Abstract
Objectives
Active surveillance (AS) protocols are designed to spare patients with ‘low risk’ prostate cancer (PCa) the potential morbidity of treatment. Our objective was to examine the treatment outcomes of men who would have been eligible for AS but rather underwent immediate radical prostatectomy (RRP).
Methods
From a prospective RRP database, we evaluated the tumor features and treatment outcomes for men who would have met one of three published AS criteria: (1) clinically localized disease, Gleason ≤ 7, and no significant comorbidities (Patel et al.) (2) T1b-T2b N0M0 disease, Gleason ≤ 7, and PSA ≤ 15 ng/ml (Choo et al.), or (3) T1c PCa (Mohler et al.).
Results
3959, 3536, and 2330 RRP patients, respectively, would have met these AS criteria. At surgery, 3–4% had a Gleason score of 8–10, 16–19% had positive surgical margins, 15–18% had extracapsular tumor extension, 3–5% had seminal vesicle invasion, and 0.4–1% had lymph node metastasis. The 5–year progression-free survival rate ranged from 84–89%. Metastasis occurred in 0.1–1.2%, and 0.1– 0.9% died from PCa. On multivariate analysis, Gleason score > 6 was the strongest predictor of biochemical progression.
Conclusions
A substantial proportion of men who might have been considered potential AS candidates had aggressive tumor features at RRP and/or progression. Biopsy Gleason score > 6 was the strongest predictor of adverse outcomes, highlighting the importance of limiting active surveillance to patients with Gleason ≤ 6. Overall, the accurate identification of patients with truly indolent PCa at the time of diagnosis remains challenging.
Keywords: active surveillance, prostate cancer, prostatectomy, candidate, outcomes
Introduction
Prostate cancer (PCa) is the most common non-cutaneous cancer among men in the United States and is the second-leading cause of cancer death.1 As a result of widespread screening efforts, PCa is increasingly detected at a localized stage. Due to the inability to reliably determine which patients diagnosed with PCa have indolent, or ‘low risk’ versus aggressive disease, there is significant debate as to which patients require immediate definitive therapy and which may be safely monitored. This difficult clinical decision is compounded by the potential for lasting side effects after intervention, including urinary incontinence and erectile dysfunction.2
To help clarify this issue, data are available from several large watchful waiting series. For example, a meta-analysis of 6 watchful waiting studies, involving patients with clinical stage T1 or T2 disease, reported that 19%, 42%, and 74% of patients with well-, moderately-, and poorly-differentiated tumors, respectively, developed metastases within 10 years.3 Similarly, Johansson et al reported on a watchful waiting cohort followed for a mean of 21 years.4 Their study concluded that although an indolent course is common for the first 10 to 15 years, patients with a 15-year life expectancy should consider definitive intervention due to the risk of late progression, metastasis, and death from prostate cancer.
The Scandinavian randomized controlled trial of watchful waiting versus RRP demonstrated that RRP led to a reduction in overall mortality, prostate cancer-specific mortality, and metastases as compared to watchful waiting.5 Although this study provides high-quality evidence in favor of surgical intervention over watchful waiting for patients with localized PCa, the majority of the participants in this study had clinical stage T2 disease. Thus, less is known about the risk to benefit ratio associated with surgery versus watchful waiting in the growing population of low-risk, stage T1c PCa seen today.
Moreover, the watchful waiting approach used in the aforementioned studies differs from some contemporary “active surveillance” protocols, in which patients with ‘low risk’ PCa undergo repeat biopsies at various intervals and definitive treatment is recommended if there is evidence of tumor progression.6 A limitation of current active surveillance programs is the lack of a clear consensus on the optimal selection or trigger for transitioning to active treatment during the so-called “window of curability”.7
A recent study by Conti et al. showed that a varying proportion of potential candidates for active surveillance had more aggressive tumor features at radical prostatectomy, depending upon the specific criteria used.8 The goal of our study was to further examine the pathological and disease-specific outcomes of patients who met one of three different criteria for active surveillance, but who elected to undergo immediate RRP.
Material and Methods
From 1983 to 2006, 4265 men underwent open RRP by a single surgeon (WJC).
We identified patients who met the criteria for three published active monitoring protocols: (1) clinically localized disease, biopsy Gleason score ≤ 7, and no significant co-morbidities (Patel et al.);9 (2) clinical stage T1b-T2b N0M0 disease, biopsy Gleason score ≤ 7, and PSA ≤ 15 ng/ml (Choo et al.)10; or (3) clinical stage T1c disease (Mohler et al.)11.
In men who met these criteria, but were treated with immediate radical prostatectomy, we examined the following pathology tumor features: pathological stage, margin status, lymph node involvement, and prostatectomy Gleason score. We also calculated the biochemical progression rates for men meeting each criterion, wherein biochemical progression was defined as a postoperative PSA level > 0.2 ng/ml confirmed by a second measurement. We also calculated the biochemical progression rate using an alternate definition of a postoperative PSA >0.4 ng/ml. Further, we calculated the postoperative PSA doubling time for men with biochemical recurrence. The Kaplan-Meier method was used to calculate 5-year progression free survival rates. Furthermore, we assessed rates of metastasis, overall survival, and 5- and 10-year prostate cancer-specific survival. Finally, we performed a multivariate analysis using Cox proportional hazards regression to identify which clinical features were associated with a greater risk of biochemical recurrence in men who met each active monitoring criterion. Probability values < 0.05 were considered significant. All statistical analysis was performed using SAS 9.1.3.
Results
Of the 4265 original patients, 4060 (95%) had data available for analysis. A total of 3959 (1), 3536 (2), and 2330 (3) men who would have met the Patel9 (1), Choo10 (2), and Mohler11 (3) criteria for active surveillance, respectively, were treated by immediate RRP. Table 1 shows the demographic information for patients meeting criteria for each of the three protocols. Most patients were white and had a biopsy Gleason score ≤ 6.
Table 1.
Patient demographic information.
| Clinically localized, biopsy Gleason score ≥ 7 and no significant comorbidities (N = 3959) | T1b-T2b N0M0, biopsy Gleason score ≤ 7 and PSA ≤ 15 ng/ml (N = 3536) | T1c PCa (N = 2330) | |
|---|---|---|---|
| Mean Age (range) | 61 (36–80) | 61 (36–80) | 60 (36–78) |
| Race (% White) | 3735 (95%) | 3336 (95%) | 2168 (93%) |
| Median PSA (range) | 5.6 (0.0–98.0) | 5.4 (0.0–15.0) | 5.5 (0.0–66.0) |
| Biopsy Gleason > 6 | 694 (18%) | 596 (17%) | 415 (18%) |
Table 2 shows the pathology tumor features and outcomes data for each subset of patients. Between 3% and 4% of patients had a prostatectomy Gleason score of 8 to 10. Positive surgical margins were present in 16 to 19%, and extra-capsular tumor extension in 15 to 18%. Seminal vesicle invasion occurred in 3 to 5%, and 0.4 to 1% had lymph node metastases. Overall, biochemical progression occurred in up to 14% of patients, with corresponding five-year progression free survival rates between 84% and 89% (Figure 1). Among men with biochemical recurrence, 11% or fewer had a PSA doubling time <12 months. Rates of metastasis were low, as were the overall and prostate-cancer specific mortality rates.
Table 2.
Pathologic and clinical outcomes among patients in our immediate prostatectomy cohort who would have met 3 criteria for active monitoring from the literature.
| Clinically localized, biopsy Gleason score ≤ 7, and no significant comorbidities (N = 3959) | T1b-T2b N0M0, biopsy Gleason score ≤ 7, and PSA ≤15 ng/ml (N = 3536) | T1c PCa (N = 2330) | |
|---|---|---|---|
| Prostatectomy Gleason Score 8–10 (%) | 4 | 4 | 3 |
| Positive Surgical Margins (%) | 19 | 18 | 16 |
| Extracapsular Extension (%) | 18 | 17 | 15 |
| Seminal Vesicle Invasion (%) | 5 | 4 | 3 |
| Lymph Node Metastasis (%) | 1 | 0.4 | 0.4 |
| Biochemical Progression (%) | |||
| >0.2 ng/ml | 14 | 11 | 8 |
| >0.4 ng/ml | 11 | 8 | 5 |
| 5-Year Progression-Free Survival Rate (%) | 84 | 88 | 89 |
| Postoperative PSA Doubling Time | |||
| <3 months (%) | 2 | 2 | 2 |
| 3– 6 months (%) | 4 | 4 | 7 |
| 6–12 months (%) | 11 | 11 | 8 |
| Metastasis (%) | 1.2 | 0.6 | 0.1 |
| 5-Year | 98 | 99 | 100 |
| Metastasis-Free Survival Rate | |||
| Deceased (%) | 6.4 | 4.3 | 2.0 |
| 5-Year Overall Survival Rate | 95 | 97 | 97 |
| PCa Death (%) | 0.9 | 0.3 | 0.1 |
| 5-Year Cancer-Specific Survival Rate (%) | 99 | 99 | 100 |
| 10-Year Cancer-Specific Survival Rate (%) | 99 | 99 | 100 |
Figure 1.
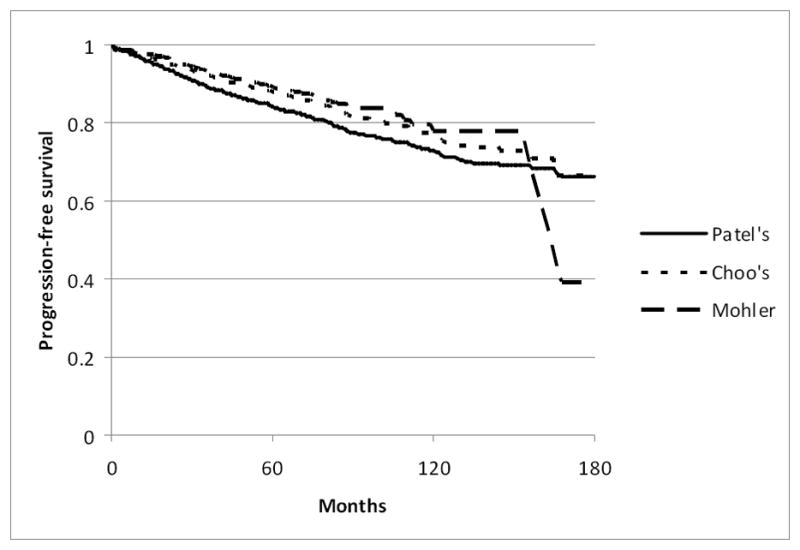
Kaplan-Meier curves for progression-free survival among men who would have met the Patel, Choo, and Mohler criteria for active surveillance.
Multivariate analysis was performed to determine if the pre-operative parameters chosen in each protocol were associated with the risk of biochemical progression (Table 3). Overall, the strongest clinical predictor of biochemical progression was a biopsy Gleason score > 6. Men with a biopsy Gleason score > 6 had a 2.74- to 3.19-fold increased hazard ratio for biochemical progression. For men who would have met the criteria by Patel et al.9 and Choo et al.10 which included both clinical stage T1 and T2, palpable disease was associated with a 1.9 -fold increased hazard ratio for biochemical progression. Finally, the PSA level at diagnosis was a statistically significant predictor of progression for patients in our cohort who would have met all three criteria.
Table 3.
Multivariate analysis of clinical predictors of biochemical progression after immediate radical prostatectomy in patients who would have met the criteria for active monitoring.
| a) Patel criteria (Clinically localized, Gleason score ≤ 7, and no significant comorbidities) | |||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Age (yrs) | 1.01 | 1.00–1.02 | 0.17 |
| PSA (ng/ml) | 1.04 | 1.03–1.04 | <0.0001 |
| Gleason > 6 | 2.74 | 2.27–3.32 | <0.0001 |
| Clinical Stage T2 vs. T1 | 1.89 | 1.56–2.30 | <0.0001 |
| b) Choo criteria (T1b-T2b N0M0, Gleason score ≤ 7, and PSA ≤ 15 ng/ml | |||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Age (yrs) | 1.00 | 0.99–1.02 | 0.68 |
| PSA (ng/ml) | 1.15 | 1.12–1.19 | <0.0001 |
| Gleason > 6 | 2.87 | 2.30–3.58 | <0.0001 |
| Clinical Stage T2 vs. T1 | 1.92 | 1.55–2.38 | <0.0001 |
| c) Mohler criteria (T1c PCa) | |||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Age (yrs) | 1.01 | 0.99–1.03 | 0.44 |
| PSA (ng/ml) | 1.06 | 1.04–1.07 | <0.0001 |
| Gleason > 6 | 3.19 | 2.35–4.35 | <0.0001 |
Comment
To limit the potential for ‘over-treating’ low-risk prostate cancer, several active monitoring protocols have been reported for patients with favorable disease characteristics at the time of diagnosis. The ideal active surveillance protocol would require: 1) an accurate pre-operative tool to predict which individuals have truly indolent disease, 2) parameters that can be serially monitored to rapidly identify individuals with disease progression, and 3) the potential for delayed treatment with the same likelihood of success as immediate therapy without any additional treatment-related morbidity.
With regard to the clinical prediction tools, investigators have retrospectively created models to help predict the presence of ‘low risk’ disease. For instance, Bastian et al. demonstrated that 91.6% of patients who met the following pre-operative criteria (i.e. Epstein criteria) had organ-confined disease at the time of prostatectomy: a prostate-specific antigen density < 0.15 ng/mL/gm, biopsy Gleason score ≤ 6, < 3 positive biopsy cores, and ≤ 50% involvement of any core with cancer.12 Similarly, Kattan et al. created a nomogram for the preoperative prediction of “indolent” prostate cancer using pretreatment PSA, clinical stage, Gleason score and other biopsy features. 13 Although such criteria are useful for patient counseling and risk assessment, they are unable to accurately predict the surgical pathology for some patients. Indeed, our data concurs with the findings of another recent study8 that despite the presence of favorable clinical characteristics, a proportion of patients with apparent low-risk disease have adverse pathology features and disease progression after undergoing immediate treatment. Overall and prostate-cancer specific survival rates are highly favorable in our patient cohort; however, it is unknown what the outcomes would have been after a period of surveillance.
With regard to assessing disease progression and the need for intervention among patients on surveillance, several different methods have been evaluated. In the study by Patel et al.,9 patients on active surveillance were followed with DRE and serum PSA every 3 months for the first year and semi-annually thereafter. In addition, biopsies were performed at baseline, 1 year, 2 to 3 years, and for any clinical indication. Based upon these serial evaluations, they defined disease progression using a point system that incorporated changes in Gleason score, PSA level, DRE, and transrectal ultrasound findings. Of 88 patients in their series, 22 (25%) had progression at a mean follow-up of 44 months, and 31 (35.2%) patients underwent treatment. Among the patients who later underwent radical prostatectomy, 88% were upgraded from the biopsy Gleason grade, and 18% had extra-capsular tumor extension. Although there were no cases of biochemical failure or metastases, the follow-up period was short (15.2 months).
In the study by Choo et al.,10 progression was initially defined as a PSADT < 2 years, an increase in palpable disease, bladder outlet obstruction, evidence of metastatic disease, or a Gleason score of 8–10 on repeat biopsy. However, among 24 patients from this protocol who underwent surgery for a PSADT of less than 2 years, 58% had pathology stage T3a–b disease, and 8% had lymph node metastases.14 With these outcomes in mind, the authors lengthened their PSADT criteria for recommending intervention to 3 years.
Finally, Mohler et al. defined progression as the development of a suspicious nodule on DRE, lower urinary tract symptoms, gross hematuria, or evidence of metastatic disease.11 Patients were also considered to have progressive disease with three consecutive rises in the serum PSA level with an overall increase of 5 ng/mL or greater. Of the 27 men in their series, 9 (33.3%) had evidence of disease progression, of which 8 occurred in the first 18 months of surveillance. Of the 4 patients who underwent radical prostatectomy, 2 had organ-confined disease, one had extra-capsular tumor extension, and one had seminal vesicle invasion. At 42, 24, 24, and 12 months of follow-up, respectively, all patients were free from biochemical recurrence.
In general, the authors of the aforementioned active monitoring studies concluded that active surveillance is a viable option for some men harboring ‘low risk’ prostate cancer.9,10 However, it is clear from their data that not all men who met their selection criteria for potentially ‘low risk’ prostate cancer truly had ‘low risk’ disease. Because most series of active surveillance for screen-detected prostate cancers are relatively recent, additional follow-up will be necessary to evaluate the long-term disease-specific outcomes. In the absence of a randomized trial involving these contemporary patients, it also remains unclear whether the treatment outcomes would have been any different with immediate intervention.
Our goal was to identify men from our surgical database who would have met criteria for active surveillance and to evaluate the outcomes of early intervention in these specific subgroups. Our results demonstrate that a proportion of patients who might be candidates for active monitoring actually have unfavorable treatment outcomes even with immediate treatment. The reported data correlates well with data from other series.15 It is not possible to determine whether a delay in treatment for these patients would have led to worse pathology, an increase in treatment-related morbidity, or missing the window for curative intervention.
Delaying PCa treatment is not the same as active surveillance; however, a recent study from the Shared Equal Access Regional Cancer Hospital (SEARCH) database evaluated patients with ‘low risk’ prostate cancer and their clinical outcomes following a surgical delay.16 Interestingly, patients who experienced a delay beyond 180 days had a significant decrease in biochemical disease-free survival after a mean follow-up of 44 months. That notwithstanding, Warlick et al. reported that the rate of “noncurable cancer” with delayed intervention after a period of surveillance in 38 patients was statistically similar to that of immediate intervention in 150 comparable low risk patients, albeit using a surrogate endpoint for “noncurability”17 Clearly, additional evidence is necessary to further elucidate this important issue.
Quality-of-life measures impact the decision making process of patients and physicians with regard to immediate versus delayed treatment for PCa. Our group recently published the complication rates of men with ‘low risk’ prostate cancer after undergoing immediate radical prostatectomy.18 Importantly, younger men with ‘low risk’ disease had favorable functional outcomes after surgery, and their outcomes were superior to those of older men. The current manuscript details the oncological outcomes in a similar patient cohort. Taken together, these references could serve as useful guides for patients and physicians with regard to complication rates and disease-specific outcomes following radical prostatectomy for ‘low risk’ PCa.
Our study has several limitations. First, as discussed, because all of the patients in our cohort underwent RRP shortly after diagnosis, it is unknown what their outcomes would have been with treatment delay or as part of the various active monitoring protocols. A second limitation of our study is the use of biochemical progression as a primary endpoint.
Next, many patients in our cohort underwent prostate biopsy at outside institutions, and the pathology from such specimens was not centrally re-reviewed. In addition, biopsy core data were not available for this cohort. Thus, we are unable to evaluate the treatment outcomes in patients meeting the criteria by Epstein and colleagues.19 However, in the recent study by Conti et al., men who met the Johns Hopkins active surveillance criteria had the lowest rates of adverse pathological features at radical prostatectomy.8 Thus, it is possible that additional information on the percentage of biopsy core involvement may have improved the preoperative prediction of disease aggressiveness. Of note, our research group has previously reported on a separate cohort of men with ≤ 2 cores of Gleason 6 prostate cancer on biopsy, and a significant proportion of these men had adverse features at radical prostatectomy.20 Future studies are warranted to identify new biomarkers that could improve patient selection for active surveillance.
Conclusions
Active surveillance protocols are currently offered to patients with ‘low-risk’ prostate cancer. We retrospectively examined the pathology features and biochemical progression-free survival rates among men who would have met three active monitoring criteria from the literature but elected active treatment. Of these men, an appreciable proportion had aggressive features in the prostatectomy specimen and/or biochemical progression. Due to the limitations of current clinical staging, some men with apparent ‘low-risk’ disease harbor aggressive tumors. Our data suggest that active surveillance should not be offered to patients with a biopsy Gleason score > 6. It is possible that detailed biopsy core information and new biomarkers may help to improve the accuracy of clinical staging.
Acknowledgments
CST acknowledges the Robert H. Lurie Comprehensive Cancer Center of Northwestern University for a Zell Family Faculty Award. Support for WJC from the Urological Research Foundation, Beckman Coulter, Inc., the Prostate SPORE grant (P50 CA90386-05S2) and the Robert H. Lurie Comprehensive Cancer Center grant (P30 CA60553).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. [Accessed March 5, 2008];Cancer Facts and Figures. 2008 http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf.
- 2.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 3.Chodak GW, Thisted RA, Gerber GS, et al. Results of conservative management of clinically localized prostate cancer. N Engl J Med. 1994;330:242–8. doi: 10.1056/NEJM199401273300403. [DOI] [PubMed] [Google Scholar]
- 4.Johansson JE, Andren O, Andersson SO, et al. Natural history of early, localized prostate cancer. Jama. 2004;291:2713–9. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]
- 5.Bill-Axelson A, Holmberg L, Filen F, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian prostate cancer group-4 randomized trial. J Natl Cancer Inst. 2008;100:1144–54. doi: 10.1093/jnci/djn255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter HB, Kettermann A, Warlick C, et al. Expectant management of prostate cancer with curative intent: an update of the Johns Hopkins experience. J Urol. 2007;178:2359–64. doi: 10.1016/j.juro.2007.08.039. discussion 2364–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dall'Era MA, Cooperberg MR, Chan JM, et al. Active surveillance for early-stage prostate cancer: review of the current literature. Cancer. 2008;112:1650–9. doi: 10.1002/cncr.23373. [DOI] [PubMed] [Google Scholar]
- 8.Conti SL, Dall'era M, Fradet V, et al. Pathological outcomes of candidates for active surveillance of prostate cancer. J Urol. 2009;181:1628–33. doi: 10.1016/j.juro.2008.11.107. discussion 1633–4. [DOI] [PubMed] [Google Scholar]
- 9.Patel MI, DeConcini DT, Lopez-Corona E, et al. An analysis of men with clinically localized prostate cancer who deferred definitive therapy. J Urol. 2004;171:1520–4. doi: 10.1097/01.ju.0000118224.54949.78. [DOI] [PubMed] [Google Scholar]
- 10.Choo R, Klotz L, Danjoux C, et al. Feasibility study: watchful waiting for localized low to intermediate grade prostate carcinoma with selective delayed intervention based on prostate specific antigen, histological and/or clinical progression. J Urol. 2002;167:1664–9. [PubMed] [Google Scholar]
- 11.Mohler JL, Williams BT, Freeman JA. Expectant management as an option for men with stage T1c prostate cancer: a preliminary study. World J Urol. 1997;15:364–8. doi: 10.1007/BF01300184. [DOI] [PubMed] [Google Scholar]
- 12.Bastian PJ, Mangold LA, Epstein JI, et al. Characteristics of insignificant clinical T1c prostate tumors. A contemporary analysis. Cancer. 2004;101:2001–5. doi: 10.1002/cncr.20586. [DOI] [PubMed] [Google Scholar]
- 13.Kattan MW, Eastham JA, Wheeler TM, et al. Counseling men with prostate cancer: a nomogram for predicting the presence of small, moderately differentiated, confined tumors. J Urol. 2003;170:1792–7. doi: 10.1097/01.ju.0000091806.70171.41. [DOI] [PubMed] [Google Scholar]
- 14.Klotz L. Active surveillance for prostate cancer: for whom? J Clin Oncol. 2005;23:8165–9. doi: 10.1200/JCO.2005.03.3134. [DOI] [PubMed] [Google Scholar]
- 15.Suardi N, Capitanio U, Chun FK, et al. Currently used criteria for active surveillance in men with low-risk prostate cancer: an analysis of pathologic features. Cancer. 2008;113:2068–72. doi: 10.1002/cncr.23827. [DOI] [PubMed] [Google Scholar]
- 16.Freedland SJ, Kane CJ, Amling CL, et al. Delay of radical prostatectomy and risk of biochemical progression in men with low risk prostate cancer. J Urol. 2006;175:1298–302. doi: 10.1016/S0022-5347(05)00646-4. discussion 1302–3. [DOI] [PubMed] [Google Scholar]
- 17.Warlick C, Trock BJ, Landis P, et al. Delayed versus immediate surgical intervention and prostate cancer outcome. J Natl Cancer Inst. 2006;98:355–7. doi: 10.1093/jnci/djj072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loeb S, Roehl KA, Helfand BT, et al. Complications of open radical retropubic prostatectomy in potential candidates for active monitoring. Urology. 2008;72:887–91. doi: 10.1016/j.urology.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Epstein JI, Walsh PC, Carmichael M, et al. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. Jama. 1994;271:368–74. [PubMed] [Google Scholar]
- 20.Griffin CR, Yu X, Loeb S, et al. Pathological features after radical prostatectomy in potential candidates for active monitoring. J Urol. 2007;178:860–3. doi: 10.1016/j.juro.2007.05.016. discussion 863. [DOI] [PubMed] [Google Scholar]


