Abstract
Objective
To test whether women with Anorexia Nervosa (AN) have increased sensitivity to punishing or rewarding stimuli, behaviors that could drive high self-control and anxious, avoidant behaviors.
Method
Sixty-four women completed the study: 33 control women (CW, mean age 19.7 years) and 31 AN (mean age 19.6 years). Participants completed diagnostic exams, questionnaires for eating disorder severity and personality, as well as the Sensitivity to Punishment/Sensitivity to Reward Questionnaire (SPSRQ).
Results
AN scored higher than CW on SPSRQ sensitivity to punishment (p<0.00001) and sensitivity to reward (p=0.005). AN women without anxiety or depression continued to have increased SPSRQ scores compared to CW.
Conclusion
This is the first study comparing the SPSRQ in AN and CW. Results suggest that reward and punishment sensitivity are increased in AN and could be potential trait markers. It is possible that harm-avoidant, anxious behaviors in AN are related to this heightened sensitivity.
Anorexia Nervosa (AN) is a severe, often persistent mental illness with the highest mortality rate among all the psychiatric disorders (1). AN is characterized by intense fear of gaining weight, food restriction and weight loss, body image distortion, and amenorrhea (2). Central to AN is the ego-syntonic nature of weight loss, as well as the high rate of treatment resistance and drop out (3). The underlying pathophysiology of AN is unknown.
AN is frequently associated with co-morbid anxiety disorders that often predate the emergence of eating disorder (ED) pathology (4). Individuals with AN are consistently characterized as highly perfectionistic and controlling (5) and show temperament traits such as high harm avoidance (HA; shy, fearful, worrying behavior, tendency to avoid perceived punishment), and lower novelty seeking (NS; the desire to explore and approach potential rewards) (4, 6-9). These characteristics raise the question whether such behaviors in AN are primary or adaptive in order to tolerate and handle the ups and downs of failures and accomplishments in daily life. While speculative, AN individuals may have heightened emotional or physiological sensitivity to experiences that are associated with reward or punishment, and that the eating disorder and associated behaviors may in some way serve to mitigate these responses by the sense of control received. We hypothesized that individuals with AN might be overly sensitive to the saliency of punishing or rewarding stimuli, and they may act to minimize exposure to such cues.
Reinforcement Sensitivity Theory (RST) (10) provides a framework that explores how differences in brain systems responsive to punishment and reward are reflected in individual personality (11-12). In the original theory, personality dimensions such as anxiety and impulsivity were thought to be controlled by two underlying neural systems: the behavioral activation system, specifically responsive to rewarding stimuli resulting in approach behavior, and the behavioral inhibition system, responsive to aversive stimuli, or punishment, leading to inhibition of behavior (11-12). Conditioned and unconditioned stimuli activate the behavior activation and inhibition system. In the most recent revision of RST, the original behavioral inhibition system has been further divided into the fight-flight-freeze system (FFFS) and the behavioral inhibition-anxiety system. RST suggests that the FFFS responds to all potentially aversive stimuli, replacing the role of the original behavioral inhibition concept, whereas the revised behavioral inhibition-anxiety system, instead, resolves approach versus avoidance of a stimulus when there is a conflict and activation of both the FFFS and behavior activation system (11, 13). Based on this revised model, personality characteristics related to impulsivity, anxiety, and fear are independently mediated by the behavioral activation system, behavioral inhibition-anxiety, and FFFS systems respectively (11).
Under the RST framework, individuals with altered sensitivities in these three systems may have an increased risk for psychological illness and distress (11), and research has shown that individuals with elevated FFFS are prone to phobia and panic, people with elevations of the behavioral activation system are prone to addictive behavior, and those with a heightened behavioral inhibition system activity in general may have an increased risk of anxiety disorders (13).
Two self-report measures exist that were designed specifically to assess reinforcement sensitivity: The Behavioral Inhibition Scale/Behavioral Activation Scale (BIS/BAS) (14) and the Sensitivity to Punishment/Sensitivity to Reward Questionnaire (SPSRQ) (15). Although the BIS/BAS has been applied the most frequently in behavioral, psychophysiological and neuro-imaging studies, some have criticized the scale’s approach towards measuring “generalized sensitivity to reward and punishment, while Gray’s theory deals with sensitivity to specific cues” (15-17). In response to that criticism, the SPSRQ was developed to test behavior inhibition and activation systems employing questions related to very specific cues and situations. Just recently, the use of the SPSRQ has been validated for the use in eating disorder populations (18) supporting the assessment of reward sensitivity in AN using this questionnaire. While aiming to test the behavioral inhibition and behavioral activation systems, the two questionnaires differ with respect to questionnaire design and item presentation.
Although there is clinical evidence of altered reward and punishment sensitivity in individuals with eating disorders, only a handful of studies have investigated the role of reinforcement sensitivity in ED populations using these measures. One group, using the BIS/BAS and SPSRQ, (18) reported that women with AN and bulimia nervosa (BN) have similar levels of behavioral inhibition reactivity; however, purging-type AN (AN-P) and BN had significantly higher levels of behavioral activation compared to restricting-type AN (AN-R), suggesting that BN and AN-P are more prone to act impulsively and approach potentially rewarding activities. That study did not include a control group, however. Another study, using only the BIS/BAS, reported higher levels of behavioral inhibition in AN-R than AN-P or controls, and lower levels of fun seeking (an aspect of behavioral activation related to the willingness to seek out potential rewards) and total behavioral activation (19). Two studies in female high school and college students using the BIS/BAS showed that heightened sensitivity to both punishment as well as reward predicted dysfunctional eating as assessed by the Eating Disorder Inventory, which suggested a possible link between Reinforcement Sensitivity and maladaptive eating behavior (20-21).
The present study is the first to compare the SPSRQ between clinically diagnosed AN participants and healthy control women (CW). In addition, study participants completed the BIS/BAS questionnaire and measures of eating disorder symptomatology. Based on our clinical observations, and in line with the work by Loxton and Dawe, we hypothesized that AN would report increased sensitivity to both punishment and reward, as well as higher levels of behavioral inhibition and behavioral activation compared to controls (20-21). Such findings could suggest the presence of a biologic vulnerability underlying the ego syntonic, over-controlling, anxious and avoidant behavior often observed in AN.
METHODS
Participants
Sixty-four women, ages 12 to 45 years, completed the study: thirty-three healthy controls (mean age = 19.67 years, standard deviation [SD] = 6.42) and thirty-one with anorexia nervosa (mean age = 19.42 years, SD = 6.98), with 9 AN binging/purging type and 22 AN restricting type. Participants with AN were recruited through the Eating Disorders Program at The Children’s Hospital in Aurora, Colorado and at the Eating Disorder Center of Denver, which included patients in inpatient, day treatment and outpatient levels of care. Control participants were recruited through local advertisements in the Denver/Metro area. For the AN group, all study procedures were completed at low weight (less than 85% ideal body weight) and meeting full AN diagnostic criteria. In general, all study procedures were completed within the first week after admission. Informed consent was obtained for each individual enrolled, and all research procedures were approved by the Colorado Multiple Institutional Review Board.
To qualify for the study, participants took part in a rigorous, multi-step screening process. Study participants completed a battery of self-report questionnaires, met individually with a doctoral level study investigator to assess medical and psychological history, and also completed a structured diagnostic interview. Healthy CW had a lifetime history of healthy body weight (between 90% and 110% of ideal body weight since menarche), did not endorse symptomatic eating or weight concerns, and were free from any major medical illness. Participants with AN met current DSM-IV-R (2) criteria for anorexia nervosa, either restricting or binge/purging subtype.
Screening
To assess for psychological symptoms in healthy individuals under 18 years (n=16), participants were interviewed with DISC Predictive Scales (DPS), a computerized tool designed to quickly and accurately screen individuals for psychopathological symptoms (22). Those who endorsed psychiatric symptoms via DPS, indicating the need for further evaluation and screening, were excluded. AN minors (n=15) completed the Clinical Diagnostic Interview Schedule for Children 4.0 (CDISC-IV), an in-depth computerized diagnostic tool to assess all major psychiatric diagnoses. Adult CW (n=17) and AN (n=16) were assessed with the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (23). Controls were excluded if they endorsed any current or past psychological symptoms. The study, however, did allow co-occurring diagnoses for the AN population, except psychotic and substance use disorders.
Measures
Once enrolled, study participants completed the following series of self-assessment questionnaires:
Temperament and Character Inventory
Cloninger’s Temperament and Character Inventory is a 240-item self-assessment questionnaire that examines personality based on 7 different dimensions. For this study, we examined harm avoidance, novelty seeking, and reward dependence specifically, which are temperament variables thought to be stable, heritable and mediated by neurotransmitter activity in the central nervous system (9).
Eating Disorder Inventory-3
The Eating Disorder Inventory-3 (EDI-3) is an expanded and improved version of the widely used Eating Disorder Inventory-2 developed by Garner (24-25). This 91-item questionnaire assesses psychological and behavioral traits related eating disorder development and maintenance. We examined the EDI-3’s Drive for Thinness (preoccupation with weight and the pursuit of thinness), Bulimia (tendency towards bulimia-like binging and purging), and Body Dissatisfaction (25). Higher scores on each of these subscales reflect greater eating disorder pathology.
Reinforcement Sensitivity Theory Scales
SPSRQ
The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) was developed directly as a means to measure Gray’s original RST theory. The SPSRQ is a 48-item scale in a yes/no format that provides a score for individual sensitivity to punishment (SP), related to Gray’s original behavioral inhibition system, and a score for sensitivity to reward (SR), related to behavioral activation system (15). Both the SP and SR have shown satisfactory test re-test reliability and convergent and discriminant validity (15). O’Connor, Colder et al. (2004) revised this original scale using confirmatory factor analysis to trim 13 assessment items that did not load appropriately the original RST construct. We employed this reduced version in the current study.
Behavioral Inhibition/Behavioral Activation Scale
Carver and White (1994) developed the Behavioral Inhibition/Behavioral Activation Scale (BIS/BAS) based on Gray’s earliest reinforcement sensitivity theory. It is a 24-item self questionnaire that yields 4 scales: one for behavioral inhibition and three inter-correlated sub-scores for the behavioral activation system - reward responsiveness (RR), drive (DR), and fun seeking (FS). RR measures response to receipt of anticipated rewards, DR measures goal-directed behavior, and FS measures one’s willingness to approach new, potentially rewarding stimuli (14). The original scale has high internal and test-retest reliability scores as well as convergent and divergent validity (14). In response to revisions of the original RST, the BIS score can also be analyzed as two separate sub-scales: a fight, flight, freeze (FFFS)-fear score and a BIS-anxiety score (26). For this study, we examined the original 4-factor BIS/BAS scale and the revised 5-factor model (26).
Both the 2-factor SPSRQ scale and the 4 and 5-factor BIS/BAS scale have been rigorously tested through confirmatory factor analysis and shown to be appropriate measures for RST in eating disorders (18).
Statistical Analyses
The SPSS statistical software package was used for data analysis. Independent-samples T-tests were used to examine BIS/BAS scores as well as SPSRQ scores between the CW and AN. In addition we explored in AN individuals with and without anxiety or depressive disorder against CW, using three-group one way ANOVA analyses and Scheefe’s post hoc test. Spearman Correlation analyses were also conducted to examine relations between the SPSRQ, BIS/BAS, demographic, and behavioral data.
RESULTS
Demographic Variables (Table 1.)
Table 1.
Demographic data, illness duration, and behavioral data as measured by the Eating Disorder Inventory-3 (EDI-3): Drive for Thinness (DT), Bulimia (B), Body Dissatisfaction (BD), and personality data from Temperament Character Inventory (TCI): Novelty Seeking (NS), Harm Avoidance (HA), and Reward Dependence (RD).
| CW (n=33) | AN (n=31) | t-stat | P-Value | |||
|---|---|---|---|---|---|---|
| mean | (SD) | mean | (SD) | |||
| Age (years) | 19.7 | (6) | 19.4 | (7) | 0.147 | 0.883 |
| Duration of Illness (years) | ------- | ------- | 4.4 | (7) | ------- | ------- |
| BMI | 21.0 | (2) | 16.3 | (1) | 11.969 | <0.00001 |
| TCI NS | 20.8 | (5) | 14.8 | (6) | 4.124 | 0.0001 |
| TCI HA | 9.4 | (44) | 22.4 | (8) | -8.350 | <0.00001 |
| TCI RD | 16.7 | (3) | 14.6 | (3) | 2.749 | 0.008 |
| EDI-3 DT | 3.0 | (4) | 20.0 | (7) | -11.381 | <0.00001 |
| EDI-3 B | 0.9 | (1) | 6.7 | (9) | -3.747 | 0.001 |
| EDI-3 BD | 4.8 | (5) | 22.7 | (11) | -8.676 | <0.00001 |
CW and AN participants were matched for age. Average BMI for CW was greater compared to AN. As measured by Cloninger’s TCI, AN participants had significantly lower levels of novelty seeking and reward dependence, but higher levels of harm avoidance. AN participants also reported significantly higher scores for EDI-3 subscales drive for thinness (DT), body dissatisfaction (BD), and bulimia (B). TCI and EDI-3 scores were consistent across age for both controls and the AN group.
SPSRQ, BIS/BAS Results (Table 2.)
Table 2.
Mean data, standard deviation, t-statistic, and p-value for SPSRQ Sensitivity to Reward (SR), Sensitivity to Punishment (SP); BIS Total, Anxiety, and Fear Scales; BAS Total, Reward Responsiveness (RR), Drive, and Fun-seeking (FS) scales.
| CW (n=33) | AN (n=31) | t-stat | P-Value | |||
|---|---|---|---|---|---|---|
| mean | (SD) | mean | (SD) | |||
| SPSRQ (SR) | 5.7 | (4) | 8.6 | (4) | -2.947 | 0.005 |
| SPSRQ (SP) | 4.3 | (3) | 11.7 | (4) | -8.512 | <0.00001 |
| BIS Total | 19.1 | (2) | 24.3 | (3) | -7.429 | <0.00001 |
| BIS Anxiety | 11.6 | (2) | 14.8 | (2) | -7.579 | <0.00001 |
| FFFS Fear | 7.5 | (2) | 9.5 | (2) | -4.193 | <0.00001 |
| BAS Total | 40.6 | (5) | 39.4 | (5) | 1.013 | 0.315 |
| BAS RR | 17.6 | (2) | 17.3 | (2) | .443 | 0.659 |
| BAS Drive | 10.4 | (2) | 10.9 | (3) | -.804 | 0.425 |
| BAS FS | 12.6 | (2) | 11.1 | (2) | 2.757 | 0.008 |
Individuals with AN scored higher than controls on the SPSRQ sensitivity to punishment (t=-8.512, p=<0.00001) as well as the SPSRQ sensitivity to reward (t=-2.947, p=0.005) scale.
For the BIS/BAS, AN scored significantly higher on the BIS total score (t=-7.43, p<0.00001), but not the BAS total score. Under the 5-factor BIS/BAS model, AN scored higher on the BIS anxiety subscale (t=-7.58, p<0.00001) and the BIS FFFS fear scale (t=-4.19, p<0.00001), but had decreased response to the BAS fun-seeking scale (t=2.76, p=0.008). Unlike previous groups, we did not find any differences between AN-R and AN-B/P subgroups. For both SPSRQ and BIS/BAS scales, only the BIS Anxiety subscale in CW yielded a significant difference between age, where healthy individuals 18 years and over reported higher scores than controls under 18 years (F=27.04, p=0.020). AN did not show this difference across age for the BIS Anxiety scale. In addition, none of the remaining scales (SPSRQ-SP, SPSRQ-SR, BIS Total, FFFS, BAS Total, RR, DR, FS) showed significant within-group differences for age. In addition, when comparing AN and CW participants under 18 years, AN continued to have increased SPSRQ-SP (p<0.0001) as well as SPSRQ-SR (p=0.05), and when comparing those 18 years and over, AN also had greater SPSRQ-SP (p<0.0001) and SPSRQ-SR (p=0.03), suggesting no significant effects of age.
Using a one-way ANOVA we also examined SPSRQ and BIS/BAS results between groups with and without co-morbid anxiety or major depressive disorders (MDD). Anxiety disorder comorbidity comparison: 14 AN met criteria for a concurrent anxiety disorder, and four AN participants had multiple anxiety diagnoses: 5 social anxiety disorder, 1 specific phobia, 6 obsessive compulsive disorder, 2 post-traumatic stress disorder, 4 generalized anxiety disorder. The SPSRQ-SP subscale showed lowest values in the CW (mean=2.51, SD=4.36) followed by AN without (mean =4.08, SD=9.88) and AN with anxiety disorder (mean =13.93, SD=3.05), and was significant between CW and AN (F=51.23) without (p<0.00001) and with anxiety disorder (p<0.00001), as well as between AN with and without anxiety disorder (p=0.003) (Figure 3). The SPSRQ-SR subscale showed significant differences (F=4.63, p=0.019) between CW (mean=5.73, SD=5.73), and AN without anxiety (mean =9.12, SD=3.69). BIS total score as well as BIS-anxiety were similar between AN with (BIS Total mean =25.50, SD=2.59; BIS anxiety mean =15.21, SD=1.19) and without anxiety disorder (BIS Total mean =23.35, SD=3.24; BIS anxiety mean=14.53, SD=1.81), but increased compared to CW (BIS Total mean=19.12, SD=2.42; BIS anxiety mean=11.61, SD=1.84) in both of those groups (F=29.61, p<0.001). FFFS was similar between CW (mean =7.52, SD=1.70) and AN without anxiety disorder (mean =8.82, SD=2.19), but increased in AN with anxiety comorbidity (mean =10.29, SD=1.54) compared to CW (F=11.98, p<0.001). Major depressive disorder (MDD) comorbidity comparison: Ten AN had comorbid MDD. AN with (mean=13.20, SD=3.12) and without (mean=11.00, SD=4.43) MDD had increased SPSRQ-SP values compared to controls (mean=4.36, SD=2.51; F=40.09, p<0.01), and there was no significant difference between the two AN groups. AN without MDD (mean=9.48, SD=3.66) had greater SPSRQ-SR compared to CW (mean=5.73, SD=3.68; F=6.26, p=0.004), but not AN with MDD (mean=6.70, SD=4.57). AN with (mean=25.60, SD=2.63) and without MDD (mean=23.71, SD=3.20) had increased BIS total score compared to CW (mean=19.12, SD=2.42; F=30.66, p<0.001), as well as increased FFFS (AN without MDD: mean=9.24, SD=1.81; AN with MDD: mean=10.00, SD=2.45; CW: mean=7.52, SD=1.698; F=9.48, CW and AN without MDD p=0.006, CW and AN with MDD p=0.002) and BIS anxiety (AN without MDD: mean=14.48, SD=1.75; AN with MDD: mean=15.60, SD=0.67; CW: mean=11.61, SD=1.84; F=30.87, p<0.001). AN with MDD (mean=35.90, SD=4.36) had decreased BAS Total scores compared to both controls (mean=40.61, SD=5.26, p=0.027) and AN without MDD (mean=41.00, SD=3.82, p=0.024), as well as decreased BAS drive scores compared to AN without MDD (p=0.018). Similarly, BAS FS was only reduced in AN with MDD (mean=10.30, SD=2.95) compared to CW (mean=12.64, SD=1.99, F=5.01, p=0.015).
Figure 3.
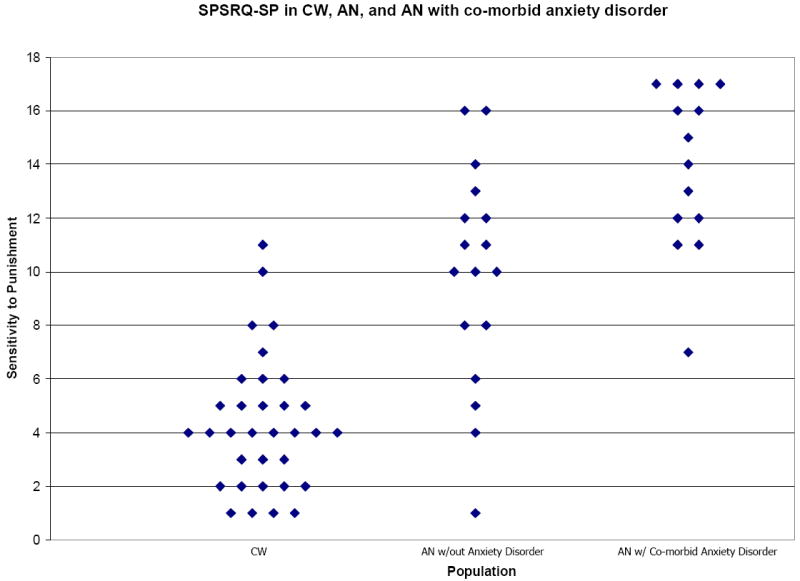
Scatter plot graph for SPSRQ Sensitivity to Punishment subscale data comparing healthy control participants (CW; n=33) to anorexia nervosa (AN) without concurrent anxiety disorder (n=17) and AN with a co-morbid anxiety diagnosis (n=14). Data yielded significant differences in SP scores between all three groups (F=51.23): CW and AN without anxiety disorder (p<0.00001) and with anxiety disorder (p<0.00001), as well as between AN with and without anxiety disorder (p=0.003).
Correlation Analyses
In CW, SPSRQ Sensitivity to Reward (SR) and SPSRQ Sensitivity to Punishment (SP) were positively associated with each other (rho=0.705, p<0.001, two-tailed). Although EDI-3 scores for controls were in the normal, non-clinical range, in CW SP was related to body dissatisfaction (rho=0.404, p<0.05). SR in CW was also correlated with both body dissatisfaction (rho=0.386, p<0.05) and drive for thinness (rho=0.398, p<0.05). In addition, in CW, all BIS scales positively correlated to reward dependence (BIS Total: rho=0.544, p<0.01, FFFS: rho=0.430, p<0.05, BIS Anxiety: rho=0.426, p<0.05). The BIS-FFFS fear scale showed positive associations with harm avoidance (rho=0.488, p=0.004), and the BAS fun seeking scale correlated with novelty seeking scores (rho=0.468, p=0.006).
In participants with AN, SP was positively associated with TCI harm avoidance subscale (rho=0.758, p<0.001), EDI-3 drive for thinness (rho=0.488, p<0.05), but negatively associated with TCI novelty seeking (rho=-0.371, p<0.05). AN participants also showed positive links between SP and all BIS scores (BISTotal rho=0.605, p<0.001; BIS anxiety rho=0.418, p=0.019; BIS FFFS rho=0.649, p<0.001) as well as between SR and BAS Total (rho=0.646, p<0.001), BAS reward responsiveness (rho=0.463, p=0.009), and BAS drive (rho=0.764, p<0.001). In addition, harm avoidance was positively associated with BIS Total score (rho=0.816, p<0.001), FFFS score (rho=0.776, p<0.001), BIS anxiety (rho=0.627, p<0.001) and negatively associated with the BAS total score (rho=0.-524, p=0.002), BAS drive (rho=-0.381, p=0.34), and BAS fun seeking (rho=-0.451, p=0.011). Drive for thinness correlated positively with BIS Total (rho=0.468, p=0.008) and BIS FFFS scales (rho=0.499, p=0.004).
DISCUSSION
This is, to our knowledge, the first study to apply the SPSRQ in individuals ill with AN compared to controls. There were two main results: women with restricting or binge-purging type AN reported higher SPSRQ sensitivity to punishment as well as higher SPSRQ sensitivity to reward scores compared to CW. This possibly suggests that this group possesses an oversensitive motivational system. These results were not determined by anxiety or depression comorbidity. Secondly, SPSRQ sensitivity to punishment and reward were related to measures for temperament and eating disorder symptomatology. BIS results were comparable to the SPSRQ-SP scale and consistent with previous studies, but lower BAS scores in the AN group were related to major depression comorbidity.
The highly significant elevation in SPSRQ sensitivity to reward as well as punishment was striking to us and may suggest an important clinical implication. AN patients are frequently difficult to engage in treatment and do not seem to respond well to positive or negative reinforcement related to eating behavior or weight gain from the environment or the treatment team. AN patients may be overwhelmed by both rewards as well as stimuli perceived as punishing, and in response may tend to avoid both. This does not mean that behavioral interventions including rewards and consequences should not be used in treatment; however, the data might advocate for making treatment extremely predictable and emphasizing the need to create a therapeutic environment that is focused on engaging the patient’s own motivation, rather than simply a system of rewards and consequences, in order to accomplish long-term recovery.
Our study population was comparable to previous studies (6) in terms of clinical assessment results, such as EDI-3 values in the clinical range together with high harm avoidance but low novelty seeking and reward dependence. The SPSRQ scores in our study were lower compared to Beck’s findings (18) but this is largely due to the fact that we reduced the total item numbers of the questionnaire in order to improve factor loading (27). Our BAS/BIS results are also comparable with Claes (19) who showed reduced BAS fun seeking but increased BIS total score in AN. Unlike this group, however, we did not find differences between AN subtypes. Similarly, only the BIS anxiety subscale in healthy controls yielded a significant age difference, where younger controls reported lower BIS anxiety. All other scales across groups were consistent for participants under 18 years compared to those 18 years and older. Furthermore, our data supports that it is important to assess comorbid anxiety and depressive disorder, since anxiety is associated with higher SPSRQ-SP, while MDD is associated with lower SPSRQ-SR.
Although both the BIS/BAS and SPSRQ were both developed as a measure of Gray’s RST, the two scales posses dissimilarities that may help explain slight differences between CW and AN participants when comparing these instruments, especially in regards to behavioral activation. The BIS/BAS scale examines reward and punishment sensitivity to general or non-specific stimuli. For example, “Even if something bad is about to happen to me, I rarely experience fear of nervousness.” The BIS/BAS does not aim to measure how one typically responds to an explicit situation, but rather how they might respond given a general circumstance; it examines non-specific reinforcement. The SPSRQ, developed more recently, examines specific reinforcement to rewards. Where the SP subscale measures the behavioral inhibition system in more ambiguous situations, like Carver and White’s BIS/BAS (14), the SR subscale measures sensitivity to overt stimuli, such as those involving money, power, sensation seeking, etc. (13).. For example, “Does the good prospect of obtaining money motivate you strongly to do some things?” Both scales attempt to capture individual differences in RST; however, it is possible the differences in question type and response format between the two scales account for disparities observed between the BIS/BAS and SPSRQ in CW and AN groups. Given that AN patients are cognitively rigid and show high verbal skills (28-29), it is possible that AN respond differently to the more specific questions in the SPSRQ. Further research is needed to confirm and explore divergence between subscales of these two instruments. The SPSRQ might be a sensitive instrument for assessing RST sensitivity. Our SPSRQ results are in line with those of Loxton and Dawe’s BIS/BAS results (20-21) who showed in a non-diagnosed college sample that both increased sensitivity to reward as well as punishment were predictive of eating problems. Also comparable to prior research (20-21) sensitivity to punishment in the present study correlated positively with EDI-3 body dissatisfaction in CW whereas sensitivity to reward correlated with drive for thinness. In AN, sensitivity to punishment correlated with drive for thinness. Harm avoidance correlated positively with SPSRQ-SP in the AN group, but not in CW. It is possible that the severity of eating disorder thoughts and behaviors could be related to or driven by higher SPSRQ scores, or that increasing severity of AN may be associated with increasing sensitivity to reward and punishment. Furthermore, the positive correlation of harm avoidance with SPSRQ-SP might support the notion that stimulus avoidance might be driven by high sensitivity to potential punishment. Duration of illness was not associated with SPSRQ or BIS/BAS scores. The positive correlations between SP and SR in the CW fit within the RST model as BIS/BAS reflect separate and distinct underlying physiological systems, each responsive to different reinforcing stimuli (11).
A few studies have found relationships between measures for reward sensitivity and brain biology. Through genetic analysis, the DRD2 A1 allele has been linked to reward sensitivity as measured by both the SPSRQ and BIS/BAS (30-31). Voxel-based morphometry methods have shown associations between sensitivity to punishment and gray matter volume in the amygdala and hippocampal area (32). Various brain alterations have been reported in AN (33) and future brain imaging studies should assess whether the increased reward and punishment sensitivity in AN in this study can be replicated and correlated with specific brain function abnormalities.
The primary study limitation was the relatively small sample size; however participant numbers were comparable with previous studies (18-19). In addition, SPSRQ SR subscale (d=0.74) and BIS Fun Seeking scale (d=-0.69) yielded medium effect sizes, and the SPSRQ SP (d=2.17) subscale, BIS Total (d=1.87), BIS Anxiety (d=1.88), and BIS FFFS scale (d=1.05) yielded large effect sizes as measured by Cohen’s d (34-35). Second, our results are taken from a self report questionnaire without objective rater or biologic assessment. Third, AN participants were not recruited from a uniform level of care (inpatient and day hospital setting). In order to acquire a uniform as possible sample, we did require that individuals enrolled with AN complete all self-assessments while at low weight status, below 85% of ideal body weight for age and height, and met full AN criteria at the time of the study. All study procedures were done within the first, and occasionally within the second week after admission. While we cannot exclude it, we do not have any indication that early weight restoration, and maybe mostly re-feeding in this case, has significant effects on our outcome measures. There were no significant correlations found between BMI and RST measures for the AN group. This is, however, an important question and we will address this question in the future with application of the questionnaires after short and long term weight restoration and recovery. Lastly, the SPSRQ scale was originally developed and presented in the Catalan language. It has been translated from this original version to English (15, 27), and possible differences between versions may affect responses collected in the two countries, although the recent validation study of the questionnaire (18) suggests its validity.
In conclusion, AN individuals appear to have elevated sensitivity to rewards and punishment based on the SPSRQ compared to healthy women. Patients with AN often present with rigid cognitive styles, perfectionism, and harm avoidant behaviors. Such behaviors could be a means of attempting to control an increased reactivity to reward and punishment. Anxious, harm-avoidant behavior, for example, may reduce one’s experience and exposure to emotionally laden stimuli, good or bad, and mediate reactivity of the Reinforcement Sensitivity system. Further research is necessary in order to explore potential relationships between SPSRQ findings and underlying brain biology, as well as implications for adapting therapeutic interventions for improved outcomes in the treatment of AN.
Figure 1.
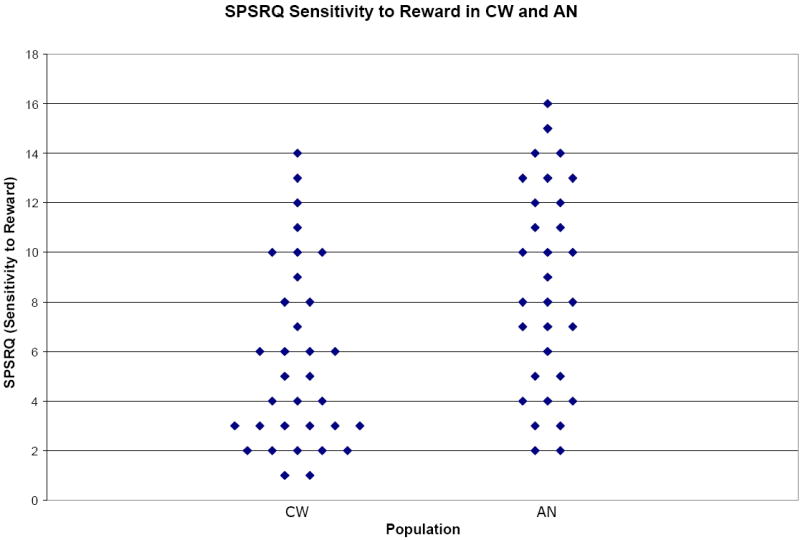
Scatter plot graph for SPSRQ Sensitivity to Reward subscale data comparing healthy control participants (CW; n=33) to individuals with anorexia nervosa (AN; n=31). Data illustrated yielded p-value p=0.005 and effect size d=0.74.
Figure 2.
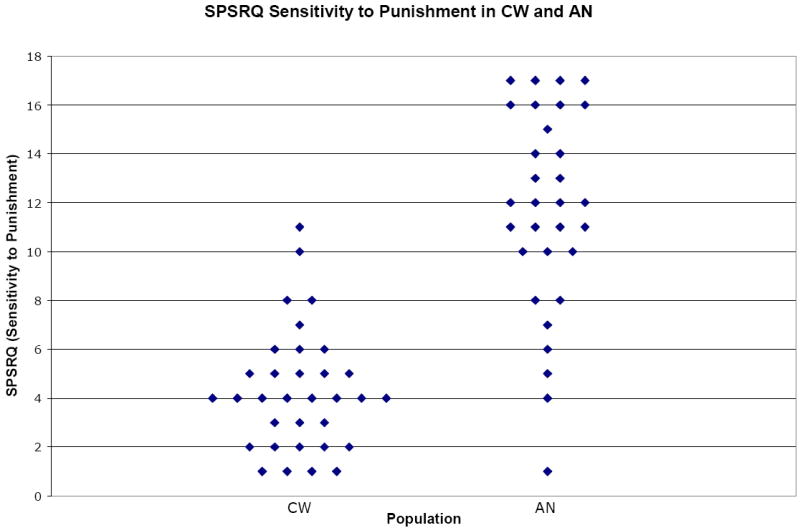
Scatter plot graph for SPSRQ Sensitivity to Punishment subscale data comparing healthy control participants (CW; n=33) to individuals with anorexia nervosa (AN; n=31). Data illustrated yielded p-value <0.00001 and effect size d=2.17.
Acknowledgments
The authors would like to thank all the individuals who participated in this study as well as The Children’s Hospital Eating Disorder Program and The Eating Disorder Center of Denver for their support and collaboration.
This work was supported by NIMH grant K23 MH080135-01A2 (GKWF), and by the Davis Foundation Award of the Klarman Family Foundation Grants Program in Eating Disorders (GKWF).
References
- 1.Sullivan PF. Mortality in anorexia nervosa. Am J Psychiatry. 1995 Jul;152(7):1073–4. doi: 10.1176/ajp.152.7.1073. [DOI] [PubMed] [Google Scholar]
- 2.APA. Diagnostic & Statistical Manual of Mental Disorders: DSM-IV-TR. 4. Washington, DC: American Psychiatric Association; 2000. Text Revision ed. [Google Scholar]
- 3.Guarda AS. Treatment of anorexia nervosa: insights and obstacles. Physiol Behav. 2008 Apr 22;94(1):113–20. doi: 10.1016/j.physbeh.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 4.Kaye WH, Bulik CM, Thornton L, Barbarich N, Masters K. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry. 2004 Dec;161(12):2215–21. doi: 10.1176/appi.ajp.161.12.2215. [DOI] [PubMed] [Google Scholar]
- 5.Cassin SE, von Ranson KM. Personality and eating disorders: a decade in review. Clin Psychol Rev. 2005 Nov;25(7):895–916. doi: 10.1016/j.cpr.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Klump KL, Strober M, Bulik CM, Thornton L, Johnson C, Devlin B, et al. Personality characteristics of women before and after recovery from an eating disorder. Psychol Med. 2004 Nov;34(8):1407–18. doi: 10.1017/s0033291704002442. [DOI] [PubMed] [Google Scholar]
- 7.Group PFC. Deriving behavioural phenotypes in an international, multi-centre study of eating disorders. Psychol Med. 2001 May;31(4):635–45. doi: 10.1017/s0033291701003671. [DOI] [PubMed] [Google Scholar]
- 8.Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Arch Gen Psychiatry. 1993 Dec;50(12):975–90. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- 9.Klump KL, Bulik CM, Pollice C, Halmi KA, Fichter MM, Berrettini WH, et al. Temperament and character in women with anorexia nervosa. J Nerv Ment Dis. 2000 Sep;188(9):559–67. doi: 10.1097/00005053-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Gray JA. The psychophysiological basis of introversion-extraversion. Behav Res Ther. 1970 Aug;8(3):249–66. doi: 10.1016/0005-7967(70)90069-0. [DOI] [PubMed] [Google Scholar]
- 11.Bijttebier P, Beck I, Claes L, Vandereycken W. Gray’s Reinforcement Sensitivity Theory as a framework for research on personality-psychopathology associations. Clin Psychol Rev. 2009 Jul;29(5):421–30. doi: 10.1016/j.cpr.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Deary V. Punishment, Reward and Personality: A review of Corry, P.J. Personality and Individual Differences. 2009;46:565–6. [Google Scholar]
- 13.Corr PJ. The Reinforcement Sensitivity Theory of Personality. Cambridge, UK: Cambridge University Press; 2008. [Google Scholar]
- 14.Carver CS, White TL. Behavioral Inhibition, Behavioral Activation, and Affective Responses to Impending Reward and Punishment: The BIS/BAS Scales. Personality and Social Psychology. 1994;67(2):319–33. [Google Scholar]
- 15.Torrubia R, Avila C, Molto J, Caseras X. The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray’s anxiety and impulsivity dimensions. Personality and Individual Differences. 2001;31:837–62. [Google Scholar]
- 16.Zinbarg R, Revelle W. Personality and conditioning: a test of four models. J Pers Soc Psychol. 1989 Aug;57(2):301–14. doi: 10.1037//0022-3514.57.2.301. [DOI] [PubMed] [Google Scholar]
- 17.Matthews G, Gilliland K. The personality theories of H.J. Eysenck and J.A. Gray; a comparative review. Personality and Individual Differences. 1999;26:583–626. [Google Scholar]
- 18.Beck I, Smits DJM, Claes L, Vandereycken W, Bijttebier P. Psychometric evaluation of the behavioral inhibition/behavioral activation system scales and the sensitivity to punishment and sensitivity to reward questionnaire in a sample of eating disordered patients. Personality and Individual Differences. 2009;47:407–12. [Google Scholar]
- 19.Claes L, Nederkoorn C, Vandereycken W, Guerrieri R, Vertommen H. Impulsiveness and lack of inhibitory control in eating disorders. Eat Behav. 2006 Aug;7(3):196–203. doi: 10.1016/j.eatbeh.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Loxton NJ, Dawe S. Reward and punishment sensitivity in dysfunctional eating and hazardous drinking women: associations with family risk. Appetite. 2006 Nov;47(3):361–71. doi: 10.1016/j.appet.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Loxton NJ, Dawe S. Alcohol abuse and dysfunctional eating in adolescent girls: the influence of individual differences in sensitivity to reward and punishment. Int J Eat Disord. 2001 May;29(4):455–62. doi: 10.1002/eat.1042. [DOI] [PubMed] [Google Scholar]
- 22.Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, et al. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. J Am Acad Child Adolesc Psychiatry. 2001 Apr;40(4):443–9. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 23.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York: Biometrics Research New York State Psychiatric Institute; 2002. [Google Scholar]
- 24.Cumella EJ. Review of the Eating Disorder Inventory-3. Journal of Personality Assessment. 2006;81(1):116–7. [Google Scholar]
- 25.Garner DM. Eating Disorder Inventory-2: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- 26.Heym N, Ferguson E, Lawrence C. An evaluation of the relationship between Gray’s revised RST and Eysenck’s REN: Distinguishing BIS and FFFS in Carver and White’s BIS/BAS scales. Personality and Individual Differences. 2008;45:709–15. [Google Scholar]
- 27.O’Connor RM, Colder CR, Hawk LW. Confirmatory factor analysis of the Sensitivity to Punishment and Sensitivity to Reward Questionnaire. Personality and Individual Differences. 2004;37:985–1002. [Google Scholar]
- 28.Maxwell JK, Tucker DM, Townes BD. Asymmetric cognitive function in anorexia nervosa. Int J Neurosci. 1984 Aug;24(1):37–44. doi: 10.3109/00207458409079532. [DOI] [PubMed] [Google Scholar]
- 29.Roberts ME, Tchanturia K, Stahl D, Southgate L, Treasure J. A systematic review and meta-analysis of set-shifting ability in eating disorders. Psychol Med. 2007 Aug;37(8):1075–84. doi: 10.1017/S0033291707009877. [DOI] [PubMed] [Google Scholar]
- 30.Lee SH, Ham B-J, Cho Y-H, Lee S-M, Shim SH. Assoication Study of Dopamine Receptor D2 Taql A Polymorphism and Reward-Related Personlaity Traits in Healthy Korean Young Females. Neuropsychobiology. 2007;56:146–51. doi: 10.1159/000115781. [DOI] [PubMed] [Google Scholar]
- 31.Davis C, Levitan RD, Kaplan AS, Carter J, Reid C, Curtis C, et al. Reward sensitivity and the D2 dopamine receptor gene: A case-control study of binge eating disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008 Apr 1;32(3):620–8. doi: 10.1016/j.pnpbp.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 32.Barros-Loscertales A, Meseguer V, Sanjuan A, Belloch V, Parcet MA, Torrubia R, et al. Behavioral Inhibition System activity is associated with increased amygdala and hippocampal gray matter volume: A voxel-based morphometry study. Neuroimage. 2006 Nov 15;33(3):1011–5. doi: 10.1016/j.neuroimage.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 33.Frank GK, Bailer UF, Henry S, Wagner A, Kaye WH. Neuroimaging studies in eating disorders. CNS Spectr. 2004 Jul;9(7):539–48. doi: 10.1017/s1092852900009639. [DOI] [PubMed] [Google Scholar]
- 34.Cohen J. Statistical Power Analysis fir the Behavioral Sciences. 2. Lawrence Erlbaum; 1988. [Google Scholar]
- 35.Cleeremans A, Content A. Current directions in implicit learning: Where is it that we were supposed to go again? Psychologica Belgica. 1998;37:1–7. [Google Scholar]


