Abstract
Retinal ganglion cells (RGCs) are normally unable to regenerate their axons after optic nerve injury or degenerative disorders, resulting in lifelong visual losses. This situation can be partially reversed by activating RGCs’ intrinsic growth state, maintaining their viability, and counteracting inhibitory signals in the extracellular environment. Advances over the past few years continue to extend the amount of regeneration that can be achieved in animal models. These findings lend hope to the possibility that clinically meaningful regeneration may become a reality within a few years provided that regenerating axons can be guided to their appropriate destinations.
Keywords: optic nerve, regeneration, trophic factor, oncomodulin, PTEN, SOCS3, KIF4
As in most CNS pathways, axons that are injured in the mature optic nerve do not grow back, leaving victims of traumatic nerve injury or degenerative diseases such as glaucoma with lifelong losses in vision. The optic nerve has long been studied for insights into the causes of regenerative failure in the CNS, focusing on such issues as the inhibitory effects of CNS myelin and the glial scar, the absence of appropriate trophic factors, the immune response to injury, cell death pathways, and the decline in neurons’ intrinsic growth capacity. The past 10–15 years have witnessed major advances in understanding why retinal ganglion cells (RGCs) normally fail to regenerate injured axons through the optic nerve and in devising ways to reverse this situation, lending hope to the possibility that functional repair might one day be possible.
Axon regeneration through the optic nerve
Under normal circumstances, damaged axons show a transient sprouting response following optic nerve injury but no long-distance regeneration. Tello, a student of Ramon y Cajal, found that if the optic nerve is cut and sutured to a segment of peripheral nerve (PN), axons will grow into the graft1. Aguayo and colleagues showed that some RGCs can regenerate axons through a PN graft that extends all the way from the cut end of the optic nerve to the superior colliculus and form synapses in the correct layer2, 3.
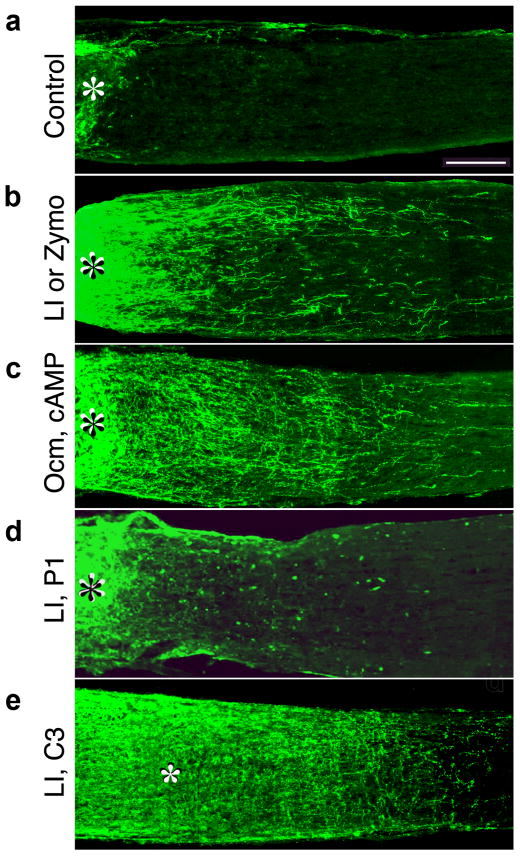
The ability of RGCs to regenerate axons through a PN graft is likely to be related in part to higher levels of growth-permissive molecules (e.g., laminin) and lower levels of growth-inhibitory molecules (e.g., NogoA) in peripheral nerves vs. the optic nerve4–6. However, it is also possible that the two differ in their ability to provide essential trophic factors. To test this latter possibility, Berry and colleagues implanted a fragment of PN into the vitreous humor and found that this stimulated RGCs to regenerate lengthy axons beyond the site of an optic nerve crush injury7. Although this growth was initially attributed to trophic factors derived from Schwann cells, the grafts contained numerous macrophages, which can enhance axon regeneration when pre-activated and placed in the optic nerve8, 9. Other methods that induce intraocular inflammation, i.e., injuring the lens or injecting the pro-inflammatory agent Zymosan into the eye, lead to even greater regeneration than PN implants (Fig. 1a,b)10, 11, 91. This regeneration is associated with a dramatic change in RGCs’ intrinsic growth state, as evidenced by a marked upregulation of proteins such as GAP-43 and SPRR1A12. Although PN implants secrete ciliary neurotrophic factor (CNTF)13, their primary effect in vivo is related to other factors associated with macrophages14.
Figure 1.
Axon regeneration in the rat optic nerve. Longitudinal sections through the rat optic nerve were stained with antibodies to the protein GAP-43 2 weeks after optic nerve injury to visualize regenerating axons. Asterisks denote the injury site. (a) Almost no regeneration occurs in the absence of further stimulation. (b) Lens injury (LI) or Zy-mosan (Zymo) induces intraocular inflammation and enables RGCs to regenerate axons through the optic nerve10, 11. (c) Ocm plus a cAMP analog, when delivered from slow-release polymeric beads, mimic the effects of lens injury16. (d) P1, an Ocm receptor antagonist suppresses the effects of lens injury17. (e) Expression of the bacterial enzyme C3 ribosyltransferase (C3) in RGCs blocks the activity of RhoA and enables axons to ignore inhibitory signals in their environment. C3 expression by itself produces only modest levels of regeneration, but greatly enhances the effects of intraocular inflammation after LI12.
Oncomodulin is a potent growth factor for RGCs
Using dissociated retinal cell cultures as a bioassay, two molecules that are present in the eye were found to stimulate mature RGCs to regenerate their axons. One is man-nose, a simple sugar that is abundant in the vitreous. Mannose stimulates RGCs to extend moderately long axons provided that cells have sufficiently high levels of intracellular cAMP ([cAMP]i)15. The second growth factor is oncomodulin (Ocm), a 12 kDa calcium-binding protein that is secreted by macrophages. Ocm accumulates rapidly in the eye following intravitreal inflammation and exhibits cAMP-dependent, high-affinity binding to a cell-surface receptor on RGCs16, 17. When released from polymeric beads placed into the vitreous, Ocm plus a cAMP analog induce nearly as much optic nerve axon regeneration as intraocular inflammation16 (Fig. 1c). Conversely, either an Ocm peptide antagonist or a neutralizing anti-Ocm antibody dramatically suppresses inflammation-induced regeneration (Fig. 1d)17. Thus, Ocm appears to mediate most of the effect of intravitreal inflammation on optic nerve regeneration. However, additional factors derived from inflammatory cells or retinal glia also appear to play a role by causing an elevation of [cAMP]I and by enhancing RGC survival17. One group failed to detect an elevation of Ocm in the eye following inflammation18 and reported that an anti-Ocm antibody does not diminish inflammation-induced regeneration19. The likely sources of these discrepant results are discussed elsewhere17, 20. Intraocular inflammation also enhances the ability of RGCs to regenerate their axons through a PN graft11, 21, and this effect is likewise blocked by an Ocm antagonist peptide17.
Altering intracellular signaling can promote optic nerve regeneration
The signaling pathways that enable RGCs to regenerate their axons are beginning to emerge. Mst3b, a purine-sensitive protein kinase, plays a central role in the signal transduction pathway through which trophic factors induce axon growth22, 23. Suppression of Mst3b expression blocks the axon-promoting effects of Ocm in culture and of inflammation-induced regeneration in vivo23, whereas expression of a constitutively active form of Mst3b enables RGCs to regenerate axons even in the absence of growth factors23. The effects of Ocm can also be blocked by an inhibitor of CaM kinases or by combining inhibitors of the PI3K, MAPK, and Jak-STAT pathways16. Conversely, deleting genes that encode suppressors of these pathways stimulates axon regeneration in vivo. Appreciable optic nerve regeneration can be stimulated by deleting the gene for PTEN, a protein- and lipid phosphatase that suppresses signaling through the PI3 kinase-Akt pathway24, and, to a somewhat lesser extent, by deleting the gene encoding SOCS3, a protein that suppresses signaling through the Jak-STAT pathway25. Deletion of either PTEN or SOCS3 leads to phosphorylation of the S6 kinase, implying that activation of the mTOR pathway plays an important role.
The intrinsic growth capacity of RGCs declines in the early postnatal period26, and is accompanied by changes in the expression of Kruppel-Like Family (KLF) transcription factors. Overexpression of KLF-4 suppresses axon growth in immature RGCs, whereas diminished KLF-4 expression increases axon growth in mature RGCs and promotes a modest amount of regeneration in vivo27. It is not yet known whether the effects of intra-ocular inflammation, PTEN deletion, or SOCS deletion are mediated through changes in the expression of any KLF transcription factors.
The role of cAMP in optic nerve regeneration
The second messenger cAMP augments axon regeneration in multiple ways, including altering the response of growth cones to inhibitory signals28, 29, stimulating the translocation of growth factor receptors to the cell surface16, 30, and altering gene expression programs31. The latter effects include down-regulation of SOCS-3 expression32 and upregulation of Arginase I (Arg I)31. Arg I is an enzyme involved in the biosynthesis of polyamines, which enhance the ability of neurons to extend axons over inhibitory substrates31. Spermidine stimulates a modest amount optic nerve regeneration33, as does elevation of cAMP16, 34, 35. As noted above, cAMP strongly enhances the effects of Ocm16, and increases the effects of intraocular inflammation36. A peptide that prevents Ocm from binding to its receptor eliminates the latter effects, showing that Ocm is the principal factor involved in both inflammation-induced regeneration and the enhancement of this phenomenon by cAMP37.
RGC survival after optic nerve injury
RGCs begin to die a few days after their axons are injured, particularly if damage occurs close to the eye38. This death can be prevented almost completely by overexpressing the anti-apoptotic Bcl family proteins Bcl-2 or Bcl-xL in RGCs39, 40. However, although axon regeneration clearly requires RGCs to remain viable, axon outgrowth and cell survival utilize different intracellular signaling pathways. This dissociation is exemplified by the failure of RGCs overexpressing Bcl-2 or Bcl-xL to regenerate axons without additional growth factors39, 41 and by the persistent enhancement of RGC survival seen after intraocular inflammation even when regeneration is suppressed by Ocm-blocking reagents17.
RGC death can be slowed, but not stopped, with a number of trophic factors, including CNTF35, 42, 43, brain-derived neurotrophic factor (BDNF)42, 44, 45, neurotrophin-4/5 (NT-4/5)46, 47, nerve growth factor (NGF)48, insulin-like growth factor-149, granulocyte-colony stimulating factor50, glial-derived neurotrophic factor (GDNF)51, 52 and neurturin53. BDNF combined with GDNF, neurturin, or intraocular inflammation has additive effects on survival, although the latter combination suppresses axon regeneration53, 54.
The death of axotomized RGCs can be slowed by preventing Caspase cleavage55–58, blocking the nuclear enzyme poly(ADP-ribose) polymerase (PARP), a substrate for caspases59, blocking nitric oxide synthase60, introducing reducing agents61 or inhibiting cell death via caspase-independent pathways62–64. Long-term prevention of RGC death after axotomy may require a combination of treatments.
Effects of other trophic factors on optic nerve regeneration
FGF2 stimulates some axon regeneration through the optic nerve65. NGF, NT-3, BDNF, and NT-4/5 do not66, 67, although a combination of FGF2, NT-3 and NGF has been reported to induce substantial regeneration67. One group has argued that CNTF mediates the effects of intravitreal inflammation on optic nerve regeneration, based primarily on the outgrowth seen using concentrations of CNTF several orders of magnitude above the established ED50 value and on the loss of axon regeneration and RGC survival seen when the genes encoding CNTF and LIF are deleted19, 36, 68. However, physiologically relevant concentrations of CNTF do not promote strong regeneration in culture10, 16, 17, 46 and many labs have failed to find strong effects of CNTF in vivo10, 25, 43, 54, 69. In addition, CNTF inhibitors have no effect10, 70 or only a mild effect19 on inflammation-induced regeneration. CNTF enhances axon regeneration through a peripheral nerve graft35, 71, but this effect is associated with intraocular inflammation and is eliminated when inflammation is suppressed72. Thus, the direct effect of CNTF on optic nerve regeneration is weak, although it may contribute to maintaining RGC survival. The axon-promoting effects of CNTF become strong when the gene that encodes SOCS3, the negative regulator of the jak-STAT pathway, is deleted25. However, optic nerve injury leads to an upregulation of SOCS in RGCs12, which may help explain the low responsiveness of axotomized RGCs to CNTF10, 25, 43, 54, 69, and intraocular inflammation amplifies axoto-my-induced SOCS upregulation greatly12, further limiting any possible contribution of CNTF to inflammation-induced regeneration20.
Growth-inhibitory signals in the optic nerve
The mature optic nerve contains many molecules that suppress axon growth, including the myelin-associated inhibitors NogoA, myelin-associated glycoprotein (MAG), and oli-godendrocyte-myelin glycoprotein (OMgp); proteoglycans that accumulate in the scar at the injury site; and additional axon-repellants (e.g., Semaphorins)73–77. Methods that counteract NogoA signaling do not lead to appreciable optic nerve regeneration on their own78. However, expression of a dominant-negative form of the nogo receptor strongly amplifies the axon-promoting effects of intraocular inflammation79. A more comprehensive way to counteract inhibition is by inactivating the small GTPase RhoA, a part of the intracellular pathway through which multiple signals inhibit axon growth. RhoA inhibition results in modest levels of axon regeneration in the injured optic nerve80, 81, but increases the amount of regeneration associated with intraocular inflammation greatly12 (Fig. 1e). Thus, although counteracting inhibitory signals is not sufficient to induce extensive optic nerve regeneration, treatments that simultaneously activate RGCs’ growth state and counteract inhibition can have dramatic effects.
Transforming RGCs into an active growth state itself enables axons to partially overcome inhibitory signals. The scar that forms at the injury site contains basement membrane components82 that are partially degraded by matrix metalloproteinases associated with growing axons83. However, as noted above, multiple other inhibitory signals remain in place, as evidenced by the dramatic effects seen when RhoA activity is suppressed in actively growing axons.
Axon guidance cues during development and regeneration
The initial development of retinal projections involves multiple cues that guide axons through the retina, optic disc, optic nerve, optic chiasm, diencephalon, and midbrain, and enable them to form a precise, topographically organized representation of visual space upon central target areas. The guidance of retinal axons during development involves many types of axon-guidance molecules, including netrins, Semaphorins, laminin, multiple members of the Ephrin/Eph families, Wnts, and Slits84–86. In view of the complex guidance mechanisms involved in the development of retinal projections, it will be important to determine whether the appropriate guidance cues persist in the mature brain to guide regenerating axons back to their correct destinations. There is evidence that at least some guidance cues remain in the mature CNS or become upregulated after optic nerve damage87–89. However, whether these cues will suffice to guide regenerating axons to their proper target areas and to re-form topographically organized maps remains to be determined.
Are we there yet?
In studies using the PN graft model, anterograde tracing and electrophysiology show that a small number of axons can regenerate all the way back to the superior colliculus2, 90. In the case of axon regeneration through the optic nerve, one group reported a re-mapping of the retina upon the SC91, but the accompanying histology raised questions as to whether the axons had been severed in the first place. In view of the scientific and clinical importance of successful regeneration, it will be important to apply strict criteria to proving one’s case, e.g., evidence that connections are forming gradually over time and are not due to spared axons92, and that any electrophysiological changes that are observed correlate with clear anatomical evidence of regeneration. In spite of these issues, the advances that have occurred over the past few years lend encouragement to the possibility that at least some RGCs will be able to regenerate their axons all the way to their central targets. The next challenges will include finding ways to optimize this regeneration and testing whether it restores functionally meaningful levels of vision.
Acknowledgments
We are grateful to the National Eye Institute of the NIH (EY05690 to LB), the Sheldon and Miriam Adelson Medical Research Foundation, and Alseres Pharmaceuticals for support of research from the authors’ laboratory.
Literature cited
- 1.Ramon y Cajal S. Degeneration and Regeneration of the Nervous System. Vol. 5. New York: Oxford University Press; 1991. [Google Scholar]
- 2.Vidal-Sanz M, Bray GM, Villegas-Perez MP, Thanos S, Aguayo AJ. Axonal regeneration and synapse formation in the superior colliculus by retinal ganglion cells in the adult rat. J Neurosci. 1987;7:2894–2909. doi: 10.1523/JNEUROSCI.07-09-02894.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguayo AJ, Rasminsky M, Bray GM, et al. Degenerative and regenerative responses of injured neurons in the central nervous system of adult mammals. Philos Trans R Soc Lond B Biol Sci. 1991;331:337–343. doi: 10.1098/rstb.1991.0025. [DOI] [PubMed] [Google Scholar]
- 4.Carbonetto S, Evans D, Cochard P. Nerve fiber growth in culture on tissue substrata from central and peripheral nervous systems. J Neurosci. 1987;7:610–620. doi: 10.1523/JNEUROSCI.07-02-00610.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savio T, Schwab ME. Rat CNS white matter, but not gray matter, is nonpermissive for neuronal cell adhesion and fiber outgrowth. J Neurosci. 1989;9:1126–1133. doi: 10.1523/JNEUROSCI.09-04-01126.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwab ME, Thoenen H. Dissociated neurons regenerate into sciatic but not optic nerve explants in culture irrespective of neurotrophic factors. J Neurosci. 1985;5:2415–2423. doi: 10.1523/JNEUROSCI.05-09-02415.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry M, Carlile J, Hunter A. Peripheral nerve explants grafted into the vitreous body of the eye promote the regeneration of retinal ganglion cell axons severed in the optic nerve. J Neurocytol. 1996;25:147–170. doi: 10.1007/BF02284793. [DOI] [PubMed] [Google Scholar]
- 8.Lazarov-Spiegler O, Solomon AS, Zeev-Brann AB, Hirschberg DL, Lavie V, Schwartz M. Transplantation of activated macrophages overcomes central nervous system regrowth failure. FASEB Journal. 1996;10:1296–1302. doi: 10.1096/fasebj.10.11.8836043. [DOI] [PubMed] [Google Scholar]
- 9.Lazarov-Spiegler O, Solomon AS, Schwartz M. Peripheral nerve-stimulated macrophages simulate a peripheral nerve-like regenerative response in rat transected optic nerve. Glia. 1998;24:329–337. [PubMed] [Google Scholar]
- 10.Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci. 2000;20:4615–4626. doi: 10.1523/JNEUROSCI.20-12-04615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin Y, Cui Q, Li Y, et al. Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci. 2003;23:2284–2293. doi: 10.1523/JNEUROSCI.23-06-02284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer D, Petkova V, Thanos S, Benowitz LI. Switching mature retinal ganglion cells to a robust growth state in vivo: gene expression and synergy with RhoA inactivation. J Neurosci. 2004;24:8726–8740. doi: 10.1523/JNEUROSCI.2774-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jo S, Wang E, Benowitz LI. CNTF is an endogenous axon regeneration factor for mammalian retinal ganglion cells. Neuroscience. 1999;89:579–591. doi: 10.1016/s0306-4522(98)00546-6. [DOI] [PubMed] [Google Scholar]
- 14.Lorber B, Berry M, Logan A. Different factors promote axonal regeneration of adult rat retinal ganglion cells after lens injury and intravitreal peripheral nerve grafting. J Neurosci Res. 2008;86:894–903. doi: 10.1002/jnr.21545. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Irwin N, Yin Y, Lanser M, Benowitz LI. Axon regeneration in goldfish and rat retinal ganglion cells: differential responsiveness to carbohydrates and cAMP. J Neurosci. 2003;23:7830–7838. doi: 10.1523/JNEUROSCI.23-21-07830.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin Y, Henzl MT, Lorber B, et al. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat Neurosci. 2006;9:843–852. doi: 10.1038/nn1701. [DOI] [PubMed] [Google Scholar]
- 17.Yin Y, Cui Q, Gilbert H, et al. Oncomodulin links inflammation to optic nerve regeneration. Proc Natl Acad Sci U S A. 2009;106:19587–19592. doi: 10.1073/pnas.0907085106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauk TG, Muller A, Lee J, Schwendener R, Fischer D. Neuroprotective and axon growth promoting effects of intraocular inflammation do not depend on oncomodulin or the presence of large numbers of activated macrophages. Exp Neurol. 2008;209:469–482. doi: 10.1016/j.expneurol.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 19.Muller A, Hauk TG, Fischer D. Astrocyte-derived CNTF switches mature RGCs to a regenerative state following inflammatory stimulation. Brain. 2007;130:3308–3320. doi: 10.1093/brain/awm257. [DOI] [PubMed] [Google Scholar]
- 20.Cui Q, Benowitz L, Yin Y. Does CNTF mediate the effect of intraocular inflammation on optic nerve regeneration? Brain. 2008;131(Pt 6):e96. doi: 10.1093/brain/awn027. [DOI] [PubMed] [Google Scholar]
- 21.Fischer D, Pavlidis M, Thanos S. Cataractogenic lens injury prevents traumatic ganglion cell death and promotes axonal regeneration both in vivo and in culture. Invest Ophthalmol Vis Sci. 2000;41:3943–3954. [PubMed] [Google Scholar]
- 22.Irwin N, Li YM, O'Toole JE, Benowitz LI. Mst3b, a purine-sensitive Ste20-like protein kinase, regulates axon outgrowth. Proc Natl Acad Sci U S A. 2006;103:18320–18325. doi: 10.1073/pnas.0605135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorber B, Howe ML, Benowitz LI, Irwin N. Mst3b, an Ste20-like kinase, regulates axon regeneration in the mature CNS and PNS. Nature Neuroscience. 2009;12:1407–1414. doi: 10.1038/nn.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park KK, Liu K, Hu Y, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith PD, Sun F, Park KK, et al. SOCS3 Deletion Promotes Optic Nerve Regeneration In Vivo. Neuron. 2009;64:617–623. doi: 10.1016/j.neuron.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldberg JL, Klassen MP, Hua Y, Barres BA. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002;296:1860–1864. doi: 10.1126/science.1068428. [DOI] [PubMed] [Google Scholar]
- 27.Moore DL, Blackmore MG, Hu Y, et al. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ming GL, Song HJ, Berninger B, Holt CE, Tessier-Lavigne M, Poo MM. cAMP-dependent growth cone guidance by netrin-1. Neuron. 1997;19:1225–1235. doi: 10.1016/s0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- 29.Song H, Ming G, He Z, et al. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- 30.Meyer-Franke A, Wilkinson GA, Kruttgen A, et al. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron. 1998;21:681–693. doi: 10.1016/s0896-6273(00)80586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai D, Deng K, Mellado W, Lee J, Ratan R, Filbin M. Arginase I and Polyamines Act Downstream from Cyclic AMP in Overcoming Inhibition of Axonal Growth MAG and Myelin In Vitro. Neuron. 2002;35:711. doi: 10.1016/s0896-6273(02)00826-7. [DOI] [PubMed] [Google Scholar]
- 32.Park KK, Hu Y, Muhling J, et al. Cytokine-induced SOCS expression is inhibited by cAMP analogue: impact on regeneration in injured retina. Mol Cell Neurosci. 2009;41:313–324. doi: 10.1016/j.mcn.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Deng K, He H, Qiu J, Lorber B, Bryson JB, Filbin MT. Increased synthesis of spermidine as a result of upregulation of arginase I promotes axonal regeneration in culture and in vivo. J Neurosci. 2009;29:9545–9552. doi: 10.1523/JNEUROSCI.1175-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monsul NT, Geisendorfer AR, Han PJ, et al. Intraocular injection of dibutyryl cyclic AMP promotes axon regeneration in rat optic nerve. Exp Neurol. 2004;186:124–133. doi: 10.1016/S0014-4886(03)00311-X. [DOI] [PubMed] [Google Scholar]
- 35.Cui Q, Yip HK, Zhao RC, So KF, Harvey AR. Intraocular elevation of cyclic AMP potentiates ciliary neurotrophic factor-induced regeneration of adult rat retinal ganglion cell axons. Mol Cell Neurosci. 2003;22:49–61. doi: 10.1016/s1044-7431(02)00037-4. [DOI] [PubMed] [Google Scholar]
- 36.Muller A, Hauk TG, Leibinger M, Marienfeld R, Fischer D. Exogenous CNTF stimulates axon regeneration of retinal ganglion cells partially via endogenous CNTF. Mol Cell Neurosci. 2009;41:233–246. doi: 10.1016/j.mcn.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Yin Y, Kurimoto T, Cen L, Gilbert H, Yang Y, Benowitz LI. Program No. 32.11. Neuroscience 2009 Abstracts. Chicago, IL: Society for Neuroscience; 2009. Oncomodulin and cAMP interact in multiple ways to promote optic nerve regeneration. Online. [Google Scholar]
- 38.Villegas-Perez MP, Vidal-Sanz M, Bray GM, Aguayo AJ. Influences of peripheral nerve grafts on the survival and regrowth of axotomized retinal ganglion cells in adult rats. J Neurosci. 1988;8:265–280. doi: 10.1523/JNEUROSCI.08-01-00265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chierzi S, Cenni MC, Maffei L, et al. Protection of retinal ganglion cells and preservation of function after optic nerve lesion in bcl-2 transgenic mice. Vision Res. 1998;38:1537–1543. doi: 10.1016/s0042-6989(97)00332-5. [DOI] [PubMed] [Google Scholar]
- 40.Malik JM, Shevtsova Z, Bahr M, Kugler S. Long-term in vivo inhibition of CNS neurodegeneration by Bcl-XL gene transfer. Mol Ther. 2005;11:373–381. doi: 10.1016/j.ymthe.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Goldberg JL, Espinosa JS, Xu Y, Davidson N, Kovacs GT, Barres BA. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron. 2002;33:689–702. doi: 10.1016/s0896-6273(02)00602-5. [DOI] [PubMed] [Google Scholar]
- 42.Mey J, Thanos S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993;602:304–317. doi: 10.1016/0006-8993(93)90695-j. [DOI] [PubMed] [Google Scholar]
- 43.Weise J, Isenmann S, Klocker N, et al. Adenovirus-mediated expression of ciliary neurotrophic factor (CNTF) rescues axotomized rat retinal ganglion cells but does not support axonal regeneration in vivo. Neurobiol Dis. 2000;7:212–223. doi: 10.1006/nbdi.2000.0285. [DOI] [PubMed] [Google Scholar]
- 44.Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci USA. 1994;91:1632–1636. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Polo A, Aigner LJ, Dunn RJ, Bray GM, Aguayo AJ. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Muller cells temporarily rescues injured retinal ganglion cells. Proc Natl Acad Sci USA. 1998;95:3978–3983. doi: 10.1073/pnas.95.7.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen A, Bray GM, Aguayo AJ. Neurotrophin-4/5 (NT-4/5) increases adult rat retinal ganglion cell survival and neurite outgrowth in vitro. J Neurobiol. 1994;25:953–959. doi: 10.1002/neu.480250805. [DOI] [PubMed] [Google Scholar]
- 47.Peinado-Ramon P, Salvador M, Villegas-Perez MP, Vidal-Sanz M. Effects of axotomy and intraocular administration of NT-4, NT-3, and brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996;37:489–500. [PubMed] [Google Scholar]
- 48.Carmignoto G, Maffei L, Candeo P, Canella R, Comelli C. Effect of NGF on the survival of rat retinal ganglion cells following optic nerve section. J Neurosci. 1989;9:1263–1272. doi: 10.1523/JNEUROSCI.09-04-01263.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kermer P, Klocker N, Labes M, Bahr M. Insulin-like growth factor-I protects axotomized rat retinal ganglion cells from secondary death via PI3-K-dependent Akt phosphorylation and inhibition of caspase-3 In vivo. J Neurosci. 2000;20:2–8. [PubMed] [Google Scholar]
- 50.Frank T, Schlachetzki JC, Goricke B, et al. Both systemic and local application of granulocyte-colony stimulating factor (G-CSF) is neuroprotective after retinal ganglion cell axotomy. BMC Neurosci. 2009;10:49. doi: 10.1186/1471-2202-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koeberle PD, Ball AK. Effects of GDNF on retinal ganglion cell survival following axotomy. Vision Res. 1998;38:1505–1515. doi: 10.1016/s0042-6989(97)00364-7. [DOI] [PubMed] [Google Scholar]
- 52.Yan Q, Wang J, Matheson CR, Urich JL. Glial cell line-derived neurotrophic factor (GDNF) promotes the survival of axotomized retinal ganglion cells in adult rats: comparison to and combination with brain-derived neurotrophic factor (BDNF) J Neurobiol. 1999;38:382–390. doi: 10.1002/(sici)1097-4695(19990215)38:3<382::aid-neu7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 53.Koeberle PD, Ball AK. Neurturin enhances the survival of axotomized retinal ganglion cells in vivo: combined effects with glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor. Neuroscience. 2002;110:555–567. doi: 10.1016/s0306-4522(01)00557-7. [DOI] [PubMed] [Google Scholar]
- 54.Pernet V, Di Polo A. Synergistic action of brain-derived neurotrophic factor and lens injury promotes retinal ganglion cell survival, but leads to optic nerve dystrophy in vivo. Brain. 2006;129:1014–1026. doi: 10.1093/brain/awl015. [DOI] [PubMed] [Google Scholar]
- 55.Kermer P, Klocker N, Labes M, Bahr M. Inhibition of CPP32-like proteases rescues axotomized retinal ganglion cells from secondary cell death in vivo. J Neurosci. 1998;18:4656–4662. doi: 10.1523/JNEUROSCI.18-12-04656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kermer P, Klocker N, Bahr M. Long-term effect of inhibition of ced 3-like caspases on the survival of axotomized retinal ganglion cells in vivo. Exp Neurol. 1999;158:202–205. doi: 10.1006/exnr.1999.7094. [DOI] [PubMed] [Google Scholar]
- 57.Kermer P, Ankerhold R, Klocker N, Krajewski S, Reed JC, Bahr M. Caspase-9: involvement in secondary death of axotomized rat retinal ganglion cells in vivo. Brain Res Mol Brain Res. 2000;85(1–2):144–150. doi: 10.1016/s0169-328x(00)00256-4. [DOI] [PubMed] [Google Scholar]
- 58.Weishaupt JH, Diem R, Kermer P, Krajewski S, Reed JC, Bahr M. Contribution of caspase-8 to apoptosis of axotomized rat retinal ganglion cells in vivo. Neurobiol Dis. 2003;13:124–135. doi: 10.1016/s0969-9961(03)00032-9. [DOI] [PubMed] [Google Scholar]
- 59.Weise J, Isenmann S, Bahr M. Increased expression and activation of poly(ADP-ribose) polymerase (PARP) contribute to retinal ganglion cell death following rat optic nerve transection. Cell Death Differ. 2001;8:801–807. doi: 10.1038/sj.cdd.4400872. [DOI] [PubMed] [Google Scholar]
- 60.Koeberle PD, Ball AK. Nitric oxide synthase inhibition delays axonal degeneration and promotes the survival of axotomized retinal ganglion cells. Exp Neurol. 1999;158:366–381. doi: 10.1006/exnr.1999.7113. [DOI] [PubMed] [Google Scholar]
- 61.Swanson KI, Schlieve CR, Lieven CJ, Levin LA. Neuroprotective effect of sulfhydryl reduction in a rat optic nerve crush model. Invest Ophthalmol Vis Sci. 2005;46:3737–3741. doi: 10.1167/iovs.05-0155. [DOI] [PubMed] [Google Scholar]
- 62.Sanders EJ, Parker E, Harvey S. Retinal ganglion cell survival in development: mechanisms of retinal growth hormone action. Exp Eye Res. 2006;83:1205–1214. doi: 10.1016/j.exer.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 63.Cheung ZH, Leung MC, Yip HK, Wu W, Siu FK, So KF. A neuroprotective herbal mixture inhibits caspase-3-independent apoptosis in retinal ganglion cells. Cell Mol Neurobiol. 2008;28:137–155. doi: 10.1007/s10571-007-9175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fitzgerald M, Payne SC, Bartlett CA, Evill L, Harvey AR, Dunlop SA. Secondary retinal ganglion cell death and the neuroprotective effects of the calcium channel blocker lomerizine. Invest Ophthalmol Vis Sci. 2009;50:5456–5462. doi: 10.1167/iovs.09-3717. [DOI] [PubMed] [Google Scholar]
- 65.Sapieha PS, Peltier M, Rendahl KG, Manning WC, Di Polo A. Fibroblast growth factor-2 gene delivery stimulates axon growth by adult retinal ganglion cells after acute optic nerve injury. Mol Cell Neurosci. 2003;24:656–672. doi: 10.1016/s1044-7431(03)00228-8. [DOI] [PubMed] [Google Scholar]
- 66.Cui Q, Lu Q, So KF, Yip HK. CNTF, not other trophic factors, promotes axonal regeneration of axotomized retinal ganglion cells in adult hamsters. Invest Ophthalmol Vis Sci. 1999;40:760–766. [PubMed] [Google Scholar]
- 67.Logan A, Ahmed Z, Baird A, Gonzalez AM, Berry M. Neurotrophic factor synergy is required for neuronal survival and disinhibited axon regeneration after CNS injury. Brain. 2006;129(Pt 2):490–502. doi: 10.1093/brain/awh706. [DOI] [PubMed] [Google Scholar]
- 68.Leibinger M, Muller A, Andreadaki A, Hauk TG, Kirsch M, Fischer D. Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J Neurosci. 2009;29:14334–14341. doi: 10.1523/JNEUROSCI.2770-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lingor P, Tonges L, Pieper N, et al. ROCK inhibition and CNTF interact on intrinsic signalling pathways and differentially regulate survival and regeneration in retinal ganglion cells. Brain. 2008;131:250–263. doi: 10.1093/brain/awm284. [DOI] [PubMed] [Google Scholar]
- 70.Lorber B, Berry M, Logan A, Tonge D. Effect of lens lesion on neurite outgrowth of retinal ganglion cells in vitro. Mol Cell Neurosci. 2002;21:301–311. doi: 10.1006/mcne.2002.1175. [DOI] [PubMed] [Google Scholar]
- 71.Cui Q, Harvey AR. CNTF promotes the regrowth of retinal ganglion cell axons into murine peripheral nerve grafts. Neuroreport. 2000;11:3999–4002. doi: 10.1097/00001756-200012180-00019. [DOI] [PubMed] [Google Scholar]
- 72.Cen LP, Luo JM, Zhang CW, et al. Chemotactic effect of ciliary neurotrophic factor on macrophages in retinal ganglion cell survival and axonal regeneration. Invest Ophthalmol Vis Sci. 2007;48:4257–4266. doi: 10.1167/iovs.06-0791. [DOI] [PubMed] [Google Scholar]
- 73.Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- 74.Cafferty WB, McGee AW, Strittmatter SM. Axonal growth therapeutics: regeneration or sprouting or plasticity? Trends Neurosci. 2008;31:215–220. doi: 10.1016/j.tins.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rossignol S, Schwab M, Schwartz M, Fehlings MG. Spinal cord injury: time to move? J Neurosci. 2007;27:11782–11792. doi: 10.1523/JNEUROSCI.3444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 77.Goldberg JL, Vargas ME, Wang JT, et al. An oligodendrocyte lineage-specific semaphorin, Sema5A, inhibits axon growth by retinal ganglion cells. J Neurosci. 2004;24:4989–4999. doi: 10.1523/JNEUROSCI.4390-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chierzi S, Strettoi E, Cenni MC, Maffei L. Optic nerve crush: axonal responses in wild-type and bcl-2 transgenic mice. J Neurosci. 1999;19:8367–8376. doi: 10.1523/JNEUROSCI.19-19-08367.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fischer D, He Z, Benowitz LI. Counteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth state. J Neurosci. 2004;24:1646–1651. doi: 10.1523/JNEUROSCI.5119-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lehmann M, Fournier A, Selles-Navarro I, et al. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J Neurosci. 1999;19:7537–7547. doi: 10.1523/JNEUROSCI.19-17-07537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bertrand J, Winton MJ, Rodriguez-Hernandez N, Campenot RB, McKerracher L. Application of Rho antagonist to neuronal cell bodies promotes neurite growth in compartmented cultures and regeneration of retinal ganglion cell axons in the optic nerve of adult rats. J Neurosci. 2005;25:1113–1121. doi: 10.1523/JNEUROSCI.3931-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Battisti WP, Shinar Y, Schwartz M, Levitt P, Murray M. Temporal and spatial patterns of expression of laminin, chondroitin sulphate proteoglycan and HNK-1 immunoreactivity during regeneration in the goldfish optic nerve. J Neurocytol. 1992;21:557–573. doi: 10.1007/BF01187117. [DOI] [PubMed] [Google Scholar]
- 83.Ahmed Z, Dent RG, Leadbeater WE, Smith C, Berry M, Logan A. Matrix metalloproteases: degradation of the inhibitory environment of the transected optic nerve and the scar by regenerating axons. Mol Cell Neurosci. 2005;28:64–78. doi: 10.1016/j.mcn.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 84.Haupt C, Huber AB. How axons see their way--axonal guidance in the visual system. Front Biosci. 2008;13:3136–3149. doi: 10.2741/2915. [DOI] [PubMed] [Google Scholar]
- 85.Erskine L, Herrera E. The retinal ganglion cell axon's journey: insights into molecular mechanisms of axon guidance. Dev Biol. 2007;308:1–14. doi: 10.1016/j.ydbio.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 86.McLaughlin T, O'Leary DD. Molecular gradients and development of retinotopic maps. Annu Rev Neurosci. 2005;28:327–355. doi: 10.1146/annurev.neuro.28.061604.135714. [DOI] [PubMed] [Google Scholar]
- 87.Wizenmann A, Thies E, Klostermann S, Bonhoeffer F, Bahr M. Appearance of target-specific guidance information for regenerating axons after CNS lesions. Neuron. 1993;11:975–983. doi: 10.1016/0896-6273(93)90126-c. [DOI] [PubMed] [Google Scholar]
- 88.Bahr M, Wizenmann A. Retinal ganglion cell axons recognize specific guidance cues present in the deafferented adult rat superior colliculus. J Neurosci. 1996;16:5106–5116. doi: 10.1523/JNEUROSCI.16-16-05106.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wizenmann A, Bahr M. Growth preferences of adult rat retinal ganglion cell axons in retinotectal cocultures. J Neurobiol. 1998;35:379–387. [PubMed] [Google Scholar]
- 90.Keirstead SA, Rasminsky M, Fukuda Y, Carter DA, Aguayo AJ, Vidal-Sanz M. Electrophysiologic responses in hamster superior colliculus evoked by regenerating retinal axons. Science. 1989;246:255–257. doi: 10.1126/science.2799387. [DOI] [PubMed] [Google Scholar]
- 91.Fischer D, Heiduschka P, Thanos S. Lens-injury-stimulated axonal regeneration throughout the optic pathway of adult rats. Exp Neurol. 2001;172:257–272. doi: 10.1006/exnr.2001.7822. [DOI] [PubMed] [Google Scholar]
- 92.Steward O, Zheng B, Tessier-Lavigne M. False resurrections: distinguishing regenerated from spared axons in the injured central nervous system. J Comp Neurol. 2003;459:1–8. doi: 10.1002/cne.10593. [DOI] [PubMed] [Google Scholar]