Abstract
Glucagon counterregulation (GCR) is a key protection against hypoglycemia compromised in insulinopenic diabetes by an unknown mechanism. In this work we present an interdisciplinary approach to the analysis of the GCR control mechanisms. Our results indicate that a pancreatic network which unifies a few explicit interactions between the major islet peptides and blood glucose (BG) can replicate the normal GCR axis and explain its impairment in diabetes. A key and novel component of this network is an α-cell auto-feedback, which drives glucagon pulsatility and mediates triggering of pulsatile GCR by hypoglycemia via a switch-off of the β-cell suppression of the α-cells. We have performed simulations based on our models of the endocrine pancreas which explain the in vivo GCR response to hypoglycemia of the normal pancreas and the enhancement of defective pulsatile GCR in β-cell deficiency by switch-off of intrapancreatic α-cell suppressing signals. The models also predicted that reduced insulin secretion decreases and delays the GCR. In conclusion, based on experimental data we have developed and validated a model of the normal GCR control mechanisms and their dysregulation in insulin deficient diabetes. One advantage of this construct is that all model components are clinically measurable, thereby permitting its transfer, validation, and application to the study of the GCR abnormalities of the human endocrine pancreas in vivo.
INTRODUCTION
Blood glucose (BG) homeostasis is maintained by a complex, ensemble control system characterized by a highly coordinated interplay between and among various hormone and metabolite signals. One of its key components, the endocrine pancreas, responds dynamically to changes in BG, nutrients, neural and other signals by releasing insulin and glucagon in a pulsatile manner to regulate glucose production and metabolism. Abnormalities in the secretion and interaction of these two hormones mark the progression of many diseases, including diabetes, but also the metabolic syndrome, the polycystic ovary syndrome, and others. Diminished or complete loss of endogenous insulin secretion in diabetes is closely associated with failure of the pancreas to respond with glucagon secretion properly not only to hyper- but also to hypoglycemia. The latter is not caused by loss of glucagon secreting α-cells, but instead is due to defects in glucagon counterregulation (GCR) signaling, through an unknown mechanism, which is generally recognized as a major barrier to safe treatment of diabetes (Cryer and Gerich, 1983 and Gerich, 1988) since unopposed hypoglycemia can cause coma, seizures, or even death (Cryer, 1999 and Cryer, 2002).
Our recent experimental (Farhy et. al., 2008) and mathematical-modeling (Farhy et. al., 2008, Farhy and McCall, 2009) results show that a novel understanding of the defects in the GCR control mechanisms can be gained if these are viewed as abnormalities of the network of intrapancreatic interactions that control glucagon secretion, rather than as defects in an isolated molecular interaction or pathway.
In particular, we have demonstrated that in a β-cell deficient rat model the GCR control mechanisms can be approximated by a simple feedback network (construct) of dose-response interactions between BG and the islet peptides. Within the framework of this construct, the defects of GCR response to hypoglycemia can be explained by loss of rapid switch-off of β-cell signaling during hypoglycemia to trigger an immediate GCR response. These results support the “switch-off” hypothesis which posits that α-cell activation during hypoglycemia requires both the availability and rapid decline of intraislet insulin (Banarer et. al., 2002). They also extend this hypothesis by refocusing from the lack of endogenous insulin signaling to the α-cells as a sole mechanistic explanation and instead focus on possible abnormalities in the general way by which the β-cells regulate the α-cells. In addition, the experimental and theoretical-modeling data collected so far indicate that the GCR control network must have two key features: a (direct or indirect) feedback of glucagon secreting α-cells on themselves (auto-feedback) and a (direct or indirect) negative regulation of glucagon by BG. In our published model these two properties are mediated by δ-cell somatostatin and we have shown that such connectivity adequately explains ours [and others (Zhou et. al., 2004)] experimental data (Farhy et. al., 2008, Farhy and McCall, 2009).
The construct we proposed recently (Farhy et. al., 2008, Farhy and McCall, 2009) is suitable for the study and analysis of rodent physiology, but the explicit involvement of somatostatin limits its applicability to clinical studies since in the human, pancreatic somatostatin cannot be reliably measured and therefore, the ability of the model to describe adequately the human physiology and its potential differences from rodent physiology cannot be verified. In the current work, we review our existing models and show that a control network in which somatostatin is not explicitly involved (but incorporated implicitly) can also adequately approximate the GCR control mechanisms. We confirm that the (new) construct can substitute for the older, more complex construct by verifying that it explains the same experimental observations already shown to be reconstructed by the older network (Farhy et. al., 2008, Farhy and McCall, 2009). We also demonstrate that the newer network can explain the regulation of the normal pancreas by BG and the gradual reduction in the GCR response to hypoglycemia during the transition from a normal to an insulin deficient state. As a result, a more precise description of the components that are the most critical for the system is provided by a model of GCR regulation. This model can be applied to study the abnormalities in glucagon secretion and counterregulation and to identify hypothetical ways to repair these not only in the rodent but also in the human.
THE MECHANISMS OF GCR DYSREGULATION IN DIABETES ARE LARGELY UNKNOWN
Studies of tight BG control in type 1 and type 2 diabetes to prevent chronic hyperglycemia-related complications have found a 3-fold excess of severe hypoglycemia (The Diabetes Control and Complications Trial Research Group, 1993; The UK Prospective Diabetes Study Group, 1998; The Action to Control Cardiovascular Risk in Diabetes Study Group, 2008). Hypoglycemia impairs quality of life and risks coma, seizures, accidents, brain injury and death. Severe hypoglycemia is usually due to overtreatment against a background of delayed and deficient hormonal counterregulation. In health, GCR curbs dangerously low BG nadirs and stimulates quick recovery from hypoglycemia (Cryer and Gerich, 1983, Gerich, 1988). However, in type 1 (Fukuda et. al., 1988; Gerich et. al., 1973; Hoffman et. al., 1994) and type 2 diabetes (Segel et. al., 2002) the GCR is impaired by uncertain mechanisms and if it is accompanied by a loss of epinephrine counterregulation it leads to severe hypoglycemia and thus presents a major barrier to safe treatment of diabetes (Cryer, 1999 and Cryer, 2002). Understanding the mechanisms that mediate GCR, its dysregulation and how it can be repaired, is therefore a major challenge in the struggle for safer treatment of diabetes.
Despite more than 30 years of research, the mechanism by which hypoglycemia stimulates GCR and how it is impaired in diabetes have yet to be elucidated (Gromada et. al., 2007). First described Gerich and coauthors (Gerich et. al., 1973), defective GCR is common after about 10 years of T1DM. The loss of GCR appears to be more rapid with a very young age of onset and may occur within a few years after onset of T1DM0. Although unproven, the appearance of defective GCR seems to parallel insulin secretory loss in these patients. The defect appears to be stimulus-specific, since α-cells retain their ability to secrete glucagon in response to other stimuli, such as arginine (Gerich et. al., 1973). Three mechanisms have been proposed as a potential source for impairment of GCR. Those that account for the stimulus-specificity of the defect include impaired BG-sensing in α-cells (Gerich et. al., 1973) and/or autonomic dysfunction (Hirsch and Shamoon, 1987 and Taborsky GJ et. al., 1998) The “switch-off” hypothesis envisions that α-cell activation by hypoglycemia requires both the availability and rapid decline of intraislet insulin and attributes the defect in the GCR in insulin deficiency to loss of a (insulin) “switch-off” signal from the β-cells (Banarer et. al., 2002).
These theories are not mutually exclusive, but they all could be challenged. For example, α-cells do not express GLUT2 transporters (Heimberg et. al., 1996) and it is unclear whether the α-cell GLUT1 transporters can account for the rapid α-cell response to variations in BG (Heimberg et. al., 2004). In addition, proglucagon mRNA levels are not altered by BG (Dumonteil et. al., 2000) and it is debatable whether BG variations in the physiological range can affect the α-cells (Pipeleers et. al., 1985). The switch-off hypothesis can also be disputed, since in the α-cell-specific insulin receptor knockout mice the GCR response to hypoglycemia is preserved (Kawamori et. al., 2009). Finally, the hypothesis for autonomic control contradicts evidence that blockade of epinephrine and acetylcholine actions did not reduce the GCR in humans (Hilsted et. al., 1991), and that the denervated human pancreas still releases glucagon in response to hypoglycemia (Diem et. al., 1990).
Recent in vivo experiments by Zhou et al. support the “switch-off” hypothesis. They have shown that, in STZ-treated rats, GCR is impaired, but can be restored if their deficiency in intraislet insulin is reestablished and decreased (switched off) during hypoglycemia (Zhou et. al., 2004). Additional in vitro and in vivo evidence to support the switch-off hypothesis has been reported (Hope et. al., 2004, Zhou et. al., 2007 a). Whether insulin is the trigger of GCR in the studies by Zhou and co-authors (Zhou et. al., 2004, Zhou et. al., 2007 a) has been challenged by results by the same group, in which zinc ions, not the insulin molecule itself, provided the switch-off signal to initiate glucagon secretion during hypoglycemia (Zhou et. al., 2007 b).
In view of the above background, the mechanisms that control the secretion of glucagon and their dysregulation in diabetes are not well understood. This lack in understanding prevents restoring GCR to normal in patients with diabetes and the development of treatments to effectively repair defective GCR to allow for a safer control of hyperglycemia. No such treatment currently exists.
APPROACH
The network underlying the GCR response to hypoglycemia includes hundreds of components from numerous pathways and targets in various pools and compartments. It would therefore be unfeasible to collect and relate experimental data pertaining to all components of this network. Nevertheless, understanding the glucagon secretion control network is vital for furthering knowledge concerning the control of GCR, its compromise in diabetes, and developing treatment strategies. To address this problem, we have taken a minimal model approach in which the system is simplified by clustering all known and unknown factors into a small number of explicit components. Initially, these components were chosen with the goal to test whether recognized physiological relationships can explain key experimental findings. In our case, the first reports describing the in vivo enhancement of GCR by switch-off of insulin (Zhou et. al., 2004) prompted us to propose a parsimonious model of the complex GCR control mechanisms including relationships between the α- and the δ-cells, BG and switch-off signals (below). According to these initial efforts (Farhy et. al., 2008) the postulated network explains the switch-off phenomenon by interpreting the GCR as a rebound. It further predicts that: (i) in β-cell deficiency, multiple α-cell suppressing signals should enhance GCR if they are terminated during hypoglycemia, and (ii) the switch-off-triggered GCR must be pulsatile. The model-based predictions motivated a series of in vivo experiments, which showed that indeed, in STZ-treated male Wistar rats, intrapancreatic infusion of insulin and somatostatin followed by their switch-off during hypoglycemia enhances the pulsatile GCR response (Farhy et. al., 2008). These experimental results confirmed that the proposed network is a good candidate for a model of the GCR control axis.
In addition to confirming the initial model predictions, our experiments also suggested some new features of the GCR control network, including indications that different α-cell suppressing switch-off signals not only can enhance GCR in β-cell deficiency, but that they do so via different mechanisms. For example, the results suggest higher response to insulin switch-off and more substantial suppression of glucagon by somatostatin (Farhy et. al., 2008). To show that these observations are consistent with our network model, we had to extend it to reflect the assumption that the α-cell activity can be regulated differently by different α-cell suppressing signals. We showed that this assumption can explain the difference in the GCR-enhancing action of two α-cell suppressing signals (Farhy and McCall, 2009).
The simulations suggest strategies to use α-cell inhibitors to manipulate the network and repair defective GCR. However, they also indicate that not all α-cell inhibitors may be suitable for that purpose, and the infusion rate of the ones that are, should be carefully selected. In this regard, a clinically verified and tested model of the GCR control axis can greatly enhance our ability to precisely and credibly simulate changes resulting from certain interventions and ultimately will assist us in defining the best strategy to manipulate the system in vivo in humans. However, the explicit involvement of somatostatin and the δ-cells in our initial network and model limits the potential for clinical applications as pancreatic somatostatin cannot be reliably measured in the human in vivo and the ability of the model to describe the human glucagon axis cannot be verified. To address this limitation we have recently reduced our initial network into a Minimal Control Network (MCN) of the GCR control axis in which somatostatin and the δ-cells are no longer explicitly involved, but their effects are implicitly incorporated in the model. Our analysis (presented below) shows that the new MCN is an excellent model of the GCR axis and can substitute the older, more complex structure. Thereby, we have developed a model that can be verified clinically and used to assist the analysis of the GCR axis in vivo in humans. Importantly, the new model is not limited to β-cell deficiency and hypoglycemia only. In fact, it describes the transition form a normal to a β-cell deficient state and can explain the failure of suppression of basal glucagon secretion in response to increase in BG observed in this state. If it is confirmed experimentally that the MCN can successfully describe both the normal and the β-cell deficient pancreas, future studies may focus on the defects of the pancreatic network not only in type 1 but also in type 2 diabetes, or more generally, in any pathophysiological condition that is accompanied by metabolic abnormalities of the endocrine pancreas.
INITIAL QUALITATIVE ANALYSIS OF THE GCR CONTROL AXIS
To understand the mechanisms of GCR and their dysregulation, the pancreatic peptides have been extensively studied and much evidence suggests that a complex network of interacting pathways modulates glucagon secretion and GCR. Some of the well documented relationships between different islet cell signals are summarized bellow.
β-cell inhibition of α-cells
Pancreatic perfusions with antibodies to insulin, somatostatin and glucagon have suggested that the blood within the islets flows from β- to α- to δ-cells in dogs, rats, and humans (Samols and Stagner, 1988; Samols and Stagner, 1990; Stagner et. al., 1988; Stagner et. al., 1989). It was then proposed that, insulin regulates glucagon, which in turn regulates somatostatin. Various β-cell signals provide an inhibitory stimulus to the α-cells and suppress glucagon. These include co-secreted insulin, zinc, GABA and amylin (Gromada et. al., 2007; Samols and Stagner, 1988; Gedulin et. al., 1997; Ito et. al., 1995; Rorsman and Hellman, 1988; Rorsman et. al., 1989; Wendt et. al., 2004; Xu et. al., 2006; Ishihara et. al., 2003; Maruyama et. al., 1984). In particular, β-cells store and secrete GABA, which can diffuse to neighboring cells to bind to localized within the islets only on α-cells (Rorsman and Hellman, 1988, Wendt et. al., 2004). Insulin can directly suppress glucagon by binding to its own receptors (Kawamori et. al., 2009) or to IGF-1 receptors on the α-cells (Van Schravendijk et. al., 1987). Insulin also translocates and activates GABAA receptors on the α-cells, which leads to membrane hyperpolarization and, ultimately, suppresses glucagon. Hence, insulin may directly inhibit the α-cells, and indirectly potentiate the effects of GABA (Xu et. al., 2006). Infusion of amylin in rats inhibits arginine-stimulated glucagon (Gedulin et. al., 1997), but not hypoglycemia GCR (Silvestre et. al., 2001); similar results were found with the synthetic amylin analog Pramlintide (Heise et. al., 2004) even though in some studies hypoglycemia was increased, but it is unclear if this is a GCR effect or is related to failure to reduce meal insulin adequately (McCall et. al., 2006). Finally, a negative effect of zinc on glucagon has been proposed (Ishihara et. al., 2003), including a role in control of GCR (Zhou et. al., 2007 b). The role of zinc is unclear as zinc ions do not suppress glucagon in the mouse (Ravier and Rutter, 2005).
δ-cell inhibition of α-cells
Exogenous somatostatin inhibits insulin and glucagon; however, the role of the endogenous hormone is controversial (Brunicardi et. al., 2001; Cejvan et. al., 2003; Klaff and Taborsky, 1987; Ludvigsen et. al., 2004; Sumida et. al., 1994; Tirone et. al., 2003; Gopel et. al., 2000 a; Brunicardi et. al., 2003; Kleinman et. al., 1994; Portela-Gomes et. al., 2000; Strowski et. al., 2000; Schuit et. al., 1989). The concept that δ-cells are downstream of α- and δ-cells favors the perception that in vivo, intraislet somatostatin cannot directly suppress the α- or β-cells through the islet microcirculation (Samols and Stagner, 1988; Samols and Stagner, 1990; Samlos et. al., 1988; Samlos et. al., 1989). On the other hand, the pancreatic α- and β-cells express at least one of the somatostatin receptors (SSTR1-5) (Ludvigsen et. al., 2004; Portela-Gomes et. al, 2000; Strowski et. al., 2000), and recent in vitro studies involving somatostatin immuno-neutralization (Brunicardi et. al., 2001) or application of selective antagonists to different somatostatin receptors suggest that α-cell somatostatin inhibits the release of glucagon (Cejvan et. al., 2003; Strowski et. al., 2000). In addition, δ-cells are in close proximity to α-cells in rat and human islets, and δ-cell processes were observed to extend into α-cell clusters in rat islets (Kleinman et. al., 1994; Kleinman et. al., 1995). Therefore, somatostatin may act via existing common gap junctions or by diffusion through the islet interstitium.
α-cell stimulation of δ-cells
The ability of endogenous glucagon to stimulate δ-cell somatostatin is supported by a study in which administration of glucagon antibodies in the perfused human pancreas resulted in inhibition of somatostatin release (Brunicardi et. al., 2001). Earlier immuno-neutralization perfusions of the rat or dog pancreas also showed that glucagon stimulates somatostatin (Stagner et. al., 1988; Stagner et. al., 1989). The glucagon receptor co-localized with 11% of immunoreactive somatostatin cells (Kieffer et. al., 1996), suggesting that the α-cells may directly regulate some of the δ-cells. Exogenous glucagon also stimulates somatostatin (Brunicardi et. al., 2001; Kleinman et. al., 1995; Epstein et. al., 1980; Utsumi et. al., 1979). Finally, glutamate, which is co-secreted with glucagon under low-glucose conditions, stimulates somatostatin release from diencephalic neurons in primary culture (Tapia-Arancibia et. al., 1988) and a similar relation could exist in the islets of the pancreas.
Glucose stimulation of β- and δ-cells
It is well established that hyperglycemia directly stimulates β-cells, which react instantaneously to changes in BG (Bell et. al., 1996; Schuit et. al., 2001; Ashcroft et. al., 1994; Dunne et. al., 1994). Additionally, it has been proposed that δ-cells have a glucose-sensing mechanism similar to those in β-cells (Gopel et. al., 2000 a; Fujitani et. al., 1996) and consequently, that somatostatin release is increased in response to glucose stimulation (Hermansen et. al., 1979; Efendic et. al., 1978), possibly via a Ca2+-dependent mechanism (Hermansen et. al., 1979).
Glucose inhibition of α-cells
Hyperglycemia has been proposed to inhibit glucagon even though hypoglycemia alone appears insufficient to stimulate high amplitude GCR (Heimberg et. al., 1995; Heimberg et. al., 1996; Reaven et. al., 1987; Rorsman and Hellman, 1988; Schuit et. al., 1997; Gopel et. al., 2000 b; Unger, 1985).
In addition to the above mostly consensus findings which show that the α-cell activity is controlled by multiple intervening pathways, there are other indirect evidences suggesting that the dynamic relationships between the islet signals are important for the regulation of glucagon secretion and GCR. For example, the concept is supported by the pulsatility of the pancreatic hormones (Genter et. al., 1998; Grapengiesser et. al., 2006; Grimmichova et. al., 2008), which implies feedback control (Farhy, 2004), and by results suggesting that: insulin and somatostatin pulses are in phase (Jaspan et. al., 1986; Matthews et. al., 1987) pulses of insulin and glucagon recur with a phase shift (Grapengiesser et. al., 2006), pulses of somatostatin and glucagon appear in antisynchronous fashion (Grapengiesser et. al., 2006), and insulin pulses entrain α- and δ-cell oscillations (Salehi et. al., 2007).
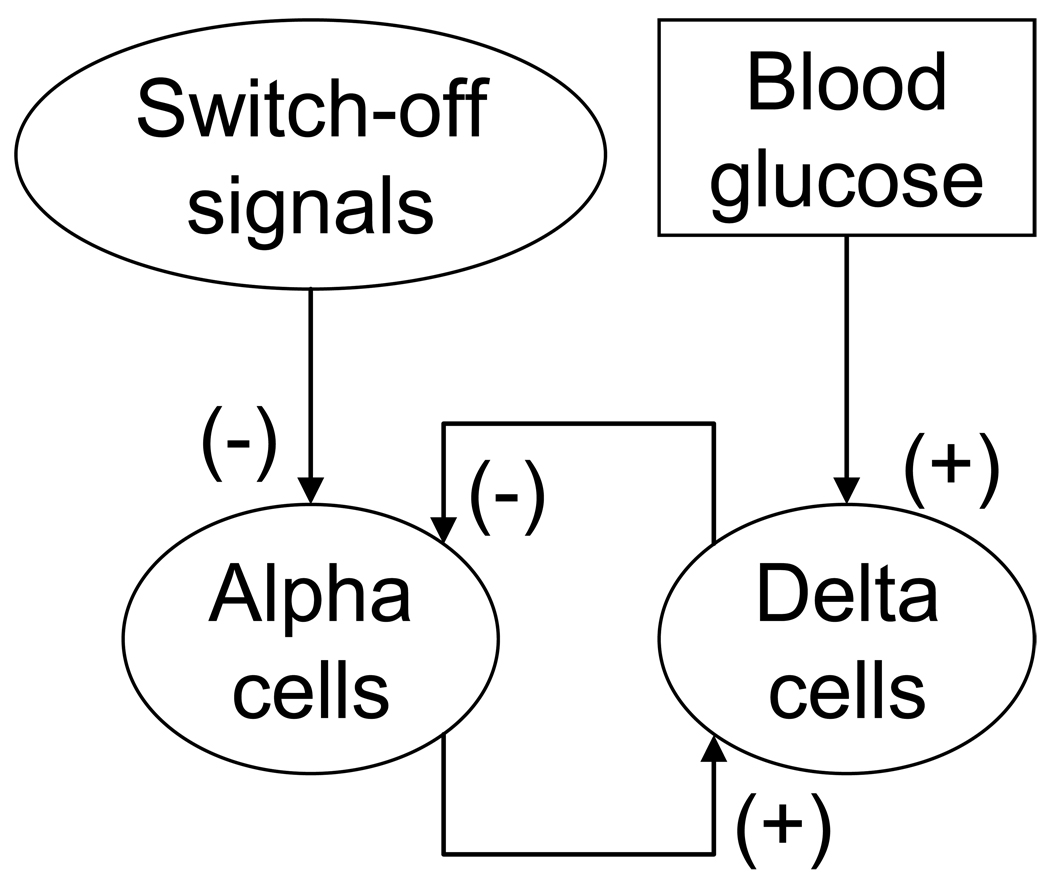
A pancreatic network consistent with these findings is shown in Figure 1. It summarizes interactions (mostly consensus) between BG, β-, α-, and δ-cells: somatostatin (or more generally the δ-cells) is stimulated by glucagon (α-cells) and BG; glucagon (α-cells) is inhibited by the δ-cells (by somatostatin) and by β-cell signals; and BG stimulates the β-cells. This network could easily explain the GCR response to hypoglycemia. Indeed, hypoglycemia would decrease both β- and δ-cell activity, which would entail increased release of glucagon from α-cells after the suppression from the neighboring β- and δ-cells is removed. However, it is not apparent whether this network can explain the defect in GCR observed in β-cell deficiency or the above mentioned restoration of defective GCR by a switch-off. This dampens the appeal of the network as a simple unifying hypothesis for regulation of GCR, and for the compromise of this regulation in diabetes. The difficulties in intuitive reconstruction of the properties of the network emerge from the surprisingly complex behavior of this system due to the α-δ-cell feedback loop.
Figure 1.
Schematic presentation of a network model of the GCR control mechanisms in STZ-treated rats.
Shortly after the first reports describing the in vivo repair of GCR by intrapancreatic infusion and switch-off of insulin (Zhou et. al., 2004), we applied mathematical modeling to analyze and reconstruct the GCR control network. These considerations, demonstrated that the network in Figure 1 can explain the switch-off effect (Farhy et. al., 2008, Farhy and McCall, 2009). We have also presented experimental evidence to support these model predictions (Farhy et. al., 2008). These efforts are described in the next section.
MATHEMATICAL MODELS OF THE GCR CONTROL MECHANISMS IN STZ-TREATED RATS
We have developed and validated (Farhy et. al., 2008, Farhy and McCall, 2009) a mathematical model of the GCR control mechanisms in the β-cell deficient rat pancreas which explains two key experimental observations: (a) in STZ-treated rats, rebound GCR which is triggered by a switch-off signal (a signal that is intrapancreatically infused and terminated during hypoglycemia) is pulsatile; and (b) the switch-off of either somatostatin and insulin enhances the pulsatile GCR. The basis of this mathematical model is the network outlined in Figure 1 which summarizes the major interactive mechanisms of glucagon secretion in β-cell deficiency by selected consensus interactions between plasma glucose, α-cell suppressing switch-off signals, α-cells, and δ-cells. We should note that the β-cells were part of the network proposed in (Farhy et. al., 2008), but not part of the corresponding mathematical model, which was designed to approximate the insulin deficient pancreas. In addition to explaining glucagon pulsatility during hypoglycemia and the switch-off responses mentioned above, this construct predicts each of the following experimental findings in diabetic STZ-treated rats:
Glucagon pulsatility during hypoglycemia after a switch-off, with pulses recurring at 15 to 20 min as suggested by the results of the pulsatility deconvolution analysis we have previously performed (Farhy et. al., 2008).
Pronounced (almost 4-fold increase over baseline) pulsatile glucagon response following a switch-off of either insulin and somatosatin during hypoglycemia (Farhy et. al., 2008);
Restriction of the GCR enhancement by insulin switch-off by high BG conditions (Zhou et. al., 2004);
Lack of a GCR response to hypoglycemia when there is no switch-off signal (Farhy et. al., 2008);
Suppression of GCR when insulin is infused into the pancreas but not switched off during hypoglycemia (Zhou et. al., 2004).
More than 30% higher GCR response to insulin vs. somatostatin switch-off (Farhy et. al., 2008);
Better glucagon suppression by somatostatin than by insulin before a switch-off (Farhy et. al., 2008).
We note that in our prior study (Farhy et. al., 2008) the comparisons between insulin and somatostatin switch-off in (vi) and (vii) were not significant. However, the difference in (vii) was close to being significant at p=0.07. Therefore one of the goals of the latter study (Farhy and McCall, 2009) was to test in silico whether the differences (vi) of a higher GCR response to insulin switch-off and (vii) a better glucagon suppression by somatostatin switch-off were likely and can be predicted by the model of the new MCN.
To demonstrate the above predictions we used dynamic network modeling and formalized the network shown in Figure 1 by a system of non-linear ordinary differential equations to approximate the glucagon and somatostatin concentration rates of change under the control of switch-off signals and BG. Then, we were able to adjust the model parameters to reconstruct the experimental findings listed in (i)–(vii) which validates the model based on the network shown in Figure 1.
The model equations are:
| Eq. 1 |
| Eq. 2 |
Here, GL(t), SS(t), BG(t), I1(t) and I2(t) denote the concentrations of glucagon, somatostatin, blood glucose, and exogenous switch-off signal(s) [acting on the pulsatile or/and the basal glucagon secretion], respectively; the derivative is with respect to the time t. The meaning of the remaining parameters is explained in the next section. We note that the presence of two terms, I1(t) and I2(t), to represent the switch-off signal in Eq. 1 reflects the assumption that different switch-off signals may have a different impact on glucagon secretion and may suppress differently the basal and/or δ-cell-regulated α-cell release.
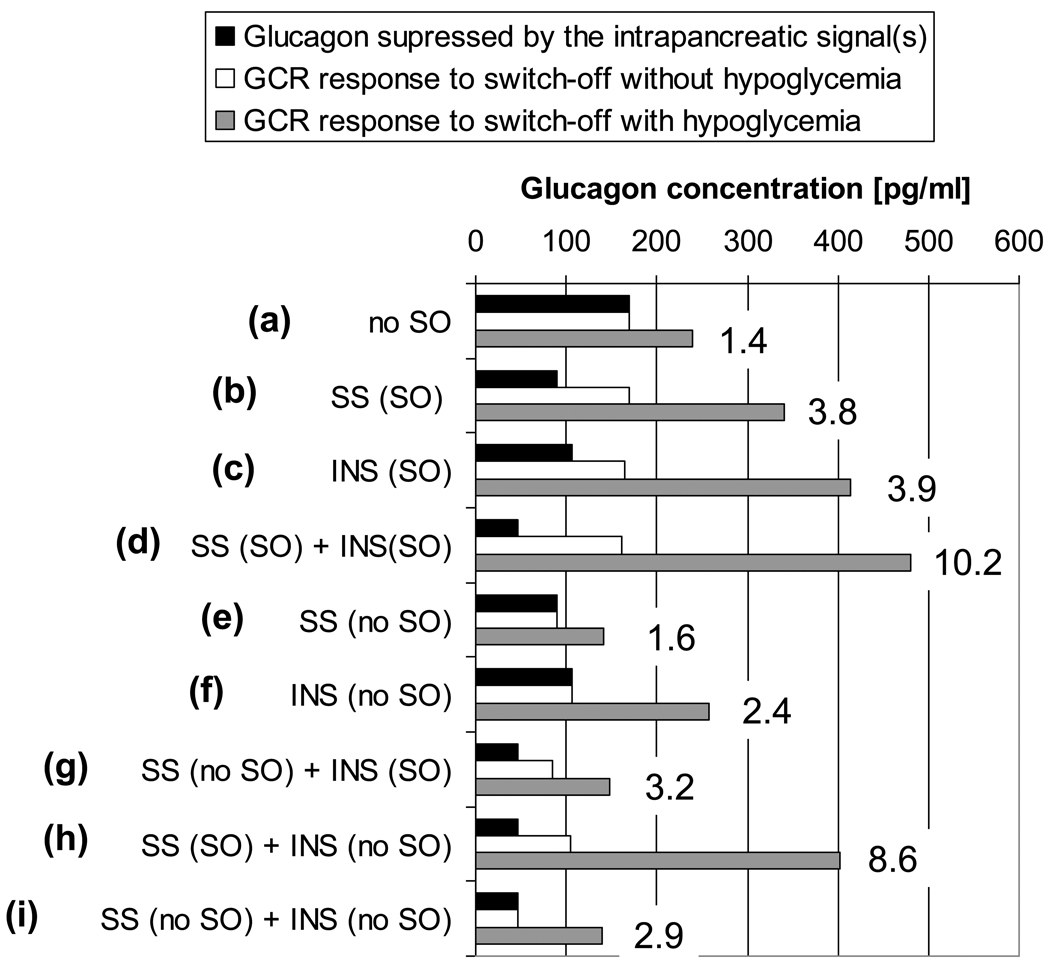
We have used the above model (Farhy and McCall, 2008) to show that the glucagon control axis postulated in Figure 1 is consistent with the experimental findings (i)–(vii) [above] and we showed that insulin and somatostatin affect differently the basal and the system-regulated α-cell activity. After the model was validated we used it to predict the outcome of different switch-off strategies and explore their potential to improve GCR in β-cell deficiency: Figure 2 (Farhy and McCall, 2008). The figure summarizes results from in silico experiments tracking the dynamic of glucagon from time t=0 h (start) to t=4 h (end). In some simulations intrapancreatic infusion of insulin or somatostatin started at t=0.5 h and was either continued to the end or was switched off at t=2.5 h. When hypoglycemia was simulated, BG=110 mg/dL from t=0 h to t=2 h, glucose decline starts at t=2 h, BG=60 mg/dL at t=2.5 h (switch-off point), at the end of the simulations (t=4 h) BG=43 mg/dL. At the top of the bar graph (a), we show baseline results without switch-off signals. The black bar illustrates the glucagon level before t=2 h which is the time where BG=110 mg/dL and glucagon would be maximally suppressed if a switch-off signal were present. The white and the grey bars illustrate the maximal glucagon response in the one hour interval from t=2.5 h to t=3.5 h without (white) and with (grey) hypoglycemia stimulus. This interval corresponds to the one hour interval after a switch-off in all other simulations. The black and white bars are the same since glucagon levels remain unchanged if there is no hypoglycemia. Each subsequent set of three bars indicates these effects with single switch-off [(b) and (c)], combined switch-off (d), no switch-off of a single signal [(e) and (f)], a mixture of switch-off and no switch-off for the two signals [(g) and (h)], and no switch-off for the combination of the two signals (i). Thus, the bar graph gives the following glucagon concentrations: glucagon suppressed by the intrapancreatic signal (black bars: the glucagon concentration immediately before the onset of BG decline at t=2h: at that time glucagon is maximally suppressed by the intrapancreatic infusion and not affected by the decline in glucose); GCR response to a switch-off if hypoglycemia was not induced (white bars: the maximal glucagon concentrations achieved within a 1 hour interval after the switch-off); and GCR response if hypoglycemia was induced (grey bars: the maximal glucagon concentrations achieved within a 1 hour interval after the switch-off). The graph also includes the maximal fold increase in glucagon in response to a switch-off during hypoglycemia relative to the glucagon levels before the onset of BG decline.
Figure 2*.
Summary of the model-predicted GCR responses to different switch-off signals with or without simulated hypoglycemia (see text for more detail). SO=switch-off; no SO= the signal was not switched off; SS=somatostatin; INS=insulin.
*Modified from (Farhy and McCall, 2009)
Thus, we concluded that the impact of an α-cell inhibitor on the GCR depends on the nature of the signal and the mode of its delivery. These comparisons between strategies of manipulating the network to enhance the GCR by a switch-off revealed a good potential of a combined switch-off to amplify the benefits provided by each of the individual signals (Farhy and McCall, 2009) and even a potential to explore scenarios in which the α-cell suppressing signal is not terminated.
NETWORK REDUCTION, APPROXIMATION OF THE NORMAL ENDOCRINE PANCREAS AND OF THE GCR ABNORMALITIES IN THE INSULIN DEFICIENT PANCREAS
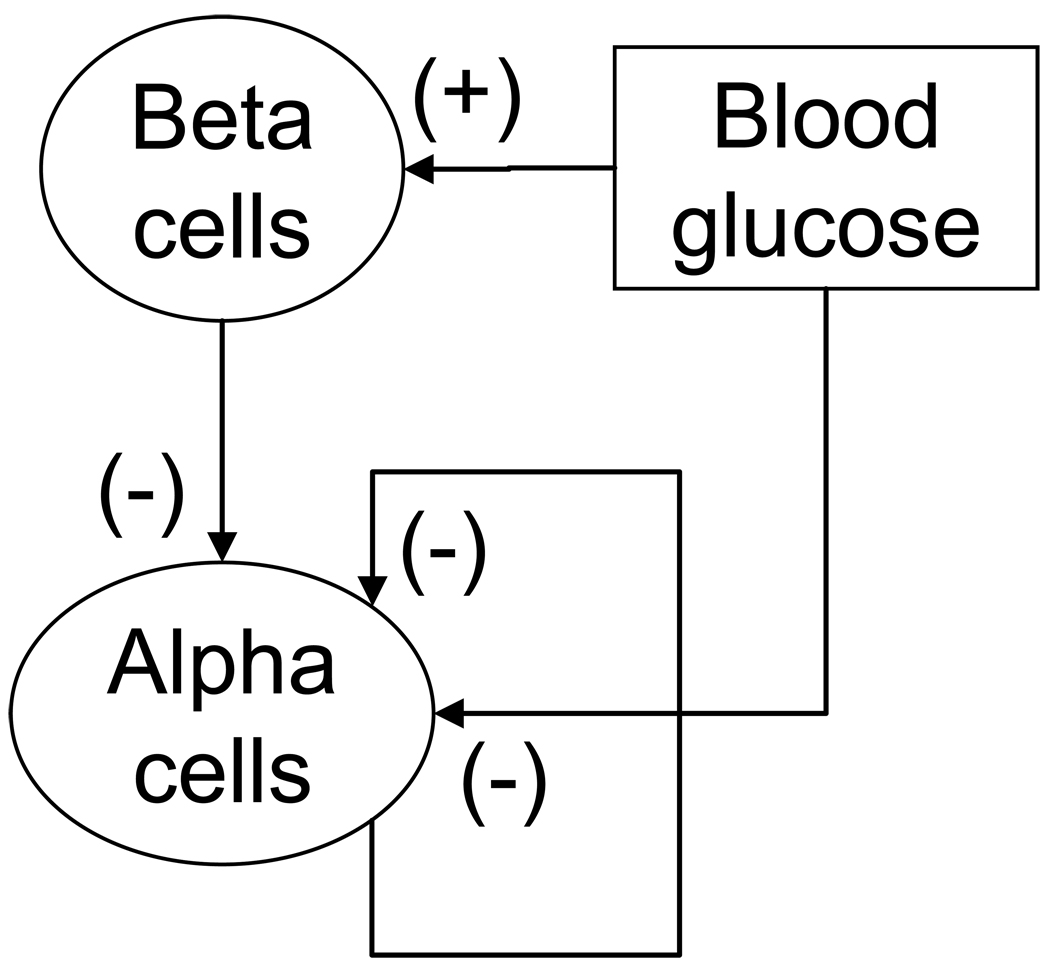
The explicit involvement of somatostatin in the model described above limits the potential clinical application as pancreatic somatostatin cannot be reliably measured in the human in vivo and the ability of the model to describe the human glucagon axis cannot be verified. It is however possible to simplify the network in a way that somatostatin is no longer explicitly involved, but is incorporated implicitly. In the original model shown in Figure 1 somatostatin appears in the following two compound pathways, the “α-cell -> δ-cell -> α-cell” feedback loop and in the “BG -> δ-cell -> α-cell” pathway. By virtue of its interactions in the “α-cell -> δ-cell -> α-cell” pathway the α-cells effectively control their own activity and therefore this pathway can be replaced by a delayed “α-cell -> α-cell” auto-feedback loop. Such regulation is also consistent with reports that glucagon may directly suppress its own release (Kawai and Unger, 1982) possibly by binding to glucagon receptors located on a subpopulation of the α-cells (Kieffer et. al., 1996) or by other autocrine mechanisms. Through the “BG -> δ-cell -> α-cell” pathway, blood glucose down-regulates the release of glucagon and the action is mediated by somatostatin. Therefore, this pathway can be simplified and substituted by the BG -> α-cell interaction. The outcome of the described procedure of network reduction is a new Minimal Control Network (MCN) of the GCR control mechanisms in which somatostatin and the δ-cells are no longer explicitly involved: Figure 3. As was originally proposed in our prior work (Farhy et. al., 2008), the β-cells of the normal pancreas are now part of the MCN (and of the mathematical model). This feature also extends the physiological relevance of the model. The β-cells are assumed to be stimulated by hyperglycemia and to suppress the activity of the α-cells. The latter action is based on an extensive data that the β-cells (co)release a variety of signals, including insulin, GABA, zinc, and amylin, all of which are known to suppress the α-cell activity: (Gedulin et. al., 1997; Ishihara et. al., 2003; Ito et. al., 1995; Reaven et. al., 1987; Rorsman and Hellman, 1988; Samols and Stagner, 1988; Van Schravendijk et. al., 1987; Wendt et. al., 2004; Xu et. al., 2006). In addition, it has been reported that the pulses of insulin and glucagon recur with a phase shift (Grapengiesser et. al., 2006) which is consistent with the postulated negative regulation of the α-cells by the β-cells. An extensive background justifying all postulated MCN relationships is presented in the previous section.
Figure 3.
A Minimal Control Network (MCN) of the interactions between BG and the α- and β-cells postulated to regulate the GCR in the normal pancreas. In this network the δ-cells are not represented explicitly.
Dynamic network approximation of the MCN
Similar to the analysis of the old network dynamic network modeling methods are used to study the properties of the MCN shown in Figure 3. In particular, two differential equations approximate the glucagon and insulin concentration rate of change:
| Eq. 3 |
| Eq. 4 |
Here, GL(t), BG(t), and INS(t) denote time-dependent concentrations of glucagon, blood glucose, and insulin (or exogenous switch-off signal in the β-cell-deficient model), respectively; the derivative is the rate of change with respect to the time t. The term Pulse in Eq. 2 denotes a pulse generator specific to the β-cells superimposed to guarantee physiological relevance of the simulations. The meaning of the parameters is defined as follows:
kGL and kINS are rates of elimination for glucagon and insulin, respectively;
rGL is BG- and auto-feedback-regulated rate of release of glucagon;
rGl,basal is glucagon basal rate of release;
rINS is BG-regulated rate of release of insulin;
rINS,basal is insulin basal rate of release;
tINS is half-maximal inhibitory dose for the negative action of insulin on glucagon;
tBG and tBG,2 are half-maximal inhibitory dose for BG (ID50);
tGL is half-maximal inhibitory dose for glucagon (ID50);
nBG, nBG,2, and nGL are Hill coefficients describing the slope of the corresponding dose-response interactions;
DGL is delay in the auto-feedback;
Determination of the model parameters
The half-life of glucagon was assumed to be approximately 2 min to match the results of our pulsatility analysis (Farhy et. al., 2008) and other published data. Therefore, we fixed the parameter kGL = 22 h−1. The half-life (t1/2) of insulin was assumed to be ~3 min as suggested in the literature (Grimmichova et. al., 2008). Therefore, to approximate insulin’s t1/2 we fixed the parameter kINS = 14 h−1. The remaining parameters used in the simulations were determined functionally and some of the concentrations presented below are in arbitrary units (specifically, those related to insulin). These units however can be easily rescaled to match real concentrations. The delay in the auto-feedback DGL = 7.2 min was functionally determined, together with the potencies tBG = 50 mg/dL, tGL = 6 pg/mL and sensitivities nBG = 5, nGL = 5 in the auto-feedback control function, to guarantee that glucagon pulses during GCR recur at intervals of 15–20 min to correspond to the number of pulses after a switch-off point detected in the pulsatility analysis (Farhy et. al., 2008). The parameters rINS = 80,000 and rINS,basal = 270, together with the amplitude of the pulses of the pulse generator and the parameters tBG,2 = 400mg/dL and nBG,2 = 3 were functionally determined to guarantee that BG is capable of stimulating more than 9-fold increase in insulin over baseline in response to a glucose bolus. The ID50, tINS = 20, was functionally determined based on the insulin concentrations to guarantee that insulin withdrawal during hypoglycemia can trigger GCR. The glucagon release (rGL = 42,750pg/mL/h) and basal secretion rate (rGL,basal = 2,128pg/mL/h) were functionally determined so that a strong hypoglycemic stimulus can trigger more than 10-fold increase in glucagon from the normal pancreas. The parameters of the pulse generator, Pulse, were chosen to generate every 6 minutes a square wave of height = 10 over a period of 36 sec based on published reports on insulin pulsatility reporting recurring insulin pulses every 4–12 min: (Pørksen, 2002). We note that insulin pulsatility was modeled to mimic the variation of insulin in the portal vein, rather than in the circulation. This explains the deep nadirs between the pulses evident in the simulations. The parameter values of the model are summarized in Table I.
Table I.
Summary of core interactive constants in the auto-feedback MCN
| Rate Constant | Dose-Response Control Functions | ||||
|---|---|---|---|---|---|
| Elimination [1/hour] |
Release [concentration/hour] |
ED50, or ID50 [concentration] |
Slope [dimension- less] |
Delay [min] |
|
| Glucagon | kGL = 22 h−1 |
rGL = 42,570pg/mL/h rGL,basal = 2,128pg/mL/h |
tGL = 85pg/mL | nGL = 5 | DGL = 7.2min |
| BG |
tBG = 50mg/dL tBG,2 = 400mg/dL |
nBG = 5 nBG,2 = 3 |
|||
| Insulin | kINS = 14h−1 |
rINS = 80,000 rINS,basal = 270 |
tINS = 20 | ||
| Pulse | periodic function: a square wave of height = 10 over a period of 36 sec recurring every 6 minutes |
||||
In silico experiments
The simulations were performed as follows:
Simulation of the glucose input to the system. We performed two different simulations to mimic hypoglycemia: (a) BG decline form 110 mg/dL to 60 mg/dL in 1 hour and (b) stepwise (1 hour steps) decline in BG from 110 to ~60 (same as in (a)), then to ~45, and then to ~42 mg/dL. The stepwise decline into hypoglycemia is intended to investigate a possible distinction between the model responses to 60 mg/dL (a) and to a stronger hypoglycemic stimulus (b); it also mimics a commonly employed human experimental conditions (staircase hypoglycemic clamp). To generate glucose profiles that satisfy (a) and (b) we used the equation BG' = −3BG + 3×step + 330, where the function step changes from 110 to 60, 45, and 42 mg/dL at 1-hour steps. Then we used the solution to the above equation in Eq. 1 and Eq. 2. Similarly, an increase of glucose was simulated by using the above equation and a step function which increases the BG levels from 110 to 240 mg/dL to mimic acute hyperglycemia.
Transition from a normal to an insulin deficient state. The simulation was performed by gradually reducing to zero the amplitude of the pulses generated by the pulse generator, Pulse.
-
Simulation of intrapancreatic infusion of different α-cell suppressing signals. These simulations were performed in the insulin deficient model (above). Eq. 2 is replaced by an equation which describes the dynamic of the infused signal:
Here, SO represents the concentration of the switch-off signal, an abrupt termination of an α-cell suppressing signal. The function Infusion describes the rate of its intrapancreatic infusion (equal to Height if the signal is infused or to 0 otherwise) and kSO its (functional) rate of elimination. Then, the terms (1+m1.SO) and (1+m2.SO) are used in Eq. 1 to divide the parameters rGL and rGl,basal, respectively to simulate suppression of the α-cell activity by the signal. Differences in the parameters m1 and m2 model unequal action of the infused signal on the basal and BG/auto-feedback-regulated glucagon secretion. In particular, to simulate an insulin switch-off we used parameters kSO = 3, Height = 55, m1 = 0.08, and m2 = 0.5; to simulate somatostatin switch-off we used kSO = 3.5, Heght = 10, m1 = 1, and m2 = 1.4. The parameters were functionally determined to explain our experimental observations (see Results) and the possible differences in the response to the two types of switch-offs (Farhy et. al., 2008). In particular, the action of exogenous insulin on BG/auto-feedback-regulated and basal glucagon secretion is distributed like a 1:6.3 ratio. Similar to our previous work (Farhy and McCall, 2009), exogenous insulin suppresses the basal more than the pulsatile glucagon release, For somatostatin, the suppressive effect is more uniform in a 1:1.4 ratio.
Validation of the MCN
To validate the new network we perform an in silico study in three steps:
-
➢
Demonstrate that the (new) MCN (Figure 3) is compatible with the mechanism of GCR and response to switch-off signals in insulin deficiency. We have already shown that our (original) network which includes somatostatin as an explicit node is consistent with key experimental data. To confirm that the (new) MCN can substitute the older more complex construct, we tested the hypothesis that it can approximate the same key experimental observations [all (i) through (vii) listed in the beginning of Methods] already shown to be predicted by the old network (Figure 1).
-
➢
Show that the mechanisms underlying the dysregulation of GCR in insulin deficiency can be explained by the MCN. To this end we demonstrated that the BG-regulated MCN can explain (i) High GCR response if the β-cells are intact and provide a potent switch-off signal to the α-cells; and (ii) Reduction of GCR following a simulated gradual decrease in insulin secretion to mimic transition from normal physiology to an insulinopenic state.
-
➢
Verify that the proposed MCN approximates the basic properties of the normal endocrine pancreas. Even though the goal of this work is to explain the GCR control mechanisms and their dysregulation, we have demonstrated that the postulated MCN can explain the increase in insulin secretion and decrease in glucagon release in response to BG stimulation.
The goal of this in silico study is to validate the MCN by demonstrating that the parameters of the mathematical model (Eqs. 1 and 2) that approximate the MCN (Figure 3) can be determined in a way that the output of the model can predict certain general features of the in vivo system. Therefore, the simulated profiles are expected to reproduce the overall behavior of the system rather than to match exactly experimentally observed individual hormone dynamics.
To integrate the equations we used a Runge-Kutta 4 algorithm and its specific implementation within the software package Berkeley-Madonna.
In silico experiments with simulated complete insulin deficiency
We demonstrate that the proposed MCN model, which has changed significantly since initially introduced (Farhy et. al., 2008; Farhy and McCall, 2009), is consistent with the experimental observations in STZ-treated rats reported by us and others (Farhy et. al., 2008, Zhou et. al., 2004).
Defective GCR response to hypoglycemia with the absence of a switch-off signal in the insulin deficient model
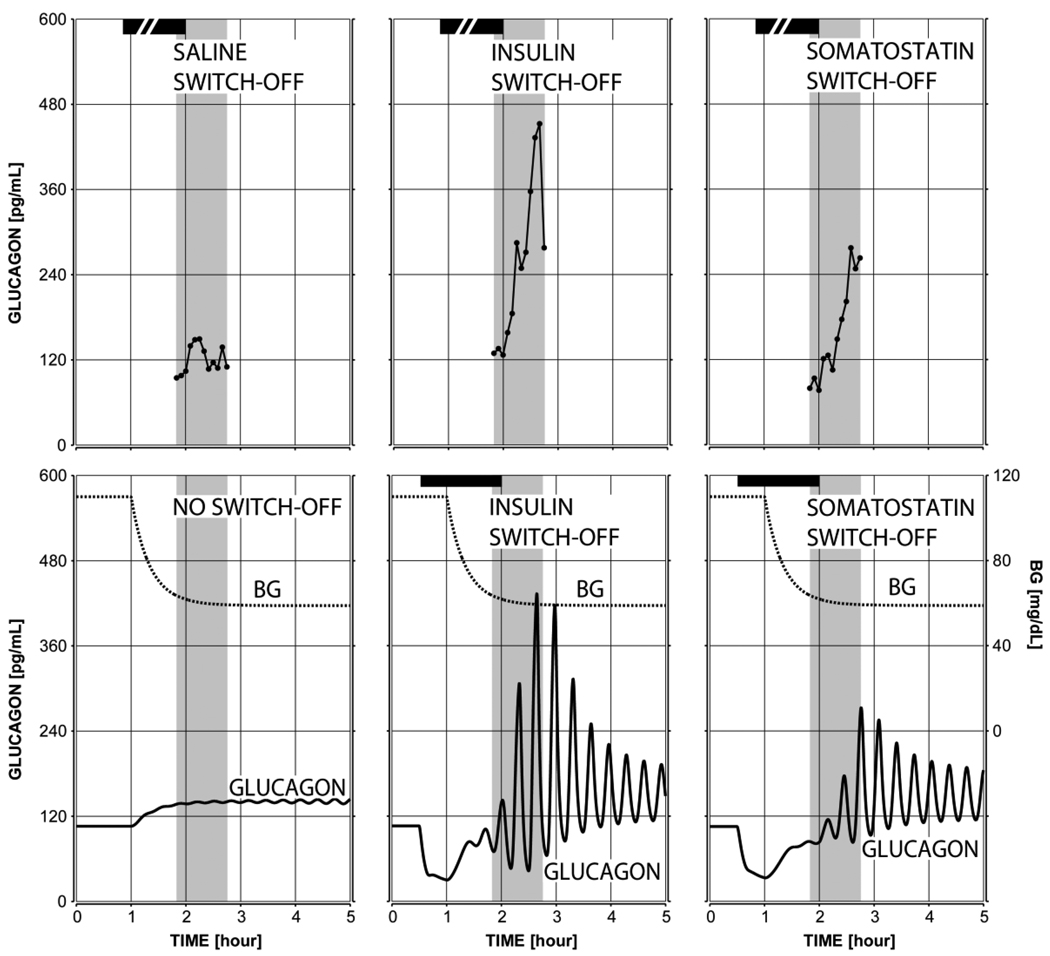
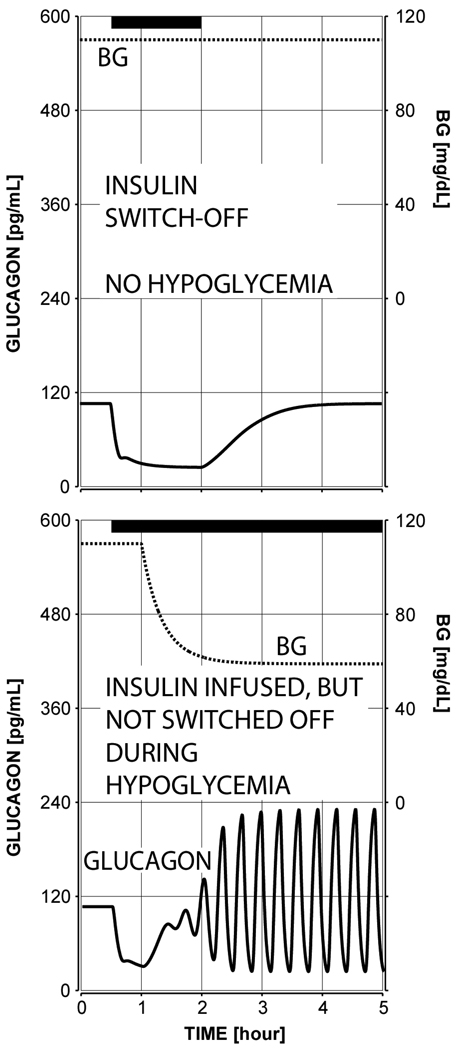
The plot in Figure 4 (bottom left panel) shows the predicted lack of glucagon response to hypoglycemia if a switch-off signal is missing – a key observation reported in our experimental study (Farhy et. al., 2008) and elsewhere (Zhou et. al., 2004; Zhou et. al., 2007 a; Zhou et. al., 2007 b). The system responds with only about 30% increase in the pulse amplitude of glucagon in the 45 min interval after BG reaches 60 mg/dl, which agrees with our experimental observations (Figure 4, top panels) and shows that the model satisfies condition (iv) from Methods (no GCR response to hypoglycemia without a switch-off signal).
Figure 4.
The mean observed (top) and model-predicted (bottom) glucagon response to hypoglycemia and saline switch-off or no switch-off (left), insulin switch-off (middle), and somatostatin switch-off (right). The shaded area marks the period monitored in our experimental study. The simulations were performed with a complete insulin deficiency.
GCR response to switch-off signals in insulin deficiency
The model response to a 1.5 hour intrapancreatic infusion of insulin or somatostatin switched off at hypoglycemia (BG=60 mg/dL) is shown in the bottom middle and right panels of Figure 4. The infusion was initiated at time t = 0.5 h (arbitrary time units) and switched-off at t = 2 h. A simulated gradual BG decline started at t = 1 h and BG = 60 mg/dL at the switch-off point. The model response illustrates a pulsatile rebound glucagon secretion after the switch-off reaching almost a 4-fold increase in glucagon in the 45 min period after the switch-off as compared to the pre-switch-off levels, which is similar to the experimental observations: Figure 4 (top middle and right panels).
Therefore, the model satisfies conditions (i) the pulsatility timing and (ii) the pulsatility amplitude increase from Methods in regard to insulin and somatostatin switch-off.
In addition, the bottom middle and right panels of Figure 4 demonstrate that the model satisfies conditions (vi) > 30% higher GCR response to insulin vs. somatostatin switch-off and (vii) better glucagon suppression by somatostatin before a switch-off compared with suppression by insulin from Methods. Of interest, the prediction that an insulin switch-off signal suppresses more potently the basal, rather than the pulsatile glucagon release (Methods) is similar to the prediction of the previous model (Farhy and McCall, 2009) and it is necessary to explain the difference between the insulin switch-off and somatostatin switch-off: Figures 4, middle vs. left panels.
Note that the experimental data shown in the top panels of Figure 4 was collected during our previous experimental study (Farhy et. al., 2008). The pulsatility of glucagon is not apparent in the plots presented in Figure 4 since they reflect averaged experimental data (n=7 in the saline group and n=6 in the insulin and somatostatin switch-off groups). In (Farhy et. al., 2008) glucagon pulsatility was confirmed on the individual profiles of glucagon measured in the circulation by deconvolution analysis and the current simulations which approximate the dynamic of glucagon in the portal circulation agree well with these results.
Reduction of the GCR response to an intrapancreatic infusion by high glucose conditions during the switch-off or by failure to terminate the signal
For comparison, Figure 5 depicts the GCR response if an insulin signal was infused and switched off, but hypoglycemia was not present (top panel) or if intrapancreatic insulin was infused, but not switched off during hypoglycemia (bottom panel). In the first simulation glucagon increases by only 60 pg/mL relative to the concentration at the switch-off point and in the second simulation the GCR response is reduced ~2-fold as compared to the response depicted in Figure 4 (middle bottom panel). This result agrees with the observations reported in (Zhou et. al., 2004) which demonstrate a lack of significant increase in glucagon in this 1 hour interval if insulin is not switched off. In an additional analysis (results not shown) we increased the simulated rate of infusion of the insulin switch-off signal 4-fold. by increasing the parameter Height from 55 to 220 (see Methods) and used a stronger hypoglycemic stimulus (~40 mg/dL). The model responded with an increase in glucagon after the switch-off which reached concentrations above 800 pg/mL in the 1 hour interval after the switch-off point. When the same signal was not terminated in this simulation, the response was restricted to a rise to only 180 pg/mL. This outcome reproduces more closely the observations in (Zhou et. al., 2004).
Figure 5.
Model-predicted minimal absolute glucagon response to insulin switch-off if the intrapancreatic signal is terminated during euglycemia (top panel; glucagon increases minimally with only 60 pg/mL greater than the concentration at the switch-off point) and to insulin intrapancreatic insulin infusion if the signal is not switched off (bottom panel: glucagon increases only 85 pg/mL greater than to the concentration at the time when BG=60 mg/dL and only about 2-fold relative to baseline)- these values are by contrast increased more than 3.5 fold when the switch off occurs –see Figure 4 the bottom middle panel. All of these simulations were performed with a complete insulin deficiency.
Thus, the model satisfies conditions (iii) the restriction of the response to an insulin switch-off by high BG conditions and (v) the absence of a pronounced GCR when no insulin switch-off is performed as detailed in the Methods.
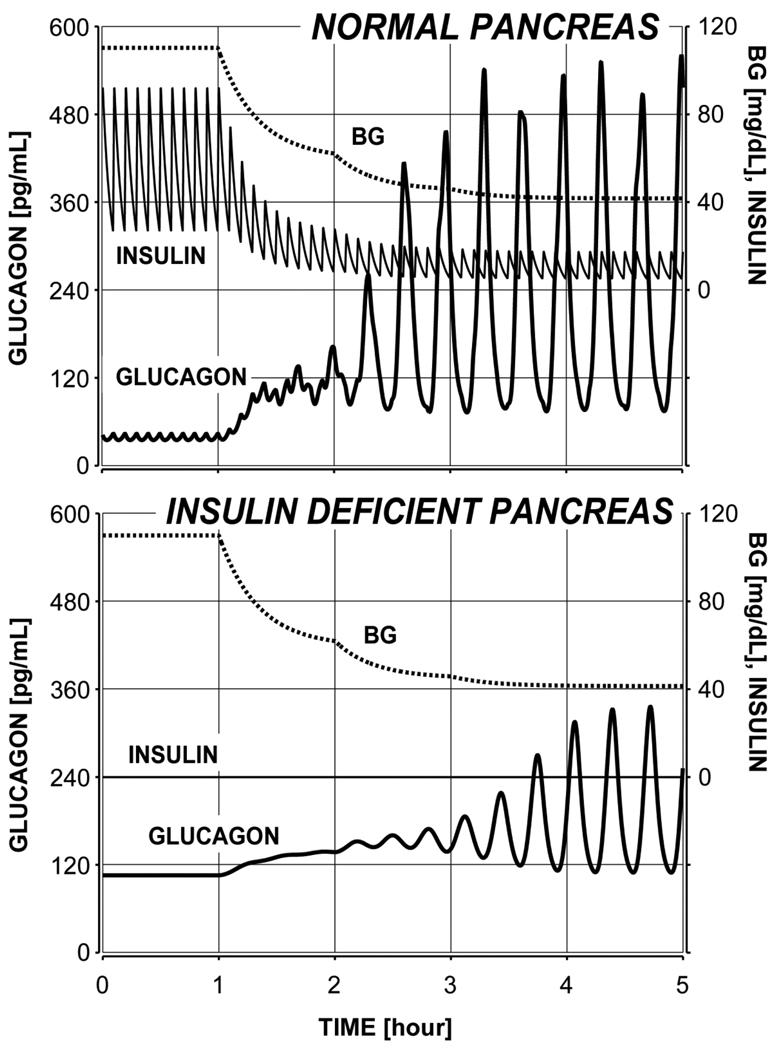
Simulated transition from a normal physiology to an insulinopenic state
One set of simulations was performed to evaluate the model-generated glucagon response to a stepwise BG decline into hypoglycemia with a normal and insulin deficient pancreas. The response of the normal model shown in Figure 6 (top panel) illustrates a pronounced glucagon response to hypoglycemia (about 4-fold increase when BG=60 mg/dL and about 14-fold increase over baseline when BG approaches 42.5 mg/dL). Of interest, the model predicts that when BG starts to fall the high-frequency glucagon pulsatility during the basal period entrained by the insulin pulses will be replaced by low-frequency oscillations maintained by the α-cell auto-feedback.
Figure 6.
Model-derived glucagon response to hypoglycemia (stepwise BG decline) in normal physiology with intact insulin release (top) and predicted decrease and delay in GCR following a simulated removal of insulin secretion to mimic a transition from a normal to an insulin deficient state.
The model also predicts that a complete absence of BG-stimulated and basal insulin release will result in the following abnormalities in the glucagon secretion and response to hypoglycemia (Figure 6, bottom panel):
-
➢
A significant reduction in the fold glucagon response to hypoglycemia relative to baseline (only about 1.3-fold increase when BG=60 mg/dL and only about 3-fold increase when BG approaches 42 mg/dL)
-
➢
A reduction in the absolute glucagon response to hypoglycemia (15% lower response when BG=60 mg/dL and 42% lower response when BG approaches 42.5 mg/dL)
-
➢
A delay in the GCR response (BG remains below 60 mg/dL for more than an hour without any sizable change in glucagon)
-
➢
A 2.5-fold increase in basal glucagon
-
➢
Disappearance of the insulin-driven high-frequency glucagon pulsatility
A comparison between the model response to hypoglycemia when BG remains at 60 mg/dL (Figure 4, lower left panel) and when it falls further to about 42.5 mg/dL in the staircase hypoglycemic clamp (Figure 6, bottom panel) reveals the interesting model prediction that a sufficiently strong hypoglycemic stimulus may still evoke some delayed glucagon release. However, additional analysis (results not shown) disclosed that if the basal glucagon release (model parameter rGL,basal) is 15–20% higher this response will be completely suppressed. Therefore, the model predicts that GCR abnormalities may be due to both the lack of an appropriate switch-off signal and to a significant basal hyperglucagonemia. The same simulations were performed also under the assumption that BG declines only to 60 mg/dL and remains at that level similarly to the experiments depicted in the lower panels of Figure 4 (results not shown). We detected that the glucagon pulses released by the normal pancreas were about 47% lower which stresses the importance of the strength of the hypoglycemic stimulus to the amount of GCR response. Under conditions of complete absence of insulin the weaker hypoglycemic stimulus evokes practically no response (this outcome has already been shown in Figure 4, lower left panel) and the concentration of glucagon was 57% lower than the response stimulated by the stepwise decline (Figure 6, bottom panel).
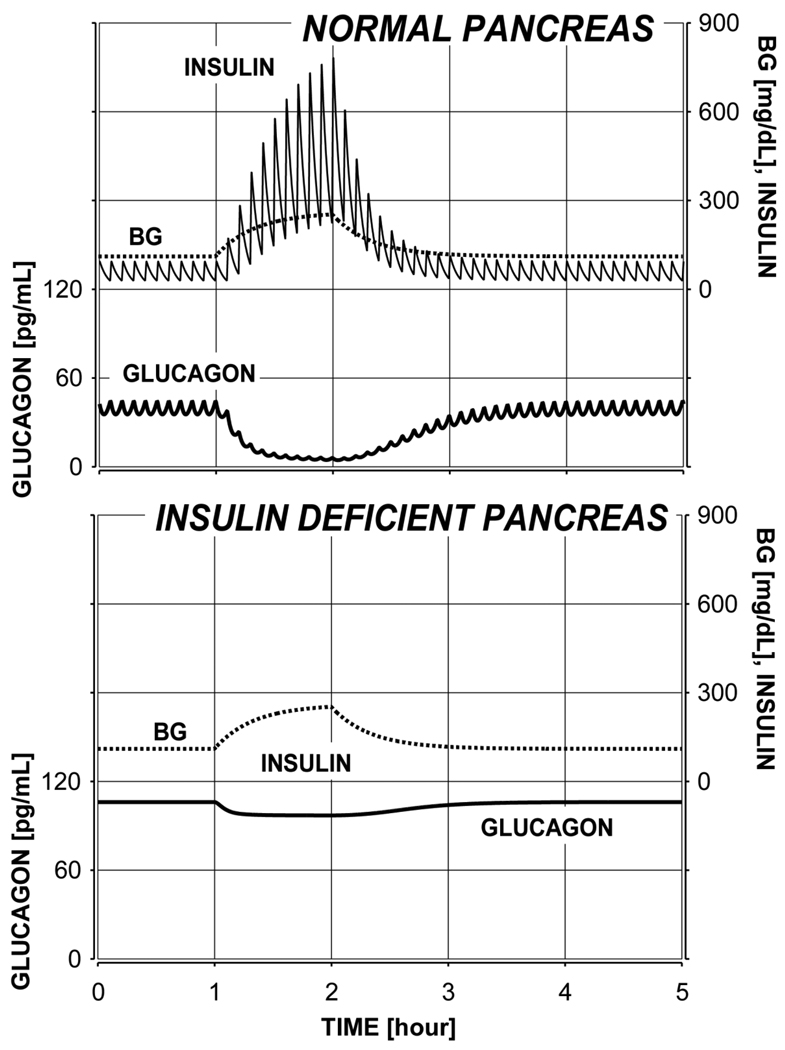
A second set of simulations was designed to test the hypothesis that the model of the MCN can correctly predict a typical increase in insulin secretion and a decrease in glucagon following an increase in blood glucose. We also monitored how these two system responses change during a transition from a normal physiology to an insulinopenic state. To this end an increase in BG was simulated (see Methods) with an elevation of the BG concentration from 110 to about 240 mg/dL in 1 hour and then a return back to normal in the next 1.5 hours. The model-predicted response of the normal pancreas is shown on the top panel of Figure 7. In this simulation the BG-driven release of insulin increased almost 9-fold which caused significant suppression in glucagon release. The bottom plot in Figure 7 illustrates the effect on the system response of 100% reduction in BG-stimulated insulin release. As expected, insulin deficiency results in an increase of glucagon and limited ability of hyperglycemia to suppress glucagon (Meier et. al. 2006).
Figure 7.
Simulated progressive decline of the ability of glucose to suppress glucagon resulting from a gradual transition (same as in Figure 6) from a normal physiology (top) to an insulinopenic state (bottom).
ADVANTAGES AND LIMITATIONS OF THE INTERDISCIPLINARY APPROACH
A key conclusion of our model-based simulations is that some of the observed system behavior (like the system response to a switch-off) emerges from interplay between multiple components. Models like the networks in Figure 1 and 3 are certainly not uncommon in endocrine research, and typically exemplify regulatory hypotheses. Traditionally, such models are studied using methods that probe individual components or interactions in isolation from the rest of the system. This approach has been taken in the majority of the published studies that investigate the GCR regulation. The limitation of this approach is that the temporal relationships between the system components and the relative contribution of each interaction to the overall system behavior cannot be properly assessed. Therefore, especially when the model contains feedbacks, the individual approach cannot answer the question of whether the model explains the system control mechanisms. The main reason for this limitation is that some key specifics of the system behavior, like its capability to oscillate and respond with a rebound to a switch-off, both require and are the result of the time-varying interactions of several components. If these are studied in isolation, little information will be gained about the dynamic behavior of this network-like mechanism. Numerous reports have documented that the glucagon control axis is indeed a complex network-like structure, and therefore it lends itself to a complex dynamic behavior analysis approach. This highlights both the significance and the necessity of the mathematical methods that we propose to use to analyze the experimental data. Using differential equations-based modeling is perhaps the only way to estimate the dynamic interplay of the pancreatic hormones and their importance for GCR control.
Mathematical models have not been applied to study the GCR control mechanisms, but have been used to explore other aspects of the control of BG homeostasis (Guyton et. al., 1978; Yamasaki et. al., 1984; Insel et. al., 2000; Steele et. al., 1974). For example, the minimal model of Bergman, Ider, Boeden, and Cobelli, proposed in 1979 for estimating insulin sensitivity (Bergman et. al., 1979), received considerable attention and further development (Bergman et. al., 1987; Cobelli et. al., 1986; Cobelli et. al., 1990; Mari, 1997; Quon et. al., 1994; Breda et. al., 2001; Toffolo et. al., 1995; Toffolo et. al., 2001). We have previously used modeling methods to successfully estimate and predict the onset of the counterregulation in T1DM patients (Kovatchev et. al., 1999; Kovatchev et. al., 2000) as well as to study other complex endocrine axes (Farhy, 2004; Farhy et. al., 2001; Farhy et. al., 2002; Farhy et. al., 2007; Farhy and Veldhuis 2003, Farhy and Veldhuis 2004, Farhy and Veldhuis 2005). However, despite the proven utility of this methodology, our recent efforts were the first to apply a combination of network modeling and in vivo studies to dissect the GCR control axis (Farhy et. al., 2008; Farhy and McCall, 2009).
The selected few MCN components cannot exhaustively recreate all signals that control the GCR. Indeed, in the normal pancreas, glucagon may control its own secretion via α/β-cell interactions. For example, human β-cells express glucagon receptors (Kieffer et. al., 1996; Huypens et. al., 2000) and exogenous glucagon stimulates insulin by glucagon- and GLP-1-receptors (Huypens et. al., 2000). One immuno-neutralization study suggests that endogenous glucagon stimulates insulin (Brunicardi et. al., 2001) while other results imply that α-cell glutamate may bind to receptors on β-cells to stimulate insulin and GABA (Bertrand et. al., 1992; Inagaki et. al., 1995; Uehara et. al., 2004).
It has been recently reported that in human islets, α-cell glutamate serves as a positive autocrine signal for glucagon release by acting on ionotropic glutamate receptors (iGluRs) on α-cells (Cabrera et. al., 2008). Thus, absence of functional β-cells may cause glutamate hypersecretion, followed by desensitization of the α-cell iGluRs, and ultimately by defects in GCR as conjectured (Cabrera et. al., 2008). Interestingly, we propose a similar hypothesis to explain the defective GCR in diabetes by an increased chronic α-cell activity due to lack of β-cell signaling. However, we suggest that hyperglucagonemia is the main reason for the GCR defects. The two hypotheses are not mutually exclusive, but ours can explain also the in vivo GCR pulsatility during hypoglycemia observed by us (Farhy et. al., 2008) and others (Genter et. al., 1998). Most importantly, the α-cell positive auto-regulation is consistent with the proposed here negative delayed α-cell auto-feedback, which could be mediated in part by iGluRs desensitization as suggested (Cabrera et. al., 2008). The autocrine regulation is implicitly incorporated in our model equations in the parameter rGL.
The β-cells may control the δ-cells, which are downstream from β-cells in the order of intraislet vascular perfusion. However, in one study, anterograde infusion of insulin antibody in the perfused rat pancreas stimulated both glucagon and somatostatin (Samlos and Stagner, 1988), while another immuno-neutralization study documented a decrease in somatostatin at high glucose concentrations (Brunicardi et. al., 2001). Suppression by insulin of the α-cells (as proposed here) could explain this apparent contradiction. It is also possible that the δ-cells inhibit the β-cells (Brunicardi et. al., 2003; Strowski et. al., 2000; Schuit et. al., 1989; Huypens et. al., 2000).
Finally, the MCN components are influenced by numerous extrapancreatic factors, some of which have important impacts on glucagon secretion and GCR, including the autonomic input, catecholamines, growth hormone, ghrelin, and incretins (Gromada et. al., 2007; Heise 2004; Havel and Ahren, 1997; Havel and Taborsky 1989). For example, the incretin GLP-1 inhibits glucagon, though the mechanism of this inhibition is still controversial (Gromada et. al., 2007). Also, there are three major autonomic influences on the α-cell: sympathetic nerves, parasympathetic nerves and circulating epinephrine, all of which are activated by hypoglycemia, and are capable of stimulating glucagon and suppressing insulin (Brelje et. al., 1989; Bolli and Fanelli, 1999; Taborsky et. al., 1998). We cannot track all signals that control the GCR and most of them have no explicit terms in our model. However, they are not omitted or considered unimportant. In fact, when we describe mathematically the MCN, we are including the impact of the nervous system and other factors, even though they have no individual terms in the equations. Thus, the MCN unifies all factors that control glucagon release based on the assumption that the primary physiological relationships that are explicit in the MCN are influenced by these factors.
The model-based simulations suggest that the postulated MCN model of regulation of GCR is consistent with the experimental data. However, at this stage we cannot estimate how good this model is, and it is therefore hard to assess the validity of its predictions. The simulations can only reconstruct the general “averaged” behavior of the in vivo system, and new experimental data are required to support the much more important property that the model can explain the GCR response in individual animals. These should involve interventional studies to manipulate the vascular input to the pancreas and analyze the corresponding changes in the output by collecting frequently sampled portal vein data for multiple hormones simultaneously. These must be analyzed by the mathematical model to estimate whether the MCN provides an objectively good description of the action of the complex GCR control mechanism. Note that with this approach we cannot establish the model-based inferences in “micro” detail, since they imply molecular mechanisms that are out of reach of the in vivo methodology. The approach cannot nor is it intended to address the microscopic behavior of the α-cells or the molecular mechanisms that govern this behavior. In this regard, insulin and glucagon (and somatostatin) should be viewed only as (macroscopic) surrogates for the activity of the different cell types under a variety of other intra and extra-pancreatic influences.
Even though it is usually not stated explicitly, simple models are always used in experimental studies and, especially in in vivo experiments, many factors are ignored or postulated to have no impact on the outcome. Using constructs like the ones described in this work to analyze hormone concentration data has the advantage that the underlying model is very explicit, incorporates multiple relationships and uses well established mathematical and statistical techniques to show its validity and reconstruct the involved signals and pathways.
CONCLUSIONS
In the current work we present our interdisciplinary efforts to investigate the system-level network control mechanisms that mediate the glucagon counterregulation (GCR) and their abnormalities in diabetes - a concept as yet almost completely unexplored for GCR. The results confirm the hypothesis that a streamlined model which omits an explicit (but not implicit) somatostatin (δ-cell) node entirely reproduces the results of our original more complex models. Our new findings define more precisely the components that are the most critical for the system and strongly suggest that a delayed α-cell auto-feedback plays a key role in GCR regulation. The results demonstrate that such a regulation is consistent not only with most of the in vivo system behavior typical for the insulin deficient pancreas, but it also explains key features, characteristic for the transition from a normal to an insulin deficient state. A major advantage of the current model is that its only explicit components are blood glucose (BG), insulin, and glucagon. These are clinically measurable which would allow the application of the new construct to the study of the control, function, and abnormalities of the human glucagon axis.
Acknowledgments
GRANTS
The study was supported by NIH/NIDDK grant R21 DK072095
Contributor Information
Leon S. Farhy, Departments of Medicine, Center for Biomathematical Technology, Center, Box 800735, University of Virginia, Charlottesville, Virginia, 22908, 434-924-2496, 434-982-3878 (fax), leon@virginia.edu.
Anthony L. McCall, Departments of Medicine, Center, Box 801407, University of Virginia, Charlottesville, Virginia, 22908, 434-243-9373, 434-982-3796 (fax), alm3j@virginia.edu.
REFERENCES
- The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of Intensive Glucose Lowering in Type 2 Diabetes. N. Engl. J. Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft FM, Proks P, Smith PA, Ammala C, Bokvist K, Rorsman P. Stimulus-secretion coupling in pancreatic beta cells. Journal of Cellular Biochemistry. 1994;55 Suppl:54–65. doi: 10.1002/jcb.240550007. [DOI] [PubMed] [Google Scholar]
- Banarer S, McGregor VP, Cryer PE. Intraislet hyperinsulinemia prevents the glucagon response to hypoglycemia despite an intact autonomic response. Diabetes. 2002;51(4):958–965. doi: 10.2337/diabetes.51.4.958. [DOI] [PubMed] [Google Scholar]
- Bell GI, Pilkis SJ, Weber IT, Polonsky KS. Glucokinase mutations, insulin secretion, and diabetes mellitus. Annual Review of Physiology. 1996;58:171–186. doi: 10.1146/annurev.ph.58.030196.001131. [DOI] [PubMed] [Google Scholar]
- Bergman RN, Ider YZ, Boeden CR, Cobelli C. Quantative estimation of insulin sensitivity. American Journal of Physiology. 1979;236:E667–E667. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- Bergman RN, Prager R, Volund A, Olefsky JM. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. Journal of Clinical Investigation. 1987;79:790–800. doi: 10.1172/JCI112886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand G, Gross R, Puech R, Loubatieres-Mariani MM, Bockaert J. Evidence for a glutamate receptor of the AMPA subtype which mediates insulin release from rat perfused pancreas. British Journal of Pharmacology. 1992;106(2):354–359. doi: 10.1111/j.1476-5381.1992.tb14340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli GB, Fanelli CG. Physiology of glucose counterregulation to hypoglycemia. Endocrinol Metab Clin North Am. 1999;28:467–493. doi: 10.1016/s0889-8529(05)70083-9. [DOI] [PubMed] [Google Scholar]
- Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes. 2001;50(1):150–158. doi: 10.2337/diabetes.50.1.150. [DOI] [PubMed] [Google Scholar]
- Brelje TC, Scharp DW, Sorenson RL. Three-dimensional imaging of intact isolated islets of Langerhans with confocal microscopy. Diabetes. 1989;38(6):808–814. doi: 10.2337/diab.38.6.808. [DOI] [PubMed] [Google Scholar]
- Brunicardi FC, Atiya A, Moldovan S, Lee TC, Fagan SP, Kleinman RM, Adrian TE, Coy DH, Walsh JH, Fisher WE. Activation of somatostatin receptor subtype 2 inhibits insulin secretion in the isolated perfused human pancreas. Pancreas. 2003;27(4):e84–e89. doi: 10.1097/00006676-200311000-00019. [DOI] [PubMed] [Google Scholar]
- Brunicardi FC, Kleinman R, Moldovan S, Nguyen TH, Watt PC, Walsh J, Gingerich R. Immunoneutralization of somatostatin, insulin, and glucagon causes alterations in islet cell secretion in the isolated per fused human pancreas. Pancreas. 2001;23(3):302–308. doi: 10.1097/00006676-200110000-00012. [DOI] [PubMed] [Google Scholar]
- Cabrera O, Jacques-Silva MC, Speier S, Yang SN, Köhler M, Fachado A, Vieira E, Zierath JR, Kibbey R, Berman DM, Kenyon NS, Ricordi C, Caicedo A, Berggren PO. Glutamate Is a Positive Autocrine Signal for Glucagon Release. Cell Metab. 2008;7(6):545–554. doi: 10.1016/j.cmet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejvan K, Coy DH, Efendic S. Intra-islet somatostatin regulates glucagon release via type 2 somatostatin receptors in rats. Diabetes. 2003;52(5):1176–1181. doi: 10.2337/diabetes.52.5.1176. [DOI] [PubMed] [Google Scholar]
- Cobelli C, Brier DM, Ferrannini E. Modeling glucose metabolism in man: theory and practice. Hormone & Metabolic Research – Supplement. 1990;24:1–10. [PubMed] [Google Scholar]
- Cobelli C, Pacini G, Toffolo G, Sacca L. Estimation of insulin sensitivity and glucose clearance from minimal model: new insights from labeled IVGTT. American Journal of Physiology. 1986;250:E591–E598. doi: 10.1152/ajpendo.1986.250.5.E591. [DOI] [PubMed] [Google Scholar]
- Cryer PE. Hypoglycemia is the limiting factor in the management of diabetes. Diabetes/Metabolism Research Reviews. 1999;15(1):42–46. doi: 10.1002/(sici)1520-7560(199901/02)15:1<42::aid-dmrr1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Cryer PE. Hypoglycemia the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia. 2002;45(7):937–948. doi: 10.1007/s00125-002-0822-9. [DOI] [PubMed] [Google Scholar]
- Cryer PE, Davis SN, Shamoon H. Hypoglycemia in Diabetes. Diabetes Care. 2003;26:1902–1912. doi: 10.2337/diacare.26.6.1902. [DOI] [PubMed] [Google Scholar]
- Cryer PE, Gerich JE. Relevance of glucose counterregulatory systems to patients with diabetes: critical roles of glucagon and epinephrine. Diabetes Care. 1983;6(1):95–99. doi: 10.2337/diacare.6.1.95. [DOI] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Diem P, Redmon JB, Abid M, Moran A, Sutherland DE, Halter JB, Robertson RP. Glucagon, catecholamine and pancreatic polypeptide secretion in type I diabetic recipients of pancreas allografts. Journal of Clinical Investigation. 1990;86(6):2008–2013. doi: 10.1172/JCI114936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumonteil E, Magnan C, Ritz-Laser B, Ktorza A, Meda P, Philippe J. Glucose regulates proinsulin and prosomatostatin but not proglucagon messenger ribonucleic acid levels in rat pancreatic islets. Endocrinology. 2000;141(1):174–180. doi: 10.1210/endo.141.1.7230. [DOI] [PubMed] [Google Scholar]
- Dunne MJ, Harding EA, Jaggar JH, Squires PE. Ion channels and the molecular control of insulin secretion. Biochemical Society Transactions. 1994;22(1):6–12. doi: 10.1042/bst0220006. [DOI] [PubMed] [Google Scholar]
- Efendic S, Nylen A, Roovete A, Uvnas-Wallenstein K. Effects of glucose and arginine on the release of immunoreactive somatostatin from the isolated perfused rat pancreas. FEBS Letters. 1978;92(1):33–35. doi: 10.1016/0014-5793(78)80715-7. [DOI] [PubMed] [Google Scholar]
- Epstein S, Berelowitz M, Bell NH. Pentagastrin and glucagon stimulate serum somatostatin-like immunoreactivity in man. J. Clin. Endocrinol. Metab. 1980;51:1227–1231. doi: 10.1210/jcem-51-6-1227. [DOI] [PubMed] [Google Scholar]
- Farhy LS. Modeling of oscillations in endocrine networks with feedback. Methods in Enzymology. 2004;384:54–81. doi: 10.1016/S0076-6879(04)84005-9. [DOI] [PubMed] [Google Scholar]
- Farhy LS, Du Z, Zeng Q, Veldhuis PP, Johnson ML, Brayman KL, McCall AL. Amplification of pulsatile glucagon secretion by switch-off of α-cell suppressing signals in Streptozotocin treated rats. American Journal of Physiology – Endocrinology and Metabolism. 2008;295:E575–E585. doi: 10.1152/ajpendo.90372.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhy LS, McCall AL. System-level control to optimize glucagon counterregulation by switch-off of α-cell suppressing signals in β-cell deficiency. Journal of Diabetes Science and Technology. 2009;3(1):21–33. doi: 10.1177/193229680900300104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhy LS, Straume M, Johnson ML, Kovatchev B, Veldhuis JD. A construct of interactive feedback control of the GH axis in the male. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2001;281(1):R38–R51. doi: 10.1152/ajpregu.2001.281.1.R38. [DOI] [PubMed] [Google Scholar]
- Farhy LS, Straume M, Johnson ML, Kovatchev B, Veldhuis JD. Unequal autonegative feedback by GH models the sexual dimorphism in GH secretory dynamics. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2002;282(3):R753–R764. doi: 10.1152/ajpregu.00407.2001. [DOI] [PubMed] [Google Scholar]
- Farhy LS, Veldhuis JD. Putative GH pulse renewal: periventricular somatostatinergic control of an arcuate-nuclear somatostatin and GH-releasing hormone oscillator. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2004;286(6):R1030–R1042. doi: 10.1152/ajpregu.00473.2003. [DOI] [PubMed] [Google Scholar]
- Farhy LS, Veldhuis JD. Joint pituitary-hypothalamic and intrahypothalamic autofeedback construct of pulsatile growth hormone secretion. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2003;285(5):R1240–R1249. doi: 10.1152/ajpregu.00086.2003. [DOI] [PubMed] [Google Scholar]
- Farhy LS, Veldhuis JD. Deterministic construct of amplifying actions of ghrelin on pulsatile growth hormone secretion. Am J Physiol Regulatory Integrative Comp Physiol. 2005;288:R1649–R1663. doi: 10.1152/ajpregu.00451.2004. [DOI] [PubMed] [Google Scholar]
- Farhy LS, Bowers CY, Veldhuis JD. Model-projected mechanistic bases for sex differences in growth-hormone (GH) regulation in the human. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1577–R1593. doi: 10.1152/ajpregu.00584.2006. [DOI] [PubMed] [Google Scholar]
- Fujitani S, Ikenoue T, Akiyoshi M, Maki T, Yada T. Somatostatin and insulin secretion due to common mechanisms by a new hypoglycemic agent, A-4166, in perfused rat pancreas. Metabolism: Clinical & Experimental. 1996;45(2):184–189. doi: 10.1016/s0026-0495(96)90051-7. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Tanaka A, Tahara Y, Ikegami H, Yamamoto Y, Kumahara Y, Shima K. Correlation between minimal secretory capacity of pancreatic beta-cells and stability of diabetic control. Diabetes. 1988;37(1):81–88. doi: 10.2337/diab.37.1.81. [DOI] [PubMed] [Google Scholar]
- Gedulin BR, Rink TJ, Young AA. Dose-response for glucagonostatic effect of amylin in rats. Metabolism. 1997;46:67–70. doi: 10.1016/s0026-0495(97)90170-0. [DOI] [PubMed] [Google Scholar]
- Genter P, Berman N, Jacob M, Ipp E. Counterregulatory hormones oscillate during steady-state hypoglycemia. American Journal of Physiology. 1998;275(5):E821–E829. doi: 10.1152/ajpendo.1998.275.5.E821. [DOI] [PubMed] [Google Scholar]
- Gerich JE. Lilly lecture: Glucose counterregulation and its impact on diabetes mellitus. Diabetes. 1988;37(12):1608–1617. doi: 10.2337/diab.37.12.1608. [DOI] [PubMed] [Google Scholar]
- Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973;182(108):171–173. doi: 10.1126/science.182.4108.171. [DOI] [PubMed] [Google Scholar]
- Gopel SO, Kanno T, Barg S, Rorsman P. Patch-clamp characterisation of somatostatin-secreting -cells in intact mouse pancreatic islets. Journal of Physiology. 2000 a;528(3):497–507. doi: 10.1111/j.1469-7793.2000.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopel SO, Kanno T, Barg S, Weng XG, Gromada J, Rorsman P. Regulation of glucagon release in mouse -cells by KATP channels and inactivation of TTX-sensitive Na+ channels. J. Physiol. 2000 b;528:509–520. doi: 10.1111/j.1469-7793.2000.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grapengiesser E, Salehi A, Quader SS, Hellman B. Glucose Induces Glucagon Release Pulses Antisynchronous with Insulin and Sensitive to Purinoceptors Inhibition. Endocrinology. 2006;147:3472–3477. doi: 10.1210/en.2005-1431. [DOI] [PubMed] [Google Scholar]
- Grimmichova R, Vrbikova J, Matucha P, Vondra K, Veldhuis P, Johnson M. Fasting Insulin Pulsatile Secretion in Lean Women with Polycystic Ovary Syndrome. Physiological Research. 2008;57:1–8. doi: 10.33549/physiolres.931493. [DOI] [PubMed] [Google Scholar]
- Gromada J, Franklin I, Wollheim CB. α-Cells of the Endocrine Pancreas: 35 Years of Research but the Enigma Remains. Endocrine Reviews. 2007;28(1):84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- Guyton JR, Foster RO, Soeldner JS, Tan MH, Kahn CB, Koncz L, Gleason RE. A model of glucose-insulin homeostasis in man that incorporates the heterogeneous fast pool theory of pancreatic insulin release. Diabetes. 1978;27:1027–1042. doi: 10.2337/diab.27.10.1027. [DOI] [PubMed] [Google Scholar]
- Havel PJ, Ahren B. Activation of autonomic nerves and the adrenal medulla contributes to increased glucagon secretion during moderate insulin-induced hypoglycemia in women. Diabetes. 1997;46:801–807. doi: 10.2337/diab.46.5.801. [DOI] [PubMed] [Google Scholar]
- Havel PJ, Taborsky GJ., Jr The contribution of the autonomic nervous system to changes of glucagon and insulin secretion during hypoglycemic stress. Endocrine Reviews. 1989;10(3):332–350. doi: 10.1210/edrv-10-3-332. [DOI] [PubMed] [Google Scholar]
- Heimberg H, De Vos A, Moens K, Quartier E, Bouwens L, Pipeleers D, Van Schaftingen E, Madsen O, Schuit F. The glucose sensor protein glucokinase is expressed in glucagon-producing alpha-cells. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(14):7036–7041. doi: 10.1073/pnas.93.14.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberg H, De Vos A, Pipeleers D, Thorens B, Schuit F. Differences in glucose transporter gene expression between rat pancreatic alpha- and beta-cells are correlated to differences in glucose transport but not in glucose utilization. Journal of Biological Chemistry. 1995;270(15):8971–8975. doi: 10.1074/jbc.270.15.8971. [DOI] [PubMed] [Google Scholar]
- Heise T, Heinemann T, Heller S, Weyer C, Wang Y, Strobel S, Kolterman O, Maggs D. Effect of Pramlintide on Symptom, Catecholamine, and Glucagon Responses to Hypoglycemia in Healthy Subjects. Metabolism. 2004;53(9):1227–1232. doi: 10.1016/j.metabol.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Hermansen K, Christensen SE, Orskov H. Characterization of somatostatin release from the pancreas: the role of potassium. Scandinavian Journal of Clinical & Laboratory Investigation. 1979;39(8):717–722. doi: 10.3109/00365517909108162. [DOI] [PubMed] [Google Scholar]
- Hilsted J, Frandsen H, Holst JJ, Christensen NJ, Nielsen SL. Plasma glucagon and glucose recovery after hypoglycemia: the effect of total autonomic blockade. Acta Endocrinologica. 1991;125(5):466–469. doi: 10.1530/acta.0.1250466. [DOI] [PubMed] [Google Scholar]
- Hirsch BR, Shamoon H. Defective epinephrine and growth hormone responses in type I diabetes are stimulus specific. Diabetes. 1987;36(1):20–26. doi: 10.2337/diab.36.1.20. [DOI] [PubMed] [Google Scholar]
- Hoffman RP, Arslanian S, Drash AL, Becker DJ. Impaired counterregulatory hormone responses to hypoglycemia in children and adolescents with new onset IDDM. Journal of Pediatric Endocrinology. 1994;7(3):235–244. doi: 10.1515/jpem.1994.7.3.235. [DOI] [PubMed] [Google Scholar]
- Hope KM, Tran PO, Zhou H, Oseid E, Leroy E, Robertson RP. Regulation of alpha-cell function by the beta-cell in isolated human and rat islets deprived of glucose: the "switch-off" hypothesis. Diabetes. 2004;53(6):1488–1495. doi: 10.2337/diabetes.53.6.1488. [DOI] [PubMed] [Google Scholar]
- Huypens P, Ling Z, Pipeleers D, Schuit F. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia. 2000;43(8):1012–1019. doi: 10.1007/s001250051484. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Kuromi H, Gonoi T, Okamoto Y, Ishida H, Seino Y, Kaneko T, Iwanaga T, Seino S. Expression and role of ionotropic glutamate receptors in pancreatic islet cells. FASEB Journal. 1995;9(8):686–691. [PubMed] [Google Scholar]
- Insel PA, Liljenquist JE, Tobin JD, Sherwin RS, Watkins P, Andres R, Berman M. Insulin control of glucose metabolism in man. A new kinetic analysis. Journal of Clinical Investigation. 1975;55:1057–1066. doi: 10.1172/JCI108006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB. Islet β-cell secretion determines glucagon release from neighboring α-cells. Nat Cell Biol. 2003;5:330–335. doi: 10.1038/ncb951. [DOI] [PubMed] [Google Scholar]
- Ito K, Maruyama H, Hirose H, Kido K, Koyama K, Kataoka K, Saruta T. Exogenous insulin dose-dependently suppresses glucopenia-induced glucagon secretion from perfused rat pancreas. Metabolism: Clinical & Experimental. 1995;44(3):358–362. doi: 10.1016/0026-0495(95)90166-3. [DOI] [PubMed] [Google Scholar]
- Jaspan JB, Lever E, Polonsky KS, Van Cauter E. In vivo pulsatility of pancreatic islet peptides. American Journal of Physiology. 1986;251(2 Pt 1):E215–E226. doi: 10.1152/ajpendo.1986.251.2.E215. [DOI] [PubMed] [Google Scholar]
- Kawai K, Unger RH. Inhibition of glucagon secretion by exogenous glucagon in the isolated, perfused dog pancreas. Diabetes. 1982;31(6):512–515. doi: 10.2337/diab.31.6.512. [DOI] [PubMed] [Google Scholar]
- Kawamori D, Kurpad AJ, Hu J, Liew CW, Shih JL, Ford EL, Herrera PL, Polonsky KS, McGuinness OP, Kulkarni RN. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab. 2009;9(4):350–361. doi: 10.1016/j.cmet.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer TJ, Heller RS, Unson CG, Weir GC, Habener JF. Distribution of glucagon receptors on hormone-specific endocrine cells of rat pancreatic islets. Endocrinology. 1996;137(11):5119–5125. doi: 10.1210/endo.137.11.8895386. [DOI] [PubMed] [Google Scholar]
- Klaff LJ, Taborsky GJ., Jr Pancreatic somatostatin is a mediator of glucagon inhibition by hyperglycemia. Diabetes. 1987;36(5):592–596. doi: 10.2337/diab.36.5.592. [DOI] [PubMed] [Google Scholar]
- Kleinman R, Gingerich R, Ohning G, Wong H, Olthoff K, Walsh J, Brunicardi FC. The influence of somatostatin on glucagon and pancreatic polypeptide secretion in the isolated perfused human pancreas. International Journal of Pancreatology. 1995;18(1):51–57. doi: 10.1007/BF02825421. [DOI] [PubMed] [Google Scholar]
- Kleinman R, Gingerich R, Wong H, Walsh J, Lloyd K, Ohning G, De Giorgio R, Sternini C, Brunicardi FC. Use of the Fab fragment for immunoneutralization of somatostatin in the isolated perfused human pancreas. American Journal of Surgery. 1994;167(1):114–119. doi: 10.1016/0002-9610(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Kovatchev BP, Farhy LS, Cox DJ, Straume M, Yankov VI, Gonder-Frederick LA, Clarke WL. Modeling insulin-glucose dynamics during insulin induced hypoglycemia. Evaluation of glucose counterregulation. Journal of Theoretical Medicine. 1999;1:313–323. [Google Scholar]
- Kovatchev BP, Straume M, Farhy LS, Cox DJ. Dynamic Network Model of Glucose Counterregulation in Subjects with Insulin-Requiring Diabetes. Methods in Enzymology. 2000;321:396–410. doi: 10.1016/s0076-6879(00)21204-4. [DOI] [PubMed] [Google Scholar]
- Ludvigsen E, Olsson R, Stridsberg M, Janson ET, Sandler S. Expression and distribution of somatostatin receptor subtypes in the pancreatic islets of mice and rats. Journal of Histochemistry & Cytochemistry. 2004;52(3):391–400. doi: 10.1177/002215540405200310. [DOI] [PubMed] [Google Scholar]
- McCall AL, Cox DJ, Crean J, Gloster M, Kovatchev BP. A novel analytical method for assessing glucose variability: using CGMS in type 1 diabetes mellitus. Diabetes Technol. Ther. 2006;8(6):644–653. doi: 10.1089/dia.2006.8.644. [DOI] [PubMed] [Google Scholar]
- Mari A. Assessment of insulin sensitivity with minimal model: role of model assumptions. American Journal of Physiology. 1997;272:E925–E934. doi: 10.1152/ajpendo.1997.272.5.E925. [DOI] [PubMed] [Google Scholar]
- Maruyama H, Hisatomi A, Orci L, Grodsky GM, Unger RH. Insulin within islets is a physiologic glucagon release inhibitor. Journal of Clinical Investigation. 1984;74(6):2296–2299. doi: 10.1172/JCI111658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DR, Hermansen K, Connolly AA, Gray D, Schmitz O, Clark A, Orskov H, Turner RC. Greater in vivo than in vitro pulsatility of insulin secretion with synchronized insulin and somatostatin secretory pulses. Endocrinology. 1987;120(6):2272–2278. doi: 10.1210/endo-120-6-2272. [DOI] [PubMed] [Google Scholar]
- Meier JJ, Kjems LL, Veldhuis JD, Lefebvre P, Butler PC. Postprandial suppression of glucagon secretion depends on intact pulsatile insulin secretion: further evidence for the intraislet insulin hypothesis. Diabetes. 2006;55(4):1051–1056. doi: 10.2337/diabetes.55.04.06.db05-1449. [DOI] [PubMed] [Google Scholar]
- Pipeleers DG, Schuit FC, Van Schravendijk CF, Van de Winkel M. Interplay of nutrients and hormones in the regulation of glucagon release. Endocrinology. 1985;117(3):817–823. doi: 10.1210/endo-117-3-817. [DOI] [PubMed] [Google Scholar]
- Pørksen N. The in vivo regulation of pulsatile insulin secretion. Diabetologia. 2002;45(1):3–20. doi: 10.1007/s125-002-8240-x. [DOI] [PubMed] [Google Scholar]
- Portela-Gomes GM, Stridsberg M, Grimelius L, Oberg K, Janson ET. Expression of the five different somatostatin receptor subtypes in endocrine cells of the pancreas. Applied Immunohistochemistry & Molecular Morphology. 2000;8(2):126–132. doi: 10.1097/00129039-200006000-00007. [DOI] [PubMed] [Google Scholar]
- Quon MJ, Cochran C, Taylor SI, Eastman RC. Non-insulin mediated glucose disappearance in subjects with IDDM. Discordance between experimental results and minimal model analysis. Diabetes. 1994;43:890–896. doi: 10.2337/diab.43.7.890. [DOI] [PubMed] [Google Scholar]
- Ravier MA, Rutter GA. Glucose or Insulin, but not Zinc Ions, Inhibit Glucagon Secretion From Mouse Pancreatic alpha Cells. Diabetes. 2005;54:1789–1797. doi: 10.2337/diabetes.54.6.1789. [DOI] [PubMed] [Google Scholar]
- Reaven GM, Chen YD, Golay A, Swislocki AL, Jaspan JB. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. Journal of Clinical Endocrinology & Metabolism. 1987;64(1):106–110. doi: 10.1210/jcem-64-1-106. [DOI] [PubMed] [Google Scholar]
- Rorsman P, Berggren PO, Bokvist K, Ericson H, Mohler H, Ostenson CG, Smith PA. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature. 1989;341(6239):233–236. doi: 10.1038/341233a0. [DOI] [PubMed] [Google Scholar]
- Rorsman P, Hellman B. Voltage-activated currents in guinea pig pancreatic alpha 2 cells. Evidence for Ca2+-dependent action potentials. Journal of General Physiology. 1988;91(2):223–242. doi: 10.1085/jgp.91.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi A, Quader SS, Grapengiesser E, Hellman B. Pulses of somatostatin release are slightly delayed compared with insulin and antisynchronous to glucagon. Regulatory Peptides. 2007;144:43–49. doi: 10.1016/j.regpep.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Samols E, Stagner JI. Intra-islet regulation. American Journal of Medicine. 1988;85(5A):31–35. doi: 10.1016/0002-9343(88)90395-6. [DOI] [PubMed] [Google Scholar]
- Samols E, Stagner JI. Islet somatostatin--microvascular, paracrine, and pulsatile regulation. Metabolism: Clinical & Experimental. 1990;39(9) Suppl 2:55–60. doi: 10.1016/0026-0495(90)90212-u. [DOI] [PubMed] [Google Scholar]
- Schuit FC, Derde MP, Pipeleers DG. Sensitivity of rat pancreatic A and B cells to somatostatin. Diabetologia. 1989;32(3):207–212. doi: 10.1007/BF00265096. [DOI] [PubMed] [Google Scholar]
- Schuit FC, Huypens P, Heimberg H, Pipeleers DG. Glucose sensing in pancreatic beta-cells: a model for the study of other glucose-regulated cells in gut, pancreas, and hypothalamus. Diabetes. 2001;50(1):1–11. doi: 10.2337/diabetes.50.1.1. [DOI] [PubMed] [Google Scholar]
- Schuit F, De Vos A, Farfari S, Moens K, Pipeleers D, Brun T, Prentki M. Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in beta cells. Journal of Biological Chemistry. 1997;272(30):18572–18579. doi: 10.1074/jbc.272.30.18572. [DOI] [PubMed] [Google Scholar]
- Segel SA, Paramore DS, Cryer PE. Hypoglycemia-associated autonomic failure in advanced type 2 diabetes. Diabetes. 2002;51(3):724–733. doi: 10.2337/diabetes.51.3.724. [DOI] [PubMed] [Google Scholar]
- Silvestre RA, Rodríguez-Gallardo J, Jodka C, Parkes DG, Pittner RA, Young AA, Marco J. Selective amylin inhibition of the glucagon response to arginine is extrinsic to the pancreas. Am J Physiol Endocrinol Metab. 2001;280:E443–E449. doi: 10.1152/ajpendo.2001.280.3.E443. [DOI] [PubMed] [Google Scholar]
- Steele R, Rostami H, Altszuler N. A two-compartment calculator for the dog glucose pool in the nonsteady state. Federation Proceedings. 1974;33:1869–1876. [PubMed] [Google Scholar]
- Stagner JI, Samols E, Bonner-Weir S. beta-alpha-delta pancreatic islet cellular perfusion in dogs. Diabetes. 1988;37(12):1715–1721. doi: 10.2337/diab.37.12.1715. [DOI] [PubMed] [Google Scholar]
- Stagner JI, Samols E, Marks V. The anterograde and retrograde infusion of glucagon antibodies suggests that A cells are vascularly perfused before D cells within the rat islet. Diabetologia. 1989;32(3):203–206. doi: 10.1007/BF00265095. [DOI] [PubMed] [Google Scholar]
- Strowski MZ, Parmar RM, Blake AD, Schaeffer JM. Somatostatin inhibits insulin and glucagon secretion via two receptors subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology. 2000;141(1):111–117. doi: 10.1210/endo.141.1.7263. [DOI] [PubMed] [Google Scholar]
- Sumida Y, Shima T, Shirayama K, Misaki M, Miyaji K. Effects of hexoses and their derivatives on glucagon secretion from isolated perfused rat pancreas. Hormone & Metabolic Research. 1994;26(5):222–225. doi: 10.1055/s-2007-1001669. [DOI] [PubMed] [Google Scholar]
- Taborsky GJ, Jr, Ahren B, Havel PJ. Autonomic mediation of glucagon secretion during hypoglycemia: implications for impaired alpha-cell responses in type 1 diabetes. Diabetes. 1998;47(7):995–1005. doi: 10.2337/diabetes.47.7.995. [DOI] [PubMed] [Google Scholar]
- Tapia-Arancibia L, Astier H. Glutamate stimulates somatostatin release from diencephalic neurons in primary culture. Endocrinology. 1988;123:2360–2366. doi: 10.1210/endo-123-5-2360. [DOI] [PubMed] [Google Scholar]
- Tirone TA, Norman MA, Moldovan S, DeMayo FJ, Wang XP, Brunicardi FC. Pancreatic somatostatin inhibits insulin secretion via SSTR-5 in the isolated perfused mouse pancreas model. Pancreas. 2003;26(3):e67–e73. doi: 10.1097/00006676-200304000-00025. [DOI] [PubMed] [Google Scholar]
- Toffolo G, Breda E, Cavaghan MK, Ehrmann DA, Polonsky KS, Cobelli C. Quantitative indices of β-cell function during graded up&down glucose infusion from C-peptide minimal models. Am J Physiol. 2001;280:2–10. doi: 10.1152/ajpendo.2001.280.1.E2. [DOI] [PubMed] [Google Scholar]
- Toffolo G, De Grandi F, Cobelli C. Estimation of beta-cell sensitivity from intravenous glucose tolerance test C-peptide data. Knowledge of the kinetics avoids errors in modeling the secretion. Diabetes. 1995;44:845–854. doi: 10.2337/diab.44.7.845. [DOI] [PubMed] [Google Scholar]
- Uehara S, Muroyama A, Echigo N, Morimoto R, Otsuka M, Yatsushiro S, Moriyama Y. Metabotropic glutamate receptor type 4 is involved in autoinhibitory cascade for glucagon secretion by alpha-cells of islet of Langerhans. Diabetes. 2004;53(4):998–1006. doi: 10.2337/diabetes.53.4.998. [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group (UKPDS) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- Unger RH. Glucagon physiology and pathophysiology in the light of new advances. Diabetologia. 1985;28:574–578. doi: 10.1007/BF00281991. [DOI] [PubMed] [Google Scholar]
- Utsumi M, Makimura H, Ishihara K, Morita S, Baba S. Determination of immunoreactive somatostatin in rat plasma and responses to arginine, glucose and glucagon infusion. Diabetologia. 1979;17:319–323. doi: 10.1007/BF01235888. [DOI] [PubMed] [Google Scholar]
- Van Schravendijk CF, Foriers A, Van den Brande JL, Pipeleers DG. Evidence for the presence of type I insulin-like growth factor receptors on rat pancreatic A and B cells. Endocrinology. 1987;121(5):1784–1788. doi: 10.1210/endo-121-5-1784. [DOI] [PubMed] [Google Scholar]
- Wendt A, Birnir B, Buschard K, Gromada J, Salehi A, Sewing S, Rorsman P, Braun M. Glucose inhibition of glucagon secretion from rat alpha-cells is mediated by GABA released from neighboring beta-cells. Diabetes. 2004;53(4):1038–1045. doi: 10.2337/diabetes.53.4.1038. [DOI] [PubMed] [Google Scholar]
- Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, Liu S, Wendt A, Deng S, Ebina Y, Wheeler MB, Braun M, Wang Q. Intraislet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab. 2006;3:47–58. doi: 10.1016/j.cmet.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y, Tiran J, Albisser AM. Modeling glucose disposal in diabetic dogs fed mixed meals. American Journal of Physiology. 1984;246:E52–E61. doi: 10.1152/ajpendo.1984.246.1.E52. [DOI] [PubMed] [Google Scholar]
- Zhou H, Tran PO, Yang S, Zhang T, LeRoy E, Oseid E, Robertson RP. Regulation of alpha-cell function by the beta-cell during hypoglycemia in Wistar rats: the "switch-off" hypothesis. Diabetes. 2004;53(6):1482–1487. doi: 10.2337/diabetes.53.6.1482. [DOI] [PubMed] [Google Scholar]
- Zhou H, Zhang T, Oseid E, Harmon J, Tonooka N, Robertson RP. Reversal of Defective Glucagon Responses to Hypoglycemia in Insulin-Dependent Autoimmune Diabetic BB Rats. Endocrinology. 2007 a;148:2863–2869. doi: 10.1210/en.2006-1375. [DOI] [PubMed] [Google Scholar]
- Zhou H, Zhang T, Harmon JS, Bryan J, Robertson RP. Zinc, Not Insulin, Regulates the Rat α-Cell Response to Hypoglycemia In Vivo. Diabetes. 2007 b;56:1107–1112. doi: 10.2337/db06-1454. [DOI] [PubMed] [Google Scholar]