain specialists, patient advocates, and the pharmaceutical industry to discuss the legitimate risks associated with long-term opioid treatment (Rosenblum et al., 2008). An important example of this is the consumer lawsuit against the manufacturers of oxycotin extended release (Oxycontin), the Purdue Frederick Company, who pleaded guilty to falsely claiming that this highly abusive drug was less addictive and less subject to abuse than other pain medications.
1.2. Individual pain management programs
Opioids are therefore beneficial analgesics for many types of pain but they carry the risk of significant side effects in some individuals. This has been the subject of many extensive reviews, such as that by Geppetti and Benemei (2009), so will not be further discussed here. However, the advantages and disadvantages of opioid therapy are briefly summarized below.
1.2.1. The arguments in favor of opioid therapy
Opioid drugs, if used properly, can be highly effective for many forms of pain and are generally of low organ toxicity (Raffa, 2006).
There is extensive literature showing that treating pain is critical in order to achieve a favorable quality of life and improve medical outcome (Haanpaa et al., 2009).
If monitored carefully and used for acute pain attenuation, addiction is a minimal side effect of opioid use in most patients (Compton and Volkow, 2006).
1.2.2. The arguments against opioid therapy
Mu opioid-directed therapeutics are not efficacious for all types of pain. For example migraines and inflammatory pain do not respond well to opiates (Bigal et al., 2008; Gatti et al., 2009).
The side effects associated with opioid analgesics may be extensive and complex. These include an array of adaptive, often-times opponent processes such as tolerance, addiction, withdrawal, diarrhea, agitation, hyperalgesia and dysphoria.
The individual nature of the arguments for and against opioid therapy suggest that there is no global answer as to whether opioid therapy should or should not be used, nor indeed which dose of a particular opioid is best for each patient. Rather the answer is specific to the patient, each requiring an individualized medication program tailored to suit their needs and match their status. Factors such as type of pain, age, immune status, gender, response to different doses of different opioids and the development, or likelihood of developing associated side-effects would determine the opioid therapy to be used. Furthermore, as these factors may change over time, constant re-evaluation and re-adjustment would be required to maintain adequate pain-relief and minimize any adverse-effects and the risk of abuse or diversion. The necessity of such individualized pain management regimes has led to a number of readily available questionnaires to screen and monitor for addiction, and guidelines to assist prescribing practitioners (Chou, 2009c).
1.2.3. Opioids for different types of pain
Without prospective studies supporting the long-term management of chronic pain with opioid analgesics, healthcare providers have applied the same principles to treat malignant, acute or chronic pain (Ballantyne and Mao, 2003), although each is different. Acute pain serves a functional purpose, and is most often self-limiting. In contrast, chronic pain is subjective, multidimensional and can arise from, or lead to, psychological distress, with no identifiable endpoint (Grichnik and Ferrante, 1991). Ideally, the treatment of the latter type of pain should consist of interdisciplinary services including a careful evaluation, medication and interventional treatments, physical therapy, behavioral interventions, psychiatric evaluation and vocational assessment and training, not just opioid analgesics. However, comprehensive programs are costly and generally not available (Ashburn and Rubingh, 1999; Ashburn and Staats, 1999).
Opioid-based individual pain regimens have shown slow, but measurable progress has been made in treating chronic nonmalignant pain, a historically undertreated population (Soin et al., 2008; Panjabi et al., 2008; Katz, 2008; Collado and Torres, 2008). This is partly because these patients are difficult to treat as their pain is often mixed with complex conditions such as mental illness, musculoskeletal problems, metabolic conditions and social stresses. Inadequate practitioner training to monitor and treat these patients has compounded the problem (Yanni et al., 2008).
The importance of opioid-based pain management of cancer patients is well recognized. Due to the nature of the disease and the accompanying pain, there is less reluctance to prescribe opioids for such pain. However, additional training and research is needed to optimize individual treatment regimens. There has been some progress in this direction, as shown by the use of controlled release opioid formulations (Hanna et al., 2009), adjunctive medication and the practice of opioid rotation when diminished analgesic efficacy occurs (Slatkin, 2009).
Whether malignant or non-malignant in nature, opioid-based management of chronic pain is becoming more acceptable and hence more frequently used. However, the risks associated with chronic opiate treatment must be recognized and tempered by an individualized program that caters to the patient and his/her changing needs during the course of their illness.
1.3. Increased pharmaceutical opioid production and sales
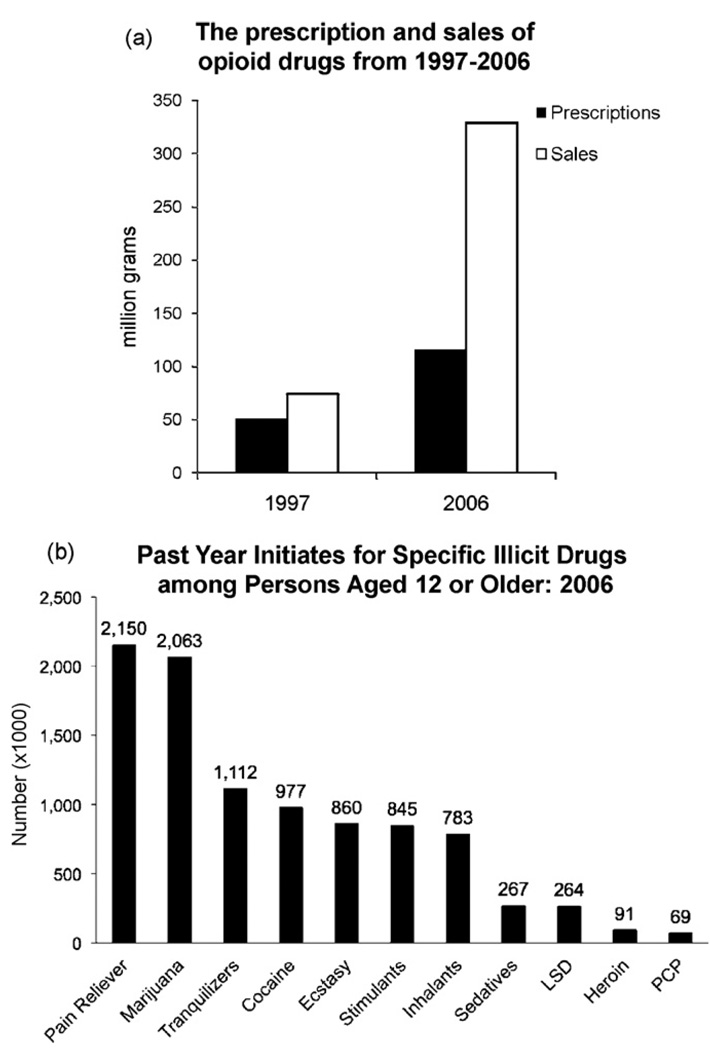
Efforts to address the under-treatment of pain have resulted in a dramatic increase in the sales and production of opioid analgesics in recent years (Kuehn, 2007) (Fig. 1). In 1997, 50.7 million grams of commonly used opioids were prescribed, and by 2006, this figure increased to 115.3 million grams, a ~130% increase over a 9 year period. However, closer analysis shows that most of this increase occurred during the last 4 years of this period. From 2002 to 2006, the number of hydrocodone prescriptions increased from 103 to 125 million, those for methadone from 2 to 4 million, and oxycodone from 28 to 38 million (Chou et al., 2009a; Drug Enforcement Administration, 2007). This increased number of prescriptions translated into a similar, if not exaggerated, increase in the sales of opioids in the United States, methadone sales increased 1177%, oxycodone 732%, and hydrocodone 244% over the same period (Manchikanti and Singh, 2008).
Fig. 1.
Indicators of increased opioid use between 1997 and 2006. (a) Prescription and sales of opioid drugs from 1997 to 2006. Data from the Drug Enforcement Administration (DEA, 2007) show an increase in prescription number as well as a dramatic increase in the amount of opiate prescribed between 1997 and 2006. (b) Past Year Initiates. Results from Substance Abuse and Mental Health Services Administration (SAMHSA) of the 2006 National Survey on Drug Use and Health indicates past year initiation of non-medical opioid pain reliever use has been significantly higher than that of marijuana (SAMHSA, 2008).
1.4. Addiction, abuse and side effects of opioid therapies
Although the increase in the number of prescriptions and the use of opioids has significantly improved the treatment of pain, this has been accompanied by an increased incidence of opioid abuse and addiction. Unfortunately no prospective studies alerted primary care providers to these side effects, nor that some population groups or individuals would be more susceptible to addiction. For example 3–16% of the population have a biogenetic vulnerability to addiction and are at greater risk of becoming addicted during long-term opioid use (Savage, 1996). This is shown by the abuse of opiates by 24% of patients suffering from back pain (Martell et al., 2007). The elderly and untreated mentally ill patients are at-risk populations, who, suffering with social isolation or other social issues, may develop substance abuse behaviors (Solomon et al., 2006; Gfroerer et al., 2003; Sullivan et al., 2005).
This underestimation of the population at risk from opioid treatment has had major consequences. Compulsive opioid use can devastate the lives of the abusers and those around them. Sufferers may have limited insight into their condition, confusing addiction with pain-relief (Robinson and Berridge, 2001). For those who find opiates rewarding, the patterns of abuse range from being occasionally problematic to severe, with worrying reports of individuals traversing the path from prescription opioid dependency to intravenous heroin (Siegal et al., 2003). A secondary problem, and one with major societal implications, is the considerable diversion of opioid therapeutics for non-medical use.
In addition to the risk of addiction there are other potential side effects associated with opioid use. These include nausea, vomiting, constipation, urinary retention, hormonal alterations and sexual dysfunction (Katz and Mazer, 2009), opioid-induced hyperalgesia (King et al., 2005b; Hay et al., 2009), immunosupression (Schwacha, 2008), sleep apnea (Walker and Farney, 2009) drowsiness, feelings of disorientation, and dizziness. Some of these, such as constipation, could, in the future, be treated with peripherally acting opioid antagonists (Webster et al., 2008). For other side effects, a strict adherence to therapeutic dosing and avoidance of contra-indicated substances such as benzodiazepines and alcohol must be followed. Opioid-induced immunosuppression has also been observed but it remains a controversial issue as to its relative role in relation to pain and stress-induced immunosuppression (Vallejo et al., 2004; Sacerdote et al., 2008; Roy and Loh, 1996).
1.5. Increase in non-medical opioid use, abuse and dependence
The increase in non-medical opioid use has paralleled the increase in opioid prescribing trends. According to the Substance Abuse and Mental Health Services Administration, the 2008 National Survey on Drug Use and Health show that 2.1% of people over age 12 report using prescription pain relievers non-medically in the past month (SAMHSA, 2008). Recent trends show that the non-medical use of pain relievers varied by age; 3.2% of 12–17 year olds abused opioids in 2002 in the month prior to the survey, this declined to 2.7% in 2007. However young adults 18–25 years old, 4.1% abused opioids in 2002 in the month prior to the survey, this increased to 4.6% in 2007. A similar increase in non-medical use was seen in the over 26 year-old adult group, which increased from 1.3 to 1.6% over the same period (Studies, 2009). Potential sources of narcotics are family and friends. Pill sharing and non-medical use of opioids is often modeled by the family members, friends and social networks (Compton and Volkow, 2006; McCabe et al., 2009). Unfortunately the misuse of prescription drugs is a growing trend worldwide and, according to the International Narcotics Control Board, will become as prevalent as that of the well-known illicit drugs (Zarocostas, 2007).
Striking a balance between medical underuse and overuse of opioids while benefiting patients is a daunting task yet essential to address. Multiple approaches targeting lay, regulatory, pharmaceutical and medical audiences must be acknowledged. Dr. Liebeskind’s goal of improved pain management education for healthcare providers must be realized in combination with education on opioid pharmacologic safety and addiction medicine principles (Chou et al., 2009b). SAMHSA and the U.S. Food and Drug Administration recently launched a public outreach effort to help ensure the safe use of methadone. The National Institute on Drug Abuse has ongoing translational studies to develop psychosocial and pharmacologic strategies and reduce prescription drug misuse while supporting the appropriate medical use. Some new directions are emerging from basic research that has shown promise in pre-clinical studies that may also address these issues.
2. Research directions seeking solutions
2.1. Optimizing opioid therapies
Although opiates are effective analgesics, in some individuals these compounds are highly effective in enhancing mood. Ultimately, novel scientific approaches coupled with creative pharmacology could separate these diverse physiological effects aiming to manage pain without affecting the reward pathways. However, such modality-specific opioids have not yet, and may never, be clearly defined. Alternately, our understanding of pain, addiction neurobiology, and the dynamic and interactive nature of receptor targets for analgesic medications seem promising and may provide a different pain management strategy. The following section reviews scientific considerations identified at the basic level with implications for improved management of pain.
2.2. The importance of opioid receptor complexes
Opioid systems are key systems for the control of pain. Importantly, opioid receptors are expressed throughout nociceptive processing circuitry and present multiple sites for inhibiting nociception. As a consequence, local application of opioid drugs induces analgesia via mu opioid receptors in the following areas: In the periphery where neurons from the dorsal root ganglia (DRG) sense painful stimuli; in the spinal cord where DRG neurons synapse with brain-bound nociceptive neurons; in the brain-stem structures, such as the Periaqueductal Gray that process nociceptive input; in the cortex and areas of the reward system that are responsible for higher processing of pain. These brain regions contain opioid receptors that, when activated, diminish the sensation of pain.
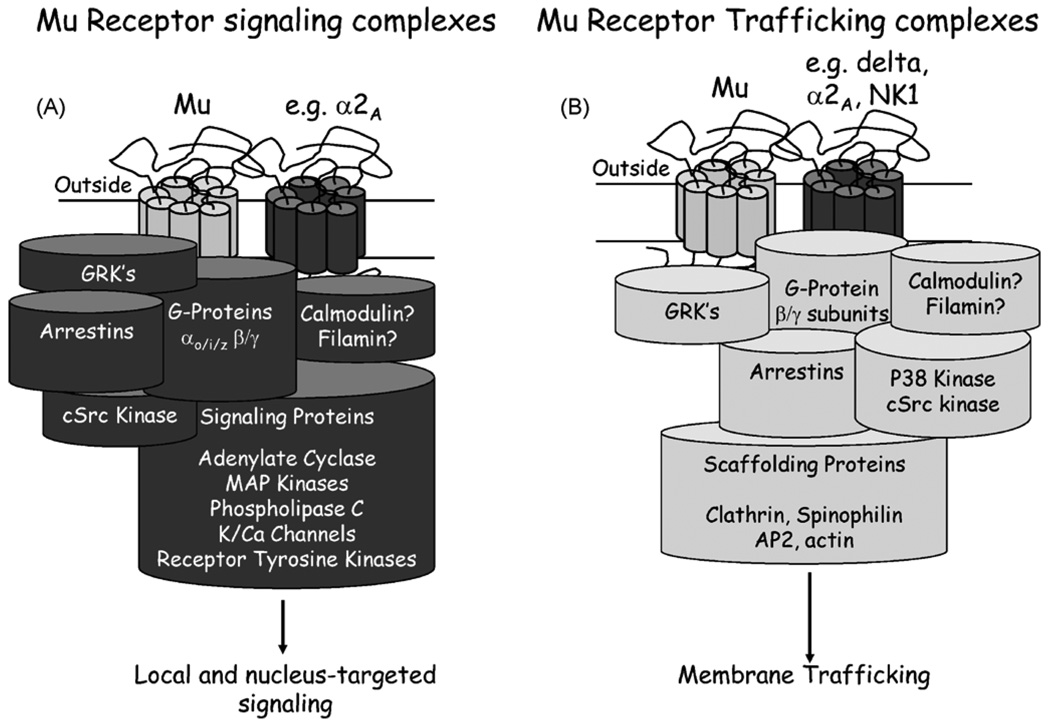
Animal, cellular and molecular models over the past decade have begun to reveal features of the “opioid receptor” that give hope for the optimization of drugs targeting opioid receptors for pain-relief without the detrimental effects of respiratory depression, addiction, constipation. The view of the opioid receptor as a signaling on/off switch has changed. The current view of the receptor is a component of a dynamic protein complex within the membrane. The receptor is capable of orchestrating the interaction of many different proteins and the final complexes formed dependent upon; the available proteins for interacting with the receptor, the history of the local environment of the receptor, and the ligand occupying the receptor (Evans, 2004). The signaling cascades that are activated depend upon the complex formed, which also dictates the trafficking and desensitization mechanisms of the receptor (Kelly et al., 2008). Fig. 2 shows the proteins that have been implicated in mu opioid receptor complexes leading to signaling, trafficking and functional regulation of the receptor and indeed other receptors that are associated with the complex.
Fig. 2.
Mu receptor signaling complexes. (A) Mu receptor signaling complexes. Agonists of the mu receptor bind to extracellular regions of this GPCR. This leads to dissociation of the cognate G-proteins from the receptor which, either through a conformational change, or physical dissociation, separate into the αi and βγ subunits. G-protein uncoupling is often followed by receptor phosphorylation, typically mediated by the GPCR kinases, GRK2 and GRK3, and potentially other kinases such as the Calcium/CaMK11 or Protein Kinase A or C. Receptor phosphorylation initiates desensitization of the receptor and leads to the recruitment of the scaffolding protein β-arrestin 2 and perhaps other non-G-protein mediated components such as by c-Src. The α or βγ subunits of the G-proteins initiate a series of signaling cascades or second messenger systems. The Gαi subunit couples with the adenylate cyclase cascade to inhibit intracellular accumulation of cAMP. The Gβγ subunits couple with ion channels such as the inwardly rectified K+ channels and the Ca2+ channels to inhibit neuronal activity and may also modulate adenylate cyclase. In addition to these pathways, mu receptor agonists activate the MAP kinase cascade in a G-protein/β-arrestin/GRK3 dependent manner, and so affect multiple processes ranging from nuclear events orchestrated by the MAP kinase cascade and membrane-localized events such as receptor desensitization and transactivation of the receptor tyrosine kinase, Epidermal Growth Factor (EGF) receptor to influence the EGF pathway. (B) Mu receptor trafficking complexes. Following mu receptor activation by ligands such as DAMGO, etorphine, fentanyl and methadone, the mu receptor internalizes in DRG neurons. As the mu receptor is a Class A receptor, it rapidly dissociates from β-arrestin 2 and is internalized into clathrin-coated pits. These pits evolve into Rab5 associated early endosomes and the receptor then recycles back to the cell membrane through either the Rab4- or Rab11-mediated early or late recycling pathways. The recycled receptor is dephosphorylated, resensitized and capable of signaling when returned to the cell membrane. Mu receptor internalization and desensitization requires an intact 3-dimensional structure, as suggested from cells lacking the actin-cytoskeleton protein, Filamin A (Onoprishvili et al., 2008). Such ligand-dependent recycling of the mu receptor is dependent on the sequence of the C-terminus. If this sequence is exchanged with that of the delta opioid receptor, β-arrestin recruitment is prolonged and the receptor is degraded rather than recycled (Walwyn et al., 2006). The internalization of the mu receptor is p38 dependent, this kinase phosphorylates the early endosomal antigen 1 and Rabenosyn5, both components of the early endosome (Mace et al., 2005). Interestingly the non-internalizing mu receptor agonist, morphine, neither activates p38 nor internalizes the mu receptor suggesting that p38 activation is a critical component of mu receptor internalization (Tan et al., 2009). Although we do not know whether ligand-dependent internalization of the mu receptor requires the non-receptor tyrosine kinase, c-Src, we have found that c-Src and β-arrestin 2 are required for ligand-independent or constitutive receptor internalization and recycling. This pathway removes constitutively, or tonically, active mu receptors from the cell membrane in a c-Src and β-arrestin 2 manner. However, if either of these 2 molecules are inhibited the receptor remains on the cell membrane with measurable physiological effects.
So why should the view of the receptor as a dynamic complex change how we view opioid therapeutics? There are two key issues: The first is that different ligands can induce the formation of different complexes that result in different signaling and trafficking cascades. The second is that different receptor environments, perhaps in different areas of brain or parts of the cell, can signal and traffic receptors in different ways in response to the same ligand. This opens up the exciting possibility for individual ligands to have distinct effects on the array of opioid behaviors and adaptive processes—effects ultimately dependent upon signaling and regulatory pathways activated by specific complexes. The on/off switch as the image of an opioid receptor has transformed to a sophisticated sensor that responds in different ways, depending on the local environment and how the sensor is manipulated.
Here we will focus on what has been learned from behavioral studies and cellular studies on dorsal root ganglia neurons from animals lacking β-arrestins, key molecules in the formation of signaling and trafficking opioid receptor complexes (Reiter and Lefkowitz, 2006). β-arrestin 1 and β-arrestin 2 are molecules named for one of their many functions, namely to bind to receptors following agonist activation and “arrest” further signaling via G-proteins. As indicated in Fig. 2, β-arrestins are implicated both in the signaling and trafficking of opioid receptors. β-Arrestins bind multiple signaling, trafficking, and regulatory proteins in addition to receptors. Thus, β-arrestins provide a hub for the formation of receptor complexes. In a classical sequence of events, agonist binding to a G-protein coupled receptor (GPCR), such as the mu receptor, promotes G-Protein activation by GDP/GTP exchange and the conformational rearrangements ultimately result in β-arrestin 1 or 2 binding to the receptor. β-Arrestin binding is often facilitated by kinases such as G-protein receptor kinases or GRK’s that phosphorylate intracellular components of the receptor (generally the C-terminal tail), and thereby increase affinity of the receptor for the β-arrestins. β-arrestins act as linkers for a series of other proteins including kinases (such as cSrc and JNK3, a c-Jun N-Terminal Kinase) and scaffolding proteins involved in trafficking (such as alpha-adaptin-2). The agonist interaction with the receptor generates a cluster of proteins in close proximity of the receptor to accomplish signaling (Fig. 2A), regulatory and trafficking (Fig. 2B) events.
Multiple studies now implicate different opioid drugs inducing different receptor-containing protein complexes. One of the initial observations was found in cell lines containing the delta opioid receptor whereby treatment with several opioid peptides and alkaloid agonists but not morphine induced loss of surface opioid binding (Von Zastrow et al., 1993). Subsequently, many agonist-selective receptor-mediated effects have been documented, including receptor phosphorylation, receptor trafficking, receptor signaling and receptor desensitization (Evans, 2004). Research indicating that the same drug can induce different complexes in different environments is less well documented, but has been implicated in a study of mu receptor trafficking in dendrites and cell bodies. In this study, morphine was found to induce effective internalization in processes but not in cell bodies (Haberstock-Debic et al., 2003).
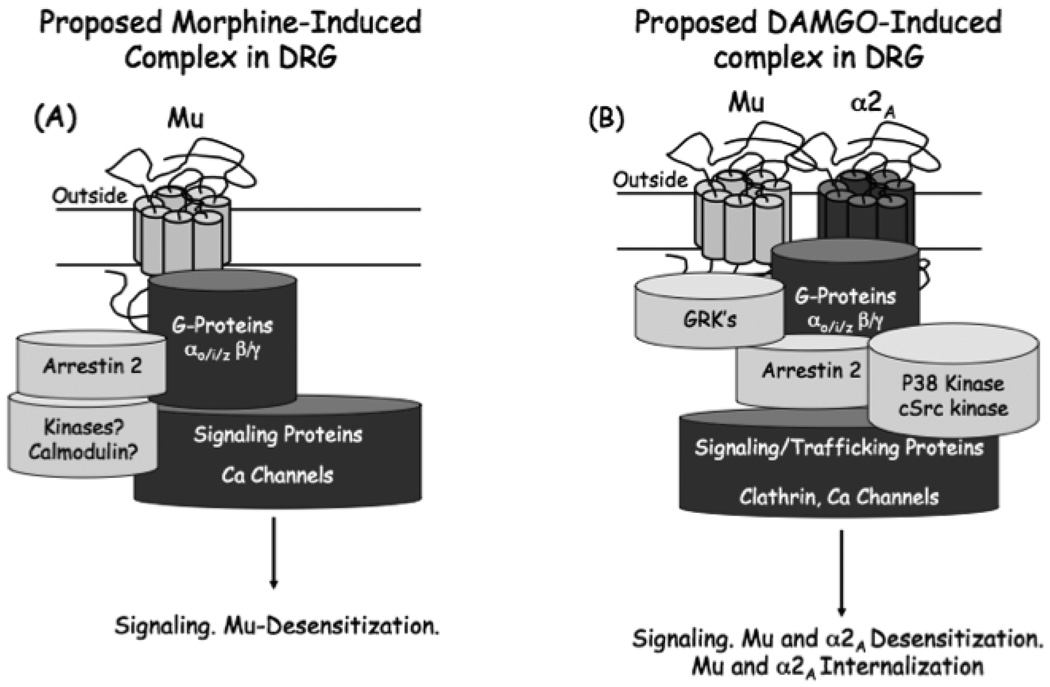
Multiple studies have shown that activation of or even just the presence of other GPCR’s can influence the pharmacology, function, and trafficking of mu receptors (Alfaras-Melainis et al., 2009). One example from our studies is the interaction of mu receptors and α2A adrenergic receptors in DRG cells (Tan et al., 2009). Primary cultures of mouse DRG neurons express multiple opioid receptors (Mu, delta and kappa) as well as α2A adrenergic receptors. Activation of α2A adrenergic receptors with an agonist such as clonidine is able to cause desensitization and internalization of α2A adrenergic receptors and mu opioid receptors. Likewise, activation of mu opioid receptors with a peptide mimetic of enkephalin ([D-Ala2,N-MePhe4, Gly-ol5]enkephalin or DAMGO) causes internalization and desensitization of both Mu and α2A adrenergic receptors. Clonidine or DAMGO-induce receptor cross-regulation can be disrupted by p38 MAP kinase inhibition. p38 inhibition also blocks DAMGO-induced mu receptor desensitization and internalization, although α2A adrenergic receptor desensitization and internalization by clonidine is unaffected. Like p38, β-Arrestin 2 also appears to be required for cross-regulation of Mu and α2A adrenergic receptors. However, unlike p38 inhibition, β-Arrestin 2 is required for α2A adrenergic receptor desensitization and not desensitization of mu receptors (Tan et al., 2009). The data clearly demonstrate that the action at one receptor can markedly influence the responsivity and trafficking of a different receptor. The hypothesized complex following DAMGO activation of mu opioid receptors in DRG neurons is indicated in Fig. 3A.
Fig. 3.
Morphine vs DAMGO receptor signaling complexes. The differential internalization, pharmacology of desensitization and signaling of the DAMGO-treated receptor (A) and morphine-treated mu receptor (B) in DRG neurons (see text) can be explained by ligand-specific receptor complex formation. An interesting difference between the morphine vs DAMGO-activated mu opioid receptors is a ligand-dependent interaction with the α2A adrenergic receptor. Our data suggest that in DRG neurons this ligand-dependent receptor-receptor interaction is p38, β-arrestin 2 and possibly internalization-dependent. DAMGO, an internalizing mu receptor agonist, results in both internalization and desensitization of the α2A receptor whereas morphine, a non-internalizing mu receptor agonist, neither internalizes nor desensitizes the α2A receptor (Tan et al., 2009).
Treatment with either morphine or DAMGO shows that different agonists lead to different signaling pathways and trafficking in DRG neurons (Tan et al., 2009). Firstly, DAMGO is found to cause internalization of mu receptors in the DRG neurons, but morphine does not. Secondly, morphine does not cause cross-desensitization or internalization of α2A adrenergic receptors as does DAMGO. And thirdly as might be anticipated, morphine does not activate p38 kinase that is observed with DAMGO treatment. However, mu receptor desensitization still occurs after treatment with morphine, and morphine-induced desensitization does not depend upon activation of p38 suggestive of different mechanisms for desensitization via morphine and DAMGO. This has been explored in other systems where morphine desensitization is shown to be PKC-dependent but not GRK-dependent, and DAMGO desensitization GRK-dependent but not PKC-dependent (Kelly et al., 2008). In DRG neurons, morphine desensitization does not appear to be PKC-dependent (unpublished observation) and it is anticipated that different cells will have different desensitization mechanisms for the same ligand activating the receptor. A hypothesis for a morphine-induced complex in DRG neurons is depicted in Fig. 2A.
The bottom line from the experiments described above in DRG neurons as well as analogous studies in other systems, is that protein complexes that form around the mu receptor are ligand-dependent, dependent on cell type and compartment within the cell, and regulated by recent history of the receptor environment. Though experiments are still in the early stages of research, it is clear that the differential formation of complexes will contribute to individual differences between drugs targeting opioid receptors and other GPCRs. It is probable that optimal complex formation for a designated treatment will become a future criteria for searching therapeutic targets and add to an already long list of requirements, including receptor specificity, receptor potency, receptor efficacy, low organ toxicity, blood brain barrier permeability, and metabolic stability.
2.3. Upregulation of Mu receptor constitutive activity
Mice lacking β-arrestin 2 have revealed another important characteristic of mu receptor trafficking. mu opioid receptors can be constitutively active and activate G-proteins in the absence of agonist ligands. In DRG neurons we have shown that constitutively active receptors are efficiently removed from the cell surface, a process that appears dependent upon cSrc and β-arrestin 2 (Walwyn et al., 2007). Thus in DRG neurons from β-arrestin 2 knockout mice or cells from wild-type mice in the presence of c-Src inhibitors, there is an increased level of surface constitutive activity of mu opioid receptors that constantly inhibits Ca2+ channels in a similar (but not identical) fashion to agonists. This constant constitutive activity appears not to have desensitized mu opioid receptor signaling, since mu agonist dose–response curves in DRG neurons from wild-type and β-arrestin 2 knockout mice are indistinguishable. Given that constitutive activity of mu receptors is enhanced in β-arrestin 2 knockout DRG neurons, we have determined if this would result in mu mediated analgesia in the absence of agonists. β-arrestin 2 knockout mice were found to have an increased nociceptive threshold in the tail immersion assay supporting previously published data (Bohn et al., 1999). Recent unpublished data from our laboratory support the notion that this opioid-mediated basal analgesia or increased nociceptive threshold in the β-arrestin 2 knockout mice is indeed due to mu-receptor constitutive activity and not due to activation of opioid receptors by endogenous opioid ligands. This finding is of potential clinical relevance since it presents a novel therapeutic target, namely the interference of mu-receptor interaction with β-arrestin 2, as a mechanism to develop analgesia that appears to not be susceptible to complete desensitization. Furthermore, the β-arrestin 2 knockout mice do not appear to have an elevated basal hedonic tone, based upon indistinguishable mu-antagonist-induced aversion in wild-type and β-arrestin 2 KO mice. Currently, we are screening for allosteric regulators and neutral antagonists that disrupt arrestin interactions yet retain constitutive activity of mu opioid receptors in hopes to discover new-non-agonist ligands of the mu receptor that use constitutive activity to produce an analgesic response.
2.4. Mu receptor splice variants
Target therapeutics differentiating behavioral effects of mu agonists could also result from drugs selective for splice variants of the mu receptor. Differential splicing of mu receptor RNA transcripts has been described resulting in receptors with different sequences at both the N- and C-terminus of the protein (Pan, 2005, review; Pasternak, 2004). These alternatively spliced mu receptors have different distributions, trafficking and desensitization properties (Koch et al., 2001; Tanowitz et al., 2008) and thus agonists selective for these different variants would be expected to generate differential behavioral profiles. However, whether drugs can be developed with sufficient selectivity in vivo for individual splice variants or that expression of selective splice variants is appropriate for differentiating reward, pain modulation and adaptive processes has not been clearly demonstrated. The differential in vivo effects that have been observed among some mu agonists have been attributed to variation in interaction with different splice variants of the receptor, although, as implicated above, there are many different mechanisms by which behavioral heterogeneity of mu agonists might be mediated.
2.5. Optimizing opioid actions by targeting other systems
One strategy to optimize opioid therapeutics that has not been sufficiently explored at the clinical level and has been revealed by rodent models using antagonists and knockout approaches is that several systems appear to work with the opioid system circuitry to control reward and opioid adaptive processes (for review see Bryant et al., 2005). Thus, the cannabinoid CB1 receptor appears to be required for opioid reward but not opioid analgesia and the substance P receptor, NK1, is required both for full morphine-reward and morphine-induced hyperalgesia that emerges during withdrawal (King et al., 2005a). The application of mixed opioid-NK1 or opioid-CB1 antagonists could be useful; especially in pain patients that are high-risk for addiction and that require opioid analgesics. However, the side effects in humans of the CB1 antagonist Rimonabant in promoting depression and suicidal behaviors (Lee et al., 2009) and the lack of a suitable NK1 antagonist has not facilitated the testing of opioid combinations with CB1 or NK1 antagonists at the clinical level. Interestingly, agents that prevent hyperalgesia also reduce tolerance demonstrating similar underlying cellular adaptations to chronic opioids. Exciting work by De-Yong Liang and colleagues suggests that different strains of mice have a greater susceptibility to develop hyperalgesia after opioid administration and polymorphisms of the β2 adrenergic receptor (β2-AR) gene were linked to these differences (Liang et al., 2006). Using the selective β2-AR antagonist butoxamine, the investigators observed a dose-dependent reversal of OIH. This study holds promise that the addition of a β2-AR antagonist to a chronic opioid regiment in humans might improve the long-term analgesic efficacy (Liang et al., 2006). Similar promising results have been found with antagonists of different components of the system such as CCK and NK1 (review; Bryant et al., 2005). The development of non-addictive opioid formulas should be a goal of pharmaceutical companies despite the lost revenue that would occur by negating abuse liability of their drug products.
2.6. Novel pharmacological targets for analgesia besides opioids
More recently there has been considerable interest in the development of non-opiate based pharmacological and non-pharmacological targets to treat pain. Some of these targets are receptors or ions channels in all or specific subsets of neurons directly involved in the pain pathways, while others target nonneuronal cell types.
2.6.1. The cholinergic system
The cholinergic system, whether modulating the levels of acetylcholine directly or activating the ligand-gated ion channel receptors or G-protein coupled muscarinic receptors, can influence nociception. Inhibitors of acetylcholinesterase, for example neostigmine, prevent the hydrolysis of acetylcholine and increase acetylcholine accumulation in the synapse. This increases the inhibition of excitatory post-synaptic neurotransmission as well as enhancing pre-synaptic inhibition, both effects decreasing pain. Although difficult to discern which types of receptors are involved, non-specific agonists of both the G-protein coupled muscarinic and nicotinic ligand-gated cholinergic ion channels are able to decrease acute, chronic, or inflammatory pain. Unfortunately, such agonists are commonly associated with unwanted side effects due to the activation of other systems making non-specific cholinergic compounds unattractive analgesics. However, more specific compounds targeting ion-channel receptor subunits involved in analgesia but not other nicotinic receptor functions, could make outstanding targets for new therapeutics (Jones and Dunlop, 2007).
2.6.2. Delta opioid receptors
Convincing pain-relieving effects mediated of the delta opioid receptor, another member of the opioid receptor family, have been difficult to show in standard animal pain models. However, in states of inflammation or after chronic morphine, a ‘priming’ effect occurs increasing delta receptor activity (Gendron et al., 2007), and delta-mediated analgesia (Cahill et al., 2001). In addition to the lack of effect in the normal state, delta receptor agonists produce serious side effects, the worst of which are life-threatening seizures, making the well-known delta compounds unlikely therapeutics. However, different delta agonists have recently been developed that do not show such unwanted side effects (Codd et al., 2009). These compounds show different phosphorylation, kinase recruitment and internalization profiles with matching differences in their physiological effects (Pradhan et al., 2009). Furthermore the delta receptor has recently been shown to be expressed in a different class of primary afferent neurons than the mu receptor suggesting that delta-based analgesics may have a different physiological effect than those targeting the mu receptor (Scherrer et al., 2009).
2.6.3. Other GPCRs
Many other G-protein coupled receptors are expressed in the pain pathways and are either well-accepted analgesics or hold promise as future analgesic targets. For example, clonidine, an agonist of the α2A adrenergic receptor is frequently used to treat post-operative pain, as is ocreotide, a somatostatin analog. A potential target is the cannabinoid system, the receptors of which are more ubiquitously expressed in the central nervous system than the opioid receptors. Cannabinoids can induce analgesia but, the psychoactive side effects of centrally acting cannabinoids limits their use. This has sparked interest in a peripherally restricted class of cannabinoid receptors to treat pain of a peripheral nature (Karst and Wippermann, 2009). Given the positive effect of the cannabinoids on analgesia and its widespread distribution, this system may offer less risk of addiction, withdrawal, and overdose than opioid drugs if the appropriate cannabinoid-like compounds can be found with minimal side-effects.
Although many of these alternative strategies targeting G-protein coupled receptors (GPCR) appear promising, many carry unwanted side effects, often a result of non-specific activation of receptors outside the nociceptive pathways. Agonists of different receptor populations, each at an ineffective analgesic dose, can reduce off-target effects and become analgesic when co-administered. Such combinatorial drug therapies, for example those targeting the cannabinoid and opioid systems, may become promising analgesics (review; Karst and Wippermann, 2009). Another approach to increase pharmacological specificity is to target receptors/molecules expressed only in the nociceptive pathway. One example is the sensory neuron-specific GPCRs (SNSRs, Dong et al., 2001) which are only expressed in a limited subset of dorsal root and trigeminal ganglia neurons. Recent evidence from an siRNA knockdown approach shows that these receptors increase the response to inflammatory pain (Ndong et al., 2009), suggesting that SNSR antagonists may be analgesic. Furthermore the appreciation that many GPCR’s form heterodimers has opened the potential of heterodimer-selective ligands. For example 6′-guanidinonaltrindole (6′-GNTI) is a reported analgesic that selectively activates spinal delta-kappa opioid receptor heterodimers (Waldhoer et al., 2005).
2.6.4. Ion channels
Manipulation of specific ion channels to treat pain is a novel and exciting area of the analgesic field that many pharmaceutical companies have adopted. Ziconotide, for example, is an N-type calcium channel inhibitor derived from the cone marine snail, that is currently available (Williams et al., 2008). However, the quest for greater specificity is an underlying issue of ion channel-mediated analgesia. Different approaches have been used to address this concern, one of which is to target spatially restricted ion channels such as the Nav1.8 and 1.9 sodium channels, the acid sensing ASIC3 channels, or the purinergic, ATP-sensing channels, all of which are expressed in dorsal root ganglia neurons, the first order sensory neurons of the pain pathway (review; Patapoutian et al., 2009). Similarly, many pharmaceutical companies are currently focusing on antagonists that block or agonists that desensitize the transient receptor potential channels, in particular the thermo-TRPV1 and TRPA1 channels expressed in the heat-sensing neurons of dorsal root ganglia (Patapoutian et al., 2009). The large pore size of the open TRPV1 channel has also been used to gain intracellular access to only these heat-sensing neurons by the lidocaine derivative, QX-413 (Patapoutian et al., 2009). In some cases, the use of partial agonists with lower efficacies and reduced side effects, such as Sazetidine-A, a partial nicotinic β2 agonist with effective analgesic properties, has been explored (Cucchiaro et al., 2008).
2.6.5. Other molecular targets
Although not often recognized for its involvement in pain, nerve growth factor (NGF) is a major mediator of inflammatory and neuropathic pain. Antibodies to this diffusible growth factor, or small peptides acting as an NGF receptor and able to absorb NGF, have been assessed as analgesics (Watson et al., 2008). Another interesting compound is an anti-TNFα antibody that has been typically used to treat inflammatory conditions such as rheumatoid arthritis and psoriasis, but may also have analgesic properties (Seadi Pereira et al., 2009).
2.7. Opioids and the immune system
Opioid-mediated analgesia may also be influenced by the peripheral release of opioid peptides, β-endorphin, met-enkephalin, and dynorphin, from specific immune cells during inflammation. These are the granulocytes, lymphocytes, and monocytes, each population releasing opioids at different stages of the inflammatory process. The granulocytes are the first to be recruited to the site of inflammation and require an intact chemokine cascade, series of adhesion molecules, and other chemo-attractants to direct their migration. Once at the site, they increase the release of opioid peptides in a p38 and PI3K dependent manner (review; Rittner et al., 2008). These opioids bind to the peripheral nerve terminals of the primary afferent neurons, which express the delta, mu, and kappa opioid receptors. This initiates the opioid signaling cascade to increase hyperpolarization and inhibit neurotransmitter release, thus reducing the sensation of pain. Such peripherally restricted release of opioids from immune cells appears not to induce tolerance, and certainly is not associated with the central side effects of the centrally acting opioids (Smith, 2008). This suggests that peripherally-restricted opioids may be more attractive alternative compounds than their centrally acting counterparts for peripheral pain.
2.8. Non-classical opioid receptors and targets
Opioids may also activate astrocytes and microglia, and accelerate the development of tolerance and the opponent process, hyperalgesia, by enhancing nitric oxide and pro-inflammatory cytokine production (Wang et al., 2009). This can partially be prevented by the ±enantiomers of naloxone or naltrexone, which bind with the toll-like 4 receptors on microglia (Hutchinson et al., 2008). Another interesting non-classical opioid receptor is the opioid-growth factor receptor present in glia and neurons, which binds with met-enkaphalin and through direct effects on DNA synthesis, affects proliferation (Zagon et al., 2002).
3. Conclusion
It must be recognized that opioid pharmaceuticals fulfill a valuable niche in the treatment of pain and that “opiophobia” can deny patients effective treatment and perhaps the opportunity for a more positive outcome. To address diversion and prescription drug misuse, increased monitoring of opioid medications, awareness of pain modalities not optimum for opioid treatments and detection of patients drug shopping or patients likely to become addicted are all measures that can be currently instated. Progress on the basic research front has focused on the reduction of analgesic tolerance yet of more clinical importance is the control of hedonic circuitry and opponent processes. Promising strategies identified in rodent models include co-administration of opioid drugs with NK1-antagonists, which reduce reward activation and nociceptive adaptations that result in hyperalgesia. The possibility of the upregulation of surface constitutively-active mu opioid receptors offers an alternative, untested, therapeutic strategy for analgesia without addiction. The identification of drugs with selective trafficking and signaling via opioid receptors that stem from differential ability to form selective receptor complexes is slowly revealing itself as an exciting new area for differentiating opioid analgesics. Drugs forming selective complexes, identifying selective preformed receptor heterodimers, or perhaps selective for specific Mu-receptor splice products have the potential to improve opioids as effective medications for pain with lessened opponent processes and addictive liability. It is anticipated that drugs targeting receptors other than opioid receptors will become analgesics of choice, which will avoid the dilemmas that individuals prescribing analgesic medications are now facing.
Acknowledgements
We thank Phoebe Chen and Jaimie Takaesu for gathering material for the review. Research reviewed from the authors laboratories was supported by DA 05010 and the Hatos Foundation.
Role of funding source
Funding for this study was provided by DA 05010 and the Hatos Foundation, Neither NIDA nor the Hatos Foundation had any further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Contributors
Much of the data mentioned in this review were completed by Wendy Walwyn who also wrote the latter section of the review on alternate targets. Karen Miotto wrote the epidemiological section in the first part of the review. Christopher Evans outlined the review, wrote the sections on the ideas and issues seeking solutions, the central theme of this manuscript, and then compiled the document. All authors read, edited and approved the final manuscript.
Conflict of interest
These authors have no conflict of interest.
References
- Alfaras-Melainis K, Gomes I, Rozenfeld R, Zachariou V, Devi L. Modulation of opioid receptor function by protein-protein interactions. Front Biosci. 2009;14:3594–3607. doi: 10.2741/3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburn MA, Rubingh CR. The role of non-opioid analgesics for the management of postoperative pain. Cancer Control. 1999;6:10–13. doi: 10.1177/107327489900602S02. [DOI] [PubMed] [Google Scholar]
- Ashburn MA, Staats PS. Management of chronic pain. Lancet. 1999;353:1865–1869. doi: 10.1016/S0140-6736(99)04088-X. [DOI] [PubMed] [Google Scholar]
- Ballantyne JC, Mao J. Opioid therapy for chronic pain. N. Engl. J. Med. 2003;349:1943–1953. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Serrano D, Reed M, Lipton RB. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology. 2008;71:559–566. doi: 10.1212/01.wnl.0000323925.29520.e7. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- Bryant CD, Zaki PA, Carroll FI, Evans CJ. Opioids and addiction: emerging pharmaceutical strategies for reducing reward and opponent processes. Clin. Neurosci. Res. 2005;5:103–115. [Google Scholar]
- Cahill CM, Morinville A, Lee MC, Vincent JP, Collier B, Beaudet A. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. J. Neurosci. 2001;21(19):7598–7607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Reuben S. More on current issues in pain management for the primary care practitioner. Acute pain: a multi-modal management approach. J. Pain Palliat. Care Pharmacother. 2005;19:69–70. doi: 10.1300/j354v19n01_13. [DOI] [PubMed] [Google Scholar]
- Chou R, Ballantyne JC, Fanciullo GJ, Fine PG, Miaskowski C. Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J. Pain. 2009a;10:147–159. doi: 10.1016/j.jpain.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Chou R, Fanciullo GJ, Fine PG, Miaskowski C, Passik SD, Portenoy RK. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J. Pain. 2009b;10:131–146. doi: 10.1016/j.jpain.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Chou R. Clinical Guidelines from the American Pain Society and the American Academy of Pain Medicine on the use of chronic opioid therapy in chronic noncancer pain: what are the key messages for clinical practice? Pol. Arch. Med. Wewn. 2009c;119:469–477. [PubMed] [Google Scholar]
- Collado F, Torres LM. Association of transdermal fentanyl and oral transmucosal fentanyl citrate in the treatment of opioid naive patients with severe chronic noncancer pain. J. Opioid Manag. 2008;4:1111–1115. doi: 10.5055/jom.2008.0016. [DOI] [PubMed] [Google Scholar]
- Codd EE, Carson JR, Colburn RW, Stone DJ, Van Besien CR, Zhang SP, Wade PR, Gallantine EL, Meert TF, Molino L, Pullan S, Razler CM, Dax SL, Flores CM. JNJ-20788560 [9-(8-azabicyclo[3.2.1]oct-3-ylidene)-9H-xanthene-3-carboxylic acid diethylamide], a selective delta opioid receptor agonist, is a potent and efficacious antihyperalgesic agent that does not produce respiratory depression, pharmacologic tolerance, or physical dependence. J. Pharmacol. Exp. Ther. 2009;329:241–251. doi: 10.1124/jpet.108.146969. [DOI] [PubMed] [Google Scholar]
- Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Cucchiaro G, Xiao Y, Gonzalez-Sulser A, Kellar KJ. Analgesic effects of Sazetidine-A, a new nicotinic cholinergic drug. Anesthesiology. 2008;109:512–519. doi: 10.1097/ALN.0b013e3181834490. [DOI] [PubMed] [Google Scholar]
- Daniel T, Thobe BM, Chaudry IH, Choudhry MA, Hubbard WJ, Schwacha MG. Regulation of the postburn wound inflammatory response by gammadelta T-cells. Shock. 2007;28:278–283. doi: 10.1097/shk.0b013e318034264c. [DOI] [PubMed] [Google Scholar]
- Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- Dunwoody CJ, Krenzischek DA, Pasero C, Rathmell JP, Polomano RC. Assessment, physiological monitoring, and consequences of inadequately treated acute pain. Pain Manag. Nurs. 2008;9:S11–S21. doi: 10.1016/j.pmn.2007.11.006. [DOI] [PubMed] [Google Scholar]
- DEA. Washington, DC: Sponsored by the Center for Substance Abuse Treatment Substance Abuse and Mental Health Services Administration; 2007. [July 20, 2007]. Summary Report of the Meeting: Methadone Mortality–A Reassessment. http://www.dpt.samhsa.gov/pdf/Methadone_Report_10%2018%2007_Brief%20w_%20attch.pdf . [Google Scholar]
- Evans CJ. Secrets of the opium poppy revealed. Neuropharmacology. 2004;47 Suppl. 1:293–299. doi: 10.1016/j.neuropharm.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Gatti A, Sabato AF, Occhioni R, Colini Baleschi G, Reale C. Controlled-release oxycodone and pregabalin in the treatment of neuropathic pain: results of a multicenter Italian study. Eur. Neurol. 2009;61:129–137. doi: 10.1159/000186502. [DOI] [PubMed] [Google Scholar]
- Gendron L, Esdaile MJ, Mennicken F, Pan H, O’Donnell D, Vincent JP, Devi LA, Cahill CM, Stroh T, Beaudet A. Morphine priming in rats with chronic inflammation reveals a dichotomy between antihyperalgesic and antinociceptive properties of deltorphin. Neuroscience. 2007;144:263–274. doi: 10.1016/j.neuroscience.2006.08.077. [DOI] [PubMed] [Google Scholar]
- Geppetti P, Benemei S. Pain treatment with opioids: achieving the minimal effective and the minimal interacting dose. Clin. Drug Investig. 2009;29 Suppl. 1:3–16. doi: 10.2165/0044011-200929001-00002. [DOI] [PubMed] [Google Scholar]
- Gfroerer J, Penne M, Pemberton M, Folsom R. Substance abuse treatment need among older adults in 2020: the impact of the aging baby-boom cohort. Drug Alcohol Depend. 2003;69:127–135. doi: 10.1016/s0376-8716(02)00307-1. [DOI] [PubMed] [Google Scholar]
- Grichnik KP, Ferrante FM. The difference between acute and chronic pain. Mt. Sinai J. Med. 1991;58:217–220. [PubMed] [Google Scholar]
- Haanpaa ML, Backonja MM, Bennett MI, Bouhassira D, Cruccu G, Hansson PT, Jensen TS, Kauppila T, Rice AS, Smith BH, Treede RD, Baron R. Assessment of neuropathic pain in primary care. Am. J. Med. 2009;122:S13–S21. doi: 10.1016/j.amjmed.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Haberstock-Debic H, Wein M, Barrot M, Colago EE, Rahman Z, Neve RL, Pickel VM, Nestler EJ, von Zastrow M, Svingos AL. Morphine acutely regulates opioid receptor trafficking selectively in dendrites of nucleus accumbens neurons. J. Neurosci. 2003;23:4324–4332. doi: 10.1523/JNEUROSCI.23-10-04324.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna M, Tuca A, Thipphawong J. An open-label, 1-year extension study of the long-term safety and efficacy of once-daily OROS(R) hydromorphone in patients with chronic cancer pain. BMC Palliat. Care. 2009;8:14. doi: 10.1186/1472-684X-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton GR, Baskett TF. In the arms of Morpheus the development of morphine for postoperative pain relief. Can. J. Anaesthesia (J. Canadien d’anesthesie) 2000;47:367–374. doi: 10.1007/BF03020955. [DOI] [PubMed] [Google Scholar]
- Hay JL, White JM, Bochner F, Somogyi AA, Semple TJ, Rounsefell B. Hyperalgesia in opioid-managed chronic pain and opioid-dependent patients. J. Pain. 2009;10:316–322. doi: 10.1016/j.jpain.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4) Eur. J. Neurosci. 2008;28:20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman RP, Purvis JM, Mallett BS. Chronic nonmalignant pain in primary care. Am. Fam Physician. 2008;78:1155–1162. [PubMed] [Google Scholar]
- Jones PG, Dunlop J. Targeting the cholinergic system as a therapeutic strategy for the treatment of pain. Neuropharmacology. 2007;53:197–206. doi: 10.1016/j.neuropharm.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Karst M, Wippermann S. Cannabinoids against pain, efficacy and strategies to reduce psychoactivity: a clinical perspective. Expert Opin. Investig. Drugs. 2009;18:125–133. doi: 10.1517/13543780802691951. [DOI] [PubMed] [Google Scholar]
- Katz WA. Opioids for nonmalignant pain. Rheum. Dis. Clin. North Am. 2008;34:387–413. doi: 10.1016/j.rdc.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Katz N, Mazer NA. The impact of opioids on the endocrine system. Clin. J. Pain. 2009;25:170–175. doi: 10.1097/AJP.0b013e3181850df6. [DOI] [PubMed] [Google Scholar]
- Kelly E, Bailey CP, Henderson G. Agonist-selective mechanisms of GPCR desensitization. Br. J. Pharmacol. 2008;153 Suppl. 1:S379–S388. doi: 10.1038/sj.bjp.0707604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T, Gardell LR, Wang R, Vardanyan A, Ossipov MH, Malan TP, Jr, Vanderah TW, Hunt SP, Hruby VJ, Lai J, Porreca F. Role of NK-1 neurotransmission in opioid-induced hyperalgesia. Pain. 2005a;116:276–288. doi: 10.1016/j.pain.2005.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T, Ossipov MH, Vanderah TW, Porreca F, Lai J. Is paradoxical pain induced by sustained opioid exposure an underlying mechanism of opioid antinociceptive tolerance? Neurosignals. 2005b;14:194–205. doi: 10.1159/000087658. [DOI] [PubMed] [Google Scholar]
- Koch T, Schulz S, Pfeiffer M, Klutzny M, Schroder H, Kahl E, Hollt V. C-terminal splice variants of the mouse mu-opioid receptor differ in morphine-induced internalization and receptor resensitization. J. Biol. Chem. 2001;276:31408–31414. doi: 10.1074/jbc.M100305200. [DOI] [PubMed] [Google Scholar]
- Kuehn BM. Prescription drug abuse rises globally. JAMA. 2007;297:1306. doi: 10.1001/jama.297.12.1306. [DOI] [PubMed] [Google Scholar]
- Lee HK, Choi EB, Pak CS. The current status and future perspectives of studies of cannabinoid receptor 1 antagonists as anti-obesity agents. Curr. Top. Med. Chem. 2009;9:482–503. doi: 10.2174/156802609788897844. [DOI] [PubMed] [Google Scholar]
- Liang DY, Liao G, Wang J, Usuka J, Guo Y, Peltz G, Clark JD. A genetic analysis of opioid-induced hyperalgesia in mice. Anesthesiology. 2006;104:1054–1062. doi: 10.1097/00000542-200605000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebeskind JC. Pain can kill. Pain. 1991;44:3–4. doi: 10.1016/0304-3959(91)90141-J. [DOI] [PubMed] [Google Scholar]
- Mace G, Miaczynska M, Zerial M, Nebreda AR. Phosphorylation of EEA1 by p38 MAP kinase regulates mu opioid receptor endocytosis. Embo J. 2005;24:3235–3246. doi: 10.1038/sj.emboj.7600799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008;11:S63–S88. [PubMed] [Google Scholar]
- Martell BA, O’Connor PG, Kerns RD, Becker WC, Morales KH, Kosten TR, Fiellin DA. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Ann. Intern. Med. 2007;146:116–127. doi: 10.7326/0003-4819-146-2-200701160-00006. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Boyd CJ, Teter CJ. Subtypes of nonmedical prescription drug misuse. Drug Alcohol Depend. 2009;102:63–70. doi: 10.1016/j.drugalcdep.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndong C, Pradhan A, Puma C, Morello JP, Hoffert C, Groblewski T, O’Donnell D, Laird JM. Role of rat sensory neuron-specific receptor (rSNSR1) in inflammatory pain: contribution of TRPV1 to SNSR signaling in the pain pathway. Pain. 2009;143:130–137. doi: 10.1016/j.pain.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Onoprishvili I, Ali S, Andria ML, Shpigel A, Simon EJ. Filamin A mutant lacking actin-binding domain restores mu opioid receptor regulation in melanoma cells. Neurochem. Res. 2008;33:2054–2061. doi: 10.1007/s11064-008-9684-y. [DOI] [PubMed] [Google Scholar]
- Pasero C. Procedure-specific pain management: prospect. J. Perianesth. Nurs. 2007;22:335–340. doi: 10.1016/j.jopan.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Pan YX. Diversity and complexity of the mu opioid receptor gene: alternative pre-mRNA splicing and promoters. DNA Cell Biol. 2005;24:736–750. doi: 10.1089/dna.2005.24.736. [DOI] [PubMed] [Google Scholar]
- Panjabi SS, Panjabi RS, Shepherd MD, Lawson MD, Johnsrud M, Barner J. Extended-release, once-daily morphine (Avinza) for the treatment of chronic nonmalignant pain: effect on pain, depressive symptoms, and cognition. Pain Med. 2008;9:985–993. doi: 10.1111/j.1526-4637.2008.00483.x. [DOI] [PubMed] [Google Scholar]
- Pasternak GW. Multiple opiate receptors: deja vu all over again. Neuropharmacology. 2004;47 Suppl. 1:312–323. doi: 10.1016/j.neuropharm.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat. Rev. Drug Discov. 2009;8:55–68. doi: 10.1038/nrd2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portenoy MD, Russell K. Opioid therapy for chronic nonmalignant pain: a review of the critical issues. J. Pain Symptom Manag. 1996;11:203–217. doi: 10.1016/0885-3924(95)00187-5. [DOI] [PubMed] [Google Scholar]
- Pradhan AA, Becker JA, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Massotte D, Gaveriaux-Ruff C, Kieffer BL. In vivo delta opioid receptor internalization controls behavioral effects of agonists. PLoS ONE. 2009;4:e5425. doi: 10.1371/journal.pone.0005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa R. Pharmacological aspects of successful long-term analgesia. Clin. Rheumatol. 2006;25 Suppl. 1:S9–S15. doi: 10.1007/s10067-006-0201-x. [DOI] [PubMed] [Google Scholar]
- Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol. Metab. 2006;17:159–165. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Rittner HL, Brack A, Stein C. Pain and the immune system. Br. J. Anaesth. 2008;101:40–44. doi: 10.1093/bja/aen078. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp. Clin. Psychopharmacol. 2008;16:405–416. doi: 10.1037/a0013628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Loh HH. Effects of opioids on the immune system. Neurochem. Res. 1996;21(11):1375–1386. doi: 10.1007/BF02532379. [DOI] [PubMed] [Google Scholar]
- SAMHSA. U.S. Department of Health and Human Services Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2008. Results from the 2008 National Survey on Drug Use and Health: National Findings (2009) http://www.oas.samhsa.gov/nsduh/2k8nsduh/2k8Results.cfm#Ch2. [Google Scholar]
- Sacerdote P, Franchi S, Gerra G, Leccese V, Panerai AE, Somaini L. Buprenorphine and methadone maintenance treatment of heroin addicts preserves immune function. Brain Behav. Immun. 2008;22:606–613. doi: 10.1016/j.bbi.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Savage SR. Long-term opioid therapy: assessment of consequences and risks. J. Pain Symptom Manag. 1996;11:274–286. doi: 10.1016/0885-3924(95)00202-2. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwacha MG. Opiates and the development of post-injury complications: a review. Int. J. Clin. Exp. Med. 2008;1:42–49. [PMC free article] [PubMed] [Google Scholar]
- Seadi Pereira PJ, Noronha Dornelles F, Santiago Santos D, Batista Calixto J, Bueno Morrone F, Campos MM. Nociceptive and inflammatory responses induced by formalin in the orofacial region of rats: effect of anti-TNFalpha strategies. Int. Immunopharmacol. 2009;9:80–85. doi: 10.1016/j.intimp.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Siegal HA, Carlson RG, Kenne DR, Swora MG. Probable relationship between opioid abuse and heroin use. Am. Fam. Physician. 2003;67(942):945. [PubMed] [Google Scholar]
- Slatkin NE. Opioid switching and rotation in primary care: implementation and clinical utility. Curr. Med. Res. Opin. 2009;25:2133–2150. doi: 10.1185/03007990903120158. [DOI] [PubMed] [Google Scholar]
- Smith HS. Peripherally-acting opioids. Pain Physician. 2008;11:S121–S132. [PubMed] [Google Scholar]
- Soin A, Cheng J, Brown L, Moufawad S, Mekhail N. Functional outcomes in patients with chronic nonmalignant pain on long-term opioid therapy. Pain Pract. 2008;8:379–384. doi: 10.1111/j.1533-2500.2008.00233.x. [DOI] [PubMed] [Google Scholar]
- Solomon DH, Avorn J, Katz JN, Weinblatt ME, Setoguchi S, Levin R, Schneeweiss S. Immunosuppressive medications and hospitalization for cardiovascular events in patients with rheumatoid arthritis. Arthritis Rheumatism. 2006;54:3790–3798. doi: 10.1002/art.22255. [DOI] [PubMed] [Google Scholar]
- Studies SOoA. Trends in Nonmedical Use of Prescription Pain Relievers: 2002–2007. The National Survey on Drug Use and Health Report. 2009
- Sullivan MD, Edlund MJ, Steffick D, Unutzer J. Regular use of prescribed opioids: association with common psychiatric disorders. Pain. 2005;119:95–103. doi: 10.1016/j.pain.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Tan M, Walwyn WM, Evans CJ, Xie CW. p38 MAPK and beta-arrestin 2 mediate functional interactions between endogenous micro-opioid and alpha2A-adrenergic receptors in neurons. J. Biol. Chem. 2009;284:6270–6281. doi: 10.1074/jbc.M806742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanowitz M, Hislop JN, von Zastrow M. Alternative splicing determines the post-endocytic sorting fate of G-protein-coupled receptors. J. Biol. Chem. 2008;283:35614–35621. doi: 10.1074/jbc.M806588200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo R, de Leon-Casasola O, Benyamin R. Opioid therapy and immunosuppression: a review. Am. J. Ther. 2004;11:354–365. doi: 10.1097/01.mjt.0000132250.95650.85. [DOI] [PubMed] [Google Scholar]
- Von Zastrow M, Keith DE, Jr, Evans CJ. Agonist-induced state of the delta-opioid receptor that discriminates between opioid peptides and opiate alkaloids. Mol. Pharmacol. 1993;44:166–172. [PubMed] [Google Scholar]
- Waldhoer M, Fong J, Jones RM, Lunzer MM, Sharma SK, Kostenis E, Portoghese PS, Whistler JL. A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9050–9055. doi: 10.1073/pnas.0501112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JM, Farney RJ. Are opioids associated with sleep apnea? A review of the evidence. Curr. Pain Headache Rep. 2009;13:20–26. doi: 10.1007/s11916-009-0021-1. [DOI] [PubMed] [Google Scholar]
- Walwyn W, Evans CJ, Hales TG. Beta-arrestin2 and c-Src regulate the constitutive activity and recycling of mu opioid receptors in dorsal root ganglion neurons. J. Neurosci. 2007;27:5092–5104. doi: 10.1523/JNEUROSCI.1157-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walwyn WM, Wei W, Xie CW, Chiu K, Kieffer BL, Ecans CJ, Maidment NT. mu opioid receptor-effector coupling and trafficking in dorsal root ganglia neurons. Neuroscience. 2006;142:493–503. doi: 10.1016/j.neuroscience.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ma W, Chabot JG, Quirion R. Cell-type specific activation of p38 and ERK mediates calcitonin gene-related peptide involvement in tolerance to morphine-induced analgesia. Faseb J. 2009 doi: 10.1096/fj.08-128348. [DOI] [PubMed] [Google Scholar]
- Watson JJ, Allen SJ, Dawbarn D. Targeting nerve growth factor in pain: what is the therapeutic potential? BioDrugs. 2008;22:349–359. doi: 10.2165/0063030-200822060-00002. [DOI] [PubMed] [Google Scholar]
- Webster L, Jansen JP, Peppin J, Lasko B, Irving G, Morlion B, Snidow J, Pierce A, Mortensen E, Kleoudis C, Carter E. Alvimopan, a peripherally acting mu-opioid receptor (PAM-OR) antagonist for the treatment of opioid-induced bowel dysfunction: results from a randomized, double-blind, placebo-controlled, dose-finding study in subjects taking opioids for chronic non-cancer pain. Pain. 2008;137:428–440. doi: 10.1016/j.pain.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Williams JA, Day M, Heavner JE. Ziconotide: an update and review. Expert Opin Pharmacother. 2008;9(9):1575–1583. doi: 10.1517/14656566.9.9.1575. [DOI] [PubMed] [Google Scholar]
- Yanni LM, Weaver MF, Johnson BA, Morgan LA, Harrington SE, Ketchum JM. Management of chronic nonmalignant pain: a needs assessment in an internal medicine resident continuity clinic. J. Opioid Manag. 2008;4:201–211. doi: 10.5055/jom.2008.0026. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Verderame MF, McLaughlin PJ. The biology of the opioid growth factor receptor (OGFr) Brain Res. Brain Res. Rev. 2002;38:351–376. doi: 10.1016/s0165-0173(01)00160-6. [DOI] [PubMed] [Google Scholar]
- Zarocostas J. Misuse of prescription drugs could soon exceed that of illicit narcotics, UN panel warns. BMJ. 2007;334:444. doi: 10.1136/bmj.39140.394410.DB. [DOI] [PMC free article] [PubMed] [Google Scholar]