Abstract
Tamoxifen (TAM) is a selective estrogen-receptor modulator that is widely used in the prevention and treatment of estrogen-receptor–positive breast cancer. Its use has significantly contributed to a decline in breast cancer mortality, since breast cancer patients treated with TAM for 5 years exhibit a 30–50% reduction in both the rate of disease recurrence after 10 years of patient follow-up and in the occurrence of contralateral breast cancer. However, in patients treated with TAM, there is substantial interindividual variability in the development of resistance to TAM therapy and in the incidence of TAM-induced adverse events, including deep-vein thrombosis, hot flashes, and the development of endometrial cancer. Aromatase inhibitors (AIs) have emerged as a viable alternative to TAM, working by inhibiting aromatase activity and blocking estrone/estrodiol biosynthesis in postmenopausal women. The current third-generation AIs, anastrozole, exemestane, and letrozole, were used initially for the treatment of metastatic breast cancer, demonstrating similar or greater benefit but less toxicity, compared with TAM, and are now being employed as adjuvant treatment for early breast cancer in postmenopausal women. This article will focus on the UDP-glucuronosyltransferases, a family of metabolizing enzymes that play an important role in the deactivation and clearance of TAM, anastrazole, and exemestane, and how interindividual differences in these enzymes may play a role in patient response to these agents.
Keywords: Breast cancer, tamoxifen, aromatase inhibitors, exemestane, anastrazole, UDP-glucuronosyltransferase, glucuronidation, metabolism, pharmacogenetics
Introduction: tamoxifen
Adjuvant endocrine therapy reduces the risk of recurrence and improves survival among women with hormone-receptor–positive breast cancer (EBCTCG, 2005). Because most breast cancers, especially those among postmenopausal women, are hormone-receptor positive, hundreds of thousands of women worldwide initiate adjuvant endocrine treatment each year.
Tamoxifen (TAM) is a nonsteroidal antiestrogen that has been commonly used for the treatment and prevention of estrogen-dependent breast cancer (Cuzick et al., 2003; Fisher et al., 1998; Howell et al., 2003; Osborne, 1998). First approved in 1977 by the U.S. Food and Drug Administration (FDA) for the treatment of women with metastatic breast cancer, TAM is currently an established hormonal treatment for all stages of estrogen-receptor (ER)-positive breast cancer. Adjuvant TAM treatment increases recurrence-free survival and overall survival in breast cancer patients with hormone-receptor–positive tumors irrespective of their nodal status, menopausal status, or age (Howell et al., 2003; Osborne, 1998). TAM is also widely used as a chemopreventive agent in women at risk for developing breast cancer (Cuzick et al., 2003; Fisher et al., 1998).
In addition to its antiestrogenic properties, which have been related to menopausal-like symptoms including hot flashes and vaginal bleeding (Osborne, 1998), TAM also exhibits seemingly tissue-dependent partial estrogen-agonistic effects that may be linked to reduced risk for ischemic heart disease and osteoporosis (McDonald and Stewart, 1991; Rutqvist and Mattsson, 1993), but may also increase the risk for endometrial cancer (Rutqvist et al., 1995; van Leeuwen et al., 1994) and venous thromboembolism (Meier and Jick, 1998). Although TAM is generally well tolerated, significant interindividual variability has been observed in the clinical efficacy as well as toxicity, of TAM. For instance, about 30% of patients acquire TAM resistance and relapse (EBCTCG, 1998). In addition, the relative risk of endometrial cancers in patients treated with TAM is estimated to be 2- to 3-fold that of controls, and the risk increases with both the duration and cumulative dose of TAM treatment (Bergman et al., 2000; Bernstein et al., 1999; Fisher et al., 1994; Rutqvist and Mattsson, 1993).
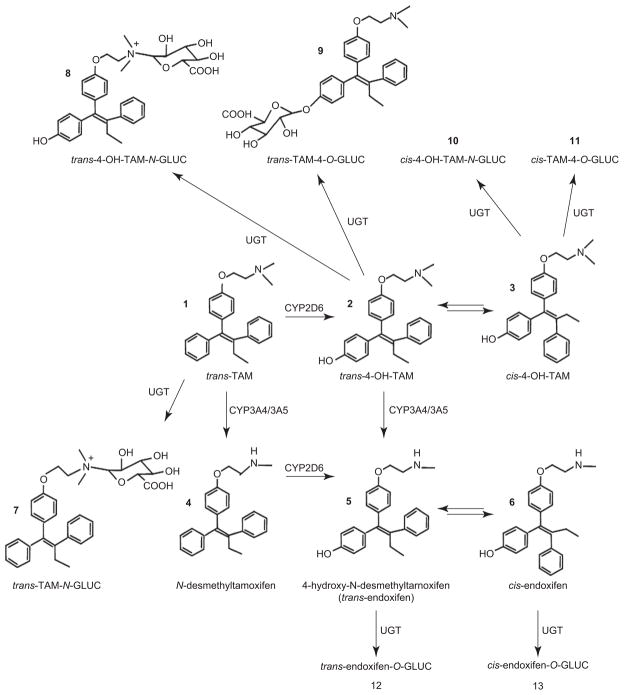
TAM acts by binding to the ER, thereby competitively inhibiting the binding of estrogen in breast tissue (Furr and Jordan, 1984). While TAM exists in both a trans and a cis configuration, the trans-isomer of TAM is the pharmaceutically manufactured form of TAM used in the treatment and prevention of breast cancer. TAM is metabolized via cytochrome P450 (CYP450)-mediated pathways into several metabolites after oral administration, including the metabolites, N-desmethylTAM (DMT), 4-hydroxyTAM (4-OH-TAM), α-hydroxyTAM (α-OH-TAM), TAM-N-oxide, N, N-didesmethylTAM (DDMT), and 4-OH-N-desmethylTAM (endoxifen; see Figure 1). CYP3A4 has been shown to be the main CYP450 enzyme involved in the metabolism of TAM to α-OH-TAM (Boocock et al., 2002; Kim et al., 2003) and to DMT (Desta et al., 2004; Jacolot et al., 1991), although CYPs 2D6, 1A1, 1A2, 1B1, 2C9, and 2C19 may also play a role in DMT formation (Crewe et al., 2002; Jacolot et al., 1991). CYP2D6 appears to be the major CYP involved in the hydroxylation of trans-TAM to trans-4-OH-TAM (Coller, 2003; Coller et al., 2002; Crewe et al., 1997, 2002; Dehal and Kupfer, 1997; Desta et al., 2004; Hu et al., 2003) and DMT to endoxifen (Desta et al., 2004). Endoxifen formation was observed to be highest in activity assays of recombinant CYP2D6 enzyme incubated with trans-4-OH-TAM, although CYP3A4 activity also correlates well with endoxifen formation in human liver microsomes (HLMs) (Desta et al., 2004).
Figure 1.
Schematic of TAM metabolism.
DMT has been established as the primary metabolite of TAM, as determined by in vitro assays performed with HLM (Desta et al., 2004) and by in vivo studies that have shown levels of steady-state plasma DMT to be greater than 70 times the levels of 4-OH-TAM in the serum (Lonning et al., 1992; Stearns et al., 2003). Steady-state plasma levels of endoxifen are ~6-fold the levels observed for 4-OH-TAM in TAM-treated subjects (Desta et al., 2004; Jin et al., 2005; Stearns et al., 2003). However, the major therapeutic contributors are hypothesized to be endoxifen and 4-OH-TAM. This is based on evidence that indicates that they exhibit up to 100-fold the levels of antiestrogenic activity as compared to TAM and other TAM metabolites (Furr and Jordan, 1984; Johnson et al., 2004; Jordan et al., 1977; Katzenellenbogen et al., 1984;L. Lim et al., 2004;Y. C. Lim et al., 2005; Stearns et al., 2003), exhibit the same relative levels of antiestrogenic activity (Johnson et al., 2004; Stearns et al., 2003), inhibit expression of β-estradiol-induced ER-dependent target genes (Johnson et al., 2004;Y. C. Lim et al., 2005), inhibit global estrogen-dependent gene expression (L. Lim et al., 2004), and exhibit high affinity for the ER (α and β) in RBA assays (Johnson et al., 2004).
Non-CYP450-mediated conjugation pathways also appear to be highly important in terms of TAM’s overall metabolism and activity profile. While the hydroxysteroid sulfotransferase, SULT1A2, is involved in sulfation of α-OH-TAM (Apak and Duffel, 2004), this enzyme does not exhibit activity against either the trans- or cis-isomers of 4-OH-TAM (Chen et al., 2002; Nishiyama et al., 2002). The phenol sulfotransferase, SULT1A1, appears to be the major sulfotransferase involved in the conjugation of both trans- and cis-4-OH-TAM in humans (Falany, 1997; Nishiyama et al., 2002).
Perhaps the most important route of elimination of TAM and its metabolites is via glucuronidation by the uridine diphosphate (UDP)-glucuronosyltranferases (UGTs). TAM is excreted predominantly through the bile, a process largely facilitated by TAM conjugation to glucuronic acid during the glucuronidation process (Lien et al., 1989), and TAM glucuronides have been identified in the urine and serum of TAM-treated patients (Lien et al., 1988, 1989; Poon et al., 1993). Most of the 4-OH-TAM and endoxifen found in the bile of TAM-treated patients was as a glucuronide conjugate (Lien et al., 1988, 1989) and TAM, 4-OH-TAM, and endoxifen are glucuronidated with very high activity by HLM (Sun et al., 2007). Although TAM metabolites are often found in their unconjugated form in feces, this is likely due to β-glucuronidase–catalyzed removal of glucuronic acid within the microflora of the small intestine (Lien et al., 1989).
Large interindividual variability in endoxifen plasma concentrations in women taking TAM have been observed and can be explained, in part, by CYP2D6 genotype (Jin et al., 2005; Stearns et al., 2003). Recent evidence demonstrates that the CYP2D6*4 deletion allele has been associated with decreased time until breast cancer recurrence, relapse-free survival, disease-free survival, and overall survival in patients treated with TAM (Borges et al., 2006; Goetz et al., 2005, 2007, 2008). In addition, variant alleles that result in low activity/expression in CYPs 2D6, 2B6, and 2C9 were correlated with levels of trans-4-OH-TAM formation in HLM from individual subjects (Coller et al., 2002). These data suggest that the levels of circulating active TAM metabolites may differ between individuals, based upon metabolizing enzyme genotype.
TAM glucuronidation
Microsomes from human liver specimens exhibit high glucuronidating activities toward TAM to form TAM-N+-glucuronide, and 4-OH-TAM to form 4-OH-TAM-N+-glucuronide and 4-OH-TAM-O-glucuronide (Kaku et al., 2004; Nishiyama et al., 2002; Sun et al., 2006). Both isomers of endoxifen are O-glucuronidated; however, unlike 4-OH-TAM, no N-glucuronidation of endoxifen isomers was detected in assays for either HLM or individually overexpressed UGTs (Sun et al., 2007), suggesting that the demethylation of the electrophilic amine on the 4-OH-TAM side chain to form endoxifen results in a lack of N-glucuronidation by UGTs.
One of the major UGTs involved in the glucuronidation of TAM and its metabolites is the hepatic enzyme, UGT1A4 (Kaku et al., 2004; Nishiyama et al., 2002; Sun et al., 2006), which catalyzes the formation of a quarternary ammonium-linked glucuronide with TAM’s and 4-OH-TAM’s N, N-dimethylaminoalkyl side chain (Kaku et al., 2004; Sun et al., 2006). This pattern of ammonium-linked glucuronidation is consistent with UGT1A4’s glucuronidation activity against primary, secondary, and tertiary amines present in a variety of carcinogenic compounds, androgens, progestins, and plant steroids (Breyer-Pfaff et al., 2000; Green and Tephly, 1996, 1998; Wiener et al., 2004a). In addition to UGT1A4, UGTs 1A1, 1A3, 1A8, 1A9, 2B7, and 2B15 overexpressing baculosomes exhibited detectable activity against 4-OH-TAM (Kaku et al., 2004). In a comprehensive characterization and kinetic analysis of the glucuronidating enzymes responsible for O-glucuronidation of TAM metabolites (Sun et al., 2007), UGTs 2B7≃1A8>1A10 exhibited the highest overall activity against trans-4-OH-TAM as determined by Vmax/Km, with the hepatic enzyme, UGT2B7, exhibiting the highest binding affinity and lowest Km (3.7 μM; Table 1). UGTs 1A10≃1A8>UGT2B7 exhibited the highest overall glucuronidating activities as determined by Vmax/Km for trans-endoxifen, with the extrahepatic enzyme, UGT1A10, exhibiting the highest binding affinity and lowest Km (39.9 μM), but with UGT2B7 again demonstrating the highest activity of the hepatic UGTs. These data suggest that several UGTs, including UGTs 1A10, 2B7, and 1A8, could play an important role in the metabolism of 4-OH-TAM and endoxifen. Interestingly, while UGT1A4 is active against TAM and 4-OH-TAM, no activity was observed for UGT1A4-overexpressing cell homogenates against endoxifen isomers, a pattern that is consistent with the lack of N-glucuronidation observed for endoxifen in HLM (Sun et al., 2007). Also, this pattern of UGT1A4 substrate selectivity is consistent with UGT1A4 activity toward the tertiary amine, imipramine, but not its N-desmethyl metabolite, desipramine (Green and Tephly, 1998).
Table 1.
Kinetic analyses of O-glucuronidation of trans-4-OH-TAM or trans-endoxifen by UGTs.a
| UGT variant |
trans-4-OH-TAM |
trans-endoxifen |
||||
|---|---|---|---|---|---|---|
| Vmax(pmol·min−1·μg−1)b | Km(μM) | Vmax/Km(μL·min−1·μg−1)b | Vmax(pmol·min−1·μg−1)b | Km(μM) | Vmax/Km(μL·min−1·μg−1)b | |
| 1A1 | 3.4 ± 0.2 | 124 ± 16 | 0.028 ± 0.004 | 2.3 ± 0.3 | 333 ± 60 | 0.007 ± 0.0005 |
| 1A3 | 1.9 ± 0.3 | 94 ± 18 | 0.02 ± 0.001 | 2.9 ± 0.4 | 158 ± 29 | 0.02 ± 0.001 |
| 1A7 | 1.2 ± 0.2 | 166 ± 27 | 0.0074 ± 0.0002 | Low activityc | ||
| 1A8 | 3.2 ± 0.2 | 23 ± 2.4 | 0.14 ± 0.02 | 12 ± 1.4 | 101 ± 13 | 0.12 ± 0.01 |
| 1A9 | 3.0 ± 0.1 | 319 ± 38 | 0.009 ± 0.001 | Low activityc | ||
| 1A10 | 4.7 ± 0.3 | 96 ± 8.0 | 0.049 ± 0.006 | 5.7 ± 0.7 | 40 ± 3.0 | 0.14 ± 0.005 |
| 2B7 | 0.55 ± 0.18 | 3.7 ± 0.6 | 0.15 ± 0.03 | 3.0 ± 0.4 | 101 ± 17 | 0.03 ± 0.004 |
| 2B17 | 0.02 ± 0.001 | 41 ± 6 | 0.001 ± 0.0001 | No detectable activityd | ||
All data are the mean ± standard deviation, based on three independent experiments.
Data are expressed per μg UGT protein, as determined by Western blot analysis.
Low activity describes the fact that although some glucuronidation activity was observed for a UGT enzyme against a particular TAM metabolite, the level of detection was below sensitivity for kinetic studies.
In addition to no detectable glucuronidation activity observed for homogenates of UGT2B17-overexpressing cells observed against trans-endoxifen, homogenates from cells overexpressing UGTs 1A6, 2B4, 2B10, 2B11, or 2B15 exhibited no detectable glucuronidating activity against trans-4-OH-TAM or trans-endoxifen.
While high antiestrogenic activity has been reported for both 4-OH-TAM and endoxifen, studies examining the effects of glucuronide conjugation of these metabolites were only recently described (Y. Zheng et al., 2007). E2-mediated induction of the gene encoding the progesterone receptor (PGR) was determined in MCF-7 cells by real-time reverse-transcriptase polymerase chain reaction (RT-PCR) for individual TAM metabolites and isomers. While E2 (1 × 10−10 M) induction of PGR mRNA was 6-fold after a 12-hour incubation, unconjugated TAM metabolites (i.e., cis- and trans-isomers of 4-OH-TAM and endoxifen) inhibited this effect. A similar dose-dependent inhibition of E2-induced PGR gene expression was found for both the trans- and cis-isomers of 4-OH-TAM and endoxifen, with maximal inhibition attained at 1 × 10−6 M of TAM metabolite. In contrast, the glucuronide conjugates of all 4-OH-TAM and endoxifen isomers exhibited no effect on E2-mediated induction of PGR expression at all concentrations of TAM metabolite conjugates examined in this study. These data indicate that isomers of both 4-OH-TAM and endoxifen exhibit roughly equipotent antiestrogenic effects on E2-induced gene expression, and that glucuronide conjugates of the same metabolites effectively negate this activity.
More recent studies have focused on ER-binding activities of TAM metabolites (Lazarus et al., 2009). Similar to that observed for PGR induction, the trans-isomers of both 4-OH-TAM and endoxifen exhibit similar relative binding activities (RBA), as compared to E2, while their glucuronide counterparts exhibited 57–130-fold decreases in RBA as compared to their unconjugated counterparts. While a similar pattern was also observed for cis TAM metabolite isomers and their glucuronides, the RBA of these unconjugated cis-isomers were 35–67-fold lower than their trans-unconjugated counterparts. These data were similar to that observed previously for 4-OH-TAM and endoxifen (Johnson et al., 2004), but was the first assessment of individual isomers and glucuronide conjugates. The trans-4-OH-TAM and trans-endoxifen isomers exhibited 30–37-fold higher RBA than trans-TAM in this study. Overall, these differences suggest that the trans-isomers of 4-OH-TAM and endoxifen are the major active antiestrogenic metabolites of TAM, that glucuronides of TAM metabolites are relatively inactive, and that cis-isomers may be inhibiting E2-induced activities by mechanisms other than competitive binding to the ER. This could have important implications in how metabolic pathways are targeted in terms of augmenting the therapeutic efficacy of TAM.
Effect of UGT polymorphisms on TAM glucuronidation activities in UGT-overexpressing cell lines
Missense polymorphisms have been identified in the UGTs active against TAM metabolites, including nonsynonymous SNPs at codons 24 and 48 of the UGT1A4 gene (Wiener et al., 2004b), at codon 268 of the UGT2B7 gene (Coffman et al., 1998), at codon 139 SNP in the UGT1A10 gene that is prevalent in African Americans (Blevins-Primeau et al., 2009; Elahi et al., 2003), and at codons 173 and 277 of the UGT1A8 gene (Huang et al., 2002). To determine whether any of these SNPs result in differential activities against the trans-isomers of 4-OH-TAM or endoxifen, in vitro kinetic analysis of HEK293 cells overexpressing the wild-type or variant isoforms of each of these four UGT enzymes was performed (Blevins-Primeau et al., 2009; Sun et al., 2006). The UGT1A8173Gly/277Cys variant exhibited no difference in overall glucuronidation activity (Vmax/Km) against trans-4-OH-TAM and exhibited a small (1.25-fold), but significant (P < 0.05), decrease in overall activity (manifested primarily by a higher Km) against trans-endoxifen, as compared to wild-type UGT1A8173Ala/277Cys (Table 2). In contrast, the UGT1A8173Ala/277Tyr variant exhibited no detectable glucuronidation against the trans-isomers of either 4-OH-TAM or endoxifen.
Table 2.
Kinetic analyses of O-glucuronidation of the trans-isomers of 4-OH-TAM and endoxifen by UGT variants.a
| UGT variant |
trans-4-OH-TAM |
trans-endoxifen |
||||
|---|---|---|---|---|---|---|
| Vmax(pmol·min−1·μg−1)b | Km(μM) | Vmax/Km(μL·min−1·μg−1)b | Vmax(pmol·min−1·μg−1)b | Km(μM) | Vmax/Km(μL·min−1·μg−1)b | |
| UGT1A8173Ala/277Cys | 2.3 ± 0.1 | 23 ± 2 | 0.10 ± 0.02 | 5.4 ± 0.2 | 98 ± 9 | 0.06 ± 0.004 |
| UGT1A8173Gly/277Cys | 5.4 ± 0.2** | 43 ± 7** | 0.13 ± 0.03 | 5.9 ± 0.4 | 135 ± 26 | 0.04 ± 0.005* |
| UGT1A8173Ala/277Tyr | No detectable activity | No detectable activity | ||||
| UGT1A424Pro/48Leu | 62 ± 5.8 | 2.2 ± 0.4 | 29 ± 2.7 | No detectable activity | ||
| UGT1A424 r/48Leu | 55 ± 11 | 1.6 ± 0.1 | 33 ± 4.9 | No detectable activity | ||
| UGT1A424Pro/48Val | 49 ± 2.8 | 1.2 ± 0.1b | 41 ± 1.4c | No detectable activity | ||
| UGT1A10139Glu | 4.7 ± 0.3 | 96 ± 8 | 0.05 ± 0.006 | 5.7 ± 0.7 | 40 ± 3 | 0.14 ± 0.005 |
| UGT1A10139Lys | 2.1 ± 0.2** | 52 ± 6** | 0.04 ± 0.006 | 1.9 ± 0.2** | 13 ± 2** | 0.14 ± 0.004 |
| UGT2B7268His | 0.55 ± 0.18 | 3.7 ± 0.6 | 0.15 ± 0.03 | 3.0 ± 0.44 | 101 ± 17 | 0.03 ± 0.004 |
| UGT2B7268Tyr | 0.54 ± 0.09* | 8.7 ± 0.8** | 0.062 ± 0.01** | 0.55 ± 0.01** | 101 ± 15 | 0.006 ± 0.001** |
All data are the mean ± standard deviation, based on three independent experiments. Homogenates from cells overexpressing UGT1A8173Ala/277Tyr exhibited no detectable activity against trans-4-OH-TAM and trans-endoxifen. Homogenates from cells overexpressing any of the UGT1A4 variants exhibited no detectable activity against trans-endoxifen.
Data are expressed per μg UGT protein, as determined by Western blot analysis.
P ≤ 0.05;
P < 0.01.
For UGT1A4, kinetic analysis demonstrated that higher N-glucuronidation activities were observed for UGT1A424Pro/48Val-overexpressing cell microsomes as compared to microsomes from wild-type UGT1A424Pro/48Leu-overexpressing cells against trans-4-OH-TAM, with a significantly (P ≤0.02) lower Km observed for trans-4-OH-TAM for the UGT1A424Pro/48Val variant (Table 2). No significant effect on enzyme kinetics was observed for the UGT1A424Thr/48Leu variant against trans-4-OH-TAM. In addition, no difference in overall glucuronidation activity was observed for the UGT1A10139Lys variant versus wild-type UGT1A10 against the trans-isomers of both 4-OH-TAM and endoxifen (Table 2).
For UGT2B7, kinetic analysis demonstrated that significantly higher glucuronidation activities were observed for the wild-type UGT2B7268His, as compared to the UGT2B7268Tyr variant, against the trans-isomers of both 4-OH-TAM (P < 0.05) and endoxifen (P < 0.01; Table 2). This was manifested by a higher Km (2.4-fold) and a lower Vmax/Km (2.4-fold) for 4-OH-TAM, as well as a lower Vmax (5.5-fold) and lower Vmax/Km (5.0-fold) for endoxifen by the UGT2B7268Tyr variant.
UGT genotypes and TAM glucuronidation phenotype in HLM
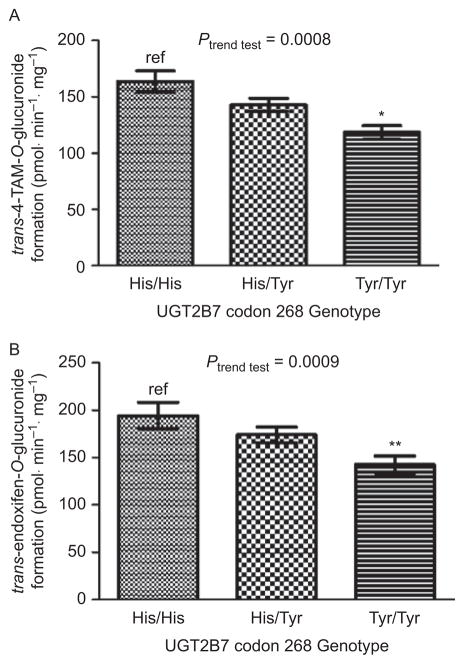
To determine the rate of O- and N-glucuronidation of trans-4-OH-TAM and trans-endoxifen, glucuronidation assays were performed for a series of HLM and analyzed by ultrapressure liquid chromatography (Blevins-Primeau et al., 2009). The mean rate of formation of TAM-4-O-glucuronide, 4-OH-TAM-N+-glucuronide, and endoxifen-O-glucuronide in 111 HLM specimens was 141 ± 45, 175 ± 52, and 168 ± 66 pmol·min−1·mg−1, respectively. A 4.5-, 10- and 17-fold range in glucuronide formation was observed for TAM-4-O-glucuronide, 4-OH-TAM-N+-glucuronide, and endoxifen-O-glucuronide, respectively. The range of the ratio of TAM-4-O-glucuronide:4-OH-TAM-N+-glucuronide in the HLM samples was 8.0-fold. These data suggest that significant differences in glucuronidation capacity exist between individual HLM against TAM metabolites. After stratifying by UGT2B7 codon 268 genotype, there was a near-significant (P = 0.059) 13% decrease in TAM-4-O-glucuronide formation in HLM with the UGT2B7 (His268Tyr) genotype and a significant (P < 0.001) 28% decrease in TAM-4-O-glucuronide formation in HLM with the UGT2B7 (Tyr268Tyr) genotype, as compared to HLM with the UGT2B7 (His269His) genotype (Figure 2, panel A). A significant (P = 0.01) 17% decrease in TAM-4-O-glucuronide formation was observed in HLM with the UGT2B7 His268Tyr genotype versus HLM with the UGT2B7 (Tyr268Tyr) genotype. A significant trend of decreasing O-glucuronidation of trans-4-OH-TAM was observed in HLM with increasing numbers of the UGT2B7268Tyr allele (P < 0.001).
Figure 2.
Analysis of glucuronidation activities against trans-4-OH-TAM and trans-endoxifen in HLM stratified by UGT2B7 genotypes. Glucuronidation assays were performed and 4-OH-TAM and endoxifen-glucuronides separated by ultrapressure liquid chromatography. (A) trans-4-OH-TAM and UGT2B7 codon 268 genotypes. (B) trans-endoxifen and UGT2B7 codon 268 genotypes. Comparative analysis was performed by using HLM from subjects with the homozygous wild-type UGT2B7268His genotype as the referent. *P < 0.001; **P < 0.002; error bars represent standard error.
Similar to that observed for trans-4-OH-TAM, a significant (P = 0.002) 27% decrease in O-glucuronidation of trans-endoxifen was observed in HLM with the UGT2B7 (Tyr268Tyr) genotype, as compared to HLM with the UGT2B7 (His268His) genotype (Figure 2, panel B). A significant trend of decreasing O-glucuronidation of trans-endoxifen was observed in HLM with increasing numbers of the UGT2B7268Tyr allele (P = 0.009). No N-glucuronidation of endoxifen was observed for any of the HLM specimens analyzed in these studies.
Aromatase inhibitors (AIs)
Aromatase, a product of the CYP19 gene, is a CYP450 enzyme complex that catalyzes the last step in several reactions for estrogen biosynthesis. Aromatase is found in many human tissues, including the ovaries (Means et al., 1991), testes (Tsai-Morris et al., 1985), adipose tissue (Bulun and Simpson, 1994), placenta (Means et al., 1991), brain (Roselli et al., 1985), muscle (Matsumine et al., 1986), skin fibroblasts (Berkovitz et al., 1987), and osteoblasts of bone (Shozu and Simpson, 1998) and facilitates the conversion of androstenedione and testosterone via three hydroxylation steps to estrone and estradiol. This conversion increases as a function of age, obesity, and aromatase activity in adipose tissue and is the major source of estrogen in postmenopausal women (Nelson and Bulun, 2001). In the past decade, a number of AIs have been developed as an alternate approach to TAM for the treatment of estrogen-receptor–positive breast cancer. The current third-generation of AIs, anastrozole, exemestane, and letrozole, are highly specific for the aromatase enzyme, with fewer side effects than previous generations of AIs and are sufficiently long acting to be administered on a daily basis. Evidence from several clinical trials indicates that AIs may be superior to TAM as first-line therapy for postmenopausal women with metastatic breast cancer (Ferretti et al., 2006). Results from at least eight major clinical trials indicate that AIs are associated with longer disease-free survival than therapy with TAM alone (Eisen et al., 2008), and this supports the use of AIs as first-line therapy or as second-line therapy after treatment with TAM. The fact that the incidence of contralateral breast cancer was significantly reduced in most of these trials is also supportive of a potential role for AIs in cancer prevention.
Serious adverse events were shown in the ATAC trial to occur more frequently with TAM than with anastrozole (Buzdar et al., 2006; Forbes et al., 2008). However, TAM was associated with reduced cholesterol, hypercholesterolemia, and other lipid metabolism disorders, as compared to AIs (Boccardo et al., 2005; Thurlimann et al., 2005). In addition, patients receiving exemestane had increased LDL levels, but lower or unchanged triglycerides, while those receiving TAM had decreased LDL levels, but higher triglycerides (Hozumi et al., 2006; Markopoulos et al., 2005). Perhaps the major toxicities associated with AIs are joint pain and bone loss, the propensity for bone fractures, and increased risk for osteoporosis (Coates et al., 2007; Coleman et al., 2007; Coombes et al., 2007; Howell et al., 2005; Howell and Group, 2006), a pattern consistent with the significantly lower hip and lumbar spine bone mineral density observed in patients on AIs (Perez et al., 2006). While not as severe as TAM, other toxicities reported for AIs include a variety of gynecological events, including vaginal bleeding or discharge and hot flashes. Variability in changes to lipid profiles, manifestation of osteoporosis, and time of recurrence were observed in patients in many of the different clinical trials (Eisen et al., 2008), and the mechanism underlying this variability in response to AIs and to their toxicities remains unclear.
AI metabolism
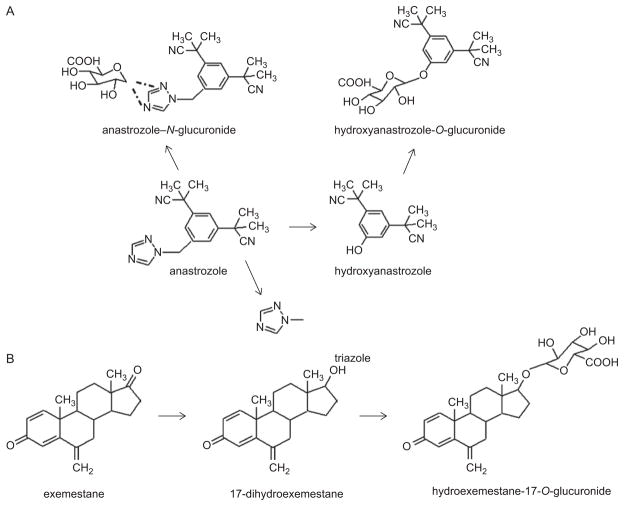
Limited studies have been performed examining AI metabolism. Anastrozole is eliminated mainly through N-dealkylation, hydroxylation via the CYP3A4 enzyme (Antoniou and Tseng, 2005; Dowsett et al., 2001), and glucuronidation via the UGT family of enzymes (Mareck et al., 2006). The major metabolites of anastrozole identified in urine and plasma are triazole, OH-anastrozole, the glucuronide of OH-anastrozole, and the N-glucuronide of anastrozole (Figure 3, panel A) (AstraZeneca, 2006). While triazole is the major circulating metabolite of anastrozole in serum of anastrozole-treated patients (AstraZeneca, 2006), 40% of excreted anastrozole is as a glucuronide. Triazole lacks pharmacologic activity (AstraZeneca, 2006); while little is known about the pharmacologic activity of OH-anastrozole, it is likely that the removal of the triazole moiety results in an inactivation of the hydroxyanastrozole derivative. Anastrozole can be directly glucuronidated to form anastrozole-N+-glucuronide in HLM (Lazarus and Sun, unpublished results). In a recent screening of cell lines individually overexpressing each of the known human UGT1A and 2B enzymes (except UGTs 1A5 and 2B28), only UGT1A4 exhibited glucuronidating activity against anastrozole, with an apparent Km of 637 ± 40 μM and a Vmax/Km of 5.6 ± 0.6 nl·min−1·μg−1 (Lazarus and Sun, unpublished results).
Figure 3.
Schematic of (A) anastrozole and (B) exemestane metabolism. Dashed lines between anastrozole-N-glucuronide and anastrozole reflect the potential binding of the glucuronide moiety to either amine group on the triazole ring.
For exemestane, studies of human liver preparations suggest that cytochrome CYP3A4 is the principal enzyme involved in exemestane oxidation (Pfizer, 2007; Anonymous, 2000), but reduction of the 17-keto group to form 17-dihydroexemestane is the major pathway for exemestane metabolism (Figure 3, panel B) (Pfizer, 2007). Unchanged exemestane is less than 1% in urine and less than 10% in plasma of the total exemestane dose (Pfizer, 2007). Urinary and fecal excretion of exemestane was similar, both around 42%, with the glucuronide of 17-dihydroexemestane (at the 17-O-position) a major metabolite found in urine (Pfizer, 2007). The glucuronidation pathway of excretion of exemestane is important, because 17-di-hydroexemestane was reported to exhibit significant antiaromatase activity in vitro (Buzzetti et al., 1993). Recent studies have shown that several UGTs exhibit activity against 17-dihydroexemestane (Table 3), with UGT2B17>UGT1A10>UGT1A8≃UGT1A4 (Lazarus and Sun, submitted). UGT2B17 exhibited the highest binding affinity against 17-dihydroexemestane, as reflected by the lowest Km (14.5 ± 2.7 μM), which is 8.5-fold lower than that of UGT1A10, and the highest overall activity, as reflected by the highest Vmax/Km (137 nl·min−1·mg−1), which is 14-fold higher than that of UGT1A8.
Table 3.
Kinetic analyses of O-glucuronidation of 17-dihydroexemestane by human UGTs.a
| UGT | Vmax(pmol·min−1·μg−1)b | Km(μM) | Vmax/Km(nl·min−1·μg−1)b |
|---|---|---|---|
| UGT1A1 | No detectable activity | ||
| UGT1A3 | No detectable activity | ||
| UGT1A4 | 0.27 ± 0.01 | 34 ± 3.9 | 8.1 ± 0.9 |
| UGT1A5 | No detectable activity | ||
| UGT1A6 | No detectable activity | ||
| UGT1A7 | No detectable activity | ||
| UGT1A8 | 0.30 ± 0.06 | 14 ± 3.9 | 22 ± 2.1 |
| UGT1A9 | No detectable activity | ||
| UGT1A10 | 12 ± 1.8 | 124 ± 15 | 100 ± 7.9 |
| UGT2B4 | No detectable activity | ||
| UGT2B7 | No detectable activity | ||
| UGT2B10 | No detectable activity | ||
| UGT2B11 | No detectable activity | ||
| UGT2B15 | No detectable activity | ||
| UGT2B17 | 2.0 ± 0.25 | 14 ± 2.7 | 137 ± 17 |
All data are the mean ± standard deviation, based on three independent experiments.
Data are expressed per μg UGT protein, as determined by Western blot analysis.
CYP3A4 was shown to metabolize letrozole to the carbinol metabolite (2° alcohol metabolite), while CYP2A6 formed this metabolite and its ketone analog (FDA, 2004). However, while glucuronidation of the alcohol metabolite was found to be the predominant species in urine (Sioufi et al., 1997), this conjugation is of inactive metabolites.
Effect of UGT polymorphisms on anastrozole and exemestane glucuronidation activities
Recent kinetic studies demonstrate that while UGT1A424Pro/48Val-overexpressing homogenates exhibited a significantly (P < 0.01) higher Km (~1.6-fold), against anastrozole as compared with the wild-type UGT1A424Pro/48Leu isoform, no differences in overall glucuronidation activity (Vmax/Km) was observed (Table 4; Lazarus and Sun, unpublished results). Similarly, no difference in anastrozole glucuronidation kinetics was observed for the UGT1A424Thr/48Leu variant, as compared to wild-type UGT1A4. This suggests that genetic variations in the hepatic UGT1A4 may not be playing a large role in variability in response to anastrozole.
Table 4.
Kinetic analyses of O-glucuronidation of anastrozole by UGT1A4 variants.a
| UGT | Vmax(pmol·min−1·μg−1)b | Km (μM) | Vmax/Km(nL·min−1·μg−1)b |
|---|---|---|---|
| UGT1A424Pro/48Leu | 3.6 ± 0.58 | 637 ± 40 | 5.6 ± 0.58 |
| UGT1A424Thr/48Leu | 3.2 ± 0.46 | 802 ± 137 | 4.1 ± 1.0 |
| UGT1A424Pro/48Val | 5.0 ± 1.3 | 1045 ± 131* | 4.8 ± 0.88 |
All data are the mean ± standard deviation, based on three independent experiments. Homogenates from cells overexpressing UGT1A8173Ala/277Tyr exhibited no detectable activity against trans-4-OH-TAM and trans-endoxifen. Homogenates from cells overexpressing any of the UGT1A4 variants exhibited no detectable activity against trans-endoxifen.
Data are expressed per μg UGT protein, as determined by Western blot analysis.
P < 0.01.
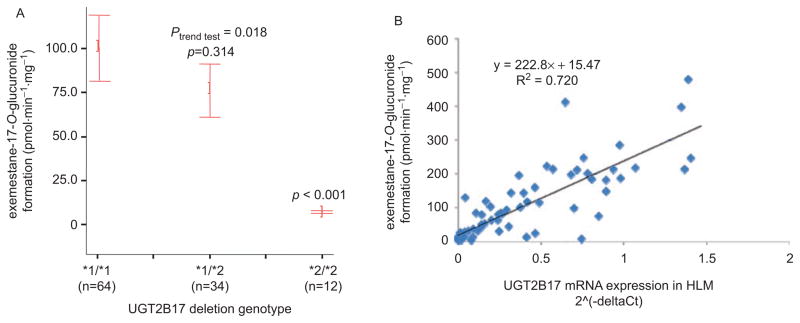
Previous studies identified a prevalent polymorphic whole-gene deletion for the UGT2B17 gene (Wilson et al., 2004). Similar to that described above for TAM glucuronidation and UGT2B7 genotype, a potential association between the UGT2B17 deletion and liver 17-dihydroexemestane glucuronidation activity was assessed in a panel of 110 HLMs. As shown in Figure 4 (panel A), there was a significant (P < 0.001) 14-fold decrease in glucuronidation activity against 17-dihy-droexemestane in HLM from subjects exhibiting the homozygous UGT2B17 deletion (*2/*2) genotype, as compared with HLM from subjects wild type (*1/*1) for UGT2B17 (Lazarus and Sun, submitted). A significant (R2 = 0.72) correlation was observed between hydroexemestane-17-O-glucuronide formation in HLM and UGT2B17 expression in the same HLM (Figure 4, panel B). In addition, the average Km for two HLMs against 17-dihydroexemestane from subjects wild type for UGT2B17 was 9 μM (Table 5), which is similar to the apparent Km for UGT2B17 against 17--dihydroexemestane in vitro (14.5 μM; see Table 3). HLM from subjects exhibiting the UGT2B17 (*2/*2) genotype exhibited an average 2.3-fold higher Km and an average 12-fold lower Vmax/KM than HLM from subjects with the UGT2B17 */*1) genotype. Together, these data suggest that the UGT2B17 deletion may significantly alter in vivo glucuronidation of a major active exemestane metabolite.
Figure 4.
HLM 17-dihydroexemestane glucuronidation activity and UGT2B17 expression stratified by UGT2B17 genotypes. (A) Exemestane-17-O-glucuronide formation versus UGT2B17 genotype in HLM. Glucuronidation assays were performed by using 9.4 μM 17-dihydroexemestane, and hydroexemestane-17-O-glucuronide was separated from parent 17-dihydroexemestane by ultraperformance liquid chromatography. The *1 and *2 alleles refer to the UGT2B17 wild-type and deleted alleles, respectively. Actual hydroexemestane-17-O-glucuronide rates are 100.2, 76.1, and 7.0 pmol·min−1.mg−1, respectively, for HLM from subjects exhibiting the UGT2B17 (*1/*1), UGT2B17 (*1/*2), and UGT2B17 (*2/*2) genotypes, respectively. Comparative analysis was performed by using HLM from subjects with the wild-type UGT2B17 (*1/*1) genotype as the referent; error bars represent standard error. (B) UGT2B17 expression versus UGT2B17 genotype in human liver. UGT2B17 expression was determined relative to PPIA as the “housekeeping” gene by real-time polymerase chain reaction by using total RNA from the same livers for whom HLMs were prepared. Comparative analysis was performed by using the UGT2B17 (*1/*1) genotype group as the referent, with the P-value shown for the UGT2B17 (*2/*2) genotype group; error bars represent standard error.
Table 5.
Kinetic analysis of 17-dihydroexemestane glucuronidation by individual HLM specimens from subjects stratified by UGT2B17 genotype.
| HLM # | UGT2B17 genotype | Vmax(pmol·min−1·mg−1) | Km(μM) | Vmax/Km(μL·min−1·mg−1) |
|---|---|---|---|---|
| 972 | *1/*1 | 51.6 | 10.6 | 4.9 |
| 1603 | *1/*1 | 20.2 | 7.4 | 2.7 |
| 4118 | *1/*2 | 63.6 | 12.1 | 5.3 |
| 1270 | *1/*2 | 7.6 | 10.2 | 0.75 |
| 416 | *2/*2 | 5.6 | 17.2 | 0.33 |
| 145 | *2/*2 | 7.5 | 23.3 | 0.32 |
UGTs and TAM/AI pharmacogenetics: focus on the hepatic UGTs
Glucuronidation plays a major role in the metabolism of TAM, anastrozole, and exemestane, with specific UGT enzymes performing either N- or O-glucuronidation of the active TAM metabolite, anastrozole, and the active exemestane metabolite, 17-dihydroexemestane. UGT2B7 appears to be the most active hepatic UGT against TAM. UGT2B7 expression has been detected in a variety of tissues, including liver, the gastrointestinal tract, and breast (Blevins-Primeau et al., 2009; Nakamura et al., 2008; Ren et al., 2000; Strassburg et al., 1999; Turgeon et al., 2001;Z. Zheng et al., 2002). Therefore, variations in UGT2B7 function or expression could potentially significantly impact individual response to drugs or chemotherapeutic agents. The O-glucuronidation of both trans-4-OH-TAM and trans-endoxifen in HLM was significantly associated with UGT2B7 genotype, with lower activities correlated with increasing numbers of the UGT2B7268Tyr allele. These data are consistent with the observation that HEK293 cells that overexpressed the UGT2B7268Tyr variant exhibited lower activity in vitro against both TAM metabolites, as compared to cells overexpressing wild-type UGT2B7268His. These results are also consistent with a functional role for this polymorphism against other substrates, including tobacco carcinogen metabolites, such as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) (Wiener et al., 2004b).
Similarly, UGT2B17 is hepatically expressed (Turgeon et al., 2001) and is the primary UGT involved in the glucuronidation of the major active exemestane metabolite, 17-dihydroexemestane. Homozygous deletion of the UGT2B17 gene is associated with significantly reduced levels of hydroexemestane-17-O-glucuronide formation in HLM. Since both the UGT2B7 codon 268 SNP and the UGT2B17 deletion are highly prevalent in the population (~0.50 and 0.30 prevalence, respectively) in Caucasians (Gallagher et al., 2007; McCarroll et al., 2006; Murata et al., 2003; Wiener et al., 2004b; Wilson et al., 2004), both polymorphisms could potentially affect overall response to these agents in a large segment of the population.
Conclusions
Additional studies examining the effect of UGT genotypes on breast microsomal glucuronidation activity against TAM and exemestane metabolites, plasma levels of TAM or exemestane metabolites, and overall patient response will be required to further examine the role of UGT polymorphisms on the therapeutic efficacy of these agents.
Footnotes
Declaration of interest: These studies were supported by Public Health Service grant R01-DE13158 (Lazarus) from the National Institutes of Health.
References
- Anonymous. Exemestane for advanced breast cancer. Med Lett Drugs Ther. 2000;42:35–36. [PubMed] [Google Scholar]
- Antoniou T, Tseng AL. Interactions between antiretrovirals and antineoplastic drug therapy. Clin Pharmacokinet. 2005;44:111–145. doi: 10.2165/00003088-200544020-00001. [DOI] [PubMed] [Google Scholar]
- Apak TI, Duffel MW. Interactions of the stereoisomers of alpha-hydroxytamoxifen with human hydroxysteroid sulfotransferase SULT2A1 and rat hydroxysteroid sulfotransferase STa. Drug Metab Dispos. 2004;32:1501–1508. doi: 10.1124/dmd.104.000919. [DOI] [PubMed] [Google Scholar]
- AstraZeneca. Arimidex anastrozole tablets. Wilming, DE; USA: 2006. [Access weblink: Jan 2007]. Available at: www1.astrazeneca-us.com/pi/arimidex.pdf.2006. [Google Scholar]
- Bergman L, Beelen ML, Gallee MP, Hollema H, Benraadt J, van Leeuwen FE. Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres’ ALERT Group. Assessment of liver and endometrial cancer risk following tamoxifen. Lancet. 2000;356:881–887. doi: 10.1016/s0140-6736(00)02677-5. [DOI] [PubMed] [Google Scholar]
- Berkovitz GD, Brown TR, Fujimoto M. Aromatase activity in human skin fibroblasts grown in cell culture. Steroids. 1987;50(1–3):281–295. doi: 10.1016/0039-128x(83)90078-8. [DOI] [PubMed] [Google Scholar]
- Bernstein L, Deapen D, Cerhan JR, Schwartz SM, Liff J, McGann-Maloney E, et al. Tamoxifen therapy for breast cancer and endometrial cancer risk. J Natl Cancer Inst. 1999;91:1654–1662. doi: 10.1093/jnci/91.19.1654. [DOI] [PubMed] [Google Scholar]
- Blevins-Primeau AS, Sun D, Chen G, Sharma AK, Gallagher CJ, Amin S, et al. Functional significance of UDP-glucuronosyltransferase variants in the metabolism of active tamoxifen metabolites. Cancer Res. 2009 doi: 10.1158/0008-5472.CAN-08-3708. Epub Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccardo F, Rubagotti A, Puntoni M, Guglielmini P, Amoroso D, Fini A, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: preliminary results of the Italian Tamoxifen Anastrozole Trial. J Clin Oncol. 2005;23:5138–5147. doi: 10.1200/JCO.2005.04.120. [DOI] [PubMed] [Google Scholar]
- Boocock DJ, Brown K, Gibbs AH, Sanchez E, Turteltaub KW, White IN. Identification of human CYP forms involved in the activation of tamoxifen and irreversible binding to DNA. Carcinogenesis. 2002;23:1897–1901. doi: 10.1093/carcin/23.11.1897. [DOI] [PubMed] [Google Scholar]
- Borges S, Desta Z, Li L, Skaar TC, Ward BA, Nguyen A, et al. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther. 2006;80:61–74. doi: 10.1016/j.clpt.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Breyer-Pfaff U, Mey U, Green MD, Tephly TR. Comparative N-glucuronidation kinetics of ketotifen and amitriptyline by expressed human UDP-glucuronosyltransferases and liver microsomes. Drug Metab Dispos. 2000;28:869–872. [PubMed] [Google Scholar]
- Bulun SE, Simpson ER. Competitive reverse transcription-polymerase chain reaction analysis indicates that levels of aromatase cytochrome P450 transcripts in adipose tissue of buttocks, thighs, and abdomen of women increase with advancing age. J Clin Endocrinol Metab. 1994;78:428–432. doi: 10.1210/jcem.78.2.8106632. [DOI] [PubMed] [Google Scholar]
- Buzdar AU, Guastalla JP, Nabholtz JM, Cuzick J, Group AT. Impact of chemotherapy regimens prior to endocrine therapy: results from the ATAC (Anastrozole and Tamoxifen, Alone or in Combination) Trial. Cancer. 2006;107:472–480. doi: 10.1002/cncr.22042. [DOI] [PubMed] [Google Scholar]
- Buzzetti F, Di Salle E, Longo A, Briatico G. Synthesis and aromatase inhibition by potential metabolites of exemestane (6-methylenandrosta-1,4-diene-3,17-dione) Steroids. 1993;58:527–532. doi: 10.1016/0039-128x(93)90029-m. [DOI] [PubMed] [Google Scholar]
- Chen G, Yin S, Maiti S, Shao X. 4-hydroxytamoxifen sulfation metabolism. J Biochem Mol Toxicol. 2002;16:279–285. doi: 10.1002/jbt.10048. [DOI] [PubMed] [Google Scholar]
- Coates AS, Keshaviah A, Thurlimann B, Mouridsen H, Mauriac L, Forbes JF, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for post-menopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol. 2007;25:486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- Coffman BL, King CD, Rios GR, Tephly TR. The glucuronidation of opioids, other xenobiotics, and androgens by human UGT2B7Y(268) and UGT2B7H(268) Drug Metab Dispos. 1998;26:73–77. [PubMed] [Google Scholar]
- Coleman RE, Banks LM, Girgis SI, Kilburn LS, Vrdoljak E, Fox J, et al. Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised, controlled study. Lancet Oncol. 2007;8:119–127. doi: 10.1016/S1470-2045(07)70003-7. [DOI] [PubMed] [Google Scholar]
- Coller JK. Oxidative metabolism of tamoxifen to Z-4-hydroxy-tamoxifen by cytochrome P450 isoforms: an appraisal of in vitro studies. Clin Exp Pharmacol Physiol. 2003;30:845–848. doi: 10.1046/j.1440-1681.2003.03921.x. [DOI] [PubMed] [Google Scholar]
- Coller JK, Krebsfaenger N, Klein K, Endrizzi K, Wolbold R, Lang T, et al. The influence of CYP2B6, CYP2C9 and CYP2D6 genotypes on the formation of the potent antioestrogen Z-4-hydroxy-tamoxifen in human liver. Br J Clin Pharmacol. 2002;54:157–167. doi: 10.1046/j.1365-2125.2002.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes RC, Kilburn LS, Snowdon CF, Paridaens R, Coleman RE, Jones SE, et al. Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomised, controlled trial. Lancet. 2007;369:559–570. doi: 10.1016/S0140-6736(07)60200-1. [DOI] [PubMed] [Google Scholar]
- Crewe HK, Ellis SW, Lennard MS, Tucker GT. Variable contribution of cytochromes P450 2D6, 2C9, and 3A4 to the 4-hydroxylation of tamoxifen by human liver microsomes. Biochem Pharmacol. 1997;53:171–178. doi: 10.1016/s0006-2952(96)00650-8. [DOI] [PubMed] [Google Scholar]
- Crewe HK, Notley LM, Wunsch RM, Lennard MS, Gillam EM. Metabolism of tamoxifen by recombinant human cytochrome P450 enzymes: formation of the 4-hydroxy, 4′-hydroxy, and N-desmethyl metabolites and isomerization of trans-4-hydroxytamoxifen. Drug Metab Dispos. 2002;30:869–874. doi: 10.1124/dmd.30.8.869. [DOI] [PubMed] [Google Scholar]
- Cuzick J, Powles T, Veronesi U, Forbes J, Edwards R, Ashley S, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361:296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- Dehal SS, Kupfer D. CYP2D6 catalyzes tamoxifen 4-hydroxylation in human liver. Cancer Res. 1997;57:3402–3406. [PubMed] [Google Scholar]
- Desta Z, Ward BA, Soukhova NV, Flockhart DA. Comprehensive evaluation of tamoxifen sequential biotrans-formation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther. 2004;310:1062–1075. doi: 10.1124/jpet.104.065607. [DOI] [PubMed] [Google Scholar]
- Dowsett M, Cuzick J, Howell A, Jackson I. Pharmacokinetics of anastrozole and tamoxifen alone, and in combination, during adjuvant endocrine therapy for early breast cancer in postmenopausal women: a sub-protocol of the Arimidex and Tamoxifen, Alone or in Combination (ATAC) Trial. Br J Cancer. 2001;85:317–324. doi: 10.1054/bjoc.2001.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Tamoxifen for early breast cancer: an overview of the randomised trials. The Early Breast Cancer Trialists’ Collaborative Group. (1998) Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- Eisen A, Trudeau M, Shelley W, Messersmith H, Pritchard KI. Aromatase inhibitors in adjuvant therapy for hormone receptor positive breast cancer: a systematic review. Cancer Treat Rev. 2008;34:157–174. doi: 10.1016/j.ctrv.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Elahi A, Bendaly J, Zheng Z, Muscat JE, Richie JP, Jr, Schantz SP, et al. Detection of UGT1A10 polymorphisms and their association with orolaryngeal carcinoma risk. Cancer. 2003;98:872–880. doi: 10.1002/cncr.11587. [DOI] [PubMed] [Google Scholar]
- Falany CN. Enzymology of human cytosolic sulfotransferases. FASEB J. 1997;11:206–216. doi: 10.1096/fasebj.11.4.9068609. [DOI] [PubMed] [Google Scholar]
- FDA. Femara. Silver Spring, Maryland, USA: U.S. Food and Drug Administration; 2004. [Access date for weblink: Feb 2007]. Available at:// www.fda.gov/cder/foi/label/2004/020726s011lbl.pdf. [Google Scholar]
- Ferretti G, Bria E, Giannarelli D, Felici A, Papaldo P, Fabi A, et al. Second- and third-generation aromatase inhibitors as first-line endocrine therapy in postmenopausal metastatic breast cancer patients: a pooled analysis of the randomised trials. Br J Cancer. 2006;94:1789–1796. doi: 10.1038/sj.bjc.6603194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher B, Costantino JP, Redmond CK, Fisher ER, Wickerham DL, Cronin WM. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst. 1994;86:527–537. doi: 10.1093/jnci/86.7.527. [DOI] [PubMed] [Google Scholar]
- Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC Trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- Furr BJ, Jordan VC. The pharmacology and clinical uses of tamoxifen. Pharmacol Ther. 1984;25:127–205. doi: 10.1016/0163-7258(84)90043-3. [DOI] [PubMed] [Google Scholar]
- Gallagher CJ, Muscat JE, Hicks AN, Zheng Y, Dyer AM, Chase GA, et al. The UDP-glucuronosyltransferase 2B17 gene deletion polymorphism: sex-specific association with urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol glucuronidation phenotype and risk for lung cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:823–828. doi: 10.1158/1055-9965.EPI-06-0823. [DOI] [PubMed] [Google Scholar]
- Goetz MP, Kamal A, Ames MM. Tamoxifen pharmacogenomics: the role of CYP2D6 as a predictor of drug response. Clin Pharmacol Ther. 2008;83:160–166. doi: 10.1038/sj.clpt.6100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz MP, Knox SK, Suman VJ, Rae JM, Safgren SL, Ames MM, et al. The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat. 2007;101:113–121. doi: 10.1007/s10549-006-9428-0. [DOI] [PubMed] [Google Scholar]
- Goetz MP, Rae JM, Suman VJ, Safgren SL, Ames MM, Visscher DW, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23:9312–9318. doi: 10.1200/JCO.2005.03.3266. [DOI] [PubMed] [Google Scholar]
- Green MD, Tephly TR. Glucuronidation of amines and hydroxylated xenobiotics and endobiotics catalyzed by expressed human UGT1.4 protein. Drug Metab Dispos. 1996;24:356–363. [PubMed] [Google Scholar]
- Green MD, Tephly TR. Glucuronidation of amine substrates by purified and expressed UDP-glucuronosyltransferase proteins. Drug Metab Dispos. 1998;26:860–867. [PubMed] [Google Scholar]
- Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) Trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- Howell A, Group AT. Analysis of fracture risk factors from the Arimadex, Tamoxifen, Alone or in Combination (ATAC) Trial: 5-year data [abstract] J Clin Oncol. 2006;24:A563. [Google Scholar]
- Howell A, Howell SJ, Evans DG. New approaches to the endocrine prevention and treatment of breast cancer. Cancer Chemother Pharmacol. 2003;52(Suppl 1):S39–S44. doi: 10.1007/s00280-003-0645-5. [DOI] [PubMed] [Google Scholar]
- Hozumi Y, Suemasu K, Takehara M, Takei H, Aihara T, Tamura M. The effect of exemestane, anastrozole, and tamoxifen on the lipidemic profile of postmeonpausal early breast cancer patients: preliminary results of NSAS (National Surgical Adjusvant Study) Breast Cancer Res Treat. 2006;100:A4051. [Google Scholar]
- Hu Y, Dehal SS, Hynd G, Jones GB, Kupfer D. CYP2D6-mediated catalysis of tamoxifen aromatic hydroxylation with an NIH shift: similar hydroxylation mechanism in chicken, rat, and human liver microsomes. Xenobiotica. 2003;33:141–151. doi: 10.1080/0049825021000042733. [DOI] [PubMed] [Google Scholar]
- Huang YH, Galijatovic A, Nguyen N, Geske D, Beaton D, Green J, et al. Identification and functional characterization of UDP-glucuronosyltransferases UGT1A8*1, UGT1A8*2 and UGT1A8*3. Pharmacogenetics. 2002;12:287–297. doi: 10.1097/00008571-200206000-00004. [DOI] [PubMed] [Google Scholar]
- Jacolot F, Simon I, Dreano Y, Beaune P, Riche C, Berthou F. Identification of the cytochrome P450 IIIA family as the enzymes involved in the N-demethylation of tamoxifen in human liver microsomes. Biochem Pharmacol. 1991;41:1911–1919. doi: 10.1016/0006-2952(91)90131-n. [DOI] [PubMed] [Google Scholar]
- Jin Y, Desta Z, Stearns V, Ward B, Ho H, Lee KH, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst. 2005;97:30–39. doi: 10.1093/jnci/dji005. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Zuo H, Lee KH, Trebley JP, Rae JM, Weatherman RV, et al. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004;85:151–159. doi: 10.1023/B:BREA.0000025406.31193.e8. [DOI] [PubMed] [Google Scholar]
- Jordan VC, Collins MM, Rowsby L, Prestwich G. A monohydroxylated metabolite of tamoxifen with potent antioestrogenic activity. J Endocrinol. 1977;75:305–316. doi: 10.1677/joe.0.0750305. [DOI] [PubMed] [Google Scholar]
- Kaku T, Ogura K, Nishiyama T, Ohnuma T, Muro K, Hiratsuka A. Quaternary ammonium-linked glucuronidation of tamoxifen by human liver microsomes and UDP-glucuronosyltransferase 1A4. Biochem Pharmacol. 2004;67:2093–2102. doi: 10.1016/j.bcp.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen BS, Norman MJ, Eckert RL, Peltz SW, Mangel WF. Bioactivities, estrogen receptor interactions, and plasminogen activator-inducing activities of tamoxifen and hydroxy-tamoxifen isomers in MCF-7 human breast cancer cells. Cancer Res. 1984;44:112–119. [PubMed] [Google Scholar]
- Kim SY, Suzuki N, Santosh Laxmi YR, Rieger R, Shibutani S. Alpha-hydroxylation of tamoxifen and toremifene by human and rat cytochrome P450 3A subfamily enzymes. Chem Res Toxicol. 2003;16:1138–1144. doi: 10.1021/tx0300131. [DOI] [PubMed] [Google Scholar]
- Lazarus P, Blevins-Primeau AS, Zheng Y, Sun D. Potential role of UGT pharmacogenetics in cancer treatment and prevention: focus on tamoxifen. Ann N Y Acad Sci. 2009;1155:99–111. doi: 10.1111/j.1749-6632.2009.04114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien EA, Solheim E, Kvinnsland S, Ueland PM. Identification of 4-hydroxy-N-desmethyltamoxifen as a metabolite of tamoxifen in human bile. Cancer Res. 1988;48:2304–2308. [PubMed] [Google Scholar]
- Lien EA, Solheim E, Lea OA, Lundgren S, Kvinnsland S, Ueland PM. Distribution of 4-hydroxy-N-desmethyltamoxifen and other tamoxifen metabolites in human biological fluids during tamoxifen treatment. Cancer Res. 1989;49:2175–2183. [PubMed] [Google Scholar]
- Lim L, Desta Z, Rae J, Flockhart D, Skaar T. Gene expression profiles of 4-hydroxy-N-desmethyltamoxifen (endoxifen) and 4-hydroxytamoxifen (4OHTAM) treated human breast cancer cells determined by cDNA microarray analysis. Clin Pharmacol Ther. 2004;75:49. [Google Scholar]
- Lim YC, Desta Z, Flockhart DA, Skaar TC. Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has antiestrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother Pharmacol. 2005;55:471–478. doi: 10.1007/s00280-004-0926-7. [DOI] [PubMed] [Google Scholar]
- Lonning PE, Lien EA, Lundgren S, Kvinnsland S. Clinical pharmacokinetics of endocrine agents used in advanced breast cancer. Clin Pharmacokinet. 1992;22:327–358. doi: 10.2165/00003088-199222050-00002. [DOI] [PubMed] [Google Scholar]
- Mareck U, Geyer H, Guddat S, Haenelt N, Koch A, Kohler M, et al. Identification of the aromatase inhibitors anastrozole and exemestane in human urine using liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:1954–1962. doi: 10.1002/rcm.2545. [DOI] [PubMed] [Google Scholar]
- Markopoulos C, Polychronis A, Zobolas V, Xepapadakis G, Papadiamantis J, Koukouras D, et al. The effect of exemestane on the lipidemic profile of postmenopausal early breast cancer patients: preliminary results of the TEAM Greek substudy. Breast Cancer Res Treat. 2005;93:61–66. doi: 10.1007/s10549-005-3783-0. [DOI] [PubMed] [Google Scholar]
- Matsumine H, Hirato K, Yanaihara T, Tamada T, Yoshida M. Aromatization by skeletal muscle. J Clin Endocrinol Metab. 1986;63:717–720. doi: 10.1210/jcem-63-3-717. [DOI] [PubMed] [Google Scholar]
- McCarroll SA, Hadnott TN, Perry GH, Sabeti PC, Zody MC, Barrett JC, et al. Common deletion polymorphisms in the human genome. Nat Genet. 2006;38:86–92. doi: 10.1038/ng1696. [DOI] [PubMed] [Google Scholar]
- McDonald CC, Stewart HJ. Fatal myocardial infarction in the Scottish adjuvant tamoxifen trial. The Scottish Breast Cancer Committee. BMJ. 1991;303:435–437. doi: 10.1136/bmj.303.6800.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means GD, Kilgore MW, Mahendroo MS, Mendelson CR, Simpson ER. Tissue-specific promoters regulate aromatase cytochrome P450 gene expression in human ovary and fetal tissues. Mol Endocrinol. 1991;5:2005–2013. doi: 10.1210/mend-5-12-2005. [DOI] [PubMed] [Google Scholar]
- Meier CR, Jick H. Tamoxifen and risk of idiopathic venous thromboembolism. Br J Clin Pharmacol. 1998;45:608–612. doi: 10.1046/j.1365-2125.1998.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata M, Warren EH, Riddell SR. A human minor histocompatibility antigen resulting from differential expression due to a gene deletion. J Exp Med. 2003;197:1279–1289. doi: 10.1084/jem.20030044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Nakajima M, Yamanaka H, Fujiwara R, Yokoi T. Expression of UGT1A and UGT2B mRNA in human normal tissues and various cell lines. Drug Metab Dispos. 2008;36:1461–1464. doi: 10.1124/dmd.108.021428. [DOI] [PubMed] [Google Scholar]
- Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol. 2001;45(3 Suppl):S116–S124. doi: 10.1067/mjd.2001.117432. [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Ogura K, Nakano H, Ohnuma T, Kaku T, Hiratsuka A, et al. Reverse geometrical selectivity in glucuronidation and sulfation of cis- and trans-4-hydroxyta-moxifens by human liver UDP-glucuronosyltransferases and sulfotransferases. Biochem Pharmacol. 2002;63:1817–1830. doi: 10.1016/s0006-2952(02)00994-2. [DOI] [PubMed] [Google Scholar]
- Osborne CK. Tamoxifen in the treatment of breast cancer. NEJM. 1998;339:1609–1618. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- Pfizer. Aromasin exemestane tablets. NY, NY, USA: 2007. [Access date: Oct, 2007]. Available at: www.pfizer.com/files/products/uspi_aromasin.pdf. [Google Scholar]
- Perez EA, Josse RG, Pritchard KI, Ingle JN, Martino S, Findlay BP, et al. Effect of letrozole versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen: a companion study to NCIC CTG MA.17. J Clin Oncol. 2006;24:3629–3635. doi: 10.1200/JCO.2005.05.4882. [DOI] [PubMed] [Google Scholar]
- Poon GK, Chui YC, McCague R, Llnning PE, Feng R, Rowlands MG, et al. Analysis of phase I and phase II metabolites of tamoxifen in breast cancer patients. Drug Metab Dispos. 1993;21:1119–1124. [PubMed] [Google Scholar]
- Ren Q, Murphy SE, Zheng Z, Lazarus P. O-glucuronidation of the lung carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), by human UDP-glucuronosyltransferases 2B7 and 1A9. Drug Metab Dispos. 2000;28:1352–1360. [PubMed] [Google Scholar]
- Roselli CE, Horton LE, Resko JA. Distribution and regulation of aromatase activity in the rat hypothalamus and limbic system. Endocrinology. 1985;117:2471–2477. doi: 10.1210/endo-117-6-2471. [DOI] [PubMed] [Google Scholar]
- Rutqvist LE, Johansson H, Signomklao T, Johansson U, Fornander T, Wilking N. Adjuvant tamoxifen therapy for early-stage breast cancer and second primary malignancies. The Stockholm Breast Cancer Study Group. J Natl Cancer Inst. 1995;87:645–651. doi: 10.1093/jnci/87.9.645. [DOI] [PubMed] [Google Scholar]
- Rutqvist LE, Mattsson A. Cardiac and thromboembolic morbidity among postmenopausal women with early-stage breast cancer in a randomized trial of adjuvant tamoxifen. The Stockholm Breast Cancer Study Group. J Natl Cancer Inst. 1993;85:1398–1406. doi: 10.1093/jnci/85.17.1398. [DOI] [PubMed] [Google Scholar]
- Shozu M, Simpson ER. Aromatase expression of human osteoblast-like cells. Mol Cell Endocrinol. 1998;139(1–2):117–129. doi: 10.1016/s0303-7207(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Sioufi A, Gauducheau N, Pineau V, Marfil F, Jaouen A, Cardot JM, et al. Absolute bioavailability of letrozole in healthy postmenopausal women. Biopharm Drug Dispos. 1997;18:779–789. doi: 10.1002/(sici)1099-081x(199712)18:9<779::aid-bdd64>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95:1758–1764. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- Strassburg CP, Strassburg A, Nguyen N, Li Q, Manns MP, Tukey RH. Regulation and function of family 1 and family 2 UDP-glucuronosyltransferase genes (UGT1A, UGT2B) in human oesophagus. Biochem J. 1999;338(Pt 2):489–498. [PMC free article] [PubMed] [Google Scholar]
- Sun D, Chen G, Dellinger RW, Duncan K, Fang JL, Lazarus P. Characterization of tamoxifen and 4-hydroxytamoxifen glucuronidation by human UGT1A4 variants. Breast Cancer Res. 2006;8:R50. doi: 10.1186/bcr1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Sharma AK, Dellinger RW, Blevins-Primeau AS, Balliet RM, Chen G, et al. Glucuronidation of active tamoxifen metabolites by the human UDP-glucuronosyltransferases. Drug Metab Dispos. 2007;35:2006–2014. doi: 10.1124/dmd.107.017145. [DOI] [PubMed] [Google Scholar]
- Thurlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. NEJM. 2005;353:2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- Tsai-Morris CH, Aquilano DR, Dufau ML. Cellular localization of rat testicular aromatase activity during development. Endocrinology. 1985;116:38–46. doi: 10.1210/endo-116-1-38. [DOI] [PubMed] [Google Scholar]
- Turgeon D, Carrier JS, Levesque E, Hum DW, Belanger A. Relative enzymatic activity, protein stability, and tissue distribution of human steroid-metabolizing UGT2B subfamily members. Endocrinology. 2001;142:778–787. doi: 10.1210/endo.142.2.7958. [DOI] [PubMed] [Google Scholar]
- van Leeuwen FE, Benraadt J, Coebergh JW, Kiemeney LA, Gimbrere CH, Otter R, et al. Risk of endometrial cancer after tamoxifen treatment of breast cancer. Lancet. 1994;343:448–452. doi: 10.1016/s0140-6736(94)92692-1. [DOI] [PubMed] [Google Scholar]
- Wiener D, Doerge DR, Fang JL, Upadhyaya P, Lazarus P. Characterization of N-glucuronidation of the lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human liver: importance of UDP-glucuronosyltransferase 1A4. Drug Metab Dispos. 2004a;32:72–79. doi: 10.1124/dmd.32.1.72. [DOI] [PubMed] [Google Scholar]
- Wiener D, Fang JL, Dossett N, Lazarus P. Correlation between UDP-glucuronosyltransferase genotypes and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone glucuronidation phenotype in human liver microsomes. Cancer Res. 2004b;64:1190–1196. doi: 10.1158/0008-5472.can-03-3219. [DOI] [PubMed] [Google Scholar]
- Wilson W, 3rd, Pardo-Manuel de Villena F, Lyn-Cook BD, Chatterjee PK, Bell TA, Detwiler DA, et al. Characterization of a common deletion polymorphism of the UGT2B17 gene linked to UGT2B15. Genomics. 2004;84:707–714. doi: 10.1016/j.ygeno.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Sun D, Sharma AK, Chen G, Amin S, Lazarus P. Elimination of antiestrogenic effects of active tamoxifen metabolites by glucuronidation. Drug Metab Dispos. 2007;35:1942–1948. doi: 10.1124/dmd.107.016279. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Fang JL, Lazarus P. Glucuronidation: an important mechanism for detoxification of benzo[a]pyrene metabolites in aerodigestive tract tissues. Drug Metab Dispos. 2002;30:397–403. doi: 10.1124/dmd.30.4.397. [DOI] [PubMed] [Google Scholar]