Abstract
OBJECTIVE
To determine whether the placement of small-calibre, rapidly absorbed prophylactic periprostatic sutures before the mobilization of the prostate could reduce blood loss during open retropubic radical prostatectomy (RRP).
PATIENTS AND METHODS
In 2007, during open RRP, we began placing prophylactic haemostatic sutures of 4-0 and 3-0 plain catgut in the anterior portions of the distal neurovascular bundles (NVBs) and lateral to the proximal NVBs and prostate pedicles before initiating the nerve-sparing dissection and mobilizing the prostate gland. To evaluate whether this reduced intraoperative blood loss, we compared estimated blood loss (EBL), non-autologous transfusion rates, and postoperative haemoglobin (Hb) levels between 100 consecutive patients treated immediately before and 100 consecutive patients treated immediately after the adoption of the prophylactic periprostatic suture technique.
RESULTS
Before the use of prophylactic haemostatic sutures, the mean intraoperative blood loss was 1285 mL, and one patient (1%) received an intraoperative non-autologous transfusion. After the adoption of prophylactic sutures, the mean EBL was 700 mL (P < 0.001), and there were no transfusions. The mean Hb concentration the morning after RRP was 10.9 g/dL before and 11.8 g/dL after the initiation of prophylactic haemostatic sutures (P < 0.001).
CONCLUSION
Prophylactic periprostatic haemostatic sutures significantly reduce intraoperative blood loss during open RRP.
Keywords: blood loss, radical prostatectomy, prophylactic, sutures, haemostasis
INTRODUCTION
A better understanding of male pelvic vascular and neural anatomy has led to reduced morbidity and more widespread patient acceptance of radical prostatectomy (RP) [1,2]. That notwithstanding, blood loss during open RP can be substantial, and any measure that helps reduce blood loss might be useful to surgeons and beneficial to patients.
The recently introduced laparoscopic and robotic-assisted laparoscopic approaches to RP have been associated with less blood loss compared to open retropubic RP (RRP) in most of the recent series, largely owing to compression of small veins and capillaries in the periprostatic tissues by the pneumoperitoneum [3–6]. For example, Jurczok et al. [7] reported an average estimated blood loss (EBL) of 200 mL in robotic RP compared to 500 mL in open RRP, with transfusion rates of 3% and 9%, respectively. Farnham et al. [8] similarly reported significantly lower intraoperative blood loss in robotic-assisted laparoscopic RP compared to RRP (191 vs 664 mL), although there was no difference in transfusion rates.
Our objective was to determine whether modifications in open RRP technique could reduce intraoperative blood loss. Specifically, we evaluated the use of rapidly absorbed (catgut), fine calibre ‘prophylactic’ (before the mobilization of the prostate) periprostatic haemostatic sutures to reduce intraoperative blood loss in a series of open RRPs.
PATIENTS AND METHODS
From 2003 to 2007, ≈ 1250 consecutive men underwent RRP by a single surgeon (W.J.C.) using a previously described technique [9–11]. Bilateral pelvic lymphadenectomy was performed in all cases, and nerve-sparing RRP was attempted when appropriate and feasible.
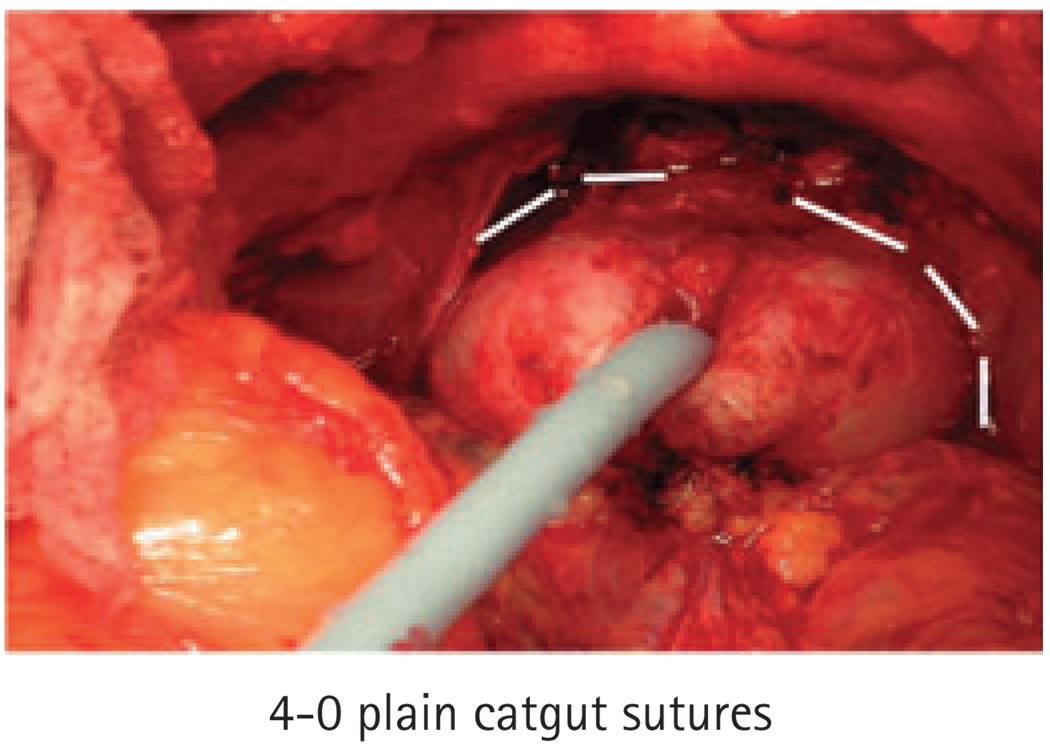
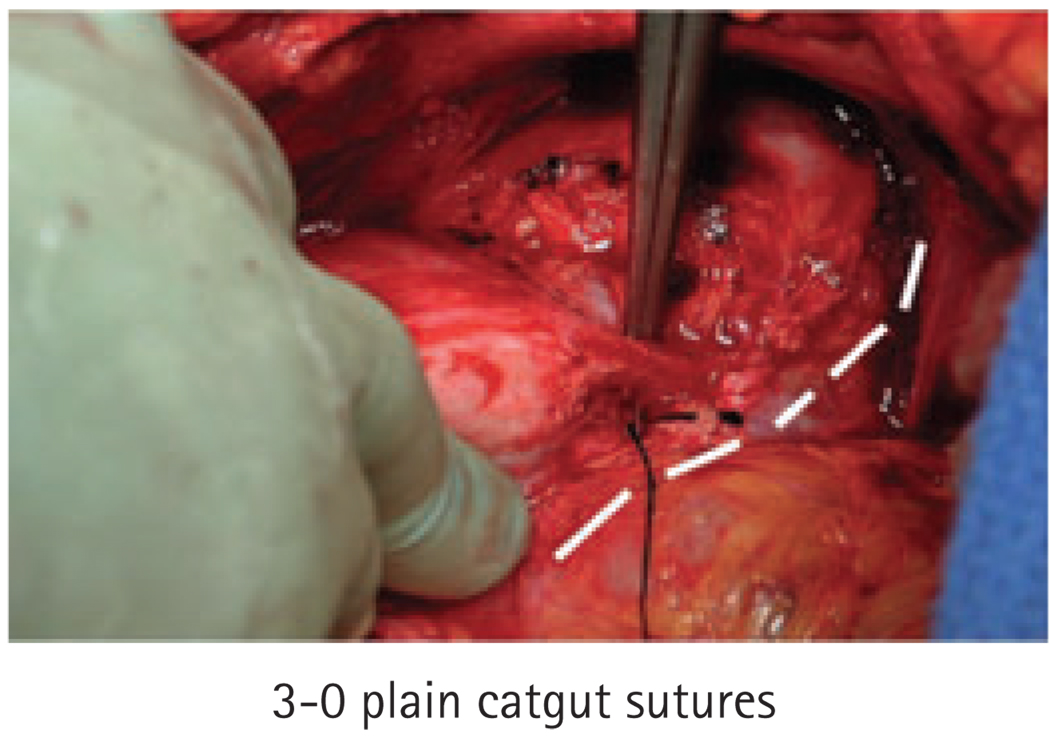
In 2007 we adopted a modification of the surgical technique, involving the routine placement of prophylactic 4-0 and 3-0 plain or chromic catgut sutures for haemostasis. Specifically, we placed 4-0 sutures in the anterior portions of the distal neurovascular bundles (NVBs) and apical prostatic pedicles immediately after the urethra was transected (Fig. 1). Then, we placed a running 3-0 haemostatic suture on each side lateral to the NVBs and continuing along the vascular pedicles of the prostate (Fig. 2). These sutures were gently tightened to give sufficient tension for haemostasis while minimizing crush injury to the incorporated tissues. We used catgut suture material due to its rapid absorption.
FIG. 1.
Placement of 4-0 prophylactic sutures (indicated by white lines).
FIG. 2.
Placement of 3-0 prophylactic sutures (indicated by white lines).
To evaluate whether this reduced intraoperative blood loss, we compared EBL, non-autologous transfusion rates, and postoperative haemoglobin (Hb) levels between 100 consecutive patients treated immediately before and 100 treated immediately after the adoption of the prophylactic periprostatic suture technique.
Although autologous blood donation was not routinely recommended before RRP, a few patients chose to do so. Intraoperative blood loss was calculated based on the amount of blood collected in the suction device and the estimated amount of blood in the laparotomy pads used during the operation. Postoperative Hb levels were routinely obtained on the morning after RRP, and the need for intraoperative non-autologous blood transfusion was recorded.
The Student’s t-test and the chi-square test were used to compare clinical characteristics, intraoperative blood loss and postoperative Hb levels between the 100 patients treated after the initiation of prophylactic sutures and the 100 consecutive patients treated before this modification.
RESULTS
The clinical and pathological characteristics of our patient population are shown in Table 1. Most patients in the suture and no suture groups were aged <60 years with serum PSA concentrations of <10 ng/mL, clinical stage T1c disease, and a biopsy Gleason score of 6. However, there were a greater proportion of men with clinical stage T1c (86% vs 73%; P = 0.04) and a biopsy Gleason score ≥7 (42% vs 27%; P = 0.04) in the prophylactic sutures group.
TABLE 1.
Comparisons between men who did and who did not receive prophylactic sutures with respect to clinicopathological features and haemostatic variables
| Prophylactic sutures |
|||
|---|---|---|---|
| Variable | No | Yes | P |
| Clinicopathological features | |||
| Mean: | |||
| Age, years | 59.6 | 59.8 | 0.89 |
| Preop. PSA, ng/mL | 6.12 | 7.11 | 0.31 |
| Clinical stage ≤T1c, % | 73 | 86 | 0.04 |
| Biopsy Gleason ≥7, % | 27 | 42 | 0.04 |
| Organ-confined, % | 81 | 77 | 0.49 |
| Prostatectomy Gleason ≥7, % | 43 | 54 | 0.15 |
| Positive surgical margins, % | 13 | 15 | 0.69 |
| Extracapsular extension, % | 19 | 22 | 0.72 |
| Seminal vesicle involvement, % | 1 | 6 | 0.06 |
| Lymph node metastases, % | 0 | 1 | 0.49 |
| Prostate weight, g | 48.9 | 50.9 | 0.54 |
| Haemostatic variables | |||
| Mean: | |||
| EBL, mL | 1285 | 700 | <0.001 |
| Postoperative Hb, g/dL | 10.9 | 11.8 | <0.001 |
At RRP, most of the men in both groups had organ-confined disease with negative (cancer-free) surgical margins. However, there was a statistically non-significant trend towards a greater proportion with seminal vesicle involvement in the prophylactic suture group (6% vs 1%; P = 0.06).
Table 1 also compares the intraoperative blood loss and postoperative Hb levels before and after the initiation of the prophylactic haemostatic suture technique. The mean EBL was significantly reduced from 1285 mL to <700 mL using these sutures (P < 0.001). Correspondingly, postoperative Hb levels were significantly higher in the prophylactic suture group (11.8 vs 10.9 g/dL, P < 0.001). One patient in the series from the no-suture group required a non-autologous intraoperative blood transfusion (1 unit).
DISCUSSION
Reiner and Walsh [1] initially described the systematic control of venous bleeding with suture ligation of the dorsal venous complex. This technique substantially decreased intraoperative bleeding during open RP, providing sufficient haemostasis in the surgical field for proper apical dissection and preservation of the NVBs. Correspondingly, this has enabled improved outcomes for the preservation of urinary continence and erectile potency [12,13]. However, intraoperative blood loss from the NVBs and prostatic pedicles has remained an issue in many contemporary open RP series [11,14,15].
The introduction of laparoscopic techniques and the more recent advent of robotic-assisted laparoscopic RP have been associated with less intraoperative blood loss than with open RRP [16]. Published series have reported an EBL ranging from < 100 mL to ≈ 1100 mL [16]. The generally lessened blood loss in laparoscopic approaches is related to the pneumoperitoneum causing compression of periprostatic capillaries and veins. Nevertheless, electrocautery or the harmonic scalpel are often used to help control bleeding during laparoscopic and robotic RP, with the potential for thermal damage to the cavernous NVBs responsible for erectile potency [3,16].
For example, Ong et al. [17] showed in a canine model that the use of thermal or electric energy sources in the proximity of the NVBs resulted in diminished erectile responses to electrical stimulation. Conversely, a careful dissection using suture ligatures did not compromise erectile response in the canine model [17]. More recently, alternate athermal techniques have been proposed to help control bleeding during laparoscopic and robotic surgery; however, the use of these methods may require additional time and technical skill [18].
Our objective was to identify ways to reduce the blood loss associated with open RRP. With carefully placed absorbable periprostatic sutures, we obtain haemostasis largely before the bleeding starts. With this technique, we have achieved a significant reduction in total blood loss and a corresponding increase in postoperative Hb levels in a contemporary open RRP series. We selected small-calibre catgut suture material for this purpose because it is rapidly absorbed and may be less deleterious to postoperative functional recovery than thermal energy in proximity to the NVBs. Notably, these sutures are tied with the least possible tension to obtain haemostasis while potentially minimizing tissue injury. The placement of these sutures did not lead to an increase in operative time, as the pre-placement of these sutures reduces the subsequent time spent achieving complete haemostasis after the prostate had been mobilized and removed.
A limitation of the present study was that although the two groups of patients were contemporary and were treated consecutively, we did not perform a randomized trial, which would be the optimal study design to evaluate the impact of a modification in surgical technique. Nevertheless, the groups with and without prophylactic sutures had similar clinical characteristics. Additionally, all operations were performed at the same hospital by the same surgeon (who had performed >4000 prior RPs), minimizing the potential for bias from variability in surgical technique, blood loss estimation, or laboratory measurement.
Another limitation of the present study was the small sample size. The low transfusion rate in both groups precludes a statistically meaningful comparison. Moreover, the physiological consequences of modest absolute differences in postoperative Hb levels are unknown.
Finally, because this technique was only recently adopted, the present follow-up is not yet sufficiently mature to examine the long-term effects on potency, continence, and cancer control. Due to the short-term advantage of reduced blood loss, we have continued to place these prophylactic sutures during open RRP. That notwithstanding, additional follow-up is necessary to determine the impact of this technical modification on functional and oncological outcomes.
In conclusion, prophylactic haemostatic periprostatic sutures significantly reduced intraoperative blood loss with the classic open RRP, and may be useful for other surgeons. Additional study is warranted to determine the implications of this surgical modification on long-term functional outcomes.
ACKNOWLEDGEMENTS
Supported in part by the Urological Research Foundation, Prostate SPORE grant (P50 CA90386-05S2) and the Robert H. Lurie Comprehensive Cancer Center grant (P30 CA60553).
Abbreviations
- (R)RP
(retropubic) radical prostatectomy
- NVB
neurovascular bundle
- EBL
estimated blood loss
- Hb
haemoglobin
Footnotes
CONFLICT OF INTEREST
William J. Catalona is associated with Beckman-Coulter and deCODE Genetics (unrelated to this article).
Contributor Information
Gustavo F. Carvalhal, Department of Urology, Northwestern University Feinberg School of Medicine, Chicago, IL
Christopher R. Griffin, Department of Urology, Northwestern University Feinberg School of Medicine, Chicago, IL
Donghui Kan, Department of Urology, Northwestern University Feinberg School of Medicine, Chicago, IL.
Stacy Loeb, Department of Urology, James Buchanan Brady Urological Institute, Johns Hopkins, Baltimore, MD, USA.
William J. Catalona, Department of Urology, Northwestern University Feinberg School of Medicine, Chicago, IL
REFERENCES
- 1.Reiner WG, Walsh PC. An anatomical approach to the surgical management of the dorsal vein and Santorini’s plexus during radical retropubic surgery. J Urol. 1979;121:198–200. doi: 10.1016/s0022-5347(17)56718-x. [DOI] [PubMed] [Google Scholar]
- 2.Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982;128:492–497. doi: 10.1016/s0022-5347(17)53012-8. [DOI] [PubMed] [Google Scholar]
- 3.Gill IS, Zippe CD. Laparoscopic radical prostatectomy: technique. Urol Clin North Am. 2001;28:423–436. doi: 10.1016/s0094-0143(05)70150-6. [DOI] [PubMed] [Google Scholar]
- 4.Guillonneau B, Vallancien G. Laparoscopic radical prostatectomy: the Montsouris technique. J Urol. 2000;163:1643–1649. doi: 10.1016/s0022-5347(05)67512-x. [DOI] [PubMed] [Google Scholar]
- 5.Menon M, Hemal AK. Vattikuti Institute prostatectomy: a technique of robotic radical prostatectomy: experience in more than 1000 cases. J Endourol. 2004;18:611–619. doi: 10.1089/end.2004.18.611. [DOI] [PubMed] [Google Scholar]
- 6.Schuessler WW, Schulam PG, Clayman RV, Kavoussi LR. Laparoscopic radical prostatectomy: initial short-term experience. Urology. 1997;50:854–857. doi: 10.1016/S0090-4295(97)00543-8. [DOI] [PubMed] [Google Scholar]
- 7.Jurczok A, Zacharias M, Wagner S, Hamza A, Fornara P. Prospective non-randomized evaluation of four mediators of the systemic response after extraperitoneal laparoscopic and open retropubic radical prostatectomy. BJU Int. 2007;99:1461–1466. doi: 10.1111/j.1464-410X.2007.06849.x. [DOI] [PubMed] [Google Scholar]
- 8.Farnham SB, Webster TM, Herrell SD, Smith JA., Jr Intraoperative blood loss and transfusion requirements for robotic-assisted radical prostatectomy versus radical retropubic prostatectomy. Urology. 2006;67:360–363. doi: 10.1016/j.urology.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 9.Catalona WJ. Nerve-sparing radical retropubic prostatectomy. Urol Clin North Am. 1985;12:187–199. [PubMed] [Google Scholar]
- 10.Catalona WJ, Carvalhal GF, Mager DE, Smith DS. Potency, continence and complication rates in 1,870 consecutive radical retropubic prostatectomies. J Urol. 1999;162:433–438. [PubMed] [Google Scholar]
- 11.Loeb S, Catalona WJ. Open radical retropubic prostatectomy. Urol Oncol. 2007;25:494–498. doi: 10.1016/j.urolonc.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Catalona WJ, Ramos CG, Carvalhal GF. Contemporary results of anatomic radical prostatectomy. CA Cancer J Clin. 1999;49:282–296. doi: 10.3322/canjclin.49.5.282. [DOI] [PubMed] [Google Scholar]
- 13.Eastham JA, Scardino PT, Kattan MW. Predicting an optimal outcome after radical prostatectomy: the trifecta nomogram. J Urol. 2008;179:2207–2211. doi: 10.1016/j.juro.2008.01.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clayman RV. Lateral pedicle control during laparoscopic radical prostatectomy: refined technique. J Urol. 2005;174:930–931. doi: 10.1097/01.ju.0000171900.48158.20. [DOI] [PubMed] [Google Scholar]
- 15.Coakley FV, Eberhardt S, Wei DC, et al. Blood loss during radical retropubic prostatectomy: relationship to morphologic features on preoperative endorectal magnetic resonance imaging. Urology. 2002;59:884–888. doi: 10.1016/s0090-4295(02)01614-x. [DOI] [PubMed] [Google Scholar]
- 16.Menon M, Tewari A, Peabody JO, et al. Vattikuti Institute prostatectomy, a technique of robotic radical prostatectomy for management of localized carcinoma of the prostate: experience of over 1100 cases. Urol Clin North Am. 2004;31:701–717. doi: 10.1016/j.ucl.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Ong AM, Su LM, Varkarakis I, et al. Nerve sparing radical prostatectomy: effects of hemostatic energy sources on the recovery of cavernous nerve function in a canine model. J Urol. 2004;172:1318–1322. doi: 10.1097/01.ju.0000139883.08934.86. [DOI] [PubMed] [Google Scholar]
- 18.Gill IS, Ukimura O, Rubinstein M, et al. Lateral pedicle control during laparoscopic radical prostatectomy: refined technique. Urology. 2005;65:23–27. doi: 10.1016/j.urology.2004.10.045. [DOI] [PubMed] [Google Scholar]