Abstract
Objectives
Antibiotic resistance is a significant global problem, but the trends in prevalence and impact of antibiotic resistance in hospitalizations in the United States are unclear. We evaluated the trends in hospitalizations associated with antibiotic-resistant infections in U.S. hospitals from 1997 to 2006.
Methods
We analyzed the National Hospital Discharge Survey (NHDS) during 1997–2006 (unweighted n=3.3 million hospitalizations; weighted n=370.3 million hospitalizations) and examined trends in prevalence of hospitalizations with antibiotic-resistant infections, length of stay, and discharge status.
Results
The number of infection-related hospitalizations with antibiotic resistance increased 359% during the 10-year period, from 37,005 in 1997 to 169,985 in 2006. The steepest rise was seen among individuals <18 years of age. The mean age of individuals with infection-related hospitalizations that had antibiotic-resistant infections decreased substantially, from 65.7 years (standard error [SE] = 2.01) in 1997 to 44.2 years (SE=1.47) in 2006. As the proportion of patients with antibiotic-resistant infections who did not have insurance increased, the length of stay for those hospitalizations had a corresponding decrease (r=0.91, p<0.01).
Conclusions
Antibiotic-resistant infections are becoming increasingly commonplace in hospitalizations in the U.S., with a steady upward trend between 1997 and 2006. Antibiotic-resistant infections are increasingly being seen in younger patients and those without health insurance.
Despite the clear benefits of antibiotic therapy for a variety of infectious diseases, the widespread use of these agents has facilitated the emergence of a variety of antibiotic-resistant pathogens.1 Because of the significant impact antibiotic resistance has on morbidity and mortality, it is considered a threat to U.S. public health and national security by the Institute of Medicine and the Infectious Diseases Society of America.2,3
Since the 1990s in the United States, the prevalence of antibiotic resistance has increased for a variety of pathogens. Increased resistance has been found for Neisseria gonorrhoeae, Salmonella ser Typhi, Escherichia coli, and Mycobacterium tuberculosis.4–7 In particular, the emergence of vancomycin-resistant enterococci (VRE) and methicillin-resistant Staphylococcus aureus (MRSA) has raised serious questions about the ability to treat some of these new resistant pathogens.8,9 At first, many of these resistant organisms were confined to hospitalized populations; however, in recent years the emergence of community-associated infections with these resistant organisms has become more common.10,11 Community-associated MRSA is particularly important because it is not simply an extension of hospital-associated MRSA into the community, but rather a different strain.
The purpose of this study was to describe the trends in hospitalizations and deaths associated with antibiotic-resistant infections in U.S. hospitals from 1997 to 2006.
METHODS
We conducted an analysis in 2010 of the National Hospital Discharge Survey (NHDS) of the years 1997–2006. The NHDS covers approximately 270,000 patients per year in 500 short-stay hospitals by using a stratified, multistage survey to create a nationally representative annual sample of discharge records. Children's and general hospitals are included; federal, military, Veterans Affairs, and institutional hospitals are not included. Each discharge record contains up to seven different International Classification of Diseases, Ninth Revision (ICD-9), Clinical Modification discharge diagnosis codes; is population-weighted on the basis of the probability of sample selection; and is adjusted for nonresponse. Nationally representative estimates of hospitalizations in the U.S. can be computed with the NHDS. We included all acute-care hospitalizations in the analysis.
Antibiotic-resistant hospitalizations
The ICD-9 stem of V09.XX represents “Infection with drug-resistant microorganisms” infections. Microbiological results were limited to the ICD-9 code and did not have actual laboratory values. This categorization does not indicate the specific pathogen (e.g., MRSA or VRE) but only if the pathogen was determined to be resistant to a specific class of antibiotics (e.g., penicillins or macrolides). For example, MRSA would be coded as an infection with microorganisms resistant to penicillins. Similarly, VRE would be coded as an infection with microorganisms resistant to vancomycin. We used the codes under this stem to represent hospitalizations with antibiotic-resistant infections.
Infections
In an effort to compare infections with antibiotic-susceptible organisms and those with antibiotic-resistant organisms, we identified hospitalizations with the diagnoses under the following ICD-9 codes: intestinal infections (00[8-9].XX); septicemia (038.XX); bacterial infections classified elsewhere (041.XX); endocarditis (421.XX); myocarditis (042.XX); pneumonia (48[1-6].XX); urinary system diseases (559.XX); peritonitis and retroperitonal infections (567.XX); kidney infections (590.XX); inflamed female pelvic organs (614.XX); carbuncle and furuncle (680.XX); cellulitis and abscess (681.XX, 682.XX); lymphadenitis (683.XX); chronic skin ulcers (707.XX); osteopathies (730.XX); systemic inflammatory response syndrome (995.XX); prosthetic device, implant, or graft infections (996.XX); and post-operative infections (998.XX).
This list of infections was classified as a group and was not used to look at specific infections. We undertook this strategy for the following reason. The V-code corresponding to antibiotic-resistant infections does not allow us to link the specific type of resistance to a specific condition because it is simply included in the list of diagnoses. For example, if someone had more than one diagnosis code (e.g., pneumonia and bacteremia), it could not be determined if the resistant code was linked to one or both or, specifically, which one. Thus, we could only count whether someone had one of the conditions and whether they did or did not have an extra diagnosis code of antibiotic-resistant infection.
Outcomes
The NHDS reported the discharge disposition. This allowed us to determine whether the hospitalization resulted in a routine discharge, discharge to a short- or long-term-care facility, or patient death. However, in terms of death, the discharge summary did not specify the cause of death. The diagnoses on the discharge abstract suggested that the disease played a role in patient death. Further, the NHDS did indicate in days the length of the hospitalization.
Analysis
The NHDS uses a complex survey design that allows the user to make population estimates. Our analysis used the appropriate sampling weights, and the analysis was conducted using SUDAAN® to account for the complex sampling designs.12 We used Chi-square analysis to compare infection-related hospitalizations with antibiotic-resistant infections with those with antibiotic-susceptible infections.
Our analysis consisted of comparing age groups as well as mean ages and proportions with the median length of stay and discharge status characterized as either death, transfer to long- or short-term-care facility, routine, or other. We performed the Anderson-Darling goodness-of-fit test on the length of stay and it indicated an exponential distribution (p<0.001). The median is a more accurate indicator for this non-normal distribution. Because of the importance of antibiotic-resistant infections in care for the elderly, we performed additional analyses stratifying by age (<18, 18–64, and ≥65 years). We performed the Anderson-Darling goodness-of-fit test for normal distribution for the ages indicating that the mean was a valid measurement for central age (p<0.001).
Although we were not able to identify the specific pathogen, because of concern about the impact of community-associated MRSA, we examined the incidence and impact of cellulitis, a diagnosis consistent with this pathogen.
We analyzed insurance data using the principal payment criteria listed in the survey and performed a correlation analysis to test the relationship between insurance status and length of stay. We also examined insurance status and length of stay. To compare length of stay, we performed a natural log transformation of the data and then computed t-tests for the mean. Finally, we felt it was important to assess the relationship between antibiotic-resistant infections and death during the hospitalization. Because death was relatively uncommon, we collapsed the data into two-year combined estimates so that we would have sufficient numbers to make reliable national U.S. population estimates for death.
RESULTS
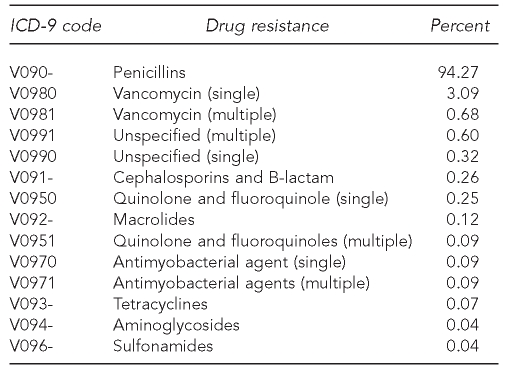
We used 10 years of weighted data totaling 370.33 million hospitalizations for the analyses. The V09 codes used to identify antibiotic resistance along with their cumulative 10-year frequency are shown in the Table. Infections resistant to penicillins, including MRSA, accounted for 94.27% of all observed antibiotic-resistant infections. Among hospitalizations with antibiotic-resistant infections, 99.63% only listed one V code, which would correspond to one class of antibiotics.
Table.
Cumulative 10-year frequency of specific antibiotic-resistant infection in hospitals: U.S., 1997–2006
ICD-9 = International Classification of Diseases, Ninth Revision
The infection diagnosis classification indicating an infection-related hospitalization accounted for 94.96% of total hospitalizations with an antibiotic-resistant infection. The percentage of total hospitalizations with an antibiotic-resistant diagnosis demonstrated a positive exponential relationship (λ=0.161, r-square=0.983) increasing from 0.12% in 1997 to 0.46% in 2006 (p<0.001). The number of hospitalizations with antibiotic-resistant infections increased 327%, from 41,581 (95% confidence interval [CI] 33,994, 49,167) in 1997 to 177,457 (95% CI 161,302, 193,611) in 2006 (data not shown).
The percentage of infection-related hospitalizations with antibiotic resistance also exhibited an exponential relationship (λ=0.151, r-square=0.983), as the yearly tangent line steadily increased and the hospitalization percentage rose from 0.66% in 1997 to 2.40% in 2006 (p=0.001), for a combined 264% increase during the 10-year span. Similarly, the number of infection-related hospitalizations with antibiotic resistance increased from 37,005 (95% CI 30,063, 43,946) in 1997 to 169,985 (95% CI 154,355, 185,614) in 2006.
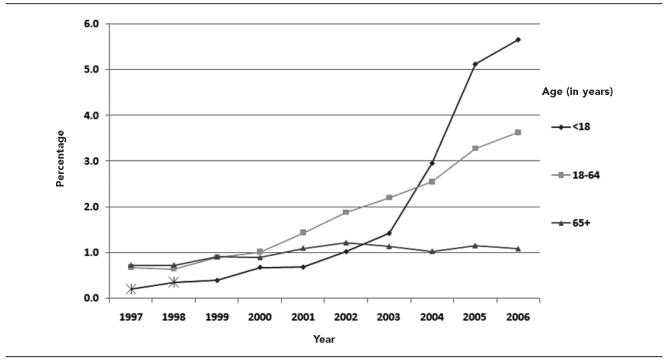
As shown in Figure 1, when stratified by age, the hospitalization percentages with antibiotic-resistant infections represent a rise in hospitalizations, with resistant infections becoming more common in younger patients, most notably those <18 years of age. The percentage of infection-related hospitalizations with antibiotic-resistant infections for subjects ≥65 years of age increased by 48.8%, with a weak linear relationship (m=0.046, r-square=0.642) from 0.731% to 1.088% (p=0.01). On the other hand, the percentage of antibiotic-resistant infections for subjects 18–64 years of age increased substantially, with an even more marked increase in patients <18 years of age. In the middle-age group, the proportion increased exponentially (λ=0.207, r-square=0.982) by 435%, from 0.68% in 1997 to 3.64% in 2006 (p=0.001). The pediatric age group indicated an even stronger exponential increase (λ=0.370, r-square=0.972), rising from 0.21% to 5.67% during the 10-year duration. The number of infection-related hospitalizations for those ≥65 years of age with antibiotic resistance increased from 23,185 (95% CI 14,134, 32,235) in 1997 to 41,942 (95% CI 24,795, 59,088) in 2006. Likewise, the number of infection-related hospitalizations for the combined younger age groups increased from 13,820 (95% CI 9,710, 17,929) in 1997 to 128,043 (95% CI 114,792, 141,293) in 2006.
Figure 1.
Percentage of infection-related hospitalizations with antibiotic-resistant microorganisms, grouped by age: U.S., 1997–2006
*Insufficient sample size to present accurate results
The mean age of individuals with infection-related hospitalizations that had antibiotic-susceptible infections increased slightly from 60.23 years (SE=0.37) in 1997 to 62.07 years (SE=0.14) in 2006. In contrast, the mean age of individuals with infection-related hospitalizations that had antibiotic-resistant infections decreased substantially, from 65.67 years (SE=2.01) in 1997 to 44.20 years (SE=1.47) in 2006 (p=0.001).
The incidence and impact of cellulitis among infection-related hospitalizations was substantial. In 1997, 10.90% of infection-related hospitalizations had a diagnosis of cellulitis with a rise to 15.77% of infection-related hospitalizations by 2006. Among hospitalizations with cellulitis diagnoses, the proportion with resistant organisms rose from 1.19% in 1997 to 8.95% in 2006. This can be contrasted with hospitalizations for infection diagnoses other than cellulitis, of which 0.60% had resistant organisms in 1997 but had risen only to 1.17% by 2006.
The median length of stay for infection-related hospitalizations without indication of antibiotic resistance remained relatively flat and linear (m=–0.05, r-square=0.93) with a bit of a decline, changing from 4.77 days (SE=0.50) in 1997 to 4.29 days (SE=0.45) in 2006. In regard to the length of stay for infection-related hospitalizations with indication of antibiotic resistance, the length of stay rose at first and then began a decline in 1999–2000, totaling an overall 40% decline from 1997 to 2006, with median stays of 6.62 (SE=0.85) and 3.97 (SE=0.24) days, respectively.
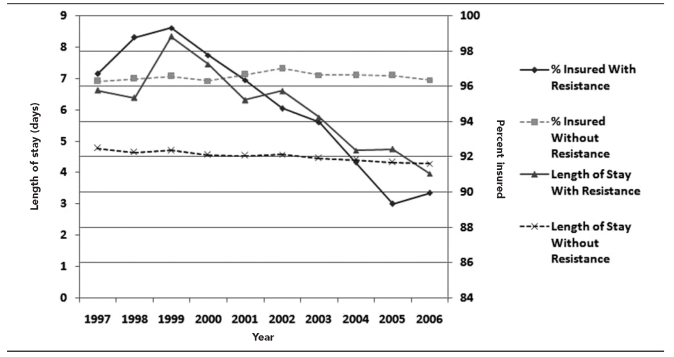
Figure 2 plots the median length of hospital stay against the presence of health insurance, suggesting that length of stay in the hospital is closely related to the presence of health insurance. As the proportion of patients with infection-related hospitalizations with antibiotic resistance who did not have insurance increased, the length of stay for those hospitalizations exhibited a corresponding decrease (r=0.91, p<0.01).
Figure 2.
Median length of stay in hospital and percent insured, with and without antibiotic resistance: U.S., 1997–2006
Patients who had infections with antibiotic-resistant organisms who did not have health insurance had shorter median lengths of stay (4.15 days, SE=0.25) during the 10-year period than did their counterparts with insurance (5.49 days, SE=0.18). The log-transformed mean length of stay was significantly shorter for those without insurance (p=0.001). A similar effect was found for these patients who had infections with susceptible organisms. Those without insurance had shorter median lengths of stay (3.25 days, SE=0.31) than patients with insurance (4.56 days, SE=0.30). Patients without insurance had a significantly shorter transformed mean length of stay (p=0.001). In terms of cellulitis hospitalizations, these patients without insurance had shorter median lengths of stay (3.01 days, SE=0.08) during the 10-year period than did patients with insurance (4.09 days, SE=0.31). Patients without insurance had a significantly shorter transformed mean length of stay (p=0.001)
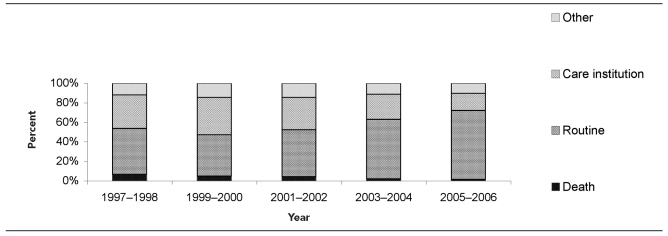
Among hospitalizations with an antibiotic-resistant infection, we examined where the patients were discharged. The percentages for 1997–1998 for routine discharge, death, other, and discharge to long- or short-term-care institution were 47.01%, 6.79%, 11.88%, and 34.32%, respectively (Figure 3). By 2005–2006, the proportion of discharges that were routine had risen to 70.62% (p<0.01), and deaths had dropped to 1.61% (p<0.01). Routine discharges increased by 50.2% during the study period, while death discharges decreased by 76.3% and discharges to care institutions declined by 49.2% during the 10-year observation period.
Figure 3.
Discharge status of hospitalizations with antibiotic-resistant infections: U.S., 1997–2006
DISCUSSION
Our findings suggest that antibiotic-resistant infections are becoming increasingly commonplace in hospitalizations in the U.S. The trends in hospitalizations with antibiotic-resistant infections showed a steady upward movement between 1997 and 2006, with an increase of 359% during the 10-year period. In terms of infection-related hospitalizations, these data indicated more than 130,000 additional hospitalizations per year with antibiotic-resistant infections in 2006 than in 1997. Infection-related hospitalizations with antibiotic-resistant infections accounted for 2.4% of all infection-related hospitalizations in 2006. This statistic continues the trend of increasing prevalence of antibiotic-resistant infections previously seen over time, and demonstrates a continued need to develop methods to contain the rise of antibiotic-resistant infections.13–15 This need is especially relevant considering the lack of new antibiotics to treat antibiotic-resistant infections, emphasizing the need to develop strategies to contain these infections that focus on limiting the development of resistance and spread of infection once antibiotic resistance is identified.1
The increase in antibiotic-resistant infections represents the changing face of individuals with these infections. Younger patients are increasingly being affected by antibiotic-resistant infections. The mean age of hospitalized patients with antibiotic-resistant infections dropped more than 20 years between 1997 and 2006. Additionally, the routine discharge of patients hospitalized with antibiotic-resistant infections demonstrated a considerable rise during the 10 years, while length of stay decreased. A lack of health insurance was closely related to the decreased length of stay among individuals with antibiotic-resistant infections. This finding is particularly troubling because it is possible that some individuals are potentially being discharged prematurely. Alternatively, individuals with insurance may be staying longer in the hospital than is warranted simply because of the ability to pay.
The shift to a younger population, with the expected decrease in mortality and morbidity, does not negate the public health impact of the rising prevalence of antibiotic-resistant infections. In addition to concerns regarding health outcomes and spread of resistant infection to more vulnerable individuals, increased health costs are also a concern. A recent study in one hospital calculated a range of medical costs from $18,588 to $29,069 per hospitalized patient attributable to antimicrobial-resistant infection, leading to societal costs of $10.7 million to $15.0 million in 2008 dollars.16 This estimate is two to three times the annual cost of $4 million to $5 million estimated by the Institute of Medicine in 1998.17 Although the length of stay for hospitalizations with antibiotic-resistant infections has decreased, costs may continue to increase as the prevalence of antibiotic-resistant infections increases, requiring the use of more expensive, newer antibiotics.18
Limitations
This study had several limitations. First, this study represents a sample of hospitalizations; however, the survey design allowed us to make reliable population estimates of hospitalizations in the U.S. Second, we could not tell if the infection arose in the hospital or if patients were colonized or infected prior to admission. However, we were still able to assess hospitalizations with a diagnosis code of a drug-resistant infection, which did provide an indication of health services for antibiotic-resistant infections in the U.S. Third, greater awareness of drug resistance among hospital coding departments may have prompted more attention to adding these codes to discharge records of patients who were relatively healthy and discharged without incident.
CONCLUSION
This study showed a substantial increase in the prevalence of antibiotic-resistant infections in hospitalized patients from 1997 to 2006. An increased prevalence of antibiotic-resistant infections was seen in younger patients, and a decreased length of stay was observed among patients without health insurance. Continued efforts to decrease the emergence of infections with antibiotic-resistant organisms and limit the spread of these infections are necessary.
Footnotes
This study was supported in part by contract HHSA290 2007 10015 from the Agency for Healthcare Research and Quality (AHRQ).
The findings and conclusions in this article are those of the authors and do not necessarily reflect the views of AHRQ.
REFERENCES
- 1.Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Sheld WM, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Disease Society of America. Clin Infect Dis. 2008;46:155–64. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 2.Smolinski MS, Hamburg MA, Lederberg J, editors. Microbial threats to health: emergence, detection, and response. Washington: Institute of Medicine; 2003. [PubMed] [Google Scholar]
- 3.Infectious Diseases Society of America. Bad bugs, no drugs: as antibiotic discovery stagnates, a public health crisis brews. Alexandria (VA): Infectious Diseases Society of America; 2004. [Google Scholar]
- 4.Wang SA, Harvey AB, Conner SM, Zaidi AA, Knapp JS, Whittington WL, et al. Antimicrobial resistance for Neisseria gonorrhoeae in the United States, 1988 to 2003: the spread of fluoroquinolone resistance. Ann Intern Med. 2007;147:81–8. doi: 10.7326/0003-4819-147-2-200707170-00006. [DOI] [PubMed] [Google Scholar]
- 5.Lynch MF, Blanton EM, Bulens S, Polyak C, Vojdani J, Stevenson J, et al. Typhoid fever in the United States, 1999–2006. JAMA. 2009;302:859–65. doi: 10.1001/jama.2009.1229. [DOI] [PubMed] [Google Scholar]
- 6.Hoopes AJ, Kammerer JS, Harrington TA, Ijaz K, Armstrong LR. Isoniazid-monoresistant tuberculosis in the United States, 1993 to 2003. Arch Intern Med. 2008;168:1984–92. doi: 10.1001/archinte.168.18.1984. [DOI] [PubMed] [Google Scholar]
- 7.Al-Hasan MN, Lahr BD, Eckel-Passow JE, Baddour LM. Antimicrobial resistance trends of Escherichia coli bloodstream isolates: a population-based study, 1988–2007. J Antimicrob Chemother. 2009;64:169–74. doi: 10.1093/jac/dkp162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deshpande LM, Fritsche TR, Moet GJ, Biedenbach DJ, Jones RN. Antimicrobial resistance and molecular epidemiology of vancomycin-resistant enterococci from North America and Europe: a report from the SENTRY antimicrobial surveillance program. Diagn Microbiol Infect Dis. 2007;58:163–70. doi: 10.1016/j.diagmicrobio.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Gerber JS, Coffin SE, Smathers SA, Zaoutis TE. Trends in the incidence of methicillin resistant Staphylococcus aureus infection in children's hospitals in the United States. Clin Infect Dis. 2009;49:65–71. doi: 10.1086/599348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Popovich KJ, Weinstein RA, Hota B. Are community-associated methicillin-resistant Staphylococcus aureus (MRSA) strains replacing traditional nosocomial MRSA strains? Clin Infect Dis. 2008;46:787–94. doi: 10.1086/528716. [DOI] [PubMed] [Google Scholar]
- 11.Maree CL, Daum RS, Boyle-Vavra S, Matayoshi K, Miller LG. Community-associated methicillin-resistant Staphylococcus aureus isolates causing healthcare-associated infections. Emerg Infect Dis. 2007;13:236–42. doi: 10.3201/eid1302.060781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Research Triangle Institute. SUDAAN®: Version 10.0. Research Triangle Park (NC): Research Triangle Institute; 2010. [Google Scholar]
- 13.Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis. 2007;13:1840–6. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (US). National Nosocomial Infections Surveillance System report, data summary from January 1990–May 1999, issued June 1999. Am J Infect Control. 1999;27:520–32. doi: 10.1016/s0196-6553(99)70031-3. [DOI] [PubMed] [Google Scholar]
- 15.Archibald LK, Banerjee SN, Jarvis WR. Secular trends in hospital-acquired clostridium difficile disease in the United States, 1987–2001. J Infect Dis. 2004;189:1585–9. doi: 10.1086/383045. [DOI] [PubMed] [Google Scholar]
- 16.Roberts RR, Hota B, Ahmad I, Scott RD, II, Foster SD, Abbasi F, et al. Hospital and societal costs of antimicrobial resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis. 2009;49:1175–84. doi: 10.1086/605630. [DOI] [PubMed] [Google Scholar]
- 17.Institute of Medicine. Antimicrobial drug resistance: issues and options. Washington: National Academies Press; 1998. [PubMed] [Google Scholar]
- 18.Sieradzki K, Roberts RB, Haber SW, Tomasz A. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N Engl J Med. 1999;340:517–23. doi: 10.1056/NEJM199902183400704. [DOI] [PubMed] [Google Scholar]