Abstract
Objectives
We described prevalence estimates of high-risk human papillomavirus (HR-HPV), HPV types 16 and 18, and abnormal Papanicolaou (Pap) smear tests among American Indian/Alaska Native (AI/AN) women compared with women of other races/ethnicities.
Methods
A total of 9,706 women presenting for cervical screening in a sentinel network of 26 clinics (sexually transmitted disease, family planning, and primary care) received Pap smears and HR-HPV type-specific testing. We compared characteristics of 291 women self-identified as AI/AN with other racial/ethnic minority groups.
Results
In our population, AI/AN and non-Hispanic white (NHW) women had similar age- and clinic-adjusted prevalences of HR-HPV (29.1%, 95% confidence interval [CI] 23.9, 34.3 for AI/AN women vs. 25.8%, 95% CI 24.4, 27.2 for NHW women), HPV 16 and 18 (6.7%, 95% CI 3.9, 9.6 for AI/AN women vs. 8.8%, 95% CI 7.9, 9.7 for NHW women), and abnormal Pap smear test results (16%, 95% CI 11.7, 20.3 for AI/AN women vs. 14.9%, 95% CI 13.7, 16.0 for NHW women). AI/AN women had a higher prevalence of HR-HPV than Hispanic women, and a similar prevalence of HPV 16 and 18 as compared with Hispanic and African American women.
Conclusions
We could not demonstrate differences in the prevalence of HR-HPV, HPV 16 and 18, or abnormal Pap smear test results between AI/AN and NHW women. This finding should improve confidence in the benefit of HPV vaccine and Pap smear screening in the AI/AN population as an effective strategy to reduce rates of cervical cancer.
In 2008, there were 11,070 new cases and approximately 3,870 deaths among women from cervical cancer in the United States.1 As a result of Papanicolaou (Pap) smear screening, the incidence of cervical cancer has been declining for the last 40 years, but the decrease has not been consistent across racial/ethnic minority groups.2 Recently published reports on cancer rates in American Indian and Alaska Native (AI/AN) populations using U.S. cancer registries with linkage to Indian Health Service (IHS) records showed a 25% higher rate of cervical cancer incidence and mortality in AI/AN women compared with non-Hispanic white (NHW) women, with a nearly twofold variation across IHS regions.2–4 One report also noted that AI/AN women diagnosed with cervical cancer were more likely to be diagnosed with non-localized disease than NHW women.3 This disparity exists even though approximately 80% of women of all races/ethnicities reported recent (within three years) Pap smear screening in a national survey of health behaviors.5
One approach to examining this discrepancy in cancer rates is to determine if there are differences in abnormal Pap smear rates by race/ethnicity, as they indicate early precursors to cervical cancer. In a large national program that offered Pap smear screening to underserved women, reported rates of abnormal Pap smear test results in the initial years of the program (1991–1998) were highest in AI/AN women during the first and second round of Pap smear screening.6 However, in subsequent years this trend reversed, with AI/AN women having one of the lowest rates of abnormal Pap smear test results in a more recent report (1995–2001).7 While this finding could reflect lower disease rates over time, it may also reflect different age and geographic inclusion criteria used during the two time periods.
Another approach to examining this disparity in cancer rates is to determine if there are differences between AI/AN and NHW women in prevalence of high-risk human papillomavirus (HR-HPV), especially of vaccine-preventable HR-HPV types 16 and 18. There are more than 40 HR-HPV types that infect the genital mucosa, and HR-HPV infection is the cause of virtually all cervical cancers. HR-HPV 16 and 18 are responsible for approximately 70% of cervical cancers worldwide.8,9 Previous studies of AI/AN in Alaska and the Northern Plains suggest that AI/AN women have a higher prevalence of non-HR-HPV 16 and 18 types.10,11
The prophylactic HPV vaccine is a significant advancement in cervical cancer prevention, as it prevents more than 90% of precancerous cervical lesions caused by HPV 16 or HPV 18 if administered to young women prior to exposure to the vaccine types.12,13 However, to impact cervical cancer rates, it must reach a broad coverage of females prior to the onset of sexual activity. Focus groups of AI/AN women have indicated their acceptance of the vaccine, but they expressed concern as to the vaccine's effectiveness in AI/AN women.14–16 Concerns voiced in these groups are that AI/AN women might have higher rates of HR-HPV types not targeted by the vaccine and that the vaccine might not be as effective in AI/AN women. These concerns must be addressed to reassure the AI/AN community that this vaccine will be effective in preventing cervical disease in their population.
To inform future cervical cancer prevention strategies, we used data from the multicenter HPV Sentinel Surveillance (HSS) project to assess the prevalence of HR-HPV, vaccine-preventable HR-HPV (types 16 and 18), and abnormal Pap smear test results identified during routine cervical screening visits among self-identified AI/AN women in this population. We then compared these prevalence estimates with those in NHW women and other racial/ethnic minority groups to assess whether any of these factors could be contributing to the disparity in cervical cancer rates between AI/AN and NHW women. We also compared the prevalence of type-specific HR-HPV found in AI/AN women in this group with that of the overall HSS group to explore whether any difference in type-specific HR-HPV infection exists that could modify the expected impact of the HPV vaccine in the AI/AN population.
METHODS
Study population
The HSS study conducted by the Centers for Disease Control and Prevention (CDC) was a large, cross-sectional study designed to measure the burden of HR-HPV infection and abnormal Pap smear test results in the U.S. cervical screening population. The enrollment, data, and specimen collection methods for this study have been previously published elsewhere.17
Briefly, women aged 18–65 years were invited to enroll when they presented for routine cervical cancer screening. Two teen clinics were included (one in Denver and one in Baltimore) that invited women aged 14–17 years to participate. Participants were recruited from 26 clinics located in six cities (Boston, Massachusetts [including two clinics in Fitchburg and Springfield, Massachusetts]; Baltimore, Maryland; New Orleans, Louisiana; Denver, Colorado; Seattle, Washington; and Los Angeles, California) beginning January 1, 2003, and ending December 31, 2005. Clinics included eight sexually transmitted disease (STD) clinics, 10 family planning clinics, and eight primary care clinics. Women excluded from the study included those with a previous hysterectomy, those with a Pap smear test or any type of cervical treatment within the 12 months prior to enrollment, those currently menstruating, and those who were pregnant. All protocols were approved by local and CDC Institutional Review Boards. Each participant gave written informed consent.
Demographic information was collected by an administered standardized questionnaire. Race/ethnicity were self-reported, and women could identify more than one race/ethnicity that included the following categories: white, Hispanic or Latina, African American/black, Asian, AI/AN, or Native Hawaiian/other Pacific Islander. Women reporting more than one race/ethnicity were included in the multiracial category. Because many AI/AN women reported more than one race/ethnicity, all women who self-identified as AI/AN were included in the AI/AN group even if they reported more than one race/ethnicity.
Measurements
HSS project staff abstracted Pap smear test results and medical record data. Providers collected cervical samples for Pap smear testing using their current local method (conventional or liquid based) at the time of pelvic examination. In addition, a second cervical sample was obtained using the Digene Cervical Sampler (Digene cervical brush and specimen transport medium; Qiagen, formerly Digene, Gaithersburg, Maryland). This sample was tested for HR-HPV DNA using the Hybrid Capture 2 assay (Qiagen). Before Hybrid Capture 2 testing, an aliquot from each cervical sample was removed and sent to CDC for HPV detection and typing based on L1 consensus polymerase chain reaction (PCR) and the prototype Roche line blot assay (reagents provided by Roche Molecular Systems, Alameda, California).
Briefly, 150 microliters (μL) were extracted with Roche MagNA Pure to a volume of 100 μL. A 10 μL aliquot was amplified and the resulting product was screened for the presence of HPV amplicons using gel electrophoresis. Samples with amplicons were hybridized to the typing strip to detect any of 37 types included on the line blot. Those that did not hybridize were examined with Sanger sequencing to detect types not included in the hybridization. Samples negative for HPV and for the endogenous control gene (β-globin) were considered inadequate. The PCR testing results are reported in this study. The prototype line blot assay detects HR-HPV DNA types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 67, 68, 69, 70, 73, 82, and IS39.
Local cytopathologists interpreted all cytology specimens using the Bethesda 2001 guidelines. In our analysis, an abnormal Pap smear test result was any cytologic result of atypical squamous cells of undetermined significance (ASC-US), atypical squamous cells—cannot exclude high-grade squamous intraepithelial lesion (ASC-H), low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), atypical glandular cells (AGC), and adenocarcinoma in situ (AIS) or cancer. None of the enrolled women had cytological evidence of invasive cervical cancer.
Statistical analysis
We calculated the overall crude prevalence of HR-HPV and crude prevalence estimates (with corresponding 95% confidence intervals [CIs]) for strata defined by age, race/ethnicity, clinic type, city, and Pap smear test results. Because the prevalence of HR-HPV infection, HPV 16- and 18-specific infection, and abnormal Pap smear test results differed significantly by age and clinic type, we indirectly standardized prevalence estimates, adjusting for age and clinic type, using the total number of people entered in the project from all cities as the standard population. We assumed a binomial distribution for all outcomes of interest and calculated 95% CIs for the prevalence estimates using the exact method. We used the Chi-square statistic to test for significant differences between proportions. We performed all analyses using SAS® version 8.2.18
RESULTS
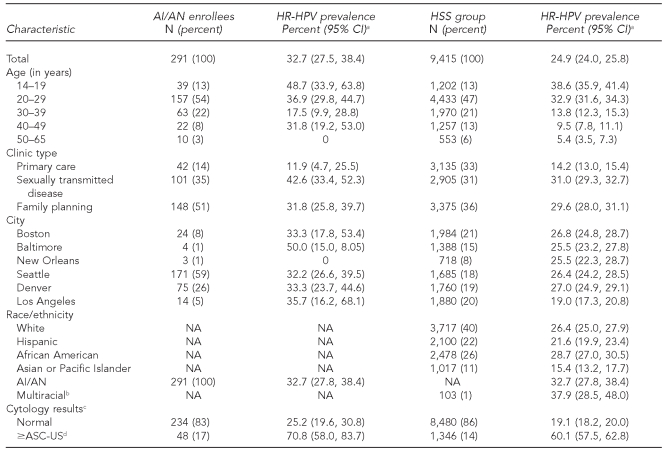
A total of 9,706 women enrolled in the HSS project were included in our analyses. Women who were positive for human immunodeficiency virus and women without valid HPV test results were excluded. Among the cohort, 291 women were classified as AI/AN, which represented 3% of the overall group (Table 1). NHW women comprised 40%, African Americans 26%, Hispanic women 22%, and Asian or Pacific Islander (API) 11% of the group.
Table 1.
Prevalence of HR-HPV by demographic characteristics and cytology results among AI/AN women compared with the entire HSS study, 2003–2005
aHR-HPV prevalence by polymerase chain reaction testing
bMultiracial refers to more than one race excluding AI/AN.
cNine AI/AN and 335 non-AI/AN women were missing Papanicolaou smear test results.
dIncludes atypical squamous cells—cannot exclude high-grade lesion, low-grade squamous intraepithelial lesion, high-grade squamous intraepithelial lesion, and atypical glandular cells results
HR-HPV = high-risk human papillomavirus
AI/AN = American Indian/Alaska Native
HSS = HPV Sentinel Surveillance
CI = confidence interval
NA = not applicable
ASC-US = atypical squamous cells of undetermined significance
Of 291 AI/AN women, 61 (21%) self-identified as AI/AN alone, 136 (47%) self-identified as AI/AN and Hispanic, 51 (18%) self-identified as AI/AN and white, 14 (5%) self-identified as AI/AN and African American, and 29 (10%) self-identified as AI/AN and other (API/Hawaiian or multiracial). Among AI/AN women alone, the crude HR-HPV prevalence was 41% (95% CI 29, 53) compared with 27% (95% CI 18, 37) among AI/AN women with an additional race and 43% (95% CI 19, 46) for AI/AN women combined with two or more races (data not shown).
Among the AI/AN women, the age range was 14–65 years, with more than half of the women (54%) in their 20s at the time of testing. About half of the women were from family planning clinics (51%), with 35% tested in STD clinics, and the remainder in primary care clinics (14%). Geographically, 59% were seen in the Seattle clinics, 26% in the Denver clinics, 8% in the Boston clinics, and the rest in the other cities (Table 1).
The overall crude prevalence of HR-HPV infection was 32.7% (95% CI 27.5, 38.4) in AI/AN women compared with 24.9% (95% CI 24.0, 25.8) in women of other racial/ethnic minority groups. Prevalence was highest among the 39 adolescents (aged 14–19 years), with 48.7% testing positive for HR-HPV. The prevalence of HR-HPV by clinic type was highest among AI/AN women attending STD clinics (42.6%) compared with family planning (31.8%) and primary care (11.9%) clinics. These findings are consistent with the overall group of women enrolled in HSS (Table 1).
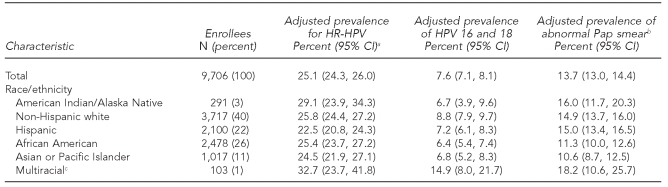
To assess the relative effects of race/ethnicity on HR-HPV, HPV 16 and 18, and abnormal Pap smear test results, we calculated age- and clinic-adjusted prevalence estimates and indirectly standardized to the total number of women entered in the study from all cities. Adjusted prevalence for HR-HPV in AI/AN women was 29.1% (95% CI 23.9, 34.3), which was similar in NHW women (25.8%, 95% CI 24.4, 27.2). Prevalence rates in African American and multiracial women were similar to AI/AN as well. Hispanic and API women had a statistically significant lower rate of HR-HPV, although the absolute difference in prevalence was small (Table 2).
Table 2.
Prevalence of HR-HPV and abnormal Pap smear test results by race/ethnicity, adjusted by age and clinic type among women in the HSS study, 2003–2005
aHR-HPV prevalence based on polymerase chain reaction testing
bAbnormal Pap smear test result defined as ≥ASC-US = atypical squamous cells of undetermined significance, ASC-H = atypical squamous cells—cannot exclude high-grade lesion, LSIL = low-grade squamous intraepithelial lesion, HSIL = high-grade squamous intraepithelial lesion, AGC = atypical glandular cells
cMultiracial consists of women who specified more than one race/ethnicity excluding American Indian/Alaska Native.
HR-HPV = high-risk human papillomavirus
Pap = Papanicolaou
HSS = HPV Sentinel Surveillance
CI = confidence interval
Among AI/AN women, 234 (83%) had a normal Pap smear test result compared with 8,480 (86%) in the non-AI/AN group (Table 1). The crude prevalence of HR-HPV in women with normal Pap smear test results was higher in AI/AN vs. non-AI/AN women (25.2% vs. 19.1%, p=0.02) but not statistically significant after adjusting for age and clinic type. Among women with an abnormal Pap smear test result, there was no difference in the crude or adjusted prevalence of HR-HPV DNA detected in cervical swab specimens among the AI/AN and non-AI/AN groups.
For the HR-HPV types targeted by the prophylactic HPV vaccine (i.e., HPV 16 and 18), adjusted prevalence estimates were not significantly different for AI/AN (6.7%, 95% CI 3.9, 9.6) and NHW (8.8%, 95% CI 7.9, 9.7) women. The rates in Hispanic, African American, and API women were also similar. Prevalence was higher at a statistically significant level for non-AI/AN multiracial women (14.9%, 95% CI 8.0, 21.7) (Table 2). Among those positive for HR-HPV, the crude estimate of HPV 16 and 18 among AI/AN women was 23% (23/95) and was similar to prevalence in non-AI/AN women (30% [720/1,625], p=0.12 for comparison) (data not shown).
Comparing racial/ethnic groups, age- and clinic-adjusted prevalence estimates of abnormal Pap smear test results (≥ASC-US), AI/AN women were no more likely to have an abnormal Pap smear test result than NHW women (16.0% vs. 14.9%) (Table 2). Similarly, no significant differences were found between AI/AN and Hispanic or multiracial women, although AI/AN women were more likely to have an abnormal Pap smear test result compared with African American and API women.
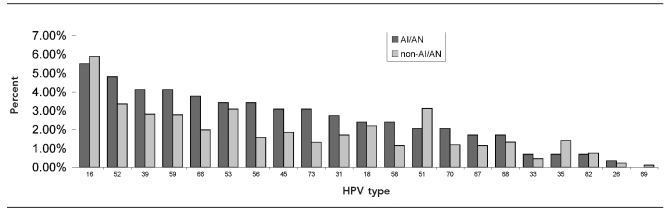
The crude prevalence of specific HR-HPV types for the AI/AN group was compared with non-AI/AN women (Figure). HPV 16 was the most prevalent (5.5%) followed in descending order by 52, 39, 59, 66, 53, 56, 45, 73, 31, 18, 58, 51,70, 67, 68, 33, 35, 82, 26, and 69. HPV IS39 was not found in the AI/AN population. While the descending order for the non-AI/AN group varied from the AI/AN group, the trend for these types was quite similar, with HPV 16 being the most prevalent and HPV 82, 26, and 69 being the least prevalent for both groups. We did not control for the presence of multiple HR-HPV DNA types in these analyses; however, the crude prevalence estimates of multiple infections were similar between AI/AN women (37.0% [36/95]) and non-AI/AN women (38.8% [909/1,436]) (p=0.86).
Figure.
Prevalence of type-specific HR-HPV DNA among women in the HSS Study, 2003–2005a
aThe prevalence estimates of types of HR-HPV DNA detected on cervical swabs by polymerase chain reaction testing in AI/AN women were compared with non-AI/AN women. Both AI/AN and non-AI/AN women had multiple infections. Thirty-six women in the AI/AN group (i.e., 154 HR-HPV infections identified among 95 women) and 909 in the non-AI/AN group (i.e., 3,732 HR-HPV infections identified among 2,344 women) had multiple infections. All identified HR-HPV infections were included in this figure. No statistical differences in HR-HPV types by AI/AN vs. non-AI/AN women were identified.
HR-HPV = high-risk human papillomavirus
HSS = HPV Sentinel Surveillance
AI/AN = American Indian/Alaska Native
DISCUSSION
In this analysis of women enrolled in the HSS project, AI/AN women had a high burden of HR-HPV, with an age- and clinic-adjusted prevalence of 29%. We found no difference between AI/AN women and NHW women with respect to prevalence of HR-HPV, HPV 16 and 18, or abnormal Pap smear test results. We did find that AI/AN women had a similar prevalence of HR-HPV to African American and multiracial women and only a slightly higher prevalence of HR-HPV compared with Hispanic and API women. Our findings do imply that prevention strategies such as routine Pap smear tests and the use of the HPV vaccine should be equally efficacious in decreasing cervical cancer rates in both AI/AN and NHW women.
The AI/AN women enrolled in our study had a higher adjusted prevalence of HR-HPV (29%) than that observed in previously published studies of AI/AN from New Mexico (7.7%), Alaska (21%), and the Northern Plains (14%).11,19,20 Potential reasons for the differences may be that the AI/AN women in this project were mainly seen in urban STD or family planning clinics rather than in primary care, gynecology, or rural outpatient clinics. Also, the HR-HPV prevalence in two of the studies19,20 were based on an earlier generation dot-blot hybridization method that is less sensitive than the PCR technology used in this study.21 The Northern Plains study used PCR technology similar to ours. Although that study had lower prevalence estimates of HR-HPV than our population, they had higher rates of HPV 16 or 18 among women who were HR-HPV positive (50%) compared with our AI/AN population (23%). It is worth noting that the Northern Plains service area has one of the highest rates of cervical cancer among AI/AN women, with rates that are 50% higher than for NHW women living in the same region.3 While further study needs to address subpopulations of AI/AN women to ascertain the reasons for identified differences seen, the high rate of HPV 16 and 18 in these populations suggests that HPV vaccination of AI/AN women is important.
The AI/AN women enrolled in our study included women who were not just AI/AN alone, but AI/AN combined with other races/ethnicities. Based on population surveys, approximately 60% of AI/AN people are of a single race/ethnicity. For those who live in urban centers, which is where our study recruitment took place, the rate is lower (54%).22,23 Because AI/AN people represent only 1.1% of the U.S. population, mortality and morbidity data on various medical conditions including cancer are often underestimated due to underreporting of the AI/AN race/ethnicity in hospital records, death certificates, and state registries.24–29 Recommendations have been made on how to classify AI/AN women who list more than one race/ethnicity, including asking for a primary race designation when multiple races/ethnicities are listed.30,31 Because this designation was not used in the HSS study, we elected to include all women who listed AI/AN as their race/ethnicity in our AI/AN group.
Our study did not show differences in age- and clinic-adjusted prevalence of abnormal cervical cytology between AI/AN women and NHW women, suggesting that higher rates of cervical cancer among AI/AN women are not due to differential rates of progression of HR-HPV infection in causing cervical dysplasia. This finding is consistent with the most recent data from the National Breast and Cervical Cancer Early Detection Program, which showed similar rates of abnormal Pap smear test results among AI/AN and NHW women.7 While rates published in U.S. Cancer Statistics series indicate that AI/AN women have the lowest rate of cervical cancer,31 rates of cervical cancer have been found to be higher in AI/AN women compared with NHW women when more extensive methods are used to limit racial/ethnic misclassification.32 A recent study compared cervical cancer rates of AI/AN women residing in or near tribal lands or IHS facilities with the rates of cervical cancer of NHW women in the same regions.3 The IHS facilities are either adjacent to or located on federally recognized tribal lands, and records from IHS and tribal data were linked to verify AI/AN race/ethnicity.
That report showed an overall 25% higher rate of cervical cancer among AI/AN women compared with NHW women. Striking regional differences were noted as well, with AI/AN women in the Northern Plains, Southern Plains, and Alaska area having 50% to 70% higher rates of cervical cancer compared with NHW women. AI/AN women in the Pacific Coast, the East, and the Southwest regions showed rates that were no different from the rates in NHW women. Interestingly, a review of cervical cancer rates and area of residence did show that women of all races/ethnicities who live in rural areas have higher rates of cervical cancer than women who live in urban areas.33 The HSS study recruited women mainly from urban areas, while the regional study used information from IHS service contract areas, which have a high number of sites that would be considered rural. Many issues including access to care and socioeconomic status also influence these rates.
We found HR-HPV 16 and 18 prevalence to be similar among AI/AN and NHW women. No significant differences were identified in the distribution of other HPV types among this AI/AN group compared with the non-AI/AN group, or in the prevalence of infection with multiple HR-HPV types. This information should be reassuring to the AI/AN community and address the concerns brought up in the focus groups with respect to the vaccine targeting correct HR-HPV types for AI/AN women. If the same HR-HPV types are responsible for similar proportions of cervical cancers in both AI/AN and NHW women (i.e., HPV 16 and 18 are responsible for 70% of cervical cancer cases in both groups), these findings suggest that the current prophylactic HPV vaccine should have a similar efficacy among AI/AN women as with other racial/ethnic minority groups. While variations in the prevalence of the non-vaccine HR-HPV types were identified, HPV 16 has been reported as the most prevalent HPV type throughout the world, with HR-HPV 16 and 18 persistence responsible for the majority of cervical cancers.34,35
Limitations
This study had a number of limitations. First, while the overall study group was evenly distributed among the cities and clinic types, the AI/AN subpopulation was not. Approximately 80% of our group was enrolled from cities located in the western half of the U.S. While this is not geographically balanced, it does somewhat reflect the AI/AN distribution in the U.S., which has about 60% of its population living in the mid- to western U.S. In addition, the majority of the AI/AN women in this study were seen in STD or family planning clinics. Although this might be considered a higher risk group than women seen in primary care clinics, it could reflect the high rate of urban AI/AN women who are uninsured (30%)23,36 and may be more likely to use an STD or family planning clinic for cervical cancer screening services. Also, we looked at a limited number of variables and did not look at important factors such as health behaviors and socioeconomic factors. Finally, the study population was limited to only 291 AI/AN women, potentially reducing the power of our findings.
CONCLUSIONS
AI/AN women attending urban STD, family planning, and primary care clinics, most of whom resided in Seattle and Denver, were no more likely to have HR-HPV, HPV 16 and 18, or abnormal Pap smear test results than NHW women. These findings are reassuring for this population because they suggest that differences in the epidemiology of HR-HPV infections among different racial/ethnic minority groups are likely to be subtle, and that preventative interventions for HPV-related cancers (including prophylactic HPV vaccination, Pap smear screening, sex partner reduction, and condom use) should work equally well in AI/AN and other racial/ethnic minority groups. However, larger regional- and tribal-based studies are needed to evaluate how knowledge, acceptability, and feasibility of HPV-related preventative interventions affect their implementation and performance in different communities of eligible women, including AI/AN women.
Acknowledgments
The authors thank Elizabeth R. Unger, PhD, MD, and David C. Swan, PhD, for clarification of testing methods of high-risk human papillomavirus types; Mona Saraiya, MD, for sharing her expertise in cervical cancer screening; and Allison Friedman, MS, and Kari Sapsis, MPH, for sharing their focus group work with American Indian/Alaska Native communities.
Footnotes
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the U.S. Census Bureau.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Espey DK, Wu XC, Swan J, Wiggins C, Jim MA, Ward E, et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110:2119–52. doi: 10.1002/cncr.23044. [DOI] [PubMed] [Google Scholar]
- 3.Becker TM, Espey DK, Lawson HW, Saraiya M, Jim MA, Waxman AG. Regional differences in cervical cancer incidence among American Indians and Alaska Natives, 1999–2004. Cancer. 2008;113(5 Suppl):1234–43. doi: 10.1002/cncr.23736. [DOI] [PubMed] [Google Scholar]
- 4.Espey D, Paisano R, Cobb N. Regional patterns and trends in cancer mortality among American Indians and Alaska Natives, 1990–2001. Cancer. 2005;103:1045–53. doi: 10.1002/cncr.20876. [DOI] [PubMed] [Google Scholar]
- 5.Kilmer G, Roberts H, Hughes E, Li Y, Valluru B, Fan A, et al. Surveillance of certain health behaviors and conditions among states and selected local areas—Behavior Risk Factor Surveillance System (BRFSS), United States, 2006. MMWR Surveill Summ. 2008;57(SS-7):1–188. [PubMed] [Google Scholar]
- 6.Benard VB, Lee NC, Piper M, Richardson L. Race-specific results of Papanicolaou testing and the rate of cervical neoplasia in the National Breast and Cervical Cancer Early Detection Program, 1991–1998 (United States) Cancer Causes Control. 2001;12:61–8. doi: 10.1023/a:1008959019019. [DOI] [PubMed] [Google Scholar]
- 7.Benard VB, Howe W, Saraiya M, Helsel W, Lawson HW. Assessment of follow-up for low-grade cytological abnormalities in the National Breast and Cervical Cancer Early Detection Program, 2000–2005. J Low Genit Tract Dis. 2008;12:300–6. doi: 10.1097/LGT.0b013e31817e308e. [DOI] [PubMed] [Google Scholar]
- 8.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 9.Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–65. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sebbelov AM, Davidson M, Kruger Kjaer S, Jensen H, Gregoire L, Hawkins I, et al. Comparison of human papillomavirus genotypes in archival cervical cancer specimens from Alaska Natives, Greenland natives and Danish Caucasians. Microbes Infect. 2000;2:121–6. doi: 10.1016/s1286-4579(00)00276-8. [DOI] [PubMed] [Google Scholar]
- 11.Bell MC, Schmidt-Grimminger D, Patrick S, Ryschon T, Linz L, Chauhan SC. There is a high prevalence of human papillomavirus infection in American Indian women of the Northern Plains. Gynecol Oncol. 2007;107:236–41. doi: 10.1016/j.ygyno.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–43. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 13.Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 14.Toffolon-Weiss M, Hagan K, Leston J, Peterson L, Provost E, Hennessy T. Alaska Native parental attitudes on cervical cancer, HPV and the HPV vaccine. Int J Circumpolar Health. 2008;67:363–73. doi: 10.3402/ijch.v67i4.18347. [DOI] [PubMed] [Google Scholar]
- 15.Sapsis K, Friedman A. Preliminary findings from exploratory and materials testing focus groups with female caregivers of American Indian 11 and 12 year olds. Paper presented at the Annual Meeting of the National Coalition of STD Directors; 2008 Oct 21–24; Phoenix, Arizona. [Google Scholar]
- 16.Indian Health Service (US) IHS child health notes. 2006. Jul, [cited 2009 Dec 22]. Available from: URL: http://www.ihs.gov/MedicalPrograms/MCH/M/documents/ICHN706.doc.
- 17.Datta SD, Koutsky LA, Ratelle S, Unger ER, Shlay J, McClain T, et al. Human papillomavirus infection and cervical cytology in women screened for cervical cancer in the United States, 2003–2005. Ann Intern Med. 2008;148:493–500. doi: 10.7326/0003-4819-148-7-200804010-00004. [DOI] [PubMed] [Google Scholar]
- 18.SAS Institute, Inc. SAS®: Version 8.2. Cary (NC): SAS Institute, Inc; 2001. [Google Scholar]
- 19.Davidson M, Schnitzer PG, Bulkow LR, Parkinson AJ, Schloss ML, Fitzgerald MA, et al. The prevalence of cervical infection with human papillomaviruses and cervical dysplasia in Alaska Native women. J Infect Dis. 1994;169:792–800. doi: 10.1093/infdis/169.4.792. [DOI] [PubMed] [Google Scholar]
- 20.Becker TM, Wheeler CM, McGough NS, Jordan SW, Dorin M, Miller J. Cervical papillomavirus infection and cervical dysplasia in Hispanic, Native American, and non-Hispanic white women in New Mexico. Am J Public Health. 1991;81:582–6. doi: 10.2105/ajph.81.5.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubbard RA. Human papillomavirus testing methods. Arch Pathol Lab Med. 2003;127:940–5. doi: 10.5858/2003-127-940-HPTM. [DOI] [PubMed] [Google Scholar]
- 22.Ogunwole SU. The American Indian and Alaska Native population: Census 2000 brief. Washington: Department of Commerce, Economics and Statistics Administration, Census Bureau (US) 2002. [cited 2009 Dec 22]. Also available from: URL: http://www.census.gov/prod/2002pubs/c2kbr01-15.pdf.
- 23.Urban Indian Health Institute. The health status of urban American Indians and Alaska Natives: an analysis of select vital records and census data sources. Seattle: Urban Indian Health Institute; 2004. [Google Scholar]
- 24.Sugarman JR, Soderberg R, Gordon JE, Rivara FP. Racial misclassification of American Indians: its effect on injury rates in Oregon, 1989 through 1990. Am J Public Health. 1993;83:681–4. doi: 10.2105/ajph.83.5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stehr-Green P, Bettles J, Robertson LD. Effect of racial/ethnic misclassification of American Indians and Alaskan Natives on Washington State death certificates, 1989–1997. Am J Public Health. 2002;92:443–4. doi: 10.2105/ajph.92.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker TM, Bettles J, Lapidus J, Campo J, Johnson CJ, Shipley D, et al. Improving cancer incidence estimates for American Indians and Alaska Natives in the Pacific Northwest. Am J Public Health. 2002;92:1469–71. doi: 10.2105/ajph.92.9.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frost F, Taylor V, Fries E. Racial misclassification of Native Americans in a surveillance, epidemiology, and end results cancer registry. J Natl Cancer Inst. 1992;84:957–62. doi: 10.1093/jnci/84.12.957. [DOI] [PubMed] [Google Scholar]
- 28.Wiggins CL, Espey DK, Wingo PA, Kaur JS, Wilson RT, Swan J, et al. Cancer among American Indians and Alaska Natives in the United States, 1999–2004. Cancer. 2008;113(5 Suppl):1142–52. doi: 10.1002/cncr.23734. [DOI] [PubMed] [Google Scholar]
- 29.Arias E, Schauman WS, Eschbach K, Sorlie PD, Backlund E. The validity of race and Hispanic origin reporting on death certificates in the United States. Vital Health Stat 2. 2008;148 [PubMed] [Google Scholar]
- 30.Swan J, Breen N, Burhansstipanov L, Satter DE, Davis WW, McNeel T, et al. Cancer screening and risk factor rates among American Indians. Am J Public Health. 2006;96:340–50. doi: 10.2105/AJPH.2004.053231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention (US) United States cancer statistics: 1999–2006 incidence and mortality Web-based report. Atlanta: Department of Health and Human Services, CDC, National Cancer Institute (US) 2010. [cited 2010 Dec 10]. Also available from: URL: http://www.cdc.gov/uscs.
- 32.Espey DK, Wiggins CL, Jim MA, Miller BA, Johnson CJ, Becker TM. Methods for improving cancer surveillance data in American Indian and Alaska Native populations. Cancer. 2008;113(5 Suppl):1120–30. doi: 10.1002/cncr.23724. [DOI] [PubMed] [Google Scholar]
- 33.Benard VB, Coughlin SS, Thompson T, Richardson LC. Cervical cancer incidence in the United States by area of residence, 1998–2001. Obstet Gynecol. 2007;110:681–6. doi: 10.1097/01.AOG.0000279449.74780.81. [DOI] [PubMed] [Google Scholar]
- 34.Clifford GM, Gallus S, Herrero R, Munoz N, Snijders PJ, Vaccarella S, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet. 2005;366:991–8. doi: 10.1016/S0140-6736(05)67069-9. [DOI] [PubMed] [Google Scholar]
- 35.Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1157–64. doi: 10.1158/1055-9965.EPI-04-0812. [DOI] [PubMed] [Google Scholar]
- 36.Ward E, Halpern M, Schrag N, Cokkinides V, DeSantis C, Bandi P, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58:9–31. doi: 10.3322/CA.2007.0011. [DOI] [PubMed] [Google Scholar]