Abstract
Background
Risk factors which predispose children with congenital diaphragmatic hernia (CDH) to recurrence remain poorly defined. We report a large series of recurrent CDH and ask whether prenatal patient factors or postnatal treatment variables better predict recurrence.
Methods
238 neonates with unilateral CDH were repaired from 1990–2006. Data were assessed by χ2 and Mann-Whitney-U tests. Multivariate regression identified independent predictors of recurrence. Statistical significance was set at P<0.05.
Results
We identified 24 recurrences (10%). Median time from repair to recurrence diagnosis was 4.9 months. Patients with recurrence were older (P=0.02) and more often required abdominal wall patches at initial repair (P=0.01) compared to non-recurrence patients. Postoperative length-of-stay (LOS)(P<0.01) and morbidity (P=0.01) were greater in patients with recurrence. Use of diaphragm patch at initial repair was greater in patients with recurrence, but only approached statistical significance (P=0.05). Only two variables independently predicted recurrence by multivariate regression: abdominal (not diaphragm) wall patch during initial repair (O.R.=3.50;P=0.04) and postoperative LOS (O.R.=1.012;P=0.01).
Conclusion
Neonates at risk for CDH recurrence are better identified by postnatal treatment variables than by prenatal patient factors. While age at repair and diaphragm patch use are greater in recurrence patients, the only factors to independently predict recurrence were postoperative LOS and abdominal wall patch use. These data can help optimize follow-up regimens.
Keywords: congenital diaphragmatic hernia, recurrence, multivariate, pediatric, neonatal
INTRODUCTION
As advances in prenatal assessment [1–3] and perinatal management [4–6] have improved outcomes for neonates with congenital diaphragmatic hernia (CDH), emphasis has shifted to the long-term follow-up of infants following surgical repair. Several centers have established multidisciplinary CDH clinics and have proposed standardized timelines for outpatient care [7–9]. Such protocols invariably provide routine screening for CDH recurrence, a well-known complication that carries significant potential morbidity. However, few systematic reviews of recurrent CDH exist [10]. The risk factors that predispose neonates with CDH to recurrence remain poorly understood.
Most available data on CDH recurrence has been limited to isolated reports and small case series [11–19], with recurrence rates ranging from 4 – 50%. Many authors suggest that postnatal treatment-related variables, such as need for ECMO and use of prosthetic diaphragm, represent the major determinants of CDH recurrence [13,15,17,19]. However, collective experience [4,20–23] has demonstrated that overall CDH outcome is highly dependent on prenatal patient factors (hernia side, functional lung parenchyma, associated anomalies, etc.). Available series rarely address the potential role of these prenatal factors in the development of recurrence. As most studies lack the statistical power to identify independent predictors of CDH recurrence, we present a large single-center experience of recurrent CDH to define which clinical variables, expressed as either prenatal patient factors or postnatal treatment variables, best predict recurrence. Improved understanding of independent risk factors contributing to recurrence may optimize development of long-term follow-up protocols.
METHODS
Our analysis was based on data from the Morgan Stanley Children’s Hospital of New York Presbyterian / Division of Pediatric Surgery CDH database and the CDH Outcomes Clinic. Approval for this report was granted by the Institutional Review Board (Protocol #AAAC8366). Data were reviewed for 313 consecutive patients treated for CDH between January 1, 1990 and December 31, 2006. Patients undergoing repair at outside institutions (n=19), bilateral hernias (n=5), Morgagni hernias (n=2), diaphragm eventrations (n=7), and cases with incomplete records (n=2) were excluded. Neonates not offered surgery due to either lethal pulmonary hypoplasia or associated anomalies incompatible with life were also excluded (n=40). 238 consecutive neonates with unilateral CDH were subsequently identified and analyzed.
Neonates with CDH were consistently managed during the study period using a strategy of pressure-limited, lung-sparing ventilation allowing for spontaneous respiration and permissive hypercapnea[4]. Patients did not receive muscle relaxants. Infants unresponsive to conventional low-rate ventilation were transitioned first to high-frequency positive-pressure ventilation, and ultimately to a high-frequency oscillator if hypoxia persisted. Surgery was delayed until ventilator support had minimized and the pre to postductal saturation gradient had subsided.
Extracorporeal membrane oxygenation (ECMO) was offered to neonates with inadequate oxygen delivery, progressive metabolic acidosis, or clinical hypoperfusion despite maximal conventional support. ECMO was only offered if patients had no lethal anomalies, no ECMO contraindications, and were able to maintain preductal saturations > 90% for at least one-hour at any time in their course; this was interpreted as evidence of adequate lung parenchyma [4,22]. If tolerated, ECMO weaning was initiated 24–48 hours following repair.
We defined patient factors as those intrinsic to prenatal presentation and not modifiable by postnatal intervention; these are: presence of prenatal diagnosis, fetal lung-to-head ratio (LHR), delivery at an outside institution, gestational age, birthweight, hernia-side, and presence of associated anomalies. Treatment-related variables describe the patient’s clinical status as expressed through postnatal intervention; these include: ECMO, age at initial repair, method of diaphragm repair (open vs. laparoscopic, primary vs. patch), type of abdominal wall closure, and postoperative length of stay (LOS). Outcome variables included survival and morbidity. Morbidity was defined as impairment in ≥1 organ system persisting beyond 6 months and was classified as gastrointestinal, respiratory, cardiovascular, neurological, renal, or miscellaneous (Table 1). Subgroup analyses on these categories were not performed. Rather, we gave morbidity a binary score (present or absent) to provide more robust statistical comparisons.
Table 1.
Classification of morbidity in neonates with CDH
| Gastrointestinal | Neurologic |
|---|---|
| Gastroesophageal reflux – medically treated | Seizures |
| Gastroesophageal reflux – surgically treated | Visual impairment / retinopathy |
| Feeding intolerance – gastrostomy tube | Hearing impairment |
| Feeding intoleratnce – jejunostomy tube | Cognitive / behavioral deficit |
| Feeding intolerance – gastrojejunostomy tube | Motor deficit |
| Other gastrointestinal problem requiring treatment | Other neurologic problem requiring treatment |
| Respiratory | Cardiovascular |
| Home oxygen requirement | Pulmonary hypertension requiring medication |
| Asthma | Arrhythmias |
| Recurrent URI / pneumonia | Systemic hypertension requiring medication |
| Other respiratory problem requiring treatment | Cardiac anomaly requiring medication or surgery |
| Other cardiovascular problem requiring treatment | |
| Renal | Miscellaneous / Other Morbidity |
| Elevated creatinine | |
| Dialysis dependent | |
| Other renal problem requiring treatment | |
Recurrence was the primary grouping variable. Continuous variables were reported as median and interquartile (IQ) ranges, and compared using nonparametric Mann-Whitney U tests. Categorical variables were assessed using χ2 tests (Fisher’s exact). Recurrence was the dependent variable for a multivariate binary logistic regression model. Parameters holding the strongest relationships with recurrence were identified as candidate predictors by univariate Fisher’s exact or Mann-Whitney U testing (P<0.1). Candidate predictors were then entered according to their univariate significance into a stepwise regression model against the dependent variable. Covariates bearing multivariate significance predictive of recurrence were included in the final model after assessment for colinearilty. Estimated odds ratios and 95% confidence intervals were provided for covariates based on the exponential of the logistic regression coefficient (eB). Statistical significance was accepted for P<0.05. Data analyses were performed using the Statistical Package for the Social Sciences (SPSS 15.0, Chicago, Ill).
RESULTS
Of 238 consecutive evaluable neonates with unilateral CDH, 24 patients (10%) developed recurrence following initial repair. The median time from initial repair to recurrence was 4.9 months (IQ range = 0.4–9.4 months; maximum = 51.3 months). 58% of all recurrences developed within the first 6 months after initial CDH repair, and 79% developed by 1 year.
Prenatal Patient-Related Factors
Comparisons of prenatal patient-related variables between neonates with and without CDH recurrence are summarized in Table 2. Prenatal CDH diagnosis was present in 17 patients with CDH recurrence and 138 patients without recurrence (71% vs. 64%, P=0.65). There was no significant difference in the rate of outside hospital deliveries among neonates with and without CDH recurrence (29% vs. 40%, P=0.51). Fetal LHR measurements obtained at 24–28 weeks gestation were available in only 43 cases (5 recurrence, 38 non-recurrence), as LHR screening was not standard practice at our center prior to 2003. The median LHR in patients developing CDH recurrence did not differ from that of non-recurrence patients (1.10 vs. 1.26; P=0.54).
Table 2.
Prenatal patient variables in neonates with and without CDH recurrence
| Recurrence (n=24) | No Recurrence (n=214) | P | |
|---|---|---|---|
| Prenatal Diagnosis | 17 (71%) | 138 (64%) | 0.65 |
| Outside Delivery | 7 (29%) | 85 (40%) | 0.51 |
| Lung-Head Ratio (LHR) † | 1.10 (0.90 – 1.57) | 1.26 (0.90 – 1.73) | 0.54 |
| Gestational Age, weeks † | 38 (37 – 40) | 38 (37 – 40) | 0.90 |
| Birthweight, grams † | 2830 (2633 – 3425) | 3130 (2750 – 3470) | 0.36 |
| Right Sided Hernias | 5 (21%) | 27 (13%) | 0.34 |
| Cardiac Anomalies | 2 (8.3%) | 20 (9.3%) | 0.99 |
| Non-cardiac Anomalies | 7 (29%) | 41 (19%) | 0.28 |
Data reported as median and interquartile range (i.e., 25th – 75th percentile).
Neonates ultimately developing CDH recurrence demonstrated a similar median gestational age (38 weeks vs. 38 weeks; P=0.90) and birthweight (2830 grams vs. 3130 grams; P=0.36) as neonates who remained recurrence-free. Right sided defects were present in 5 cases of recurrence compared to 27 right sided defects among children without CDH recurrence (21% vs. 13%; P=0.34). We detected no difference in the rate of associated cardiac (8.3% vs. 9.3%; P=0.99) and noncardiac (29% vs. 19%; P=0.28) congenital anomalies between recurrence and non-recurrence patients.
Postnatal Treatment-Related Factors
Comparisons of postnatal treatment-related variables between neonates with and without CDH recurrence are summarized in Table 3. Patients developing recurrence had similar ECMO requirements as those without recurrence (25% vs. 19%; P=0.42). However, the median time from birth to initial CDH repair was greater in patients with recurrence (5.5 days vs. 4.0 days; P=0.02). A Gore-tex (W.L. Gore, Flagstaff, AZ) prosthesis (1 mm) was required in a greater percentage of patients who developed recurrence compared to non-recurrence patients, but this difference only approached statiscal significance (67% vs. 44%; P=0.05). Importantly, 10 of 24 repairs which recurred required an abdominal wall prosthesis compared to 39 of 214 repairs in the non-recurrence group (42% vs. 18%; P=0.01). Only 7 neonates included in this study underwent complete thoracoscopic CDH repair. The recurrence rate following thoracoscopic repair was 2 of 7, compared to 22 of 231 open transabdominal repairs (29% vs. 9.5%; P=0.15). Regardless of operative approach, the median postoperative LOS following initial CDH repair was longer in children who would later develop recurrence (44 days vs. 20 days; P<0.01).
Table 3.
Postnatal treatment variables in neonates with and without CDH recurrence
| Recurrence (n=24) | No Recurrence (n=214) | P | |
|---|---|---|---|
| ECMO | 6 (25%) | 40 (19%) | 0.42 |
| Thoracoscopic Repair | 2 (8.3%) | 5 (2.3%) | 0.15 |
| Diaphragm Prosthesis | 16 (67%) | 94 (44%) | 0.05 |
| Age at 1st Repair, days † | 5.5 (3.0 – 8.8) | 4.0 (2.0 – 6.0) | 0.02 |
| Abdominal Wall Prosthesis | 10 (42%) | 39 (18%) | 0.01 |
| Postoperative LOS, days † | 44 (22 – 153) | 20 (10 – 36) | <0.01 |
| Morbidity* | 15 (62%) | 77 (36%) | 0.01 |
| Survival | 21 (88%) | 187 (87%) | 0.99 |
Data reported as median and interquartile range (i.e., 25th – 75th percentile).
As defined in Methods – organ system dysfunction persisting beyond 6 months of age.
Twenty-one of 24 CDH recurrences were repaired. Patients not undergoing reoperation included one child with recurrence 5 days following repair of a right CDH while on ECMO who expired due to lethal pulmonary hypoplasia. A second neonate developed recurrence 12 days following left CDH repair, but was withdrawn from ventilatory support due to underlying Fryns syndrome. The third recurrence not repaired was discovered incidentally on chest x-ray six years after initial repair of a right CDH, with the family declining surgery for this small defect in their asymptomatic child. Of the 21 CDH recurrences that proceeded to surgery, 12 (57%) required a new diaphragm prostheses; this included 8 patients with a patch already in place, and 4 patients who had undergone primary closure of the diaphragm during their initial repair.
Outcomes
As defined by study inclusion criteria, all evaluable neonates survived the perinatal period and underwent initial CDH repair. There were 3 deaths in the recurrence group (12%), two of which (described above) occurred before repair of the recurrence could be performed. The third death occurred as an intraoperative respiratory arrest during repair of a left CDH recurrence two months following the initial operation in a neonate with a prolonged ICU course complicated by severe pulmonary hypertension and right ventricular failure. The overall survival in this series was therefore 88% in the recurrence group, which was comparable to the 87% post-repair survival rate among children without CDH recurrence (P=0.99). Morbidity, as defined in Methods and Table 1, was present in 15 of 24 recurrence patients as compared to only 77 of 214 non-recurrence patients (62% vs. 36%; P=0.01).
Multivariate Regression Model
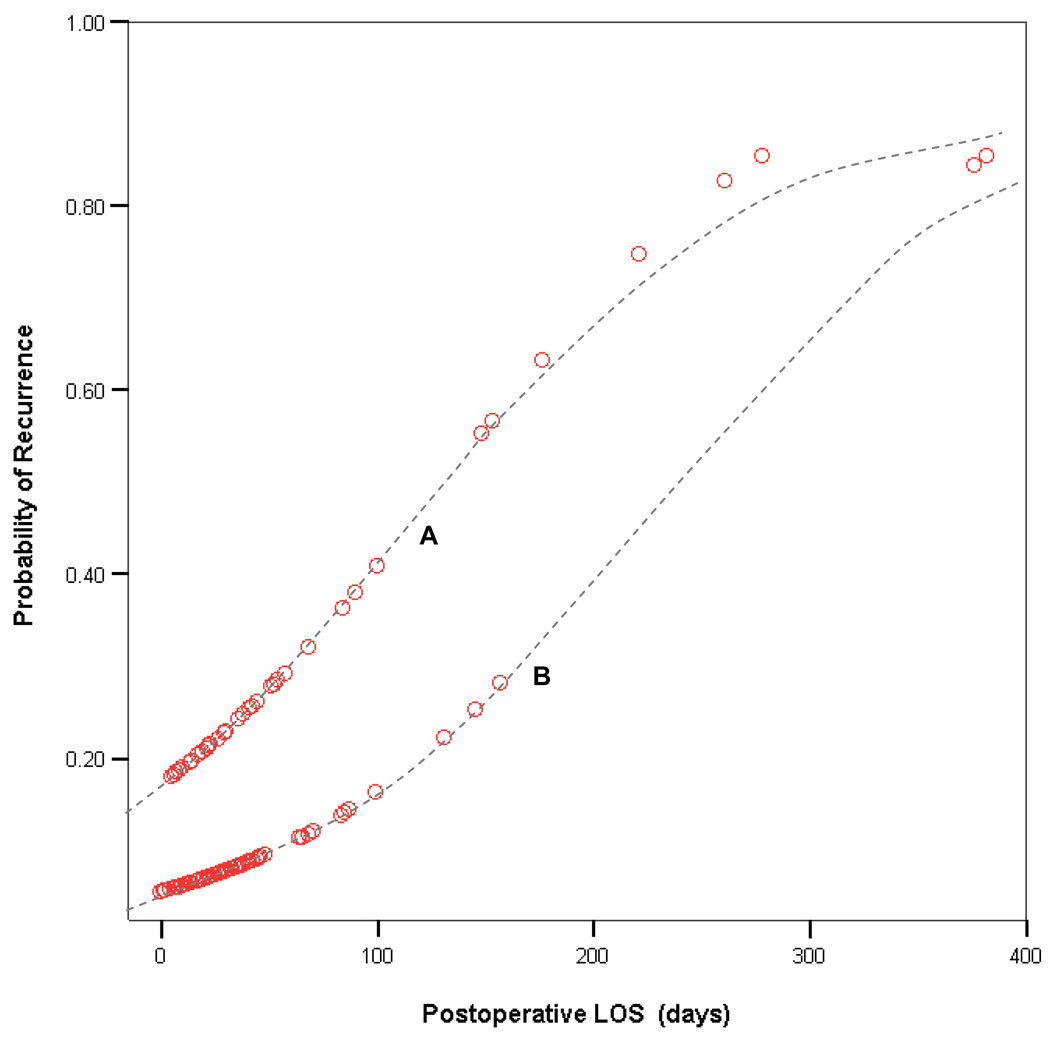
Variables from Tables 2 & 3 associated with P-values <0.1 were then entered into a stepwise logistic regression model to assess their multivariate validity in predicting recurrence following initial CDH repair. Multivariate regression identified abdominal wall patch use and postoperative LOS as the only two independent predictors influencing recurrence (Table 4). Probability of recurrence based on these variables can be calculated as follows:  Graphical representation showing the effect of postoperative LOS on the probability of recurrence, as derived from this logit equation, is shown in Figure 1.
Graphical representation showing the effect of postoperative LOS on the probability of recurrence, as derived from this logit equation, is shown in Figure 1.
Table 4.
Multiple logistic regression of factors influencing recurrence in CDH patients
| Variable | Coefficient (B) |
Odds Ratio |
95% Confidence Interval for Odds Ratio |
P value |
|---|---|---|---|---|
|
Intercept of Regression |
− 2.811 | --- | --- | --- |
| Abdominal Wall Patch Use |
1.253 | 3.500 | 1.051–11.657 | 0.041* |
| Postoperative Length of Stay |
0.012 | 1.012 | 1.003–1.022 | 0.009* |
| Time from Birth to Initial Repair |
0.006 | 1.006 | 0.980–1.034 | 0.645 |
| Long-term Morbidity |
− 0.351 | 0.704 | 0.215–2.301 | 0.561 |
| Diaphragmatic Patch Use |
0.436 | 1.547 | 0.465–5.151 | 0.477 |
Statistically significant independent risk factors by multiple logistic regression.
Figure 1. Predicting CDH recurrence based on postoperative LOS.
Graphical representation of the effect of postoperative LOS on the probability of recurrence. The clustering of datapoints along two separate curves reflects the influence of abdominal wall closure type on the probability of recurrence. The upper curve (A) represents patients with an abdominal wall prosthesis (higher probability of recurrence), while the lower curve (B) represents patients who underwent a primary abdominal closure (lower probability of recurrence).
DISCUSSION
Previous studies on recurrent CDH appear insufficiently powered to identify independent predictors of hernia recurrence (Table 5). Attempts at pooling these data through meta-analysis would not be practical due to the heterogeneity in CDH management reported by multiple centers. Our institutional approach to managing neonatal CDH has remained constant over the last two decades [4,22]. This has permitted us to query a large homogeneous study population for the independent predictors of CDH recurrence. We have investigated whether the risk factors traditionally associated with recurrence (i.e., use of prosthetic diaphragm, ECMO), as well as any prenatal patient-related variables, bear multivariate significance in predicting CDH recurrence across a large cohort of neonates.
Table 5.
Summary of published data on CDH recurrence
| Study | Total CDH Survivors |
Recurrences | Potential Risk Factors | |
|---|---|---|---|---|
| Cohen and Reid [11] | 1981 | 58 | 13 (22%) | None; all primary repairs |
| Lally, et al [26] | 1992 | 18 | 9 (50%) | |
| Van Meurs, et al [18] | 1993 | 18 | 4 (22%) | |
| Koot and Molenaar [14] | 1993 | 24 | 5 (21%) | Diaphragm patch |
| DeKort and Bax [12] | 1996 | 23 | 1 (4%) | Use of lypholized dura |
| Nobuhara, et al [9] | 1996 | 78 | 4 (5%) | Diaphragm patch |
| Ssemakula, et al [17] | 1997 | 60 | 5 (8%) | |
| Hajer, et al [13] | 1998 | 66 | 9 (14%) | ECMO, right side, diaphragm patch |
| Moss and Harrison [15] | 2001 | 29 | 12 (41%) | ECMO, diaphragm patch |
| Saltzman, et al [16] | 2001 | 39 | 5 (13%) | Diaphragm patch |
We did not detect significant differences between neonates with and without CDH recurrence in any previously identified patient-related variable. Rates of prenatal diagnoses, outside hospital deliveries, right-sided hernias, and associated anomalies were all similar in the recurrence and non-recurrence groups. The median prenatal LHR, birthweights, and estimated gestational ages at birth were also equivalent regardless of recurrence status. This suggests that just prior to birth, all surviving neonates may carry a similar risk of recurrence. While many large-volume studies have emphasized the roles of hernia-side, LHR, and associated anomalies in determining CDH survival and long-term morbidity [1,3,20,21,23–25], these prenatal patient factors do not appear to specifically influence recurrence in this series.
Several variables that were not identified by previous studies on CDH recurrence were able to distinguish children with recurrence from those who remained recurrence-free: time from birth to initial repair, morbidity, abdominal wall patch use, and postoperative LOS. These factors are best classified as postnatal treatment variables. The median time from birth to initial CDH repair was 1.5 days longer in neonates who would ultimately recur. As described in Methods, initial CDH repair occurred when ventilator requirements were minimized and the pre/postductal saturation gradient resolved. This finding suggests that patients who ultimately recurred were initially sicker and required longer preoperative stabilization. While initiation of ECMO often delays repair, this is unlikely to account for the longer time to surgery in the recurrence group because ECMO requirements were similar in both groups. Earlier studies have not reported correlation between time to surgery and risk of recurrence.
Morbidity was statistically more prevalent among patients developing recurrence compared to non-recurrence patients. To simplify our analysis, we have presented our morbidity data as a binary outcome (i.e., morbidity is present or absent), rather than stratify morbidity by organ-system. With only 24 recurrence patients, analysis of morbidity subgroups is unlikely to be statistically valid. These data cannot conclude whether the presence of recurrence directly affects morbidity, or whether development of recurrence is merely a marker of a more physiologically-impaired infant who is at risk for long-term morbidity; we suspect the latter.
Use of an abdominal wall prosthesis and postoperative LOS were the only two variables independently predictive of CDH recurrence. Previous studies have not identified either of these as risk factors for recurrence. It is not surprising that patients with a longer postoperative LOS had increased risk of recurrence, as duration of their ICU stay likely reflected a combination of underlying disease severity, nutritional status, and comorbidity, all of which likely play a role in determining recurrence. An abdominal prosthesis, even when promptly removed by a staged closure, may predispose to recurrence by interfering with the skeletal muscle integrity of the diaphragm as it relates to the anterior abdominal wall. Additionally, DeKort and Bax [12] have discussed abdominal wall patch use and recurrence in the context of reduced peritoneal volume from too small a diaphragmatic patch, and advocate a larger prosthesis to improve compliance and reduce both the intra-abdominal pressure, need for abdominal wall prosthesis, and subsequent recurrence. Our data do not directly support this hypothesis, as we routinely use large diaphragm prostheses to facilitate compliance, and did not appreciate correlation between diaphragm and abdominal wall patch use among recurrence patients (3/10 abdominal prostheses had primary diaphragm repairs; 9/14 primary abdominal closures required a diaphragm patch). More likely, an abdominal wall patch closure also represents a “sicker” patient and does not directly cause or predispose to recurrence.
A higher percentage of patients in the recurrence group required a diaphragm prosthesis during initial repair compared to the non-recurrence group. While this difference just failed to reach univariate significance (P=0.05), the borderline correlation vanished after multivariate analysis (P=0.48). Many previous studies identify diaphragm patches as a risk factor for recurrence [9,13–16]. Some reports [13,18,19] suggest that high recurrence rates seen with prosthetic repairs reflect improved survival with ECMO use, as most children in the pre-ECMO era requiring patches did not survive long enough to recur. Later studies hypothesized that use of too small a patch, as well as different institutional thresholds for using prosthetic diaphragm, may account for the increased recurrence rates [13,15]. We favor using a prosthetic diaphragm when primary closure would result in tension, with many repairs involving primary closure of the medial diaphragm and a small patch to bridge the lateral aspect of the defect.
Taken together, these data suggest that neonates in this study at risk for CDH recurrence were best identified by postnatal treatment variables that describe a more severe clinical manifestation of CDH (delayed preoperative stabilization, longer recovery time, more abdominal patches, higher morbidity). This recurrence risk appears independent of prenatal patient factors, and is not influenced by the use of prosthetic diaphragm. While earlier studies have reported that recurrence can be predicted by conventional postnatal treatment variables (i.e., ECMO, diaphragm patch use), we have identified new treatment variables that may better predict recurrence (i.e., abdominal wall patch use, postoperative LOS). We suspect that abdominal prostheses and prolonged ICU courses do not directly predispose to CDH recurrence, but rather represent an alternative method of describing a more severe clinical manifestation of CDH.
The multivariate analysis has generated a convenient nomogram (Figure 1) against which risk of CDH recurrence at our institution can be plotted based on postoperative LOS (x axis) and use of an abdominal wall prosthesis (curve A vs. B). Assigning a percentage-risk of recurrence based on this nomogram at time of discharge can facilitate improved discussions with families regarding the sequelae of CDH repair. More importantly, a multi-disciplinary CDH clinic can use these data to design follow-up algorithms specific to the patient’s risk of recurrence, providing more frequent imaging to high-risk patients while avoiding unnecessary radiation in those with negligible recurrence risk. Accumulation of additional follow-up data will allow us to optimize this multivariate model for predicting recurrence, and provide prospective validation of its efficacy in guiding the long-term care of CDH survivors.
REFERENCES
- 1.Deprest J, Jani J, Van Schoubroeck D, et al. Current consequences of prenatal diagnosis of congenital diaphragmatic hernia.[see comment] Journal of Pediatric Surgery. 2006;41:423–430. doi: 10.1016/j.jpedsurg.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 2.Graham G, Devine PC. Antenatal diagnosis of congenital diaphragmatic hernia. Semin Perinatol. 2005;29:69–76. doi: 10.1053/j.semperi.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Laudy JAM, Van Gucht M, Van Dooren MF, et al. Congenital diaphragmatic hernia: an evaluation of the prognostic value of the lung-to-head ratio and other prenatal parameters. Prenat Diagn. 2003;23:634–639. doi: 10.1002/pd.654. [DOI] [PubMed] [Google Scholar]
- 4.Boloker J, Bateman DA, Wung J-T, et al. Congenital diaphragmatic hernia in 120 infants treated consecutively with permissive hypercapnea/spontaneous respiration/elective repair.[see comment] Journal of Pediatric Surgery. 2002;37:357–366. doi: 10.1053/jpsu.2002.30834. [DOI] [PubMed] [Google Scholar]
- 5.Cacciari A, Ruggeri G, Mordenti M, et al. High-frequency oscillatory ventilation versus conventional mechanical ventilation in congenital diaphragmatic hernia. Eur J Pediatr Surg. 2001;11:3–7. doi: 10.1055/s-2001-12204. [DOI] [PubMed] [Google Scholar]
- 6.Morini F, Goldman A, Pierro A. Extracorporeal membrane oxygenation in infants with congenital diaphragmatic hernia: a systematic review of the evidence. Eur J Pediatr Surg. 2006;16:385–391. doi: 10.1055/s-2006-924751. [DOI] [PubMed] [Google Scholar]
- 7.American Academy of Pediatrics Section on S, American Academy of Pediatrics Committee on Fetus and N. Lally KP, et al. Postdischarge follow-up of infants with congenital diaphragmatic hernia. Pediatric. 2008;121:627–632. doi: 10.1542/peds.2007-3282. [DOI] [PubMed] [Google Scholar]
- 8.Chiu P, Hedrick HL. Postnatal management and long-term outcome for survivors with congenital diaphragmatic hernia. Prenat Diagn. 2008;28:592–603. doi: 10.1002/pd.2007. [DOI] [PubMed] [Google Scholar]
- 9.Nobuhara KK, Lund DP, Mitchell J, et al. Long-term outlook for survivors of congenital diaphragmatic hernia. Clin Perinatol. 1996;23:873–887. [PubMed] [Google Scholar]
- 10.Rowe DH, Stolar CJ. Recurrent diaphragmatic hernia. Semin Pediatr Surg. 2003;12:107–109. doi: 10.1016/s1055-8586(02)00020-3. [DOI] [PubMed] [Google Scholar]
- 11.Cohen D, Reid IS. Recurrent diaphragmatic hernia. J Pediatr Surg. 1981;16:42–44. doi: 10.1016/s0022-3468(81)80113-3. [DOI] [PubMed] [Google Scholar]
- 12.J Pediatr Surg LM, Bax KM. Prosthetic patches used to close congenital diaphragmatic defects behave well: a long-term follow-up study. Eur J Pediatr Surg. 1996;6:136–138. doi: 10.1055/s-2008-1066490. [DOI] [PubMed] [Google Scholar]
- 13.Hajer GF, vd Staak FH, de Haan AF, et al. Recurrent congenital diaphragmatic hernia; which factors are involved? Eur J Pediatr Surg. 1998;8:329–333. doi: 10.1055/s-2008-1071226. [DOI] [PubMed] [Google Scholar]
- 14.Koot VC, Bergmeijer JH, Molenaar JC. Lyophylized dura patch repair of congenital diaphragmatic hernia: occurrence of relapses. J Pediatr Surg. 1993;28:667–668. doi: 10.1016/0022-3468(93)90027-i. [DOI] [PubMed] [Google Scholar]
- 15.Moss RL, Chen CM, Harrison MR. Prosthetic patch durability in congenital diaphragmatic hernia: a long-term follow-up study. J Pediatr Surg. 2001;36:152–154. doi: 10.1053/jpsu.2001.20037. [DOI] [PubMed] [Google Scholar]
- 16.Saltzman DA, Ennis JS, Mehall JR, et al. Recurrent congenital diaphragmatic hernia: A novel repair. J Pediatr Surg. 2001;36:1768–1769. doi: 10.1053/jpsu.2001.28818. [DOI] [PubMed] [Google Scholar]
- 17.Ssemakula N, Stewart DL, Goldsmith LJ, et al. Survival of patients with congenital diaphragmatic hernia during the ECMO era: an 11-year experience. J Pediatr Surg. 1997;32:1683–1689. doi: 10.1016/s0022-3468(97)90506-6. [DOI] [PubMed] [Google Scholar]
- 18.Van Meurs KP, Robbins ST, Reed VL, et al. Congenital diaphragmatic hernia: long-term outcome in neonates treated with extracorporeal membrane oxygenation. J Pediatr. 1993;122:893–899. doi: 10.1016/s0022-3476(09)90013-0. [DOI] [PubMed] [Google Scholar]
- 19.Atkinson JB, Poon MW. ECMO and the management of congenital diaphragmatic hernia with large diaphragmatic defects requiring a prosthetic patch. J Pediatr Surg. 1992;27:754–756. doi: 10.1016/s0022-3468(05)80109-5. [DOI] [PubMed] [Google Scholar]
- 20.Azarow K, Messineo A, Pearl R, et al. Congenital diaphragmatic hernia--a tale of two cities: the Toronto experience. Journal of Pediatric Surgery. 1997;32:395–400. doi: 10.1016/s0022-3468(97)90589-3. [DOI] [PubMed] [Google Scholar]
- 21.Fisher JC, Jefferson RA, Arkovitz MS, et al. Redefining outcomes in right congenital diaphragmatic hernia. J Pediatr Surg. 2008;43:373–379. doi: 10.1016/j.jpedsurg.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 22.Stolar C, Dillon P, Reyes C. Selective use of extracorporeal membrane oxygenation in the management of congenital diaphragmatic hernia. Journal of Pediatric Surgery. 1988;23:207–211. doi: 10.1016/s0022-3468(88)80723-1. [DOI] [PubMed] [Google Scholar]
- 23.Wilson JM, Lund DP, Lillehei CW, et al. Congenital diaphragmatic hernia--a tale of two cities: the Boston experience. Journal of Pediatric Surgery. 1997;32:401–405. doi: 10.1016/s0022-3468(97)90590-x. [DOI] [PubMed] [Google Scholar]
- 24.Colvin J, Bower C, Dickinson JE, et al. Outcomes of congenital diaphragmatic hernia: a population-based study in Western Australia.[erratum appears in Pediatrics. 2006 May;117(5):1870] Pediatrics. 2005;116:e356–e363. doi: 10.1542/peds.2004-2845. [DOI] [PubMed] [Google Scholar]
- 25.Metkus AP, Filly RA, Stringer MD, et al. Sonographic predictors of survival in fetal diaphragmatic hernia. Journal of Pediatric Surgery. 1996;31:148–151. doi: 10.1016/s0022-3468(96)90338-3. discussion 151-142. [DOI] [PubMed] [Google Scholar]
- 26.Lally KP, Paranka MS, Roden J, et al. Congenital diaphragmatic hernia. Stabilization and repair on ECMO. Ann Surg. 1992;216:569–573. doi: 10.1097/00000658-199211000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]