Abstract

In this report, it is demonstrated that chiral vinyl aziridines can be stereospecifically ring expanded. This synthetic approach allows controlled access to chiral 2,5-cis or 2,5-trans-3-pyrroline products from starting materials with the appropriate aziridine geometry. Twenty three ring expansion examples, most of which feature a stereospecific cyclization, are presented.
A cursory review of the structural motifs of the top 200 top selling drugs1 reveals that around 90% contain at least one nitrogen atom and approximately 65% are decorated with a heterocycle. Not surprisingly, the majority of these heterocycles are nitrogenous, with pyrrolidines a commonly occurring heterocyclic scaffold. Given the success of chiral pyrrolidines as important pharmaceutical building blocks it follows that a range of practical synthetic methods are needed2 to provide access to any targeted structural and stereochemical pyrrolidine pattern.
We have chosen to tackle the challenge of developing useful pyrrolidine forming methods by revisiting the ring expansion of vinyl aziridines, first reported by Atkinson.3 Surprisinigly, despite the potential usefulness of converting a vinylaziridine into a 3-pyrroline, there had only been a single study focused on using metal catalysts to aid the rearrangement prior to our contribution to this field. 4 Oshima and coworkers found that tosylated dieneaziridines could be ring expanded in the presence of a palladium catalyst to the 3-pyrrolines. Both the N-tosyl group and the diene moiety were reported to be essential for the success of this rearrangement. Simple non-dienic vinyl aziridines did not ring expand, furnishing instead a complex mixture of products. In our recent report, we demonstrated that this significant substrate limitation could be solved using Cu(hfacac)2 as a catalyst.5 The substrate scope of this new transformation, which we demonstrated for a range of tosyl (Ts) and N-phthalimide (NPhth) protected vinyl aziridines, was shown to be quite broad. In this report we expand these investigations further and focus our attention on stereospecific vinyl aziridine ring expansions and application of this new methods towards accessing chiral pyrroline products.
In order to maximize the synthetic potential of our method for preparing chiral pyrroline products, it is essential that reliable, asymmetric, convergent, and scalable routes be available to access the requisite starting materials (chiral vinyl aziridines). 6 The union of an imine and suitably activated nucleophile quickly emerged as the optimal approach (Scheme 1). The imine based retrosynthetic analysis is highlighted for chiral pyrroline 3, which we envisioned would originate from the copper catalyzed ring expansion of a trans- or cis-vinyl aziridine (4 and 5). These isomeric vinyl aziridines could be accessed by treating imines 7 and 8 with nucleophiles 6 and 9, respectively. An attractive feature of this disconnection approach is that either the imine or the nucleophile could serve the role of chiral auxiliary.
Scheme 1.
Retrosynthetic Analysis for Chiral Pyrrolines
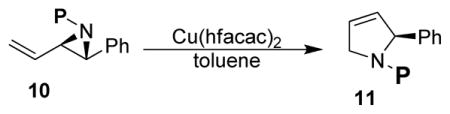
Prior to assembling the requisite chiral aziridines it was of critical importance to learn what other nitrogen protecting groups (P) besides Ts and Phth, might also be suitable for this reaction (Table 1). We realized that aryl and acyl substituted aziridines would be particularly challenging substrates, given their known tendency to undergo competing Claisen rearrangements or intramolecular displacement reactions.7 This prediction turned out to be true as in the case of N-benzyl (entry d) a known hydride shift occurred instead,8 while for N-phenyl (entry c) the expected Claisen rearrangement was observed.9 Boc protected aziridine 10 (entry e) did ring expand to the desired 3-pyrroline,10 while in contrast when a benzoyl group (entry j) was present, the ring expansion failed and instead a mixture of five and seven membered heterocycles was formed.11 When vinyl aziridine 10 was not protected (NH, entry i) it rapidly oligomerized when subjected to the reaction conditions. Curiously, the t-butyl sulfinamide substituted vinyl aziridine (entry f) formed 1-phenyl butadiene in high yield rather than the expected 3-pyrroline product (11).12 We were delighted to find that 4-nitrobenzylsulfonamide (Ns) and Bus t-butylsulfonamide (Bus) groups, in addition to p-toluenesulfonamide (entry a), were compatible with the ring expansion conditions. These studies suggest that sulfonamides are especially well suited for our ring expansion reaction.
Table 1.
Ring Expansion: Nitrogen Substituent Tolerance
 | |||
|---|---|---|---|
| entry | P = | time (h) | yield of 11 |
| a | Ts | 5 | 92% |
| b | NPhtha,b | 2 | 97% |
| c | Pha | 2 | 0%c |
| d | Bna | 2 | 0%d |
| e | Boc | 5 | 60% |
| f | S(O)tBu | 1 | 0%e |
| g | Bus | 5 | 86% |
| h | Ns | 5 | 94% |
| i | H | 2 | 0%f |
| j | Bz | 5 | 0%g |
Conditions: 5 mol % Cu(hfacac)2, 150 °C
racemic aziridine used,
cis/trans mixture,
mixture of azepine and 2-pyrroline products,
(Z)-N-benzylidene-1-phenylbut-2-en-1-amine isolated in 86% yield,
(E)-buta-1,3-dien-1-ylbenzene formed in 69% yield,
Polymer formed,
Five and seven membered ring products observed.
Armed with insights into what aziridine protecting groups are compatible with our reaction conditions we turned our attention to the design of a scalable asymmetric route to vinyl aziridine substrates. The use of proline derivatives as organocatalysts13 and as key building blocks of many pharmaceuticals and natural products14 ensures its status as a privileged structural motif. Chiral 2,3-dehydroproline (3-pyrroline) products commonly originate from natural 3-hydroxy proline,15 a neglected family of chiral proline products emerged as an ideal target for our new methodology. Retrosynthetic analysis for 2,5-dihydropyrrolidine 15 (Scheme 2) suggests that the chiral vinyl aziridine ring expansion substrate (14) should originate from a Darzens reaction16 between a bromo acetate (12) and a chiral conjugated Ellman type imine17 (13). These chiral imines are attractive substrates because they are trivial to make, are very stable, available in both enantiomeric forms and easy to handle. Since our nitrogen substituent ring expansion compatibility study (Table 1) had unfortunately revealed that the immediate products of this union, N-sulfinamide protected vinyl aziridines, did not ring expand we needed to resort to oxidizing the Darzen reaction products prior to ring expansion in order to have a compatible substrate (Bus-protected aziridines). This was readily accomplished using m-CPBA. Interestingly, we have learned that this oxidation can also be accomplished with ammonium molybdate tetrahydrate in excellent yield.18
Scheme 2.
Darzen Reaction Route to Chiral Proline Products
Twelve chiral aziridines substrates shown in Table 2 (R = Bus) were prepared using the strategy detailed above. The Darzen reaction step was in general high yielding and the cis/trans-aziridine ratios were in the modest 2:1–4:1 range. In order to better understand the scope of the ring expansion, the resulting cis- and trans-vinyl aziridines were separated and evaluated individually. Both cis- and trans-vinyl aziridines readily ring expand to 3-pyrrolines in excellent yields in the presence of catalytic amounts of Cu(hfacac)2. Entries e–h highlight the methodoloies ability to offer two suitable retrosynthetic choices for accessing a particular enantiomer (entries e–f give the (S)-enantiomer and g–h the (R)-enantiomer). Entries i–k demonstrate how control of a desired 2,5-pyrroline substitution pattern can be achieved simply by starting with the appropriate cis- or trans-aziridine precursor.19 Deprotection of Bus-protected cyclic dialkylamines containing an adjacent electron withdrawing group like the 3,4-dehydroproline products in Table 2 is well documented to proceed cleanly without any epimerization.20
Table 2.
Ring Expansion of Bus-Protected Vinyl Aziridines
| entry | substrate (14) | product (15) | yield |
|---|---|---|---|
| a |  |
72% | |
| b |  |
 |
77% |
| c |  |
 |
87% |
| d |  |
 |
86% |
| e |  |
 |
90% |
| f |  |
90% | |
| g |  |
 |
81% |
| h |  |
83% | |
| i |  |
 |
67% |
| j |  |
 |
87% |
| k |  |
 |
59% |
| l |  |
 |
50% |
Conditions: 5 mol % Cu(hfacac)2, toluene, 5 h, 150 °C. Cy = cyclohexyl. R = Bus (14 a-l) R = S(O)tBu (16 a-l).
A complementary convergent asymmetric approach to chiral vinyl aziridines en route to 3-pyrroline products employs a chiral sulfide in place of a chiral imine (Scheme 3). Although the use of chiral nucleophiles versus chiral electrophiles for accessing aziridines is currently far less developed, this approach would avoid the oxidation step prior to ring expansion. Aggarwal21 and coworkers have shown that chiral sulfonium nucleophiles can be used to synthesize aziridines in a highly selective manner. We chose to employ the limonene based chiral auxiliary (19)22 for additions to sulfonamide imines (18). The Aggarwal chiral auxiliary is easily prepared in either enantiomeric form. This route provided us with the chiral ring expansion precursors (20) in a single step. Yields for this step were mostly high and the cis/trans-aziridine product ratios were in the 6:1–20:1 range. Interestingly, the bis-aryl vinyl aziridines products (20a–e) were obtained as a single trans-isomer (dr >20:1) while the cyclohexyl-substituted aziridines (20f–k) were obtained with slightly lower selectivity (dr = 6:1–11:1).
Scheme 3.
Asymmetric Synthesis of Tosyl Protected Aziridines
Chiral 2-aryl-substituted pyrrolidines are important structures and have received increased attention in recent years.23 We were delighted to find that all of the chiral vinyl aziridine substrates 20a–k could be ring expanded stereospecifically using Cu(hfacac)2 as catalyst in uniformly excellent yields to corresponding 2-aryl-3-pyrrolines 21a–k (Table 3). A single stereoisomer was obtained in each case. Both electron donating and withdrawing substitutents are well tolerated, including aryl bromides, chlorides, iodides and fluorides. The broad tolerance of aryl substituents should serve those researchers interested in applying this new methodology to complex natural products, pharmaceuticals, or organocatalytic architectures well. Impressively, the corresponding chiral cis-vinyl aziridines stereospecifically afford the trans-fused 3-pyrroline products as single stereoisomeric products (entries j–k).
Table 3.
Ring Expansion of Tosyl-protected Vinyl Aziridines
| entry | Substrate (20) | product (21) | t(h) | yield |
|---|---|---|---|---|
| a | R1 = H R2 = Ph R3 = 4-Cl-C6H4 |
2 | 84% | |
| b | R1 = H R2 = Ph R3 = 4-F-C6H4 |
 |
2 | 91% |
| c | R1 = H R2 = Ph R3 = 4-Br-C6H4 |
2 | 92% | |
| d | R1 = H R2 = Ph R3 = 4-Br-C6H4 |
 |
2 | 87% |
| e | R1 = H R2 = Ph R3 = 2-OMe-C6H4 |
2 | 81% | |
| f | R1,R2 = -(CH2)4- R3 2-Br-C6H4 |
 |
2 | 97% |
| g | R1,R2 = -(CH2)4- R3 = C6H5 |
 |
2 | 92% |
| h | R1,R2 = -(CH2)4- R3 = 4-CF3-C6H4 |
 |
2 | 93% |
| i | R1,R2 = -(CH2)4- R3 = 4-NO2-C6H4 |
 |
5 | 96% |
| j | R1,R2 = -(CH2)4- R3 = 4-CF3-C6H4 |
 |
4 | 92% |
| k | R1,R2 = -(CH2)4- R3 = 4-NO2-C6H4 |
 |
5 | 71% |
Conditions: 5 mol % Cu(hfacac)2, toluene, 150 °C.
In summary, we have demonstrated two new practical complimentary routes to access valuable chiral 3-pyrroline products from readily accessible vinyl aziridine precursors. This was accomplished by coupling an imine with an appropriate nucleophile and then catalytically ring expanding the resulting chiral vinyl aziridine using Cu(hfacac)2. The substrate scope and functional group tolerance of this new synthesis is very broad. The excellent chirality transfer demonstrated by the examples presented also serves to underscore the mechanistic uniqueness of this new catalytic reaction and advantage over all other aziridine ring expansion routes aiming to form 3-pyrrolines. It is important to note, that an added advantage to both of our asymmetric 3-pyrroline routes is that they provide a double bond handle for further functionalization, which can be readily converted to more complex pyrrolidine products in a substrate controlled manner. As better asymmetric aziridine methods are developed more opportunities will arise for applications of this new method.
Supplementary Material
Acknowledgments
We would like to thank NSF (CHE-0848324) and the NIH (training grant, T32GM008500, to M. B.) for financial support. Special thanks to Dr. Emil Lobkovsky for obtaining all X-Ray crystal structures.
Footnotes
Supporting Information Available Full experimental details for all new compounds reported in this article including X-ray data for compounds 15d, 15j, 15l, 21d and 21f are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.The structures of the top 200 selling drugs can be downloaded from: http://cbc.arizona.edu/njardarson/group. McGrath NA, Brichacek M, Njardarson JT. J Chem Educ. 2010;87:1348–1349.
- 2.Royer Jacques., editor. Asymmetric Synthesis of Nitrogen Heterocycles. Wiley-VCH; Weinheim: 2009. [Google Scholar]
- 3.a) Atkinson RS, Rees CW. Chem Commun. 1967:1232–1232. [Google Scholar]; b) Hudlicky T, Frazier JO, Kwart LD. Tetrahedron Lett. 1985;26:3523–3526. [Google Scholar]; c) Pearson WH. Tetrahedron Lett. 1985;26:3527–3530. [Google Scholar]; d) Hudlicky T, Reed JW. Angew Chem Int Ed. 2010;49:4864–4876. doi: 10.1002/anie.200906001. [DOI] [PubMed] [Google Scholar]; e) Scheiner P. J Org Chem. 1967;32:2628–2630. [Google Scholar]; f) Hirner S, Somfai P. Synlett. 2005:3099–3102. [Google Scholar]
- 4.a) Fugami K, Morizawa Y, Oshima K, Nozaki H. Tetrahedron Lett. 1985;26:857–860. [Google Scholar]; b) Fugami K, Miura K, Morizawa Y, Oshima K, Utimoto K, Nozaki H. Tetrahedron. 1989;45:3089–3098. [Google Scholar]
- 5.Brichacek M, Lee D, Njardarson JT. Org Lett. 2008;10:5023–5026. doi: 10.1021/ol802123e.For a review of 3-pyrroline synthetic approaches: Brichacek M, Njardarson JT. Org Biomol Chem. 2009;7:1761–1770. doi: 10.1039/b900236g.
- 6.Pellisier H. Tetrahedron. 2010;66:1509–1555. [Google Scholar]
- 7.Pommelet JC, Chuche J. Can J Chem. 1976;54:1571–1581. [Google Scholar]
- 8.Somfai P, Ahman J. Tetrahedron Lett. 1995;36:1953–1956. [Google Scholar]
- 9.Fantauzzi S, Gallo E, Caselli A, Piangiolino C, Ragaini F, Re N, Cenini S. Chem Eur J. 2009;15:1241–1251. doi: 10.1002/chem.200801148. [DOI] [PubMed] [Google Scholar]
- 10.Mishra A, Rice SN, Lwowski W. J Org Chem. 1968;33:481–486. [Google Scholar]
- 11.Heine HW, Fetter ME, Nicholson EM. J Am Chem Soc. 1959;81:2202–2204. [Google Scholar]
- 12.We have not been able to find other examples of this type of deamination reaction for sulfinamide protected aziridines. Furthermore, we have tested this on several other substrates and observed the same deamination behavior. Aziridine deaminations are not without precedence, see: Clard RD, Helmkamp GK. J Org Chem. 1964;29:1316–1320.Muller RK, Felix D, Schreiber J, Eschenmosher A. Helv Chim Acta. 1970;53:1479–1484.
- 13.a) Kotsuki H, Ikishima H, Okuyama A. Heterocycles. 2008;75:493–529. [Google Scholar]; b) Kotsuki H, Ikishima H, Okuyama A. Heterocycles. 2008;75:757–797. [Google Scholar]
- 14.Mauger AM. J Nat Prod. 1996;59:1205–1211. doi: 10.1021/np9603479. [DOI] [PubMed] [Google Scholar]
- 15.a) Meyer SC, Ramanjulu J, Vera MD, Pfizenmayer AJ, Joullie MM. J Org Chem. 1994;59:5192–5205. [Google Scholar]; b) Greenwood ES, Hitchcock PB, Parsons PJ. Tetrahedron. 2003;59:3307–3314. [Google Scholar]
- 16.Davis FA, Liu H, Zhou P, Fang T, Reddy GV, Zhang Y. J Org Chem. 1999;64:7559–7567. doi: 10.1021/jo991389f. [DOI] [PubMed] [Google Scholar]
- 17.a) Liu G, Cogan DA, Ellman JA. J Am Chem Soc. 1997;119:9913–9914. [Google Scholar]; b) Ellman JA, Owens TD, Tang TP. Acc Chem Res. 2002;35:984–995. doi: 10.1021/ar020066u. [DOI] [PubMed] [Google Scholar]; c) Davis FA, Reddy RT, Reddy RE. J Org Chem. 1992;57:6387–6389. [Google Scholar]; d) Davis FA. J Org Chem. 2006;71:8993–9003. doi: 10.1021/jo061027p. [DOI] [PubMed] [Google Scholar]
- 18.This cheap, practical and simple oxidation protocol, which is commonly employed for accessing Julia olefination substrates, has not been reported previously for converting sulfinamides to sulfonamides: Blakemore PR. J Chem Soc Perkin Trans. 2002;1:2563–2585.
- 19.Entry 1 is an exception as this highly congested system scrambled prior to ring expansion.
- 20.a) Koep S, Gais HJ, Raabe G. J Am Chem Soc. 2003;125:13243–13251. doi: 10.1021/ja030324y. [DOI] [PubMed] [Google Scholar]; b) Gunter M, Gais HJ. J Org Chem. 2003;68:8037–8041. doi: 10.1021/jo030171x. [DOI] [PubMed] [Google Scholar]; c) Tiwari SK, Gais HJ, Lindenmaier A, Babu GS, Raabe G, Reddy LR, Kohler F, Gunter M, Koep S, Iska VBR. J Am Chem Soc. 2006;128:7360–7373. doi: 10.1021/ja061152i. [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal VK, Vasse JL. Org Lett. 2003;5:3987–3990. doi: 10.1021/ol035554w. [DOI] [PubMed] [Google Scholar]
- 22.Illa O, Arshad M, Ros A, McGarrigle EM, Aggarwal VK. J Am Chem Soc. 2010;132:1828–1830. doi: 10.1021/ja9100276. [DOI] [PubMed] [Google Scholar]
- 23.For recent approaches to chiral 2-pyrrolines consult: Campos KR, Klapars A, Waldman JH, Dormer PG, Chen C. J Am Chem Soc. 2006;128:3538–3539. doi: 10.1021/ja0605265.Davis FA, Song M, Augustine A. J Org Chem. 2006;71:2779–2786. doi: 10.1021/jo052566h.Fang YQ, Jacobsen EN. J Am Chem Soc. 2008;130:5660–5661. doi: 10.1021/ja801344w.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





