Abstract
Cartilage morphology change is an important biomarker for the progression of osteoarthritis. The purpose of this study was to assess the accuracy of in vivo cartilage thickness measurements from MR image-based 3D cartilage models using a laser scanning method and to test if the accuracy changes with cartilage thickness. Three-dimensional tibial cartilage models were created from MR images (in-plane resolution of 0.55 mm and thickness of 1.5 mm) of osteoarthritic knees of ten patients prior to total knee replacement surgery using a semi-automated B-spline segmentation algorithm. Following surgery, the resected tibial plateaus were laser scanned and made into 3D models. The MR image and laser-scan based models were registered to each other using a shape matching technique. The thicknesses were compared point wise for the overall surface. The linear mixed-effects model was used for statistical test. On average, taking account of individual variations, the thickness measurements in MRI were overestimated in thinner (<2.5 mm) regions. The cartilage thicker than 2.5 mm was accurately predicted in MRI, though the thick cartilage in the central regions was underestimated. The accuracy of thickness measurements in the MRI-derived cartilage models systemically varied according to native cartilage thickness.
Keywords: cartilage, thickness, accuracy, osteoarthritis, MRI
1 Introduction
A substantial portion of the elderly population suffers from degenerative joint disease (osteoarthritis). However, the primary treatments address the symptoms rather than the disease since the causes for initiation and progression of osteoarthritis are not well understood [1]. Quantitative measurement of cartilage thickness offers an attractive method for learning more about the disease and can be used to track the progression of osteoarthritis [2]. In particular, semi-automatic image segmentation methods have been used to delineate cartilage boundaries of knee MR images and to create 3D cartilage models [3–6]. While these 3D models have advanced our understanding of morphological cartilage changes associated with osteoarthritis [2,7–10], the evaluation of new disease modifying interventions would benefit from a rigorous assessment of the condition influencing the spatial accuracy of these models.
Assessing the regional accuracy of cartilage morphometric measurements using 3D models derived from MRI is particularly important for the evaluation of disease modifying interventions designed for treating osteoarthritis at an early stage since the lesion is typically localized to a specific region of the cartilage. Thus more global measurements such as cartilage volume might not detect regional changes that are critical to the assessment of the disease process. Thus there is a need for additional work to complement existing studies (Table 1) of 3D cartilage models; that have assessed the accuracy of volume measurement of a 3D cartilage model using a water displacement method for cartilage in specimens from amputated knees and total knee replacement (TKR) surgeries [3,8,10,11]; and surface area of a 3D cartilage model using an image of aluminum foil covering the cartilage from total TKR surgery [10]. An accurate stereophotogrammetric method has also been used to make precise measurements of the 3D shape of ex vivo cartilage [12] and to verify cartilage thickness measurements. However, that study was conducted using cadaveric cartilage from MR images [13] and the relationship of these measurements taken from in vivo MRI has not been established. While thickness accuracy has also been evaluated using anatomical sections [8,10,11], it was unclear whether these studies controlled for thickness changes to tissue dehydration in open air.
Table 1.
Summary of previous studies on the accuracy of MRI-derived 3D cartilage models
| Validation method | Specimens | No. of specimens | Error quantification |
|---|---|---|---|
| Cartilage volume | Femoral, tibial, and patellar cartilage from amputated knees, and patellar cartilage from TKR surgery [3] Tibial cartilage from TKR surgery [8] Tibial and patellar cartilage from TKR surgery [10] Femoral, tibial, and patellar cartilage from cadaveric human knees [12] |
3+3 8 Half of 21 8 |
8.2% and 5.9% for two MRI methods rms average 5.5% qMRI overestimation in patella (5.1%) and lateral tibia (3.6%), and underestimation in medial tibia (−3.1%) 3.6%, 3.8%, 4.2%, and 1.1% for patellar, femoral, medial, and lateral cartilage, respectively |
| Cartilage surface area | Tibial and patellar cartilage from TKR surgery [10] | 21 | 4.6% to 9.1% |
| Cartilage thickness | Tibial cartilage from TKR surgery [8] Tibial and patellar cartilage from TKR surgery [10] Femoral, tibial, and patellar cartilage from cadaveric human knees [12] |
8 Half of 21 8 |
rms average 6.2% 4.3% for patella and 12.3% for medial tibia For patella, 84.5% of the surface <1 mm error |
Thus while many aspects of the accuracy of morphometric have been addressed some of the conditions limiting the accuracy of morphometric measurements derived from MRI including partial volume effects, chemical shift, and geometric distortion [14] have not been evaluated. The partial volume effect is particularly important when considering the sensitivity of the accuracy of thickness measurements to the native cartilage thickness. The accuracy was predicted to be reduced for thinner cartilage due to a partial volume effect and MR image voxel anisotropy [15,16].
There remain unanswered questions regarding the sensitivity of cartilage thickness measurements to the native thickness of the cartilage. Considering the importance of local regional cartilage thickness measurement in diagnosing the progression of osteoarthritis, there remains a need to evaluate the sensitivity of cartilage thickness measurements to the native thickness of the cartilage. Thus, the purpose of this study was to assess the accuracy of in vivo cartilage thickness measurements from MR image-based 3D cartilage models using a laser scanning method [17,18] and to test the hypothesis that the accuracy of the thickness measurement changes with cartilage thickness.
2 Methods
2.1 Materials
Approval to conduct this study was obtained from the institutional review board. Total knee replacement patients who were scheduled for surgery within 1 month were recruited. Subjects with reasonable amount of cartilage in at least one compartment in the osteoarthritic knee were included. After informed consent was obtained, MR images of the osteoarthritic knee were performed on ten subjects (all male, age 69±8 years, height 173±6 cm, weight 99±17 kg, and body mass index 33±5 kg/m2). To minimize the effect of loading history on articular cartilage morphology, the MR images were taken early in the morning and the subjects were instructed to avoid loading knees before MR imaging. These subjects were to be operated on within 2 weeks (an average of 6 days) after the MR images were obtained.
2.2 MR Imaging Knee Cartilage
Knee MR images were obtained prior to surgery using a fat-saturated 3D spoiled gradient recalled echo in sagittal plane. This sequence had TR (repetition time) =60 ms, TE (echo time) =5 ms, flip angle =40 deg, receiver bandwidth of +/−15.63 kHz, FOV (field of view) of 140 × 140 mm, slice thickness of 1.5 mm, 60 slices, and matrix of 256 × 256. So the in-plane resolution was 0.55 × 0.55 mm. Scan time was 10 min and 18 s. Images were acquired on a 1.5T MRI scanner (GE Healthcare, Milwaukee, WI and SIGNA LX, software rev. 12.4) with a standard transmit-receive extremity coil. This sequence has previously been used to assess cartilage morphology [6,8,19,20].
2.3 Cartilage Model From MR Images
The MR images were processed using custom software [6]. A single experienced observer performed all segmentations to achieve maximum reproducibility [6]. The cartilage was segmented from each image of a set of MR images using a semi-automatic B-spline snake method [5,21]. Boundaries from the initial segmentation were manually corrected to increase accuracy. The boundaries obtained from the segmentation were reconstructed into a 3D polygon model using a surface rendering method [22]. The reproducibility of this method was tested in our previous study [6].
2.4 Cartilage Model From Actual Tissue Using Laser Scan
Following total knee replacement surgery, the resected tibial plateaus were kept in isotonic saline solution for less than 2 h until they were laser scanned. The actual cartilage thickness was measured using a 3D laser-scan based method. The proximal tibia was attached to a platform made of acrylonitrile butadiene styrene (ABS) using instant epoxy glue and left in air for 10 min for adhesive fixation. The cubical ABS platform was designed with two cylindrical bars to exactly register multiple laser-scan data in order to reconstruct the 3D shape. The fluid on the cartilage surface was dried in air during the adhesive fixation time for 10 min and the shiny and semitransparent surface was treated with white spray powder (starch/acrylates/acrylamide copolymer) to increase opacity and decrease the laser-scan errors [16].
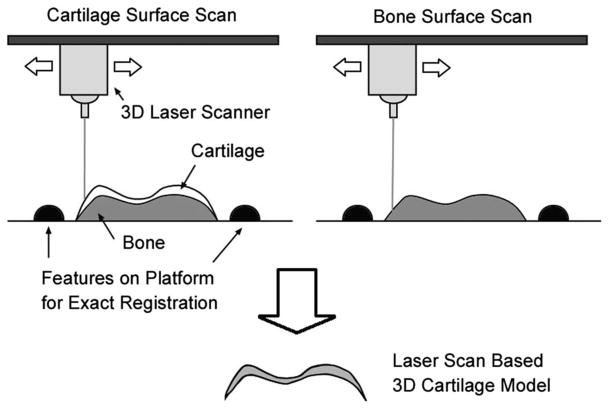
The cartilage surface was scanned on a rotating table using a 3D desktop laser scanner (Model-15, Cyberware, Monterey, CA). The resolution of the laser scanner was 50 μm in depth. The field of view of the laser scanning was 80 × 80 mm2 and points were sampled every 300 μm. The scanning was repeated at 24 different angles and registered using the program CYDIR (Cyberware, Monterey, CA). This overlapping multiscan technique increased the overall accuracy to 50 mm. The laser scan to obtain a 3D surface geometry took 12 min. After the articular surface of the cartilage had been scanned, the proximal tibia piece was submerged in 6.0% sodium hypochlorite solution for up to 24 h to digest the cartilage [6]. After the cartilage was removed, the laser scanner was again used to find the subchondral bone. The two 3D laser scans of cartilage and subchondral bone surfaces were registered to each other using the features in the ABS platform by rigid body rotation and translation [23] (Fig. 1). The registered surfaces were reconstructed into a 3D model of actual cartilage (Fig. 1) using the RAPIDFORM software (Inus Technology, Seoul, South Korea) [6].
Fig. 1.
The schematic description of creating 3D cartilage models from two laser-scan data sets. Cartilage surface and bone surface were scanned along with features on platform and registered to each other using the platform features to obtain actual shape of cartilage.
2.5 Thickness Comparison Between Two Models
Thicknesses were calculated for all the points on the articular surface by finding the closest point on the cartilage-subchondral bone interface surface. The thickness information was encoded on the cartilage model [5,6] as a thickness map. The 3D cartilage models from laser-scan data and MR images were registered to each other using a surface matching technique [23]. The thickness maps were projected onto planes and compared point wise as shown in Fig. 2. The difference map was calculated by subtracting thickness values of the laser-scan based 3D model from the MR image-based 3D model.
Fig. 2.
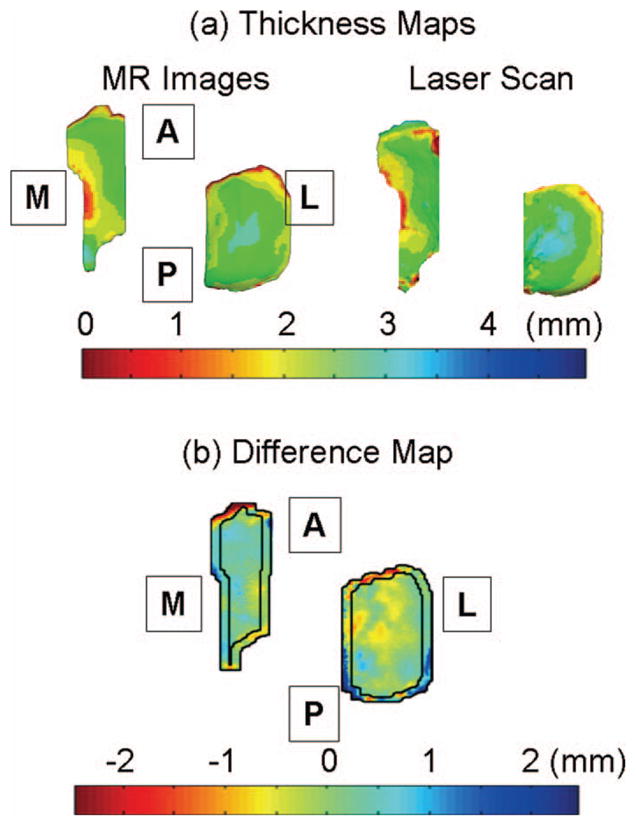
(a) Thickness maps calculated from a MR image-based 3D model and a laser-scan based 3D models. Dark blue represents the thickest cartilage and dark red represents the thinnest cartilage as shown in the color bar. (b) Difference maps were calculated by subtracting the thickness maps from the laser-scan based 3D model from the MR image-based 3D model. Thus blue regions represent thickness overestimation in MR image-based 3D models, green regions show the same estimation, and red regions represent underestimation. The letters A, P, M, and L in boxes represent anterior, posterior, medial, and lateral, respectively.
2.6 Linear Mixed-Effects Model
We hypothesized that the errors of the thickness measurements varied linearly with actual cartilage thicknesses. The statistical model to test this hypothesis included the effects of not only actual thicknesses but also variations between specimens on the errors because the data were obtained from ten specimens [24].
In the formula, yij is the error of jth measurement in ith specimen and xij is its actual thickness. β0 and β1 represent the fixed-effects interpreted as population parameters for the intercept and the slope, respectively. b0i and b1i represent the random-effects, which are deviations from population parameter of ith specimen for the intercept and the slope, respectively. εij is the residual. The null hypothesis that β1 = 0 was tested which means that the error is not the linear function of actual thickness. The 95% confidence interval of the fitted line was also calculated to test if error is different from 0.
2.7 Error Assessment in Measuring Actual Cartilage Shape
The sensitivity of thickness change due to dehydration was confirmed in a manner similar to a previous study that reported the dehydration rate of articular cartilage in the open air and provided an approximation function of cartilage thickness reduction over time [25]. We confirmed that the previously reported approximation function for the type of specimen in this study by evaluating the volume change due to dehydration over a 24 h period. The results were consistent with the previous study. The time the cartilage specimens were exposed to air were carefully controlled to 10 min of preparation and 12 min of laser scanning. Using the approximation function the thickness change for 22 min of exposure to air was less than 1%.
3 Results
The cartilage thickness based on 3D cartilage models derived from MRI tends to be overestimated for thin cartilage and the error decreased with the increase in the cartilage thickness for all specimens (Fig. 3).
Fig. 3.
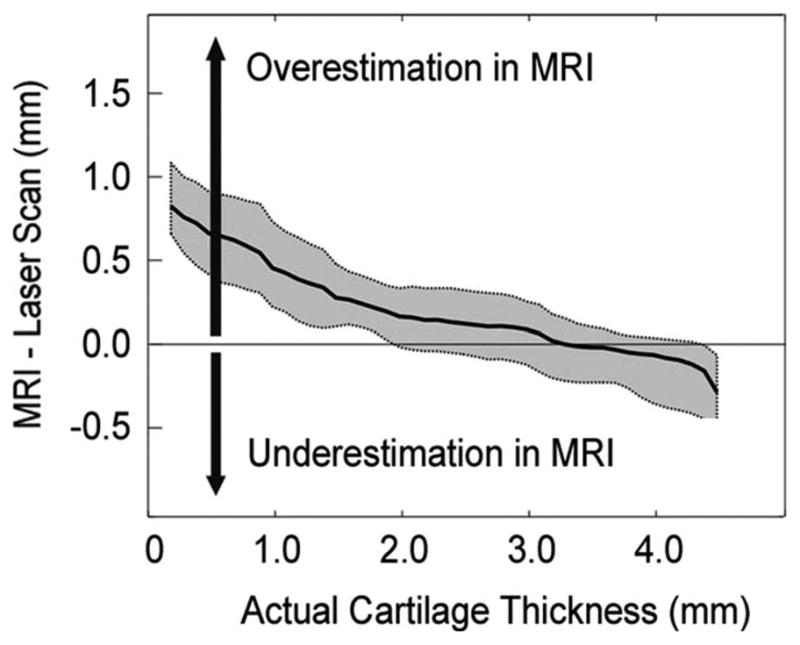
Differences in thickness measurement between MRI based 3D models and laser-scan based 3D models were calculated point by point for each of the specimens. This graph is from one of the tested specimens. Positive values represent thickness overestimation in MR image-based measurements. The median of the difference was drawn as solid lines, and 25% and 75% quartiles were drawn as dotted lines.
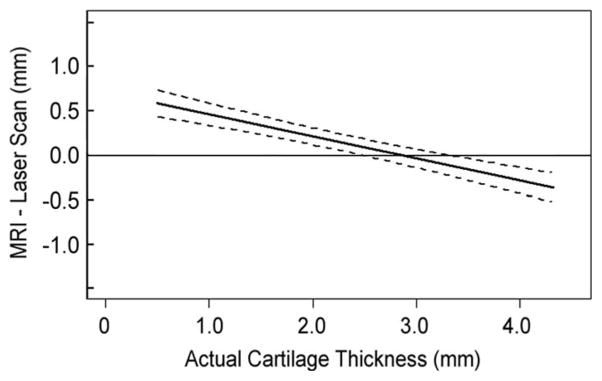
The accuracy systematically varied with the actual cartilage thickness at the significance level of 0.05 according to the linear mixed-effects model. The fitted line along with 95% confidence interval for the fixed-effects was obtained from a linear mixed-effects model (Fig. 4). The slope and intercept from the linear mixed-effect model was −0.25 and 0.71, respectively. The variations of slope and intercept between individual specimens ranged from −0.44 to −0.07, and from 0.23 to 1.00, respectively. The residuals of the mixed effects models were evenly distributed along the fitted values in a visual check.
Fig. 4.
Variations of individual specimens are shown for ten specimens with gray lines. Positive and negative values represent thickness overestimation and underestimation in MR image-based 3D models, respectively. The linear mixed-effects model was used to find a fitting line of the data from ten specimens. The black lines represent the fitted line from the linear mixed-effects model and the 95% confidence interval.
On average, taking account of individual variations, the thickness measurements in MRI were overestimated in thinner (< 2.5 mm) regions. The differences approached zero at the thickness between 2.5 mm and 3.3 mm, where the 95% confidence interval included the zero line. In thicker (>3.3 mm) regions the thicknesses were underestimated.
4 Discussion
The results showed that the accuracy of cartilage thickness measurements in the cartilage models derived from MRI varies systematically. Thin cartilage tends to be overestimated in the cartilage models. The thickness measurement was accurate for the cartilage whose thickness was between 2.5 mm and 3.3 mm.
The linear mixed-effects model could explain the effects of actual thicknesses (fixed effect) and specimens (random effect) on the errors in measuring cartilage thickness in MR image-based 3D models. A significant linear relationship between the accuracy and the actual cartilage thickness was found. This result supports the previous simulation studies [15,16] that the thickness of thin objects such as articular cartilage is overestimated due to partial volume effect and MR imaging voxel anisotropy.
The study showed that the accuracy of thickness measurement of articular cartilage in MRI-derived cartilage models is affected by its actual thickness. In our MR images, tibial cartilage orientation was almost vertical to sagittal plane thus the orientation effect was negligible but the MR image voxel anisotropy was 2.7 (=slice thickness/in-plane resolution), which is in the range that thin cartilage less than 2.5 mm can be affected [15,16].
This study implies that the accuracy of cartilage thickness measurements in cartilage models should be considered in the context of the native thickness of the articular cartilage specimens. It is expected that the error would be lower in measuring cartilage thickness of healthy articular cartilage.
While the previous simulation studies showed that the error approached zero in thick cartilage [15,16], the linear mixed-effects model in this study suggested that the thick cartilage (>3.3 mm) can be underestimated. One of the possible explanations should be due to our assumption of the linear change in the accuracy and testing thin osteoarthritic cartilage specimens, which may have governed the slope and the intercept of the linear mixed-effects model. While weight bearing was minimized prior to testing it is possible that the thickness in the central regions of cartilage was underestimated due to unknown residual compressive deformation in the central regions of the articular cartilage resulting from weight bearing prior to the test or passive joint capsule tension [26,27].
The specimens were from osteoarthritic knees, so some portions of the specimens had surface fibrillation. Though it did not change the surface shape visibly and was not detectable in MRI, we did not quantify the effect of this surface fibrillation on the laser scanning technique.
A thickness change due to dehydration of the cartilage in open air is an important consideration in studies of this type. The specimens were carefully controlled to be in the open air for 22 min from the starting of the set up to the completion of the laser scanning, 10 min for fixation on the platform and 12 min for scanning. While there could have been a small amount of dehydration during the 22 min there was no observable change in the shape and based on a study by Pham et al. [25] the cartilage thickness reduction over 22 min was less than 1%. Thus while small this effect should be considered when evaluating the results presented here or in other studies of this type.
We tested the accuracy of only one MR protocol and one resolution. There are many variables in MR imaging such as sequence, image resolution, and magnet strength. Recently, more efficient protocols such as steady state free precession (SSFP) [28] and fluctuating equilibrium MR imaging [29] have been developed for cartilage imaging and 3.0 Tesla machines are being adapted in clinics. This would save imaging time, increase image resolution, and signal to noise ratio (SNR). Currently, 3.0 Tesla magnets can achieve higher resolution such as in-plane resolution of 0.31 mm or less [30,31]. For the purpose of this study we assumed that the semi-automated B-spline snake method would give the best segmentation results. However, there exist other segmentation schemes such as anatomical template deformation or more automated segmentation techniques [32]. It may be that for other MR protocols and segmentation methods, the accuracy of cartilage thickness measurement will be different than what we found in this study. Thus, additional studies with different settings in the future would help generalize this result.
In conclusion, the accuracy of cartilage thickness measurement from MRI-derived cartilage models varied according to cartilage thickness. The thickness of thin cartilage less than 2.5 mm was significantly overestimated in MRI, with MRI in-plane resolution of 0.55 mm and thickness of 1.5 mm when processed using semiautomated B-spline snake method. The cartilage thicker than 2.5 mm was accurately estimated in MRI though the thick cartilage in the central regions was underestimated.
Acknowledgments
We would like to thank all subjects who contributed their time and tissues, and the orthopedic doctors in Palo Alto VA hospital, Barbara Elspas and David Fisher. This study was funded by NIH Contract Nos. AR049792 and EB002524.
Contributor Information
Seungbum Koo, Email: skoo@cau.ac.kr, School of Mechanical Engineering, Chung-Ang University, Seoul 156-756, South Korea.
Nicholas J. Giori, Department of Orthopedic Surgery, Stanford University, 450 Broadway Street, Pavilion C, 4th Floor Redwood City, CA 94063-6342; VA Palo Alto Healthcare System, 3801 Miranda Avenue, Palo Alto, CA 94304-1290.
Garry E. Gold, Department of Radiology, Stanford University, Stanford, CA 94305.
Chris O. Dyrby, VA Palo Alto Healthcare System, 3801 Miranda Avenue, Palo Alto, CA 94304-1290; Department of Mechanical Engineering, Stanford University, 496 Lomita Mall, Durand Building, Room 061, Stanford, CA 94305-4038.
Thomas P. Andriacchi, Department of Orthopedic Surgery, Stanford University, 450 Broadway Street, Pavilion C, 4th Floor, Redwood City, CA 94063-6342; VA Palo Alto Healthcare System, 3801 Miranda Avenue, Palo Alto, CA 94304-1290; Department of Mechanical Engineering, Stanford University, 496 Lomita Mall, Durand Building, Room 061, Stanford, CA 94305-4038.
References
- 1.Andriacchi TP, Mündermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A Framework for the In Vivo Pathomechanics of Osteoarthritis at the Knee. Ann Biomed Eng. 2004;32(3):447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 2.Cicuttini FM, Wluka AE, Wang Y, Stuckey SL. Longitudinal Study of Changes in Tibial and Femoral Cartilage in Knee Osteoarthritis. Arthritis Rheum. 2004;50(1):94–97. doi: 10.1002/art.11483. [DOI] [PubMed] [Google Scholar]
- 3.Peterfy CG, van Dijke CF, Janzen DL, Gluer CC, Namba R, Majumdar S, Lang P, Genant HK. Quantification of Articular Cartilage in the Knee With Pulsed Saturation Transfer Subtraction and Fat-Suppressed MR Imaging: Optimization and Validation. Radiology. 1994;192(2):485–491. doi: 10.1148/radiology.192.2.8029420. [DOI] [PubMed] [Google Scholar]
- 4.Solloway S, Hutchinson CE, Waterton JC, Taylor CJ. The Use of Active Shape Models for Making Thickness Measurements of Articular Cartilage From MR Images. Magn Reson Med. 1997;37(6):943–952. doi: 10.1002/mrm.1910370620. [DOI] [PubMed] [Google Scholar]
- 5.Stammberger T, Eckstein F, Englmeier KH, Reiser M. Determination of 3D Cartilage Thickness Data From MR Imaging: Computational Method and Reproducibility in the Living. Magn Reson Med. 1999;41(3):529–536. doi: 10.1002/(sici)1522-2594(199903)41:3<529::aid-mrm15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 6.Koo S, Gold GE, Andriacchi TP. Considerations in Measuring Cartilage Thickness Using MRI: Factors Influencing Reproducibility and Accuracy. Osteoarthritis Cartilage. 2005;13(9):782–789. doi: 10.1016/j.joca.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Raynauld JP, Martel-Pelletier J, Berthiaume MJ, Labonte F, Beaudoin G, de Guise JA, Bloch DA, Choquette D, Haraoui B, Altman RD, Hochberg MC, Meyer JM, Cline GA, Pelletier JP. Quantitative Magnetic Resonance Imaging Evaluation of Knee Osteoarthritis Progression Over Two Years and Correlation With Clinical Symptoms and Radiologic Changes. Arthritis Rheum. 2004;50(2):476–487. doi: 10.1002/art.20000. [DOI] [PubMed] [Google Scholar]
- 8.Burgkart R, Glaser C, Hyhlik-Durr A, Englmeier KH, Reiser M, Eckstein F. Magnetic Resonance Imaging-Based Assessment of Cartilage Loss in Severe Osteoarthritis: Accuracy, Precision, and Diagnostic Value. Arthritis Rheum. 2001;44(9):2072–2077. doi: 10.1002/1529-0131(200109)44:9<2072::AID-ART357>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Koo S, Dixit AN, Alexander EJ, Gold G, Goodman S, Dyrby CO, Giori NJ, Andriacchi TP. Morphological Variations of Femoral Cartilage are Influenced by Gait Characteristics in Healthy and Osteoarthritic Knees. 50th Annual Meeting of the Orthopaedic Research Society; San Francisco, CA. Mar.2004. [Google Scholar]
- 10.Graichen H, von Eisenhart-Rothe R, Vogl T, Englmeier KH, Eckstein F. Quantitative Assessment of Cartilage Status in Osteoarthritis by Quantitative Magnetic Resonance Imaging: Technical Validation for Use in Analysis of Cartilage Volume and Further Morphologic Parameters. Arthritis Rheum. 2004;50(3):811–816. doi: 10.1002/art.20191. [DOI] [PubMed] [Google Scholar]
- 11.Eckstein F, Gavazzeni A, Sittek H, Haubner M, Losch A, Milz S, Englmeier KH, Schulte E, Putz R, Reiser M. Determination of Knee Joint Cartilage Thickness Using Three-Dimensional Magnetic Resonance Chondro-Crassometry (3D MR-CCM) Magn Reson Med. 1996;36(2):256–265. doi: 10.1002/mrm.1910360213. [DOI] [PubMed] [Google Scholar]
- 12.Ateshian GA, Soslowsky LJ, Mow VC. Quantitation of Articular Surface Topography and Cartilage Thickness in Knee Joints Using Stereophotogrammetry. J Biomech. 1991;24(8):761–776. doi: 10.1016/0021-9290(91)90340-s. [DOI] [PubMed] [Google Scholar]
- 13.Cohen ZA, McCarthy DM, Kwak SD, Legrand P, Fogarasi F, Ciaccio EJ, Ateshian GA. Knee Cartilage Topography, Thickness, and Contact Areas From MRI: In-Vitro Calibration and In-Vivo Measurements. Osteoarthritis Cartilage. 1999;7(1):95–109. doi: 10.1053/joca.1998.0165. [DOI] [PubMed] [Google Scholar]
- 14.Sumanaweera T, Glover G, Song S, Adler J, Napel S. Quantifying MRI Geometric Distortion in Tissue. Magn Reson Med. 1994;31(1):40–47. doi: 10.1002/mrm.1910310106. [DOI] [PubMed] [Google Scholar]
- 15.Sato Y, Kubota T, Nakanishi K, Sugano N, Nishii T, Ohzono K, Nakamura H, Ochi T, Tamura S. Three-Dimensional Reconstruction and Quantification of Hip Joint Cartilages From Magnetic Resonance Images. Lect Notes Comput Sci. 1999;1679:338–347. [Google Scholar]
- 16.Sato Y, Tanaka H, Nishii T, Nakanishi K, Sugano N, Kubota T, Nakamura H, Yoshikawa H, Ochi T, Tamura S. Limits on the Accuracy of 3-D Thickness Measurement in Magnetic Resonance Images—Effects of Voxel Anisotropy. IEEE Trans Med Imaging. 2003;22(9):1076–1088. doi: 10.1109/TMI.2003.816955. [DOI] [PubMed] [Google Scholar]
- 17.Haut TL, Hull ML, Howell SM. A High-Accuracy Three-Dimensional Coordinate Digitizing System for Reconstructing the Geometry of Diarthrodial Joints. J Biomech. 1998;31(6):571–577. doi: 10.1016/s0021-9290(98)00049-9. [DOI] [PubMed] [Google Scholar]
- 18.Sommers MB, Martin JK, Erne OK, Bottlang M. Laser Displacement Sensor Reports Are Affected by Surface Color and Opacity. 27th Annual Meeting of American Society of Biomechanics; Toledo, OH. Sept.2003. [Google Scholar]
- 19.Disler DG, McCauley TR, Wirth CR, Fuchs MD. Detection of Knee Hyaline Cartilage Defects Using Fat-Suppressed Three-Dimensional Spoiled Gradient-Echo MR Imaging: Comparison With Standard MR Imaging and Correlation With Arthroscopy. AJR, Am J Roentgenol. 1995;165(2):377–382. doi: 10.2214/ajr.165.2.7618561. [DOI] [PubMed] [Google Scholar]
- 20.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, Kothari M, Lu Y, Fye K, Zhao S, Genant HK. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the Knee in Osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Kass M, Witkin A, Terzopoulos D. Snakes: Active Contour Models. Int J Comput Vis. 1987;1(4):321–331. [Google Scholar]
- 22.Lorensen WE, Cline HE. Marching Cubes: A High Resolution 3D Surface Construction Algorithm. Comput Graph (ACM) 1987;21(4):163–169. [Google Scholar]
- 23.Chen Y, Medioni G. Object Modeling by Registration of Multiple Range Images. Proceedings of the IEEE Conference on Robotics and Automation.1991. [Google Scholar]
- 24.Pinheiro JC, Bates DM. Mixed Effects Models in S and S-Plus. 1. Springer; New York: 2002. [Google Scholar]
- 25.Pham A, Hull ML. Dehydration Rates of Meniscus and Articular Cartilage In Vitro Using a Fast and Accurate Laser-Based Coordinate Digitizing System. J Biomech. 2007;40(14):3223–3229. doi: 10.1016/j.jbiomech.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 26.Eckstein F, Tieschky M, Faber S, Englmeier KH, Reiser M. Functional Analysis of Articular Cartilage Deformation, Recovery, and Fluid Flow Following Dynamic Exercise In Vivo. Anat Embryol (Berl) 1999;200(4):419–424. doi: 10.1007/s004290050291. [DOI] [PubMed] [Google Scholar]
- 27.Waterton JC, Solloway S, Foster JE, Keen MC, Gandy S, Middleton BJ, Maciewicz RA, Watt I, Dieppe PA, Taylor CJ. Diurnal Variation in the Femoral Articular Cartilage of the Knee in Young Adult Humans. Magn Reson Med. 2000;43(1):126–132. doi: 10.1002/(sici)1522-2594(200001)43:1<126::aid-mrm15>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 28.Hargreaves BA, Gold GE, Beaulieu CF, Vasanawala SS, Nishimura DG, Pauly JM. Comparison of New Sequences for High-Resolution Cartilage Imaging. Magn Reson Med. 2003;49(4):700–709. doi: 10.1002/mrm.10424. [DOI] [PubMed] [Google Scholar]
- 29.Gold GE, Hargreaves BA, Vasanawala SS, Webb JD, Shimakawa AS, Brittain JH, Beaulieu CF. Articular Cartilage of the Knee: Evaluation With Fluctuating Equilibrium MR Imaging—Initial Experience in Healthy Volunteers. Radiology. 2006;238(2):712–718. doi: 10.1148/radiol.2381042183. [DOI] [PubMed] [Google Scholar]
- 30.Eckstein F, Buck RJ, Wyman BT, Kotyk JJ, Le Graverand MP, Remmers AE, Evelhoch JL, Hudelmaier M, Charles HC. Quantitative Imaging of Cartilage Morphology at 3.0 Tesla in the Presence of Gadopentate Dimeglumine (Gd-DTPA) Magn Reson Med. 2007;58(2):402–406. doi: 10.1002/mrm.21290. [DOI] [PubMed] [Google Scholar]
- 31.Eckstein F, Buck RJ, Burstein D, Charles HC, Crim J, Hudelmaier M, Hunter DJ, Hutchins G, Jackson C, Kraus VB, Lane NE, Link TM, Majumdar LS, Mazzuca S, Prasad PV, Schnitzer TJ, Taljanovic MS, Vaz A, Wyman B, Le Graverand MP A9001140 Study Group. Precision of 3.0 Tesla Quantitative Magnetic Resonance Imaging of Cartilage Morphology in a Multi Center Clinical Trial. Ann Rheum Dis. 2008;67:1683–1688. doi: 10.1136/ard.2007.076919. [DOI] [PubMed] [Google Scholar]
- 32.Pham DL, Xu C, Prince JL. Current Methods in Medical Image Segmentation. Annu Rev Biomed Eng. 2000;2:315–337. doi: 10.1146/annurev.bioeng.2.1.315. [DOI] [PubMed] [Google Scholar]