Abstract
Here we develop an injectable composite system based for repeated ultrasound-triggered on-demand drug delivery. An in situ-cross-linking hydrogel maintains model drug (dye)-containing liposomes in close proximity to gas-filled microbubbles that serve to enhance release events induced by ultrasound application. Dye release is tunable by varying the proportions of the liposomal and microbubble components, as well as the duration and intensity of the ultrasound pulses in vitro. Dye is minimal at baseline. The composite shows minimal cytotoxicity in vitro, and benign tissue reaction after subcutaneous injection in rats,. Ultrasound application also triggers drug release for two weeks after injection in vivo.
Keywords: on-demand, triggered-release, ultrasound, drug-delivery, hydrogels
1. Introduction
The development of on-demand drug delivery systems would allow patients to determine the frequency and intensity of drug administration for a variety of medical applications. For example, on-demand drug delivery systems may allow the patient to personally modulate the location, timing and extent of drug release to alleviate pain. A wide range of approaches has been used including, among others, triggers such as near-infrared light [1], magnetically induced heating [2], and ultrasound [3]. Similarly, a variety of formulations has been employed ranging from the particulate [1, 3], polymer matrixes[4] to the macroscopic [5, 6]. The principal limitations of many approaches has been that the particulate ones frequently result in a single drug release event, while more macroscopic devices that can release multiple doses often require surgical implantation and/or contact with external electrical or other equipment.
Our goal was to develop an injectable depot system to provide sustained, on-demand drug delivery. We selected liposomes as the drug-containing component, as they are widely known to be excellent systems for delivering a range of compounds [7, 8]. Systemically-delivered liposomes can be triggered to release drugs at specific locations by ultrasound [3, 9], which is a convenient method for noninvasive triggering where repeated pulses are desired [3]. Here, the liposomes are injected within an in situ cross-linking hydrogel matrix. The hydrogel network forms around the liposomes, protecting them from surrounding inflammatory cells and preserving their close proximity to co-suspended microbubbles, which might otherwise separate from them due to their different buoyancy. Microbubbles are gas-filled lipid monolayers that have been used extensively as ultrasound contrast agents [10] and recently as drug delivery vehicles [11, 12]. Our hypothesis is that adding microbubbles to the formulation will enhance drug release from the liposomes in response to ultrasound, increasing the difference between baseline and peak release rates – a potentially important goal in many biomedical applications. The combined system is injectable and offers several well controlled parameters to optimize ultrasound sensitivity and the drug release profile.
2. Materials and methods
2.1 Material
Phenazine methosulfate, trypan blue, sodium chloride, methanol, chloroform, cholesterol, Dioctadecyldimethylammonium bromide (DODAB), CMC (medium viscosity), Dextran (100 kDa) adipic dihydrazide (ADH), 1-ethyl-3-[3-(dimethylamino)propyl]-carbodiimide (EDC), hydroxybenzotriazole (HOBt), sodium periodate, ethylene glycol, tert-butyl carbazate (t-BC), sodium bicarbonate, sodium chloride, acetic acid and octyl β-D-glucopyranoside (OGP) were from Sigma (St. Louis, MO). HA (Mw = 490 kDa and 1.4 MDa), 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), and 1,2-distearoyl-sn-glycero-3-phosphatidylglycerol, sodium salt (DSPG) were purchased from Genzyme (Cambridge, MA). Tert-butanol was purchased from Riedel-de Haën (Seelze, Germany).
2.2 Liposome preparation and characterization
Liposomes were prepared by modified thin lipid film hydration [13]. Lipids were selected to produce relatively solid liposomes at 37°C (phase transition temperatures, Tm; DSPC, DSPG = 56°C). DSPC:DSPG:cholesterol, negatively charged, DSPC:DODAB:cholesterol, positively charged, (molar ratio 3:1:2) and DSPC:cholesterol, neutral, (molar ratio 4:2) were dissolved in t-butanol and lyophilized. The lyophilized cake was hydrated with the dyes phenazine methosulfate (10 mg/mL) or trypan blue (10 mg/mL), at 55-60 °C. The suspension was homogenized at 10,000 rpm with a 3/8” Mini-Micro workhead on a L4RT-A Silverson Laboratory Mixer (East Longmeadow, MA) for 10 minutes followed by ten freeze-thaw cycles. Excess free dye was removed by centrifugation (4000 rpm, 4° C for 20 minutes) and replaced by 2 mL PBS and then dialyzed against PBS in 50 kDa molecular weight cut-off dialysis bags for 48 hours. Dye-free liposomes were prepared by the same procedure, omitting the dye.
2.2.1.Liposome characterization
Liposomes were sized with a Beckmann Coulter Counter Multisizer 3 (Fullerton, CA). Zeta potentials were measured using Brookhaven Instruments Corporation ZetaPALS and ZetaPlus software (Holtsville, NY). Liposome drug concentrations were determined following disruption of the liposomes with OGP. Phenazine methosulfate and trypan blue were quantified by SpectraMax 384 Plus fluorometer (Molecular Devices, Sunnyvale, CA) at 387 and 607 nm, respectively. Lipid concentrations were determined by colorimetry by the Bartlett assay [14].
2.3 Microbubble Preparation and characterization
Microbubbles were prepared using techniques described previously by Feshitan et al [23]. Distearoyl-phosphatidylcholine (DSPC; Avanti Polar Lipids, Alabaster, AL) and polyoxyethylene-40 stearate (PEG40S; Sigma-Aldrich, St. Louis, MO) lipids were dissolved in chloroform at a molar ratio of 9:1, transferred to a scintillation vial, and evaporated with a steady nitrogen stream while vortexing for ten minutes followed by several hours under house vacuum. A 0.01 M solution of phosphate buffered saline (PBS) (Sigma-Aldrich) was filtered using 0.2-μm pore size polycarbonate filters (VWR, West Chester, PA). The dried lipid film was hydrated with filtered PBS and mixed with PEG40S to a final lipid concentration of 2.0 mg/mL, and sonicated with a 20-kHz probe (model 250A, Branson Ultrasonics; Danbury, CT) at low power (power setting dialed to 3/10; 3 Watts) above the main phase transition temperature of the phospholipid (~55 °C for DSPC). Perfluorobutane gas (PFB; Fluoromed, Round Rock, TX) was introduced to the surface of the lipid suspension, and high power sonication (power setting dialed to 10/10; 33 Watts) was applied to the suspension for about 10 seconds at the gas-liquid interface to generate microbubbles. The microbubble suspension was collected into 30-mL syringes (Tyco Healthcare, Mansfield, MA). Washing and concentrating by centrifugation was performed with a bucket-rotor centrifuge (model 5804, Eppendorf, Westbury, NY), with a radius of approximately 16.1 cm from the center to the syringe tip, at 300 G for 5 minutes to collect the microbubbles from the suspension into a cake. The remaining suspension (infranate) was recycled for the next batch of microbubbles and resulting cakes were pooled and stored in sealed, 2-mL serum vials.
2.3.1 Microbubble characterization
Microbubble size distribution was determined by laser light obscuration and scattering (Accusizer 780A, NICOMP Particle Sizing Systems, Santa Barbara, CA). 2-μL samples of each microbubble suspension were diluted into the 30-mL flask under mild mixing. Microbubble size distribution was also determined by the electrozone sensing method (Multisizer 3, Beckman Coulter, Opa Locka, FL). A 4-μL sample of microbubble suspension was diluted into a 60-mL flask and stirred continuously to prevent flotation-induced error. A 30-μm aperture (size range of 0.6-18 μm) was used for the measurements. All samples were measured in triplicate and analyzed for both number- and volume-weighted distributions.
2.4 Preparation of Hydrogels and characterization
dextran-CHO/CMC-ADH hydrogel: dextran aldehyde was prepared as previously described[15] ; 1.5 g dextran were dissolved in 150 mL of distilled water overnight, to which 802.1 mg of sodium periodate was added and stirred for 2 hours. 400 μL of ethylene glycol was added at 2 h to stop the reaction and was left to stir for an additional 1 h. Hydrazide was prepared as previously described[15] ; 0.5 g CMC was dissolved in 100 mL of distilled water, and reacted with 1.5 g of ADH in the presence of 240 mg EDC and 240 mg HOBt at pH 6.8 overnight at room temperature. The products were purified by exhaustive dialysis for 3 days, and then lyophilized. The purified product was freeze-dried and kept at 4 °C. The degree of fictionalization was quantified using 1H NMR[16] for CMC-ADH and using hydroxylamine reaction for dextran- CHO. In brief, 100mg dextran-CHO was stirred in 25 mL of 0.25M hydroxylamine solution for 3 hours. The pH was measured and sodium hydroxide (0.1 M) was added in aliquots of 100 or 200 μl with the change in pH being recorded after every addition. A plot of dpH/dV against the total volume of NaOH was drawn, with the maximum peak corresponding to the number of moles of aldehyde groups present in the 100 mg of dextran-CHO.
2.4.1 Preparation of hydrogel disks with liposomes and microbubbles
the hydrogels were formed using a double-barreled syringe (Baxter: Deerfield, IL). For the first syringe 1 mL of 2.5% CMC–ADH solution in phosphate buffered saline (PBS) was loaded. For the second syringe 6% dextran- CHO was mixed in PBS with different amounts of liposomes (0, 100, 200 and 500 uL) and microbubbles (0, 200, 400 uL and 1 mL); in all cases the final concentration of dextran-CHO was brought to 6% and the total volume kept at1 mL. The two solutions were merged by injection into a rubber mold sandwiched between two slide glasses, resulting in a dextran-CHO/CMC-ADH hydrogel. The diameters and the thicknesses of the prepared hydrogels were 1.2 cm and 3.5 mm, respectively.
2.4.2.Hydrogel Characterization
Similar HA-ADH/HA-CHO (2.5%/2.5%) and HA-ADH/dextran-CHO (6%/2.5%) were prepared, analyzed and compared to dextran-CHO/CMC-ADH (6%/2.5%) in terms of swelling ratio (%) and stability in vitro, in PBS and cell Medium (DMEM, Gibco) at 37°C. The time course of swelling of gel disks was measured gravimetrically as follows: the weight of the hydrogels after gelation time was measured up to 5 weeks after immersion in both solutions (every day for the first week and then every 3 days thereafter), by separating the portions of the hydrogels that remained intact from the degraded material by transferring intact disks into fresh wells of solution before each measurement. The swelling ratio was calculated as the weight at a given time point divided by the initial weight of the hydrogel. Gelation time was measured as described before [12]. Aqueous o.1 mL CMC-ADH (2.5%) solution was mixed with aqueous 0.1 mL dextran-CHO (6%) solution with stirring at 155 rpm using a Corning model PC-320 hot plate/stirrer. The time until the mixture formed gel was measured five times for each hydrogel no additive, liposomes (200 μL = 1.5% liposomes by volume), microbubbles (800 μL = 12.9% microbubbles by volume) and liposomes with microbubbles (200 μL + 800 μL (1.5% + 12.9% liposomes and microbubbles by volume) the measurements were done at 25 and 37°C.
2.5 Drug release
2.5.1 In vitro drug release without ultrasound application
2.5.1.1 Liposome formulation
One mL of liposomes or compounds in solution was inserted into the lumen of a SpectraPor 1.1 Biotec Dispodialyzer (Spectrum Laboratories, Rancho Domingeuz, CA) with a 50,000 MW cut-off. The dialysis bag was placed in a test tube with 12 mL phosphate buffered saline and incubated at 37 °C on a tilt-table (Ames Aliquot, Miles). At predetermined intervals, the dialysis bag was transferred to a new test tube with fresh phosphate buffered saline that was pre-warmed to 37°C. Concentrations of compounds were quantified as above.
2.5.1.2 Drug release from gels
Disk-shaped dextran-CHO/CMC-ADH hydrogels with dye (trypan blue or phenazine methosulfate), either free in solution or encapsulated in liposomes (0, 100, 200, 300, 400 and 500 μL), and with or without microbubbles (0, 200, 400 and 800 μL), were prepared in a rubber mold sandwiched between two glass slides. The hydrogel disks were weighed and placed in 12-well plates with an insert for ease of gel transfer. 4 mL of phosphate buffered saline (PBS) was added to each well and the gels were incubated at 37°C with constant rotation. Release medium was sampled (0.5 mL) at different time points and replaced with 4 mL of fresh PBS. The release samples were frozen until spectrophotometer analysis.
2.5.2 In vitro drug release following ultrasound application
A 20 KHz low-frequency ultrasonic processor (VC400, Sonics & Materials, Newtown, CT) was used. The ultrasonic probe (13 mm diameter) was immersed in a 12-well plate with insert. The hydrogel disks were placed into the inserts and submerged in a total of 4 mL solution per well. The probe was adjusted to rest at the top of the solution without directly contacting the hydrogels (2 mm distance). Irradiation was conducted at a full duty cycle at varying intensities (0, 4, 6, 7, 8 and 10 W/cm2) and durations (0, 5, 7, 10 and 15 sec). The samples were kept in a temperature-controlled water bath, and its temperature was monitored (37 °C) throughout the experiment to prevent heat-induced liposomal drug release.
2.6 Rheological testing
Rheological measurements were made with an AR-G2 controlled stress rheometer (TA Instruments, New Castle, DE) using a 20-mm parallel plate geometry and 1.1 mm gap with adhesive-backed 600 grit silicon carbide sandpaper to ensure surface contact between the hydrogels and the bounding surfaces (McMaster-Carr, Elmhurst, IL). Dynamic oscillatory modes were used to measure the elastic (G’), loss (G”). An initial strain sweep from 0.1-10% strain was performed for each gel at a frequency of 3 rad/s (18.8 Hz) to determine a range corresponding to the linear viscoelastic regime of our material (data not shown). A strain of 0.1%, was chosen within this range and all subsequent frequency sweeps were performed at this strain over a range of frequencies up to 15 rad/s (94.2 Hz), at 25° C. Four hydrogel types were tested for their relative mechanical characteristics: plain dextran-CHO/CMC-ADH (6%/2.5%) hydrogel, hydrogel with liposomes encapsulating trypan blue (200 μL liposome solution per 1mL total volume, i.e. volume fraction φ≈1.5%), hydrogel with microbubbles (800 μL microbubble solution per 1mL total volume, i.e. volume fraction φ≈12.9%), and hydrogel with 200 μL solution of encapsulating liposomes plus 800 μL solution of microbubbles. All sets of rheological data were collected twice using different blends of prepared microcomposite hydrogels. For each formulation, n=4 different samples were tested resulting in standard deviations generally <5% for elastic modulus G’, confirming the high reproducibility of the rheological analysis.
2.7 Cytotoxicity assay
Microcomposite hydrogels (dextran-CHO/CMC-ADH, liposomes and microbubbles) were investigated in a human mesothelial cell line (CRL-9444: American Type Culture Collection (ATCC), Manassas, VA) and macrophage cell line J774.A1 (TIB-67TM: ATCC) using the MTT assay (MTT kit, Promega G4100 Madison, WI). Briefly, mesothelial cells were grown and maintained in the complete growth medium (Medium199 with Earle’s BSS, 0.75 mM L-glutamine and 1.25 g/L sodium bicarbonate supplemented with 3.3 nM epidermal growth factor, 400 nM hydrocortisone, 870 nM insulin, 20 mM HEPES and 10% fetal bovine serum (Gibco)) at 37 °C in 5% CO2. Macrophages were grown and maintained in DMEM (Gibco, Carlsbad, CA) with 10% fetal bovine serum. Disk hydrogels, containing blank liposomes and microbubbles were placed in each well and floated in the culture medium 24 h after seeding the cells. MTT assay was performed 48, 72 and 96 hr and then every 7 days for up to 3 weeks after adding the hydrogels, for macrophages and mesothelial cells, respectively. Results were normalized to cells cultured without test compounds.
2.8 In vivo application of dextran-CHO/CMC-ADH
Animals were cared for in compliance with protocols approved by the Massachusetts Institute of Technology Committee on Animal Care, in conformity with the NIH guidelines for the care and use of laboratory animals (NIH publication #85-23, revised 1985).
2.8.1 Subcutaneous application
Male CD-1 mice (Charles River Laboratories, Wilmington, MA), (20-28 g) were anesthetized with 2–3% isoflurane in oxygen. The backs were shaved with an electric animal hair clipper and the shaved areas were cleaned with 70% ethanol solution. 0.1 mL of dextran-CHO/CMC-ADH hydrogels containing free trypan blue, or liposomes encapsulating trypan blue, with or without microbubbles, were injected from a double-barreled syringe (0.05 ml of 2.5% CMC- ADH in one syringe, 0.05 mL of 6% dextran-CHO with liposomes encapsulating trypan blue, with or without microbubbles, in the other). Hydrogels with trypan blue liposomes and microbubbles were prepared as described above. Ultrasound probe was applied to the shaven site above cold water, and the mice were exposed for a 1 minute cylce to ultrasound (6 W/cm2, 10 sec pulse 1 sec on/1 sec pulse off). Pictures were taken before and after ultrasound application (n=8, for each group) for 20 days, intensity of the color was analyzed using Adobe Photoshop pipette toll sampler CMYK color detection, color area was normalized to nearby area with no hydrogel in order to normalize the results to reduce the interference of light and the exposure time of the camera based on the operator. Second animal group, n=8 in each group were sacrificed for examination of the tissue reaction (6 and 14 days after injection, n=4 for each group in each time point). Tissues were processed for hematoxylin-eosin sections with standard techniques.
2.9 Statistics
Data are presented as means ± standard deviations (n = 4 in release kinetics, cell work, and n = 8 for in vivo studies). To take multiple comparisons into account, all statistical comparisons were done with the Tukey-Kramer test, using InStat software (GraphPad, San Diego CA). A P-value <0.05 was considered to denote statistical significance.
3. Results
3.1 Hydrogel preparation and characterization
Hydrogels that cross-link in situ by formation of hydrazone bonds were evaluated as candidates for our system based on their stability with and without application of ultrasound. Their compositions are denoted as follows: hyaluronic acid, HA; carboxymethylcellulose, CMC; aldehyde modification, -CHO; adipic hydrazide modification, -ADH. Three hydrogels were tested: HA-CHO/HA-ADH, dextran-CHO/HA-ADH, and dextran-CHO/CMC-ADH. Polymer concentrations of 6% (wt/vol) for all dextran-CHO and 2.5% for all of the modified hyaluronic acid and carboxymethylcellulose components were chosen based on previous work, as they allow rapid gel formation [15, 17].
HA-CHO/HA-ADH and dextran-CHO/HA-ADH underwent visually obvious degradation in PBS and in cell culture medium by 5 and 10 days respectively, while dextran-CHO/CMC-ADH (6%/2.5%) maintained its shape in vitro for 4 weeks with no observable degradation. The application of a short, low-intensity (10 sec, W/cm2) pulse of low-frequency ultrasound (20 kHz) 1 hr after the formation of gels caused visually obvious destruction of the HA-ADH/HA-CHO (2.5%/2.5%) and dextran-CHO/HA-ADH (6%/2.5%) gels after 6 and 10 pulses, respectively, while dextran-CHO/CMC-ADH maintained its shape for 16 pulse applications. The dextran-CHO/CMC-ADH hydrogel also had the lowest swelling ratio: 50 ± 7% compared to 220 ± 14% for HA-CHO/HA-ADH and 120 ± 23% for dextran-CHO/ HA-ADH in PBS at 37 °C. These findings suggested that dextran-CHO/CMC-ADH would be the best candidate hydrogel for this application. Consequently all subsequent experiments used dextran-CHO/CMC-ADH hydrogels. 1H NMR spectra of CMC-ADH [17] demonstrated that 51.6% of the N-acetyl-D-glucosamine residues were modified, as calculated from the ratio of the area of the peak for the N-acetyl-D-glucosamine residue of CMC (singlet peak at 2.0 ppm) to that for the methylene protons of the adipic dihydrazide at 1.62 ppm. Analysis of aldehyde groups formed by the oxidation of dextran with hydroxylamine yielded a 33.1% degree of oxidation [18].
3.2 Liposome preparation and characterization
To produce liposomes suitable for hydrophilic drugs with a range of charges, lipids were prepared in three formulations containing cholesterol together with (i) the neutral lipid DSPC, (ii) DSPC and the negatively charged DSPG, or (iii) DSPC and the positively charged DODAB (Table 1). These lipids were combined in ratios previously determined to produce stable liposomes at 37°C [19, 20]. The median volume-weighted diameter of the multi-lamellar liposomes produced was approximately 4.0 μm for all three formulations. Lipid concentrations for all liposome suspensions were in the range of 50-60 mg/mL, resulting in volume fractions of approximately 7.5% (Table 1). The liposomes are referred to as “neutral, “negative” and “positive” respectively based on measured median zeta potential charges of 0 mV, −35 mV and +31 mV.
Table 1.
Liposome characterization
| Liposome type | Model drug | Drug loading (mg/m L) |
Lipid conc. (mg/mL) |
Dye entrapmentg (%) |
Mean diameter (μm) |
Volume fractionh |
Zeta potential (mV) |
|---|---|---|---|---|---|---|---|
| Positivea | Trypan blueee | f | – | <2% | – | – | – |
| Phenazine methosulfated |
3.4 ±0.02 | 59±5 | 34± 2 | 4.0± 2.5 | 7.4±0.63 | +31± 2.1 | |
| Neutralb | Trypan bluee | 3.2±0.03 | 60±4 | 32± 3 | 4.2± 1.1 | 7.5±0.05 | −0.4± 0.3 |
| Phenazine methosulfated |
2.5 ±0.04 | 60±3 | 25± 4 | 4.2± 1.1 | 7.5±0.37 | −0.9± 0.5 | |
| Negativec | Trypan bluee | 2.8 ±0.04 | 58±3 | 28± 4 | 4.2± 1.1 | 7.3±0.34 | −35.1 ± 1.3 |
| Phenazine methosulfated |
– | <5% | – | – | – |
Data are means ± SD, n = 4.
DSPC:cholesterol::DODAB::3:2:1.
DSPC:cholesterol::2:1.
DSPC:cholesterol:DSPG::3:2:1.
Positively charged.
Negatively charged.
Not stable: particles aggregated.
Percentage of initial concentration.
Percentage of volume displaced in solution.
3.3 Model drug encapsulation
Liposomes were loaded with two dyes used as model hydrophilic drugs: cationic phenazine methosulfate and anionic trypan blue. Both drugs achieved acceptable levels of encapsulation in like-charged or neutral liposomes, but loading was unsuccessful in oppositely charged liposomes owing to particle aggregation (Table 1). Consequently, further experiments were conducted using dyes in liposomes with neutral charge or the same charge as the dye.
3.4 Release kinetic
3.4.1 Liposomal formulation stability
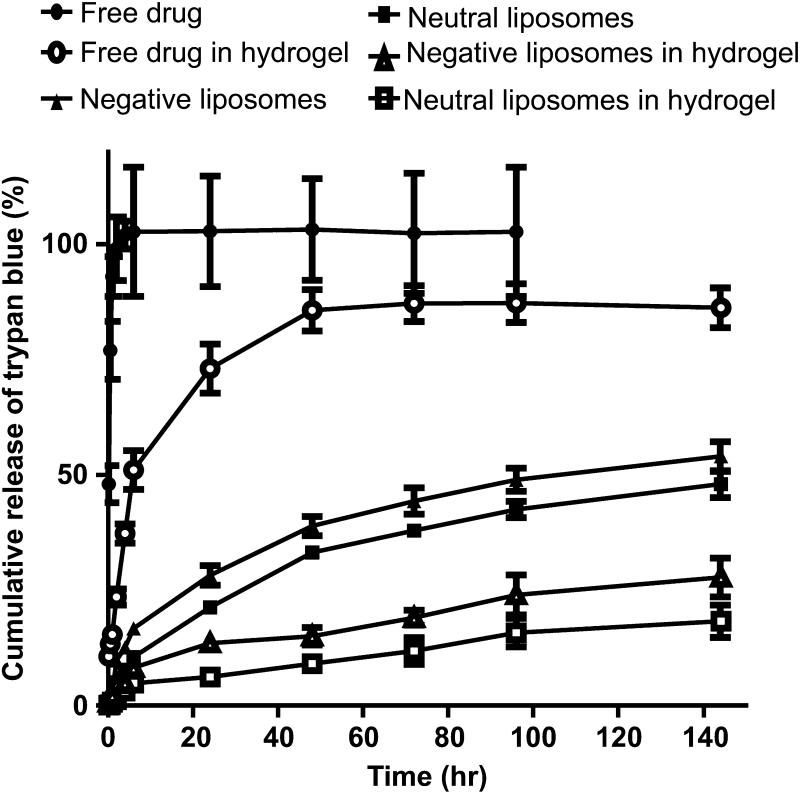
To identify the formulations with the lowest baseline (non-triggered) release, release kinetic studies were performed with dye-containing liposomes contained in dialysis membranes (50,000 molecular weight cutoff) submerged in PBS at 37 °C. Free phenazine methosulfate and free trypan blue in PBS were used as controls. Release of trypan blue was slightly slower from neutral liposomes than from negative liposomes (e.g. p<0.05 at 72 h, Fig. 1). Similarly, the release of phenazine methosulfate was slightly slower from positively charged liposomes than from neutral liposomes (e.g. p<0.05 at 72 h, data not shown). Based on these results, only positive phenazine-methosulfate liposomes and neutral trypan blue liposomes were incorporated into hydrogels for further experimentation.
Figure 1.
In vitro release of trypan blue from dextran-CHO/CMC-ADH hydrogels, either free in the gel matrix or encapsulated in negatively charged and neutral lipid-based liposomes in PBS at 37 °C. Data are means with standard deviations (n = 4). Statistical comparisons are discussed in the text.
In all subsequent in vitro experiments, both trypan blue and phenazine-methosulfate were tested. Very similar results were obtained for both. In the interest of brevity, we will only show data for trypan blue.
3.4.2 Release kinetics of liposomes in hydrogel
Dye-containing liposomes dispersed in dextran-CHO (400 μL liposome solution per 1 mL total volume, with 6% dextran-CHO per total volume) were mixed with an equal volume of 2.5% CMC-ADH via a double-barreled syringe in a rubber mold sandwiched between two glass slides. The resulting cross-linked hydrogel disks containing liposomes were weighed and placed into inserts submerged in 12-well plates filled with PBS at 37°C to monitor release kinetics.
Comparable concentrations of free dyes (1.36 mg/mL dye; the same amount as in 400 μL of liposomes) were incorporated into discs used as liposome-free controls. The release medium was sampled at predetermined time points and the gel inserts were transferred into plates with fresh PBS. Incorporation into hydrogels slowed release of free dyes in solution (e.g. p < 0.01 at 72 hr., Fig. 1) and even more significant with liposome-encapsulated dyes (e.g. p<0.001 at 72 hr., Fig 1). In all experiments, the final concentration of cross-linked polysaccharides was kept constant, irrespective of the varying amounts of other components added. (These were added as liquid suspensions to the dried polymers.) Similarly, the concentration of dyes was maintained constant, whether the dyes were free or encapsulated.
3.4.3 Release kinetics after incorporation of microbubbles
Microbubbles composed of neutral DSPC: PEG40s lipid monolayers encapsulating perfluorobutane gas were incorporated into the composite gel discs (see methods). The median volume-weighted diameter of the microbubbles was approximately 4.0 μm, and were suspended in a solution at 16.1 ± 7.6% volume fraction (Table 2). Hydrogel discs were made as above, so as to contain two different amounts of microbubble solution (250 μL or 500 μL) and dye-containing liposome solution (400 μL). Release kinetics were monitored as above. Incorporation of either amount of microbubbles did not change the baseline (untriggered) release kinetics of the dyes (data not shown).
Table 2.
Microbubble characterization
| Microbubble composition (molar ratio) |
Total compositiona |
Volume fractionb |
Mean diameter (μm) |
|---|---|---|---|
| DSPC:PEG40S (9:1) | 8.4 × 109± 2 × 109 | 16.1 ± 7.6 | 2.3± 0.1 |
Data are means ± SD, n = 3.
Particles/mL.
% volume displaced in solution.
3.5 Ultrasound-triggered release kinetics
We investigated the effect of ultrasound on the release kinetics of dextran-CHO/CMC-ADH hydrogel discs formulated with both types of dye-containing liposomes, with or without microbubbles added. Gel discs containing equal amounts of free dye were used as controls.
To apply the ultrasound pulse to each gel, the tip of an ultrasound probe was submerged 1.2 mm beneath the surface of the PBS but not in direct contact with the gels. The distance between the transducer and the gel was kept at 2 mm. At each time point, the release media were sampled before and after the ultrasound pulse was applied, then the gel inserts were transferred to a fresh well of PBS in preparation for the next time point. All gels were incubated at 37°C between time points. The ultrasound was operated at 20 kHz, which was previously shown to be optimal for perturbing the lipid shells of liposomes [3, 9, 21]. We determined that 20 kHz disrupted microbubbles as well: microbubbles in solution were exposed to 20 kHz ultrasound at 4, 6 and 8 W/cm2 for 10 sec, after which the size and number concentration were measured using a Beckmann Coulter Counter Multisizer 3. After the first pulse, the number concentration of the microbubbles was reduced by 57, 78 and 95 % at pulse intensities of 4, 6 and 8 W/cm2, respectively. After the second pulse no microbubbles could be detected at any intensity. The temperature of the PBS was monitored to ensure it remained 37 °C during ultrasound application.
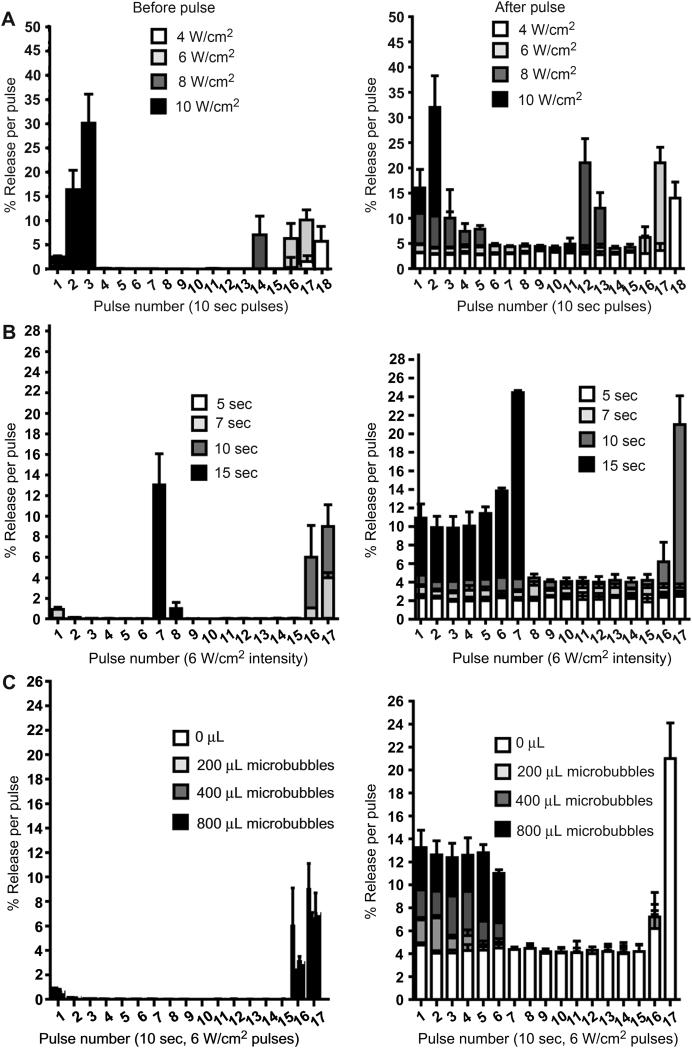
3.5.1 Effect of pulse intensity on release kinetics
Using a pulse duration of 10 sec, dye release was measured at pulse intensities of 4, 6, 8, and 10 W/cm2. These pulse intensities resulted in the visual destruction of the gels after the 18th, 16th, 12th and 2nd pulse, respectively (Fig. 2A), with a concurrent marked release of dye. Of the intensities that did not result in rapid gel destruction, 8 W/cm2 gave the greatest magnitude of release (*p< 0.001, for each of the first 5 pulses), but relatively poor reproducibility. Both 4 and 6 W/cm2 provided reproducible release; release at 6 W/cm2 was higher (Fig. 2A).
Figure 2.
The effect of low-frequency ultrasound pulse intensity (A) and duration (B) on in vitro release of trypan blue from 200 μL of negative liposomes in dextran-CHO/CMC-ADH hydrogels at 37 °C. (C) Effect of microbubble concentration (0, 200, 400 and 800 μL added to the hydrogel) using a 10-sec pulse at 6 W/cm2. For each parameter, dye release before (left panel) and after (right panel) each pulse is shown. Data are means with standard deviations (n = 4) of the percentage of total dye released. Statistical comparisons are discussed in the text.
3.5.2 Effect of pulse duration on release kinetics
Using a pulse intensity of 6 W/cm2, dye release was measured at pulse durations of 5-15 seconds. At pulse duration of 5, 7, and 10 seconds, the magnitude of % release correlated very closely with pulse duration (the average R2 = 0.976; Fig. 2B, “after” panel). With 15-second pulses, the gels began to disintegrate after the 6th pulse (note Fig. 2B “before” panel), and they were completely destroyed after the 9th.
3.5.2 Effect of liposome and microbubble concentrations on triggered release
Using 6 W/cm2 in 10 sec pulses, increasing the liposomal content of the hydrogel discs from 100 to 500 μL dye-loaded liposomes per 1 mL dextran-CHO resulted in the same relative % release of the dye per pulse. However, the absolute amount of dye released increased with liposomal content (R2 = 0.996). For example, increasing from 200 to 400 μL increased dye release from 0.141 to 0.279 mg/mL after the second pulse (*p<0.001, comparing gels with 200, 400 and 800 μL of liposomes, for the first 4 pulses). Increasing liposome content did not increase the number of cycles of triggered release that could be obtained. We chose 200 μL of liposomes for further experimentation for reasons of convenience – that concentration yielded data that were already in the optimal absorbance range of our spectrophotometer.
We then evaluated the effect of adding different amounts of microbubbles to the hydrogels (Fig. 2c). The addition of microbubbles lead to a linear increase in release magnitude (R2 = 0.99) and the number of cycles over which that increase was seen. For example, 200 μL of microbubbles caused release to increase from 4.5 to 7% and lasted for the first 2 pulses (*p<0.001); 400 μL increased the release to 9% and lasted for 4 pulses, and 0.8 mL of microbubbles increased the release to 12.5% (an increase of 3.1 fold, p<0.001) that lasted for 6 pulses . All hydrogels released 3.8-4% of dye content per pulse during pulses 7 to 15, irrespective of microbubble loading (Fig. 2C). The incorporation of microbubbles lead to more rapid depletion of dye in the gels, as seen in the fact that only the 0 and 200 μL groups showed triggered release at pulses 16 and 17, when the gels were disintegrating. Increasing the amount of microbubbles in the hydrogel also increased the number of cycles that showed enhancement of release.
3.6 Effect of liposome and microbubble incorporation on hydrogel properties
We examined the effects of incorporating microbubbles and liposomes on the gelation time and viscoelasticity of the formulation. Gel discs were prepared as above (using neutral trypan blue liposomes), but with a stir bar rotating at ~150 rpm inside the plastic mold during injection, The gelation time was considered the time at which stir bar could no longer rotate inside the gels. The average gelation time of dextran-CHO/CMC-ADH gels was ~30 sec at 25 °C, and was accelerated by the incorporation of liposomes and microbubbles, and by increasing the temperature from 25 to 37 °C (p<0.001 between all groups tested and between the groups at different temperatures, Table 3).
Table 3.
Gelation times of CMC–ADH 2.5%/DEX–CHO 6% with added components
| Temp (°C) | Gelation time (s) |
|||
|---|---|---|---|---|
| No additive |
With liposomes |
With microbubbles |
With liposomes and microbubbles |
|
| 25 | 32.4 ± 0.6 | 25.6± 0.9 | 19.3±0.5 | 11.4 ± 0.9 |
| 37 | 23± 0.7 | 17 ±1.4 | 10.6±0.55 | 6.4± 0.6 |
Data are means ± SD (n = 5).
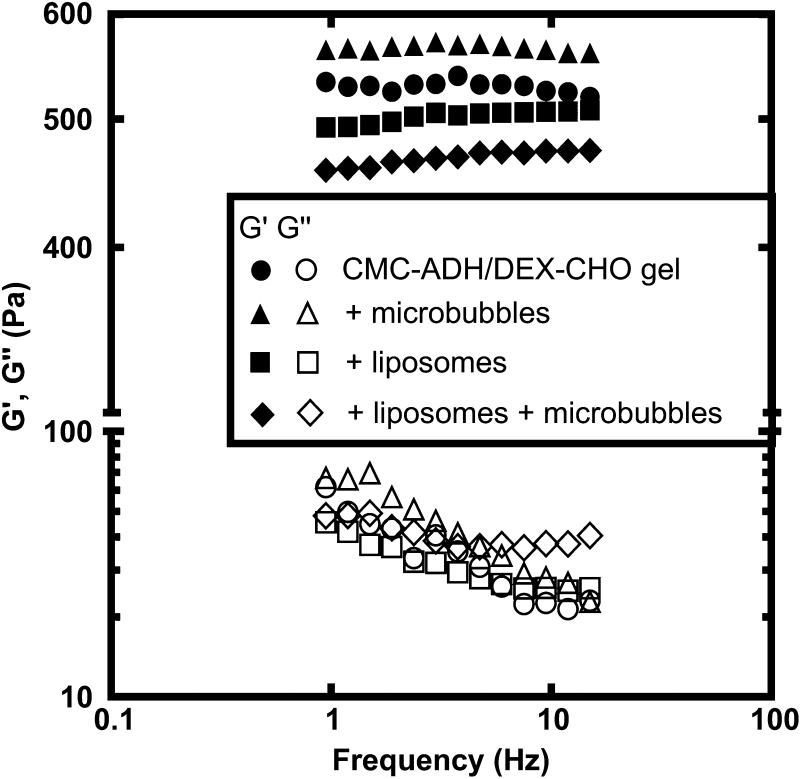
The viscoelastic properties were measured for dextran-CHO/CMC-ADH hydrogels with and without the inclusion of liposomes (at volume fraction φ≈1.5%) and microbubbles (at volume fraction φ≈12.9%). Tested samples exhibited similar elastic (G’) and viscous (G”) moduli within the linear viscoelastic regime (Fig. 3). All gels were predominantly elastic (G’ >> G”), and the elastic moduli were essentially independent of frequency for the range examined here. The viscous contribution to the total response is quantified by the loss tangent, tan δ= G”/G’, which is small for all of our trials (tan δ<0.05).
Figure 3.
Elastic modulus (G’, filled symbols) and viscous modulus (G”, open symbols) as a function of oscillation frequency for dextran-CHO/CMC-ADH (6%/2.5%) hydrogels with and without the inclusion of liposomes (at volume fraction φ≈1.5%) and microbubbles (at volume fraction φ≈16%). Liposomes encapsulating trypan blue are added via 200 μL liposome solution per 1 mL total volume. Microbubbles are added via 800 μL microbubble solution per 1 mL total volume. The gels were predominantly elastic, as reflected by G’ > G”. The addition of the liposomes and microbubbles had relatively little effect on the viscoelastic properties of the hydrogels.
The incorporation of microbubbles increased the elastic modulus slightly, whereas the addition of liposomes (with or without microbubbles) decreased the elastic modulus. The coefficient of variation (standard deviation as a percentage of mean) of the elastic moduli of the various groups were generally < 5%. While statistically significant, the differences between groups were modest (~10%) . All formulations behaved as elastic solids in the linear viscoelastic regime explored here, with minimal effects from the addition of liposomes and/or microbubbles.
3.7 Cytotoxicity assay
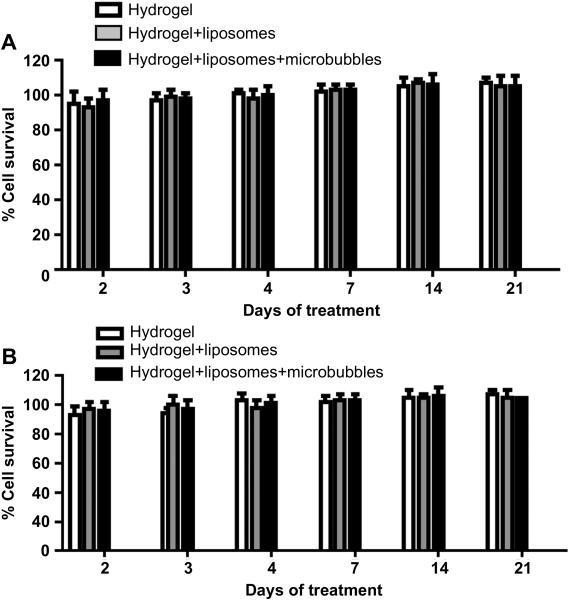
Mesothelial (CRL-9444) and macrophage (J774.A1) cell lines were exposed to the hydrogel formulations, with or without liposomes and microbubbles, for up to 3 weeks with media changes every 3 days. We used dye-free liposomes since the subject of inquiry is the delivery vehicle, not the dye used as a model drug. For the 3 weeks tested, both mesothelial (Fig. 4A) and macrophage (Fig. 4B) cell lines showed viability greater than 90% of that of cells not exposed to any formulation. The incorporation of liposomes and microbubbles into the hydrogels did not affect viability.
Figure 4.
Effect on cell viability (MTT assay) over time of exposure to the dextran-CHO/CMC-ADH hydrogel, DSPC:DODAB:cholesterol-based liposomes, and hydrogel-liposome-DSPC-based microbubble composites. A. Mesothelial cells (CRL-9444 cell line). B. Macrophages (J774.A1 cell line). Data are means (of % survival of untreated cells) with standard deviations (n = 4). There were no significant differences between groups.
3.8 Ultrasound-triggered release in vivo
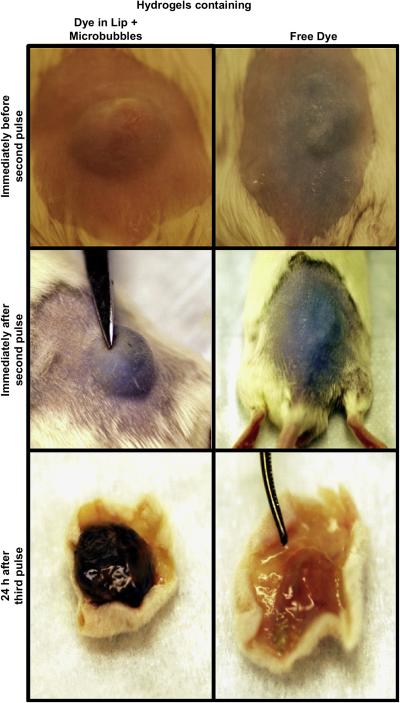
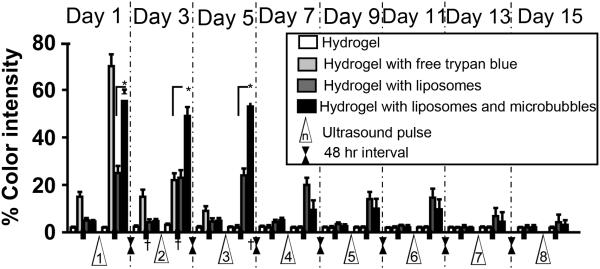
To confirm the efficacy of our system in vivo, we injected composite gels containing trypan blue, either free or in neutral liposomes (200 μL), with or without microbubbles (800 μL), subcutaneously in CD-1 white mice. The injected region of the skin and the ultrasound probe were immersed in a water-filled cylinder, and the tip of the ultrasound was positioned at a distance less than 2 mm above but not in direct contact with the skin in order to avoid causing irritation or burns. The appearance of trypan blue in the usually white-tan mouse skin was evaluated to determine the in vivo feasibility of controlling the dye release as an on-and-off, on-demand application (Fig. 5). To quantitate the intensity of blueness before and after each pulse of the ultrasound, digital pictures of the injection site were taken, and the intensity of the released blue in the center of the injection site was analyzed as in Methods, n=8 for each group (Fig. 6). The skin of mice injected with hydrogels containing free trypan blue developed bluish discoloration within 20 min after injection even without the application of ultrasound. The blue became more intense with the application of a first 1 min ultrasound pulse of 1 min. (This pulse duration was developed empirically, to avoid causing burns, and consisted of six 10-sec bursts separated by 1-sec off at 6 W/cm2. Pulses were administered 48 hr apart.) Skin color returned to baseline after 24 hr. A second 1 min pulse at 48 hr caused the blueness to return; the color was gone by 24 hr thereafter. No dye could be detected after a third pulse. In contrast, hydrogels containing trypan blue in liposomes both with and without microbubbles showed minimal discoloration of the skin for more than 2 weeks in the absence of ultrasound application. With application of ultrasound, dye-containing liposomes showed on-demand release for two weeks. Twenty-four hours after each pulse the intensity of the dye on the skin returned to a background level of 5%. An increase in released dye afterultrasound aplicationwas seen for 6 pulses, after which it was hard to detect visually. The addition of microbubbles resulted in a visually obvious higher release of trypan blue in response to ultrasound. For example, after the first pulse, the measured blue intensity was 23 ± 3% for hydrogels with liposomes, and 56 ± 2.7% for hydrogels with liposomes and microbubbles (p < 0.001).
Figure 5.
Representative photographs of injection sites following injection of hydrogels containing free dye, or dye in liposomes with associated microbubbles. The top panels show the skin surface before the second pulse (24 h after the first pulse). The middle panels are immediately after the second pulse. The bottom panel shows the inner face of the skin (subcutaneous tissue, the injection site) on necropsy 24 h after the third pulse. The time points at which pictures were taken are denoted by “†” in Fig. 6.
Figure 6.
The ultrasound-triggered release profile of trypan blue from neutral liposomes suspended with microbubbles in dextran-CHO/CMC-ADH hydrogels, injected subcutaneously into mice. Ultrasound was applied for 1 min at 6 W/cm2, with a pulse cycle 10 sec on and 1 sec off. “% color intensity” is a measure of the intensity of blue color obtained from pictures of the injection site taken before and after each pulse, as in Methods. Data are means with standard deviations (n = 8). * p<0.001. † = time points referred to in Fig. 5.
3.8.1 Necropsy
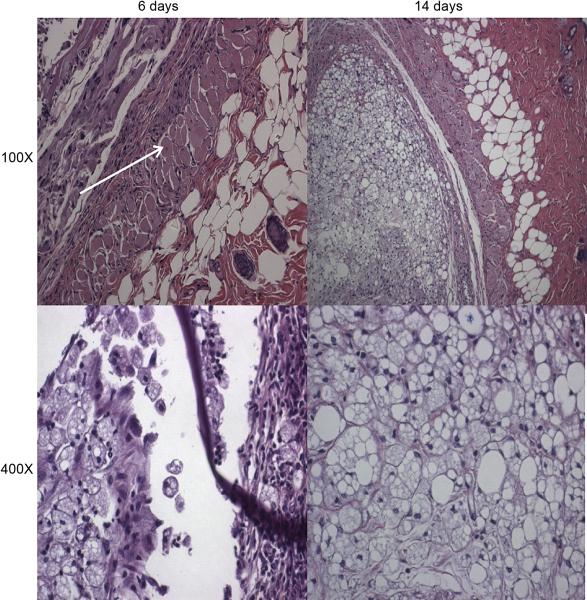
Hydrogels were injected subcutaneously (n=8 for each group). Half were excised on day 6, 24 h after the third pulse, and the rest on day 14. In all cases, the hydrogels were still localized at the site of injection. Hydrogels containing liposomes were very blue in color on day 6 (Fig. 5), in contrast to the hydrogels containing free-dye, which were almost clear. Tissue reaction was mild, with little matting or apparent inflammation. Light microscopy (Fig. 7) revealed a mild-to-moderate inflammatory reaction, with some foamy macrophages (the highly lucent cells seen in the bottom panels of Fig. 7), suggesting ingestion of the formulation. There were occasional centralized nuclei in muscle cells, suggesting mild muscle injury in the immediate vicinity of the gels. No difference in tissue reaction was observed between the tested groups.
Figure 7.
Representative light microscopy of hematoxylin-eosin stained sections of the injection sites of the hydrogels, 6 and 14 days after injection. The 400X views focus on the foamy macrophages. The arrow denotes a centralized nucleus.
4. Discussion
We have developed an injectable, multi-component system for providing on-demand, ultrasound-triggered drug release. Each component served a specific purpose: the liposomes carried the model drugs (the dyes) and prevented their premature release, the microbubbles enhanced the drug release by increased cavitation, and the hydrogel maintained both particles in close proximity to each other and in a relatively constrained location so that they could be affected by the ultrasound beam. Importantly for many applications, the liposomal component, which contained the drugs, was engineered so that the baseline rate of drug release would be minimal. This was successful with the hydrophilic dyes used here, which have difficulty leaving the aqueous liposome interior by crossing the lipid bilayer. Partly as a result of the low baseline rate of drug release, these formulations were able to maintain triggerability for at least 14 days. This system could allow a broad range of total drug payloads and patterns of release, depending on the liposomal drug loading, liposomal concentration, microbubble loading, pulse time, and pulse intensity. The particular combination of parameters used here was geared toward minimizing tissue injury [21] by using a short pulse at relatively low intensity. The pulse cycles used here (10-sec in vitro and 1-min pulsed cycles, 10-sec on, 1-sec off in vivo, see Methods) are short compared to those used in previous reports [3, 9, 21]. We note that in addition to allowing for temporal control of dose administration (i.e. on-demand delivery), these formulations would allow for precise determination of the magnitude of the drug dose to be delivered, by modulating either the intensity or the duration of the ultrasound pulse, or the formulational parameters mentioned above.
One key feature of this system is the combination of injectability and mechanical integrity. In that regard, it was reassuring that the incorporation of liposomes and microbubbles at these concentrations had little effect on the mechanical properties of the hydrogel (Fig. 3). The resulting hydrogels remain viscoelastic solids with the addition of liposomes and/or microbubbles. Once injected, the device would be well suited for applications where high concentrations of drug are desired, such as local anesthesia. Chemotherapeutic applications can also be envisioned. However, there is no reason why it could not also be adapted to delivering systemically distributed drugs, such as narcotics. Such applications with drugs with relatively poor therapeutic indices would have to be designed carefully so as to ensure that overdoses could not occur easily. Local tissue reaction will also be important. The formulations were minimally cytotoxic in vitro, and tissue reaction was mild, suggesting that biocompatibility is unlikely to be problematic. The presence of microbubbles could minimize tissue injury for ultrasound application by reducing the intensity or duration of ultrasound required to achieve a given level of drug release. However, it is possible that high local concentrations of drugs could cause local tissue injury, even if reaction to the base formulation is relatively benign [22].
5. Conclusions
We have developed a composite triggered release system consisting of in situ cross-linking hydrogels containing liposomes and microbubbles, with numerous parameters that allow tunability of drug content and release. Drug release could be controlled by changing the concentration and proportion of microbubbles and liposomes, and by modulating the duration and intensity of ultrasound pulses. Triggerability was maintained for at least two weeks, in vitro and in vivo. The formulation showed minimal cytototoxicity in the absence of encapsulated compounds, and tissue reaction was relatively benign
Acknowledgment
This work was support by: GM073626 (to DSK) from NIGMS (National Institute of General Medical Sciences).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wu G, Mikhailovsky A, Khant HA, Fu C, Chiu W, Zasadzinski JA. Remotely triggered liposome release by near-infrared light absorption via hollow gold nanoshells. J Am Chem Soc. 2008;130(26):8175–7. doi: 10.1021/ja802656d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hoare T, Santamaria J, Goya GF, Irusta S, Lin D, Lau S, et al. A magnetically triggered composite membrane for on-demand drug delivery. Nano Letters. 2009;9(10):3651–7. doi: 10.1021/nl9018935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schroeder A, Avnir Y, Weisman S, Najajreh Y, Gabizon A, Talmon Y, et al. Controlling liposomal drug release with low frequency ultrasound: mechanism and feasibility. Langmuir. 2007;23(7):4019–25. doi: 10.1021/la0631668. [DOI] [PubMed] [Google Scholar]
- [4].Kost J, Leong K, Langer R. Ultrasound-enhanced polymer degradation and release of incorporated substances. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(20):7663–6. doi: 10.1073/pnas.86.20.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Santini JT, Jr., Cima MJ, Langer R. A controlled-release microchip. Nature. 1999;397(6717):335–8. doi: 10.1038/16898. [DOI] [PubMed] [Google Scholar]
- [6].Ciolino JB, Hoare TR, Iwata NG, Behlau I, Dohlman CH, Langer R, et al. A drug-eluting contact lens. Invest Ophthalmol Vis Sci. 2009;50:3346–42. doi: 10.1167/iovs.08-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–60. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- [8].Schwendener RA. Liposomes in biology and medicine. Advances in experimental medicine and biology. 2007;620:117–28. doi: 10.1007/978-0-387-76713-0_9. [DOI] [PubMed] [Google Scholar]
- [9].Schroeder A, Honen R, Turjeman K, Gabizon A, Kost J, Barenholz Y. Ultrasound triggered release of cisplatin from liposomes in murine tumors. J Control Release. 2009;137(1):63–8. doi: 10.1016/j.jconrel.2009.03.007. [DOI] [PubMed] [Google Scholar]
- [10].Lindner JR. Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov. 2004;3(6):527–32. doi: 10.1038/nrd1417. [DOI] [PubMed] [Google Scholar]
- [11].Kheirolomoom A, Dayton PA, Lum AF, Little E, Paoli EE, Zheng H, et al. Acoustically-active microbubbles conjugated to liposomes: characterization of a proposed drug delivery vehicle. J Control Release. 2007;118(3):275–84. doi: 10.1016/j.jconrel.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sirsi SR, Borden MA. Microbubble compositions, properties and biomedical applications. Bubble Science, Engineering and Technology. 2009;1(1/2):3–17. doi: 10.1179/175889709X446507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Szoka F, Jr., Papahadjopoulos D. Comparative properties and methods of preparation of lipid vesicles (liposomes) Annu Rev Biophys Bioeng. 1980;9:467–508. doi: 10.1146/annurev.bb.09.060180.002343. [DOI] [PubMed] [Google Scholar]
- [14].Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem. 1959;234(3):466–8. [PubMed] [Google Scholar]
- [15].Ito T, Yeo Y, Highley CB, Bellas E, Kohane DS. Dextran-based in situ cross-linked injectable hydrogels to prevent peritoneal adhesions. Biomaterials. 2007;28(23):3418–26. doi: 10.1016/j.biomaterials.2007.04.017. [DOI] [PubMed] [Google Scholar]
- [16].Ito T, Yeo Y, Highley CB, Bellas E, Benitez CA, Kohane DS. The prevention of peritoneal adhesions by in situ cross-linking hydrogels of hyaluronic acid and cellulose derivatives. Biomaterials. 2007;28(6):975–83. doi: 10.1016/j.biomaterials.2006.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yeo Y, Highley CB, Bellas E, Ito T, Marini R, Langer R, et al. In situ cross-linkable hyaluronic acid hydrogels prevent post-operative abdominal adhesions in a rabbit model. Biomaterials. 2006;27(27):4698–705. doi: 10.1016/j.biomaterials.2006.04.043. [DOI] [PubMed] [Google Scholar]
- [18].Hudson SP, Langer R, Fink GR, Kohane DS. Injectable in situ cross-linking hydrogels for local antifungal therapy. Biomaterials. 2009;31(6):1444–52. doi: 10.1016/j.biomaterials.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Epstein H, Gutman D, Cohen-Sela E, Haber E, Elmalak O, Koroukhov N, et al. Preparation of alendronate liposomes for enhanced stability and bioactivity: in vitro and in vivo characterization. The AAPS journal. 2008;10(4):505–15. doi: 10.1208/s12248-008-9060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Epstein-Barash H, Shichor I, Kwon AH, Hall S, Lawlor MW, Langer R, et al. Prolonged duration local anesthesia with minimal toxicity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(17):7125–30. doi: 10.1073/pnas.0900598106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Schroeder A, Kost J, Barenholz Y. Ultrasound, Liposomes, and Drug Delivery: Principles for Using Ultrasound to Control the Release of Drugs from Liposomes. Chem Phys Lipids. 2009;162(1/2):1–16. doi: 10.1016/j.chemphyslip.2009.08.003. [DOI] [PubMed] [Google Scholar]
- [22].Jia X, Colombo G, Padera R, Langer R, Kohane DS. Prolongation of sciatic nerve blockade by in situ cross-linked hyaluronic acid. Biomaterials. 2004;25(19):4797–804. doi: 10.1016/j.biomaterials.2003.12.012. [DOI] [PubMed] [Google Scholar]