Lyme disease (LD) is the most commonly reported vector-borne disease in the United States. Between 2003 and 2005, 64,328 cases of LD were reported in the U.S. Of those cases, 93% were reported from 10 endemic states: Connecticut, Delaware, Maryland, Massachusetts, Minnesota, New Jersey, New York, Pennsylvania, Rhode Island, and Wisconsin.1 In Connecticut, 1,810 reported cases met the surveillance case definition of LD in 2005, resulting in an incidence rate of 53 per 100,000 population.2
Reducing the number of blacklegged ticks (Ixodes scapularis), the vector for LD should in theory reduce the incidence of LD in an endemic area. One such strategy targets white-tailed deer (Odocoileus virginianus). Although deer are not competent reservoir hosts for the LD causative agent, Borrelia burgdorferi, they are the primary hosts for adult blacklegged ticks and are an important tick transport mechanism.3,4 One study showed that more than 95% of adult female ticks feed on white-tailed deer.5 Nymphal ticks will also feed on deer.6,7 Therefore, deer-targeted interventions could provide a large-scale method for controlling tick populations by reducing the number and movement of blacklegged ticks. Two proposed deer-targeted interventions have included a topical acaricide applied using a four-poster device and deer-reduction programs. However, few studies have evaluated whether deer-targeted interventions resulted in decreased LD incidence, particularly in non-island settings.
The four-poster device is a passive topical treatment system developed by the U.S. Department of Agriculture, Agricultural Research Services. A central bin stores and dispenses bait to attract deer. As the deer feeds, rollers apply an acaricide directly to the head, ears, and neck, which is transferred to other body areas by self-grooming.8 Studies of this device have shown a significant decrease in blacklegged tick abundance on deer and in the surrounding area.8–11
In 1997, the Northeast Area Tick Control Project (NEATCP) was launched in five states (Rhode Island, Connecticut, New York, New Jersey, and Maryland) to evaluate the effectiveness of the four-poster treatment devices in controlling ticks.12 Core treatment areas (CTAs) were approximately 25 kilometers squared (km2) each, and the four-poster devices were to be deployed at a rate of 25 devices per CTA (or approximately one device/0.2 km2). This calculation was based on pretrial populations of approximately 44 deer in a CTA, though this number varied slightly with locality.12,13 The CTA that served as the basis for this rate was located in Old Lyme, Connecticut, where 24 devices were maintained from 1997 to 2002. Tick abundance was monitored at all sites for two years following the removal of the four-poster devices.14 However, the effect on LD incidence was not evaluated at any of the NEATCP sites.
Another deer-targeted intervention aims to reduce deer populations through a controlled deer hunt. Two studies showed that complete, or near complete, elimination of deer was effective in reducing tick populations on an inhabited peninsula and islands off the northeastern U.S. coast. However, it is unclear whether deer-reduction strategies will translate to mainland settings where deer cannot be eliminated completely.15,16 Computer simulations have suggested that reducing deer density to 7.5 deer/km2 could decrease infected nymphal tick density by 40% within four years, while near elimination of deer is needed to lower infected nymphal tick density by >99%.17 However, it is unclear to what extent deer numbers must be reduced to significantly impact LD incidence, especially in mainland settings.
In 2000, an annual controlled deer hunt began in the coastal Mumford Cove community of Groton, Connecticut. The herd was initially reduced by 92%, and has subsequently been maintained at the same low level, approximately 3.8 deer/km2.18,19 A community survey identified a large decrease in self-reported LD cases one year after the initial hunt.18 However, self-reported data have various inherent limitations.
We evaluated two deer-targeted tick-control strategies for their effects on LD in southeastern Connecticut: four-poster acaricide treatment and deer reduction in a non-island setting. This study aimed to determine if deer-targeted interventions had a significant effect on LD incidence (1) before and after treatment and (2) in treatment areas vs. control regions.
METHODS
Study area
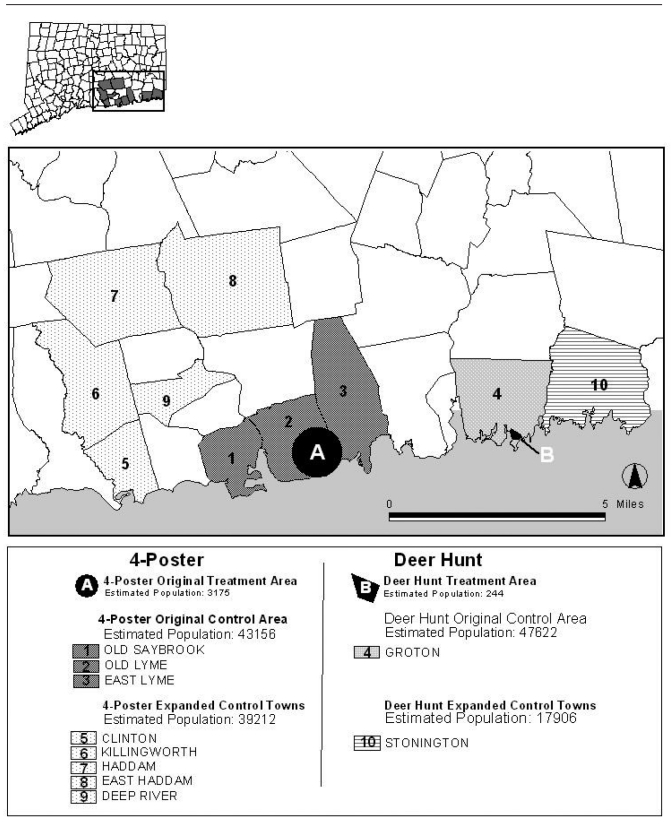
The study area is depicted in Figure 1. The 25 km2 four-poster original treatment area was based on the Connecticut NEATCP protocol's CTA and encompassed a portion of the towns of Old Lyme and East Lyme. For the purposes of this study, the original control area comprised the remaining areas of Old Lyme and East Lyme as well as Old Saybrook, which was the NEATCP tick sampling control area.12 The five towns of Clinton, Deep River, East Haddam, Haddam, and Killingworth were selected as expanded control towns to provide an additional measure of control. Due to their close proximity to the original treatment and original control areas, these towns would be expected to have a similar tick exposure risk.
Figure 1.
Connecticut catchment area for a deer-targeted intervention study, 1992–2006
The deer hunt original treatment area was the community of Mumford Cove, a residential community of approximately 112 homes located on a 1.9 km2 peninsula along the Connecticut coast in New London County in Groton.18,20 Mumford Cove has approximately 0.38 km2 residential development and 41 km2 woodland area. The remainder of Groton served as the original control area. The nearby town of Stonington served as the expanded control town.
Case data
We identified cases from Connecticut Department of Public Health (CT DPH) LD surveillance records. For the purposes of this evaluation, we defined a case as a resident in the study area who had a physician-diagnosed erythema migrans (EM) rash ≥5 centimeters, and an onset date during the years 1992–2006. EM rash cases were used as a surrogate for incident LD cases.
Case addresses were geocoded for classification into original treatment or original control area using ArcGIS® software.21 We obtained town boundaries and Topologically Integrated Geographic Encoding and Referencing (TIGER) system road data from the University of Connecticut Map and Geographic Information Center.22 We excluded post office boxes and missing or incomplete street addresses. We did not geocode addresses from cases in the expanded control towns; these cases were assigned to their respective towns.
Data analysis
We obtained U.S. Census block 2000 population data from ESRI®.23 Census block data were overlaid with original treatment and original control areas using ArcGIS. We calculated incidence as total EM rash cases per 100,000 population for every study year in each area.
To examine the effect of each intervention, we designated a “before treatment” and “after treatment” period. Due to the two-year tick lifecycle, the interventions would not be expected to significantly affect tick populations and disease incidence until two years after implementation. For the four-poster analysis, we classified “before treatment” as 1992–1998 and “after treatment” as 1999–2006. For the deer hunt analysis, we classified “before treatment” as 1992–2001 and “after treatment” as 2002–2006.
We analyzed the data using SAS® version 9.1.3.24 For both the four-poster and deer hunt original treatment areas, we compared the mean EM rash incidences before and after treatment to determine if the treatment produced a statistically significant difference. We used a student's t-test to analyze normal data. We analyzed non-normal data that could not be transformed using the Wilcoxon Rank Sum test.
To account for the general decreasing trend in EM rash incidence in the study area since 1992,25 we also compared the EM rash incidence in the original treatment areas with the incidence in both the original control areas and expanded control towns. For each study year, we calculated the relative rate of original treatment area incidence vs. original control area incidence, and the relative rate of original treatment area incidence vs. expanded control town incidence. We then compared the mean relative rates before and after treatment using the statistical tests previously described. This study was approved by the Human Investigation Committees at both the Yale University School of Medicine and CT DPH.
RESULTS
Between 1992 and 2006, 1,296 EM rash cases were reported from the original treatment and original control areas; 212 reports (16%) had missing addresses, incomplete addresses, or post office boxes. Of the remaining 1,084 cases, 943 (87%) were automatically geocoded and 83 (8%) were interactively geocoded. Upon inspection, 50 (4.6%) were outside their respective catchment areas and, thus, excluded. Of the 976 geocoded cases, 702 (72%) cases were in the four-poster areas and 274 (28%) cases were in the deer hunt areas. The median age of cases was 39 years in the four-poster original treatment area (range: 1–82 years); 36 years in the four-poster original control area (range: one day to 86 years); 47 years in the deer hunt original treatment area (range: 3–64 years); and 38 years in the deer hunt original control area (range: seven months to 92 years). During the study period, 1,356 EM rash cases were reported in the expanded control towns: 956 (71%) in four-poster expanded control towns and 400 (29%) in the deer hunt expanded control town. The estimated population for each study area is shown in Figure 1.
Four-poster
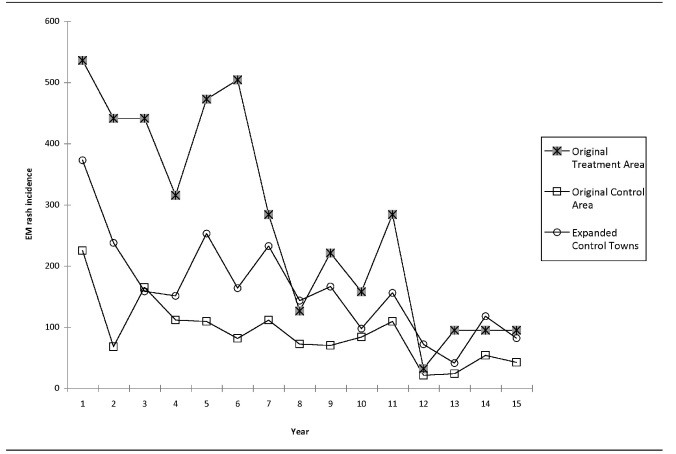
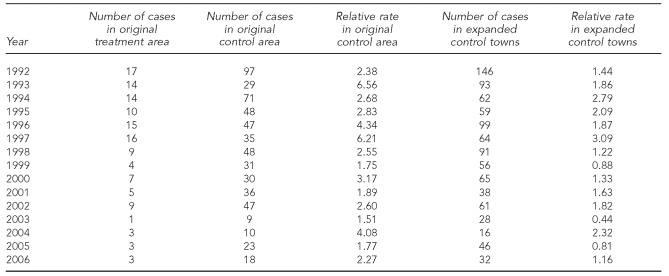
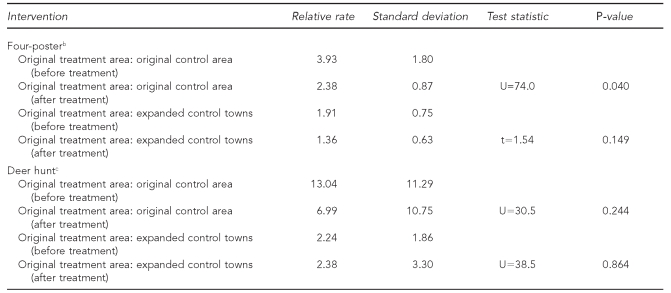
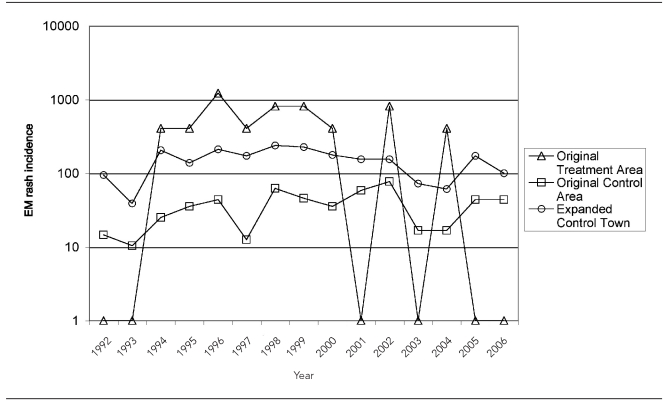
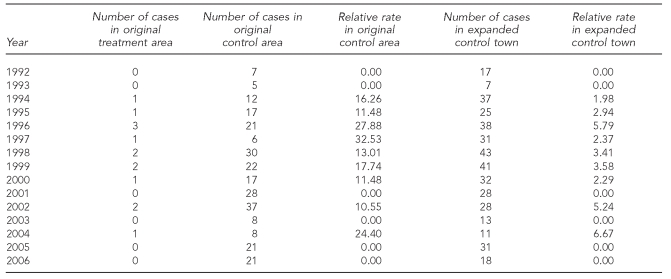
The results of the four-poster analysis showed a general decreasing trend in EM rash incidence in all areas (Figure 2). The annual number of cases and the relative rates of EM rash incidence between the original treatment area and original control area, and the original treatment area and expanded control towns are shown in Table 1. In the original treatment area, the mean incidence before treatment was 427.5 cases per 100,000 population (standard deviation [SD] = 94.2); after treatment, the mean incidence was 137.8 cases per 100,000 population (SD=80.6). The mean incidence was significantly different before and after treatment (t=6.35, p<0.001). The mean relative rate between the original treatment area and the original control area was significantly higher after treatment; however, the mean relative rate of the original treatment area compared with the expanded control town was not significantly different before and after treatment (Table 2).
Figure 2.
Annual incidence of Lyme disease cases per 100,000 population in the four-poster original treatment area, original control area, and expanded control towns:a Connecticut, 1992–2006b
aThe original treatment area is a four-poster treatment area; the original control area is a similar area in Old Saybrook, Connecticut; expanded control towns include surrounding towns of Clinton, Deep River, East Haddam, East Lyme, Haddam, Killingworth, Old Lyme, and Old Saybrook.
bThe after treatment period is 1999–2006.
EM = erythema migrans
Table 1.
Number of cases and relative rates of Lyme disease in the four-poster original treatment area vs. original control area,a and the original treatment area vs. expanded control towns: Connecticut, 1992–2006b
aThe original treatment area is a four-poster treatment area; the original control area is a similar area in Old Saybrook, Connecticut; expanded control towns include surrounding towns of Clinton, Deep River, East Haddam, East Lyme, Haddam, Killingworth, Old Lyme, and Old Saybrook.
bThe after treatment period is 1999–2006.
Table 2.
Results from a comparison analysis of deer-targeted interventions between the original treatment and original control areas and expanded control towns, and before and after treatment: Connecticut, 1992–2006a
aThe after treatment period is 1999–2006.
bThe original treatment area is a four-poster treatment area; the original control area is a similar area in Old Saybrook, Connecticut; expanded control towns include surrounding towns of Clinton, Deep River, East Haddam, East Lyme, Haddam, Killingworth, Old Lyme, and Old Saybrook.
bThe original treatment area is Mumford Cove, the original control area is the rest of Groton, and the expanded control town is Stonington, Connecticut.
Deer hunt
The deer hunt analysis did not show a clear decreasing trend in EM rash incidence in the original treatment area (Figure 3). The annual number of cases and the relative rates of EM rash incidence between the original treatment area and original control area, and the original treatment area and expanded control towns are shown in Table 3. In the original treatment area, the mean incidence rate before treatment was 450.8 cases per 100,000 population (SD=407.6); after treatment, the mean incidence rate was 245.9 cases per 100,000 population (SD=366.6). The mean incidence rate was not significantly different before and after treatment (U=32.5, p=0.432). Neither the mean relative rate between the original treatment area and original control area, nor the mean relative rate between the original treatment area and expanded control towns was significantly different before and after treatment (Table 2).
Figure 3.
Annual incidence of Lyme disease cases per 100,000 population for the deer hunt original treatment area, original control area, and expanded control town:a Connecticut, 1992–2006b
aThe original treatment area is Mumford Cove, the original control area is the rest of Groton, and the expanded control town is Stonington, Connecticut.
bIn 1992, 2001, 2003, 2005, and 2006, no cases were reported from the treatment area. Symbols below the x-axis represent no cases per 100,000 population. The after treatment period is 2002–2006.
EM = erythema migrans
Table 3.
Number of cases and relative rates of Lyme disease in the deer hunt original treatment area vs. original control area,a and the original treatment area vs. expanded control town: Connecticut, 1992–2006b
aThe original treatment area is Mumford Cove, the original control area is the rest of Groton, and the expanded control town is Stonington, Connecticut.
bThe after treatment period is 2002–2006.
DISCUSSION
We evaluated the effectiveness of two deer-targeted interventions on reducing EM rash incidence in an LD endemic area. We found some evidence that the four-poster devices may have reduced the mean EM rash incidence in the original treatment area. The deer hunt was not shown to have had a statistically significant effect on mean EM rash incidence in the original treatment area.
The four-poster results are consistent with other studies that showed the device was effective at reducing tick populations8–11 and with computer simulations suggesting that acaricidal treatment of deer would prevent the most cases of LD.26 Meta-analyses for all the states indicated that the NEATCP effectively reduced the relative density of nymphs and provided 71% nymphal control by the sixth treatment year. The entomologic risk for LD was reduced 68% overall in treated vs. control areas among the five study sites by the study's end.13,27,28
A decrease in EM rash incidence was seen both before and after treatment and compared with the original control area. This finding could indicate that the decrease observed was not only the result of a general decrease in EM rash in the area. However, when the original treatment area was compared with the expanded control towns, the decrease in mean relative rates of EM rash was not significant. This inconsistency could indicate that the results may be due to random variation, a general decreasing trend in EM rash incidence, or from lower relative reductions in tick abundance in Old Lyme compared with other NEATCP sites.13,14
In Mumford Cove, reducing the number of deer did not have a significant effect on the reported EM rash incidence despite a significant decrease in tick abundance (Unpublished data, Kilpatrick HJ, LaBonte AM, Stafford KC 3rd. Effects of hunting on deer density, tick abundance, and human cases of Lyme disease in a residential community. 2010). There was a 45% decrease in mean EM rash incidence after treatment in the original treatment area and a 46% decrease after treatment in the relative rate of the original treatment area compared with the original control area. This rate was less than the community survey reported by Kilpatrick and LaBonte, which showed a decrease from 33 cases in 2000 to five cases in 2001.18 One explanation for the discrepancy was the source of EM rash reports: CT DPH vs. self-report. Underreporting of cases likely impacted our results.29 It's also possible that self-reported LD cases were overreported in the community survey. Finally, cases were reported at the household level in the Kilpatrick study vs. the individual level in this study. However, none of our cases was reported from the same household, so this effect was likely limited. The small population size and low number of EM rash cases reported from Mumford Cove limited this study's power and likely prevented this analysis from detecting a significant effect. This study highlights some of the difficulties in measuring the impact of tick management on disease incidence as opposed to tick abundance and the assumed LD risk.
While our results on reported LD incidence were not consistent with previous studies of deer reduction,15,16 islands and other geographically isolated tracts allow for the elimination, or substantial reduction, in deer numbers greater than may be possible for mainland deer populations. In computer simulations, LD disappears when deer are eliminated. There may, however, be a transient increase in cases in the first two years due to availability of unattached ticks and an increase in the proportion of infected ticks.17,26 Additionally, estimating mainland deer populations may be difficult within an urban forest landscape, which can reduce deer visibility, resulting in larger populations than expected.30
For both the four-poster and deer hunt analyses, the standard surveillance case definition increased the likelihood that the cases were consistent between treatment and control areas as well as over time. The interventions were also evaluated with respect to the ultimate outcome: LD as measured by EM rash, rather than an intermediate step in the exposure pathway: tick density. Finally, the control populations helped account for EM rash trends in the areas over time.
Limitations
The study design had several limitations. One limitation was that the LD surveillance system in Connecticut changed during the study period. Old Lyme, Old Saybrook, and the four-poster expanded control towns were under active surveillance for the entire study period. However, Groton and East Lyme were only under active surveillance from 2001 to 2006, likely influencing reporting rates in those areas. Stonington did not have active LD surveillance during the study period. However, EM rash incidence in Stonington was similar to towns with active surveillance, so the effect may have been minimal. Changes in physician reporting patterns over time could have also affected the results if reporting patterns differed between treatment and control areas over time.
The four-poster population had an additional limitation. We assumed the populations in the census blocks that were intersected by the treatment boundary were evenly distributed across that census block in the original treatment area. If residences were congregated in one portion of the census block, then the actual population in the original treatment area may have been different depending on the residences' true distribution. However, 78.9% of the census blocks in the original treatment area were completely contained within the original treatment area, so this likely had a minimal impact.
Additionally, we excluded cases in the original treatment area and original control area that could not be accurately geocoded, assuming an equal proportion of cases were geocoded in the original treatment area and the original control area. If the addresses that were excluded due to geocoding issues were not evenly distributed throughout the study area, then the incidence estimates may be inaccurate. The expanded control towns' evaluation had an additional limitation because cases in the expanded control towns did not need to be geocoded to calculate incidence. The expanded control towns likely contained more cases than the original treatment area, as no cases were excluded from the expanded control towns on the basis of address. Therefore, the relative rates of the original treatment area to expanded control towns may not be an accurate representation of the true relative rates.
Finally, we did not survey individuals, so other confounding factors may have been present. Behavioral, environmental, and socioeconomic factors may have affected the results if there were differences between the original treatment area and original control area. In particular, because the Mumford Cove residents voted to decrease the deer population, they may have been more aware of LD and, thus, practiced more protective behaviors before the hunt. Additional studies may help reduce the effect of potential confounders and further evaluate the significance of our findings.
CONCLUSIONS
Our findings suggest that the four-poster device was effective in decreasing the incidence of EM rash in an endemic area. Despite a decrease in EM rash incidence, however, we did not find a statistically significant effect of the deer hunt on EM rash incidence, probably due to LD reporting issues, study design limitations, and the small population size. Further study is necessary to conclusively evaluate the effect of deer-targeted tick-control interventions on LD incidence. A prospective, multiyear study designed with consistent surveillance methods, a large population, and established control areas could provide reliable data for analyzing the effectiveness of deer-targeted interventions. A combination of deer-targeted and other interventions, such as education, may ultimately prove to be successful in preventing LD in endemic areas.
Acknowledgments
The authors thank the Jan A.J. Stowijk Fellowship for providing funding for this project; and Jim Meek and Nicole Stabach for reviewing the article.
Footnotes
Jennifer Garnett is a former MPH Candidate at the Yale School of Public Health, Connecticut Emerging Infections Program, in New Haven, Connecticut, and a current student at the Albert Einstein College of Medicine in Bronx, New York. Neeta Connally is an Associate Research Scientist at the Yale School of Public Health, Connecticut Emerging Infections Program. Kirby Stafford, III, is Vice Director, Chief Entomologist, and State Entomologist in the Department of Entomology at the Connecticut Agricultural Experiment Station in New Haven. Matthew Cartter is a State Epidemiologist with the Connecticut Department of Public Health in Hartford, Connecticut.
REFERENCES
- 1.Lyme disease—United States, 2003–2005. MMWR Morb Mortal Wkly Rep. 2007;56(23):573–6. [PubMed] [Google Scholar]
- 2.Ertel SH, Esponda B, Nelson R. Lyme disease—Connecticut 2005. Connecticut Epidemiologist. 2006;26:13–4. [Google Scholar]
- 3.Piesman J, Spielman A, Etkind P, Ruebush TK, II, Juranek DD. Role of deer in the epizootiology of Babesia microti in Massachusetts, USA. J Med Entomol. 1979;15:537–40. doi: 10.1093/jmedent/15.5-6.537. [DOI] [PubMed] [Google Scholar]
- 4.Wilson ML, Adler GH, Spielman A. Correlation between abundance of deer and that of the deer tick Ixodes dammini (acari: ixodidae) Ann Entomol Soc Am. 1986;78:172–6. [Google Scholar]
- 5.Wilson ML, Litwin TS, Gavin TA, Capkanis MC, Maclean DC, Spielman A. Host-dependent differences in feeding and reproduction of Ixodes dammini (acari: ixodidae) J Med Entomol. 1990;27:945–54. doi: 10.1093/jmedent/27.6.945. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JF, Magnarelli LA. Vertebrate host relationships and distribution of ixodid ticks (acari: ixodidae) in Connecticut, USA. J Med Entomol. 1980;17:314–23. doi: 10.1093/jmedent/17.4.314. [DOI] [PubMed] [Google Scholar]
- 7.Keirans JE, Hutcheson HJ, Durden LA, Klompen JS. Ixodes (ixodes) scapularis (acari: ixodidae): redescription of all active stages, distribution, hosts, geographical variation, and medical and veterinary importance. J Med Entomol. 1996;33:297–318. doi: 10.1093/jmedent/33.3.297. [DOI] [PubMed] [Google Scholar]
- 8.Pound JM, Miller JA, George JE, Lemeilleur CA. The “4-poster” passive topical treatment device to apply acaricide for controlling ticks (acari: ixodidae) feeding on white-tailed deer. J Med Entomol. 2000;37:588–94. doi: 10.1603/0022-2585-37.4.588. [DOI] [PubMed] [Google Scholar]
- 9.Pound JM, Miller JA, George JE. Efficacy of amitraz applied to white-tailed deer by the “4-poster” topical treatment device in controlling free-living lone star ticks (acari: ixodidae) J Med Entomol. 2000;37:878–84. doi: 10.1603/0022-2585-37.6.878. [DOI] [PubMed] [Google Scholar]
- 10.Solberg VB, Miller JA, Hadfield T, Burge R, Scheck JM, Pound JM. Control of Ixodes scapularis (acari: ixodidae) with topical self-application of permethrin by white-tailed deer inhabiting NASA, Beltsville, Maryland. J Vector Ecol. 2003;28:117–34. [PubMed] [Google Scholar]
- 11.Schulze TL, Jordan RA, Schulze CJ, Healy SP, Jahn MB, Piesman J. Integrated use of 4-poster passive topical treatment devices for deer-targeted acaricide applications, and Maxforce TMS bait boxes to rapidly suppress populations of Ixodes scapularis (acari: ixodidae) in a residential landscape. J Med Entomol. 2007;44:830–9. doi: 10.1603/0022-2585(2007)44[830:iuoppt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Pound JM, Miller JA, George JE, Fish D. The United States Department of Agriculture Northeast Area-wide Tick Control Project: history and protocol. Vector Borne Zoonotic Dis. 2009;9:365–70. doi: 10.1089/vbz.2008.0182. [DOI] [PubMed] [Google Scholar]
- 13.Pound JM, Miller JA, George JE, Fish D, Carroll JF, Schulze TL, et al. The United States Department of Agriculture's Northeast Area-wide Tick Control Project: summary and conclusions. Vector Borne Zoonotic Dis. 2009;9:439–48. doi: 10.1089/vbz.2008.0200. [DOI] [PubMed] [Google Scholar]
- 14.Stafford KC, 3rd, Denicola AJ, Pound JM, Miller JA, George JE. Topical treatment of white-tailed deer with an acaricide for the control of Ixodes scapularis (acari: ixodidae) in a Connecticut Lyme borreliosis hyperendemic community. Vector Borne Zoonotic Dis. 2009;9:371–9. doi: 10.1089/vbz.2008.0161. [DOI] [PubMed] [Google Scholar]
- 15.Wilson ML, Telford SR, 3rd, Piesman J, Spielman A. Reduced abundance of immature Ixodes dammini (acari: ixodidae) following elimination of deer. J Med Entomol. 1988;25:224–8. doi: 10.1093/jmedent/25.4.224. [DOI] [PubMed] [Google Scholar]
- 16.Rand PW, Lubelczyk C, Holman MS, Lacombe EH, Smith RP., Jr. Abundance of Ixodes scapularis (acari: ixodidae) after the complete removal of deer from an isolated offshore island, endemic for Lyme disease. J Med Entomol. 2004;41:779–84. doi: 10.1603/0022-2585-41.4.779. [DOI] [PubMed] [Google Scholar]
- 17.Mount GA, Haile DG, Daniels E. Simulation of management strategies for the blacklegged tick (acari: ixodidae) and the Lyme disease spirochete, Borrelia burgdorferi. J Med Entomol. 1997;34:672–83. doi: 10.1093/jmedent/34.6.672. [DOI] [PubMed] [Google Scholar]
- 18.Kilpatrick HJ, LaBonte AM. Deer hunting in a residential community: the community's perspective. Wildlife Soc Bull. 2003;31:340–8. [Google Scholar]
- 19.Connecticut Department of Environmental Protection. Managing urban deer in Connecticut: a guide for residents and Communities. 2nd ed. Hartford (CT): CT DEP; 2007. Bureau of Natural Resources. [Google Scholar]
- 20.Kilpatrick HJ, Stober WA. Effects of temporary bait sites on movements of suburban white-tailed deer. Wildlife Soc Bull. 2002;30:760–6. [Google Scholar]
- 21.ESRI. ArcGIS®: Version 9.1. Redlands (CA): ESRI; 2005. [Google Scholar]
- 22.University of Connecticut. MAGIC: Map and Geographical Information Center—Connecticut town coverages. [cited 2007 Oct 10]. Available from: URL: http://magic.lib.uconn.edu.
- 23.ESRI. ArcData: Download Census 2000 TIGER/Line® data. [cited 2007 Nov 15]. Available from: URL: http://arcdata.esri.com/data/tiger2000/tiger_download.cfm.
- 24.SAS Institute, Inc. SAS®: Version 9.1.3 for Windows. Cary (NC): SAS Institute, Inc; 2007. [Google Scholar]
- 25.Connecticut Department of Public Health. Lyme disease statistics. [cited 2008 Apr 11]. Available from: URL: http://ct.gov/dph/cwp/view.asp?a=3136&q=399694.
- 26.Hayes EB, Maupin GO, Mount GA, Piesman J. Assessing the prevention effectiveness of local Lyme disease control. J Public Health Manag Pract. 1999;5:84–92. doi: 10.1097/00124784-199905000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Brei B, Brownstein JS, George JE, Pound JM, Miller JA, Daniels TJ, et al. Evaluation of the United States Department of Agriculture Northeast Area-wide Tick Control Project by meta-analysis. Vector Borne Zoonotic Dis. 2009;9:423–30. doi: 10.1089/vbz.2008.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoen AG, Rollend LG, Papero MA, Carroll JF, Daniels TJ, Mather TN, et al. Effects of tick control by acaricide self-treatment of white-tailed deer on host-seeking tick infection prevalence and entomologic risk for Ixodes scapularis-borne pathogens. Vector Borne Zoonotic Dis. 2009;9:431–8. doi: 10.1089/vbz.2008.0155. [DOI] [PubMed] [Google Scholar]
- 29.Meek JI, Roberts CL, Smith EV, Jr, Cartter ML. Underreporting of Lyme disease by Connecticut physicians, 1992. J Public Health Manag Pract. 1996;2:61–5. doi: 10.1097/00124784-199623000-00017. [DOI] [PubMed] [Google Scholar]
- 30.Jordan RA, Schulze TL, Jahn MB. Effects of reduced deer density on the abundance of Ixodes scapularis (acari: ixodidae) and Lyme disease incidence in a northern New Jersey endemic area. J Med Entomol. 2007;44:752–7. doi: 10.1603/0022-2585(2007)44[752:eorddo]2.0.co;2. [DOI] [PubMed] [Google Scholar]