Abstract
We have recently reported that the toll-like receptor 3 (TLR3) agonist poly(I:C) induces adjuvant effects to post vaccination CD8+ T cells responses through rapid induction of innate mediators, including NK cells, macrophages, dendritic cells (DCs), and inflammatory cytokines. However, whether this TLR3 agonist directly targets CD8+ T cells needs to be carefully investigated. In this study, we found that optimal post vaccination CD8+ T cell responses to ex vivo DC-based vaccination requires triggering of TLR3 signaling pathway in DCs in vitro as well as in the recipient host, indicating a role for other cell types. Real-time PCR analysis revealed that TLRs (TLR1–TLR13) are expressed in purified (>99% pure) CD4+ and CD8+ T cells from C57BL/6 and BALB/c mice, where the magnitude of the expression was strain and cell type dependent. In vitro, treatment of these purified T cells with poly(I:C) modulated the expression of TLRs including TLR3. Furthermore, non-specific and antigen-specific stimulation of CD8+ T cells by phorbol myristate acetate and MHC class I peptide-pulsed splenocytes, respectively, modulated TLR expression in purified CD4+ and CD8+ T cells. Importantly, brief conditioning of purified naïve TCR transgenic OT-1 (CD8+) T cells in vitro with poly(I:C) induced activation of these cells in absence of antigen stimulation. Interestingly, when these in vitro poly(I:C)-conditioned OT-1 cells were adoptively transferred into naïve recipient followed by peptide vaccination, they showed superior expansion and activation to their naïve counterparts. These results suggest that CD8+ T cells can be activated by triggering their TLR3. Furthermore, the data support the notion of direct involvement of TLRs in adaptive immune responses.
Keywords: Adoptive transfer, BALB/c, CD4, CD8, TLR, C57B6/L, OT-1, OVA peptide, Poly(I:C), TLR3 agonist, TLR3 ligand, T cells, Vaccination
1. Introduction
Bridging innate and adaptive immunity is critical to generate functional immune responses [1–3]. The direct and immediate recognition of pathogens is primarily mediated by a set of germline-encoded receptors known as pattern recognition receptors (PRRs), and represents a potential means to link innate and adaptive immunity. PRRs, which include Toll-like receptors (TLRs), are able to recognize pathogen-associated molecular patterns (PAMPs) that are unique to pathogenic microorganisms and induce specific immune responses against them [4]. In contrast to pathogenic microbes, however, cancer cells do not encode PAMPs. Therefore, one potential approach to link innate and adaptive arms of immunity against cancer would be by triggering TLRs expressed on innate immune cells. However, to design efficacious approaches based on bridging innate and adaptive immunity, it would be helpful to explore whether adaptive immune cells also express functional TLRs.
TLRs are members of a family of transmembrane proteins with an extracellular leucine-rich domain and a conserved cytoplasmic domain homologous to that of the interleukin-1 receptor (IL-1R), termed the Toll/IL-1R homology (TIR) domain [5]. This structure allows TLRs to recognize PAMPs and activate, via the TIR domain, a series of downstream pathways that result in immune and inflammatory responses. After binding to their specific ligands on innate immune cells, TLRs dimerize and undergo conformational changes which are required for the recruitment of adaptor molecules to the TIR domain [6–11]. Once the adaptors have been recruited, a complex of IL-1R-associated kinases (IRAks), TRAF6 and IRF-5 is formed that results in the downstream phosphorylation of IκB which in turn frees NF-κB. Unbound NF-κB translocates into the nucleus where it directly regulates the transcription of pro-inflammatory genes [12]. When triggered, TLR signaling induces pro-inflammatory mediators, including cytokines and chemokines, and maturation of dendritic cells (DCs) [13–18]. These mediators in combination with mature DCs, activate cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells, promoting adaptive immunity [16,17,19,20]. In this context, we and others have reported that addition of certain TLRLs to different immunization regimens leads to marked adjuvant effects to post vaccination CD8+ T cell responses, coinciding with anti-tumor and anti-viral immunity [20–27].
Traditionally, TLR expression has been associated with professional antigen presenting cells. This leads to the concept that the adjuvant effects of TLR/TLRL signaling occurred mainly in innate immune cells, in particular DCs and NK cells. However, this concept has been challenged by recent studies reporting TLR expression in T cells and on non-lymphoid cells such as fibroblasts and epithelial cells [28–33]. Furthermore, changes in the levels of TLR expression in response to infection have been shown in human CD4+ and CD8+ T cells [34], suggesting that TLR function is not limited to innate responses, but can play a direct role in adaptive immunity as well. Therefore, a better understanding of the functionality of TLRs on CD8+ T cells could allow a better design of anti-cancer immunotherapy. In the present study, we asked whether T cells express TLRs. We found that both CD4+ and CD8+ T cells express different arrays of TLRs. Interestingly, we found that TLR3 is functional since its engagement by the TLR3L poly(I:C) led to significant antigen-specific CD8+ T cell responses. Our results provide the rationale design of efficacious immunotherapeutic approaches by targeting TLR expressed on both innate and adaptive immune cells.
2. Materials and methods
2.1. Mice
B6.SJL, C57BL/6, and BALB/c wild type mice and OT-1 TCR transgenic mice on C57BL/b background were purchased from Jackson Laboratory (Bar Harbor, ME). OT-1 mice (Ly5.2) were bred with B6.SJL (Ly5.1) mice to generate Ly5.1+/Ly5.1+ mice heterozygous for the OT-1 TCR (Vα2/Vβ5) transgene. Transgene expression was confirmed by flow cytometry with mAb specific for Vα2Vβ5. All animals were housed under specific pathogen-free conditions in accordance with institutional and federal guidelines at the Medical University of South Carolina.
2.2. Antibodies and reagents
Anti-CD16/CD32, and FITC-, PE-, APC-, and cychrome-conjugated mAbs, including anti-CD4, anti-CD8, anti-CD11b, anti-CD11c, anti-CD25, anti-CD69, anti-CD62L, anti-B220, anti-Ly6G (Gr1), anti-Ly5.1, and anti-NK1.1 were purchased from Pharmingen (San Diego, CA). MHC class-I OVA albumin SIINFEKL peptide (OVAp) (American Peptide Company, Inc., Sunnyvale, CA) was dissolved in 10% DMSO (Sigma, St. Louis, MO) and diluted in PBS. Phorbol myristate acetate (PMA) (Sigma) was dissolved in PBS at high concentration and stored at −20 °C until used. Recombinant murine cytokines, including GM-CSF and IL-4 (R&D), were stored as a lyophilized powder at −20 °C, and reconstituted immediately prior to use in 0.1% bovine serum albumin in phosphate buffered solution (PBS). The TLR3L poly(I:C) (InvivoGen, San Diego, CA) was reconstituted according to the manufacture description.
2.3. Generation of DCs
Bone marrow (BM)-derived DCs were generated as we previously described [35]. Briefly, bone marrow was flushed from the femurs and tibias of mice and then depleted of red blood cells by lysis with ACK buffer (Biofluids, Camarillo, CA). Cells were suspended in complete RPMI and then supplemented with murine GM-CSF (20 ng/ml) and murine IL-4 (20 ng/ml) and plated out in six-well plate at 1 × 106 cells/ml. On day four of culture, complete RPMI medium containing the same amount of cytokines was added to increase the total volume by 50%. On day seven, non-adherent and loosely adherent DC were harvested, washed and, except in control conditions, pulsed with 5 μg/ml OVAp for 3 h, then washed three times in complete RPMI medium. The maturity and phenotype of the enriched DCs (CD11c+CD80+, CD86+) were confirmed by the flow cytometry.
2.4. Adoptive transfer of OT-1 T Cells and immunization
OT-1 transgenic cells that express TCR specific for an H-2Db-restricted CD8+ T cell eiptope from OVAp were used. Spleen and lymph nodes from OT-1 TCR transgenic mice were harvested, homogenized, and washed in HBSS (Cellgro). Pooled cells were then passed over a CD8+ selection column from R&D Systems (Minneapolis, MN). Naïve or in vitro poly(I:C)-treated CD8+Ly5.1+ OT-1 T cells (1.5 × 106/mouse) were adoptively transferred into naïve congenic C57BL/6 Ly5.2+ recipient mice and monitored by flow cytometry with anti-Ly5.1 and anti-CD8+ mAb. After adoptive transfer, this CD8+ T-cell population represents approximately 0.05–0.2% of cells in the lymphoid organs. Recipient mice were rested for 24 h after OT-1 adoptive transfer and then vaccinated with 100 μg/mouse OVAp. When mentioned, peptides were mixed with 200 μg/mouse poly(I:C). In some experiments, recipient mice were vaccinated by i.v. injection of 1 × 106 OVAp-pulsed DCs prepared as above.
2.5. Flow cytometry
Cell surface analysis and apoptosis were measured by flow cytometry as we described before [36]. In brief, fresh leukocytes (1 × 106) were treated with anti-CD16/CD32 for 5 min on ice and then stained with the indicated conjugated mAbs, and incubated for 30 min on ice. The cells were washed twice and re-suspended in 0.3 ml of 0.5% BSA, 0.02% sodium azide solution. Cell apoptosis was measured by annexin-V binding assay. Cell surface immunofluorescence was measured using a FACSCalibur flow cytometer and was analyzed with CellQuest software.
2.6. T cell proliferation and cytokine production
Spleens of vaccinated mice were harvested on day seven post vaccination, and fresh splenocytes (responder) were prepared in complete RPMI 1640 medium and 5 × 105 cells were co-cultured with 5 × 105 naïve irradiated (2000 rad) splenocytes (stimulator) pulsed previously with 5 μg/ml OVAp for 2 h at 37 °C. For T cell proliferation, cultured cells were pulsed with 3H-thymidine for the last 18 h, and then cells were harvested to measure 3H uptake by γ-counter as we previously described [37]. For cytokine production, culture supernatants were harvested after 24 h of cell culture and cytokine levels were determined by flow cytometric bead array as we previously described [38].
2.7. Real-time RT-PCR
Total cellular RNA was isolated using TRIZOL reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Final RNA pellets were dissolved in RNA secure buffer (Ambion, Austin, TX). To eliminate the genomic DNA contamination, total RNA (5 μg) was digested with DNase (Promega, Madison, WI). Complementary DNA (20 μl) was made from total RNA using M-MLV reverse transcriptase (Promega, Madison, WI) primered with oligo dT. Real-time RT-PCR was performed on a Gene Amp 5700 Sequence Detection System (PE Biosystems, Foster City, CA). Real-time PCR analysis was performed as we previously described [37]. The standard reaction volume was 10 μl and contained 1X QuantiTect SYBR Green PCR Master Mix (Qiagen, Valencia, CA), 0.002U AmpErase (Applied Biosystems, Foster City, CA), 0.35 μl cDNA template, and 200 nM of oligonucleotide primer. Initial steps of RT-PCR were 2 min at 50 °C for AmpErase activation, followed by a 15 min hold at 95 °C. Cycles (n = 40) consisted of a 15 s melting at 95 °C, followed by a 1 min annealing/extension at 60 °C. The final step was a 60°C incubation for 1 min. All reactions were performed in triplate. The primer-pairs for the gene analysis are listed in the Table 1. The sequences of TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, and TLR9 were designed as previously described in [29]. The sequences of TLR8, TLR11, TLR12 and TLR13 were designed in our laboratory. For a given real-time RT-PCR sample, the RNA expression level was calculated from cycle threshold (Ct) value with the Rockit program. In our analysis, we normalized the results to a reference control gene, β2-microglobin, and reported as the expression level as mean normalized expression (MNE).
Table 1.
Primers used in the quantitation of mouse TLR gene expression.
| Genbank no. | Primer sequence | (5′–3′) |
|---|---|---|
| NM_009735 | B2M F | 5′-TGTCTCACTGACCGGCCTGTAT-3′ |
| B2M R | 5′-GTTCAGTATGTTCGGCTTCCCA-3′ | |
| NM_030682 | Tlr1F | 5′-TTCCGTGATGCACAGCTCCTT-3′ |
| Tlr1R | 5′-TCTGCTCGCCTGAGTTCTTCA-3′ | |
| NM_011905 | Tlr2 F | 5′-CCAAGAGGAAGCCCAAGAAAG-3′ |
| Tlr2 R | 5′-AGGCATCATAGCAAACGTCCC-3′ | |
| NM_126166 | Tlr3 F | 5′-CTTGCGTTGCGAAGTGAAGAA-3′ |
| Tlr3 R | 5′-CCAATTGTCTGGAAACACCCC-3′ | |
| NM_021297 | Tlr4 F | 5′-AGCAGGTGGAATTGTATCGCC-3′ |
| Tlr4 R | 5′-CCCATTCCAGGTAGGTGTTTCT-3′ | |
| NM_016928 | Tlr5 R | 5′-ATATCCACCGAAGACTGCGATG-3′ |
| Tlr5 R | 5′-AGTGACCGTGCACAGGATGAA-3′ | |
| NM_011604 | Tlr6 F | 5′-GAATGTGACCCTCCAGCACAT-3′ |
| Tlr6 R | 5′-AGTTTAACCGAGCACTTCCAGG-3′ | |
| NM_133211 | Tlr7 F | 5′-CTGGAGTTCAGAGGCAACCATT-3′ |
| Tlr7 R | 5′-GTTATCACCGGCTCTCCATAGAA-3′ | |
| NM_133212 | Tlr8 F | 5′-GCCTCAGAGCCTCCAAGAGTTA-3′ |
| Tlr8 R | 5′-CCAGCAAGTGAAGGTGAGGAA-3′ | |
| NM_031178 | Tlr9 F | 5′-AGCTGAACATGAACGGCATCT-3′ |
| Tlr9 R | 5′-TGAGCGTGTACTTGTTGAGCG-3′ | |
| NM_205819 | Tlr11 F | 5′-CTTGCATTTCCTCTCCCTTGTG-3′ |
| Tlr11 R | 5′-AGGTCAAGTGCACGAAGCTCA-3′ | |
| NM_205823 | Tlr12 F | 5′-CCAAATACGGATGAGCCCAGA-3′ |
| Tlr12 F | 5′-AGGAACAATACTGCCGGAGCA-3′ | |
| NM_205820 | Tlr13 F | 5′-TTGTCACCTGCTCGGAAACCTA-3′ |
| Tlr13 F | 5′-GCTGCTTAATGCCCTCTGCAT-3′ |
2.8. Western blot analysis of TLR3
Cellular extracts were prepared as described, and protein samples were mixed in Laemmli loading buffer, boiled for 5 min, and then subjected to 14% SDS-PAGE. After electrophoresis, proteins were transferred onto PVDF membrane (Millipore, Billerica, MA). The blots were incubated with goat anti-mouse TLR3 polyclonal antibody (Pharmingen) overnight at 4 °C. Membranes were washed with 0.05% (v/v) Tween 20 in PBS (pH 7.6) and incubated with a 1:3000 dilution of Horseradish peroxidase (HRP) linked with anti-goat IgG secondary antibody (Pharmingen) for 60 min at room temperature. Protein bands were visualized by ECL substrate (Pierce).
2.9. Statistics
Statistical analyses were performed using the Student’s t-test as appropriate. All P values were two sided, with P < 0.05 considered significant.
3. Results
3.1. Triggering TLR3 signaling in DCs is not sufficient to augment memory CD8+ T cell responses
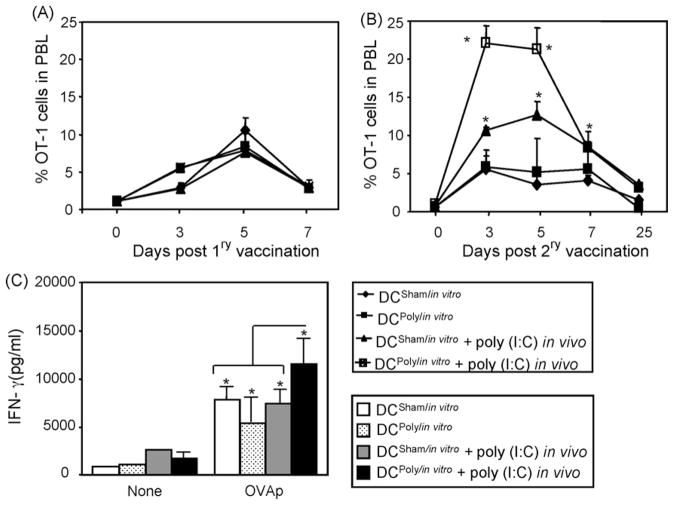
It has been recently reported by our group that concomitant administration of the TLR3 agonist poly(I:C) with OVAp immunization results in marked adjuvant effects to T cell responses, where NK cells played partial but significant roles [36,38]. Other cells, in particular DCs and T cells, also might play a critical role. Although the main aim of this study is to test the contribution of T cells upon triggering TLR3 signaling, it was also tested whether triggering this signaling pathway of this TLR3 in DCs is sufficient to augment CD8+ T cell responses. To test the direct effects of poly(I:C) on DCs, it was confirmed that BM-generated DCs expressed TLRs (TLR1–13) and that these TLRs are functional as evidenced by their acquisition of the activation phenotype CD80+, CD86+, and CD40+ when stimulated with different TLR agonists (data not shown). Next, DCs were utilized to determine the impact of poly(I:C) on their adjuvant effects to T cells. Thus, DCs were generated from BM and then treated with or without 50 μg poly(I:C) to generate DCsham/in vitro and DCpoly/in vitro, respectively. Cells were then pulsed with OVAp and injected into naïve mice that were adoptively transferred one day before with naïve OT-1 T cells followed with or without poly(I:C) treatment. Under this setting, poly(I:C) was added to DCs in vitro or injected in vivo upon vaccination with OVAp-pulsed sham-conditioned or poly(I:C)-conditioned DCs. Analysis of the number of effector cells in the peripheral blood revealed that vaccination with DCsham/in vitro or DCpoly/in vitro induced similar OT-1 cell expansion, which was slightly augmented when poly(I:C) was concomitantly administered with either of these DC vaccination (Fig. 1A). Analysis of memory responses to OVAp revaccination, however, showed that when poly(I:C) was co-administered along with antigen priming with DCsham/in vitro or DCpoly/in vitro, the expansion of memory OT-1 cells was much higher than those obtained after vaccination with DCsham/in vitro or DCpoly/in vitro alone. Importantly, conditioning with poly(I:C) both in vitro and in vivo significantly (P < 0.05) induced higher OT-1 cell expansion (Fig. 1B). The recall responses of the OT-1 cells correlated with increased levels of IFN-γ (Fig. 1C) and TNF-α (data not shown). Of note, the levels of IFN-γare always much higher than TNF-α. These data indicate that triggering of TLR signaling in DCs alone is not sufficient to augment memory T cell responses, and that TLR signaling in cells other than DCs might be critical.
Fig. 1.
Triggering TLR3 signaling in DCs is not sufficient to augment memory CD8+ T cell responses: (A) DCs were generated from bone marrow mononuclear cells of C57BL/6 mice and pulsed with 1 μg/ml OVAp for 3 h at 37 °C. Then, cells were co-cultured with naïve OT-1 cells at different ratio to establish their capability to present OVAp to OT-1 cells reflected by the degree of OT-1 cell proliferation. (B) Freshly prepared DCs were treated with medium or 50/ml μg poly(I:C) to generate DCsham/in vitro and DCpoly/in vitro, respectively. These cells were then washed and pulsed with 1 μg/ml for 3 h at 37 °C and injected (1 × 106) into naïve C57BL/6 (Ly5.2) female mice (n = 4/group) adoptively transferred 24 h before with naïve 1 × 106 OT-1 T cells from B6 SJL (Ly5.1) mice. Half of the DC-vaccinated mice were i.p. injected immediately with 200 μg/mouse. Mice were bled at the indicated time points and the numbers of the Ag-specific OT-1 cells were measured by flow cytometry. (C) Mice were re-vaccinated with 100 μg/mouse OVAp two weeks post DC-vaccination, and the numbers of memory OT-1 cells were measured in peripheral blood samples at the indicated time points. (D) 25 days post secondary vaccination, spleens were harvested. Spleen cell suspensions were stimulated with 1 μg/ml OVAp overnight and the levels of IFN-γ produced by OT-1 cells were determined in supernatants by flow cytometric beads array. *P < 0.05 as compared to control counterparts.
3.2. TLR expression in CD4+ and CD8+ T cells
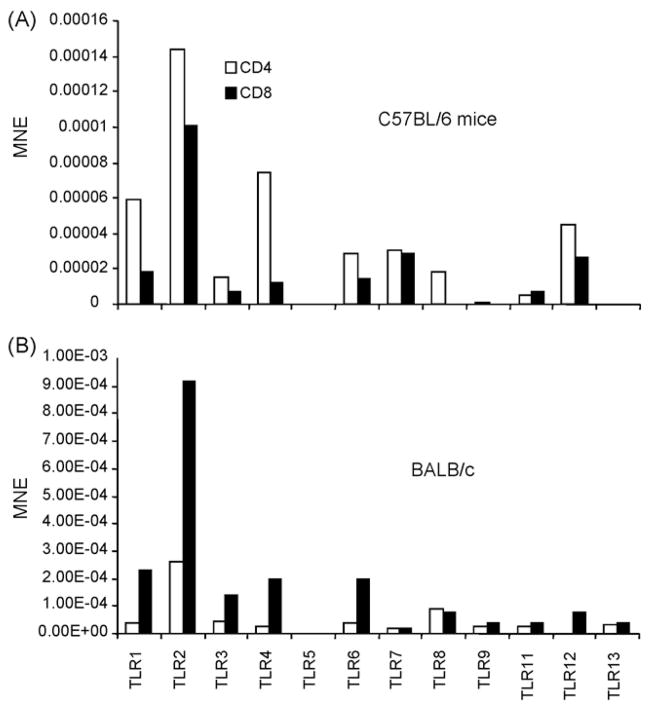
To determine whether poly(I:C) acts directly on T cells, TLR expression in these cells was analyzed. Because OT-1 cells are on C57BL/6 (H2b) background and because there might be strain-dependent quantitative and qualitative differences in TLR expression, the expression of TLR2, TLR3, TLR4, TLR5, TLR6, TLR7 and TLR9 was analyzed in purified subsets of CD4+ or CD8+ T cells from C57BL/6 or BALB/c mice using real time RT-PCR. Similar levels of expression of TLR4 and TLR6 were observed among CD4+ T cells from C57BL/6 or BALB/c mice. CD4+ T cells from C57BL/6 mice expressed higher levels of TLR2, TLR5 and TLR7 (Fig. 2A), while CD4+ cells from BALB/c mice expressed higher levels of TLR3 and TLR9 (Fig. 2B). CD8+ T cells from C57BL/6 (Fig. 3A) mice expressed considerably lower levels of TLR3, TLR4, TLR6, and TLR9 than CD8+ T cells from BALB/c (Fig. 3B). These differences in TLR expression might explain in part the differences in susceptibility to certain pathogens between different strains of mice.
Fig. 2.
Strain differences in the expression of TLRs on murine CD4+ T cells. CD4+ T cells were sorted from spleen of naïve from C57BL/6 (A) or BALB/c (B) mice. cDNA was prepared from these cells and TLR expression was determined by real Time RT-PCR. TLR expression is shown as mean normalized expression (MNE) relative to the expression of β-actin.
Fig. 3.
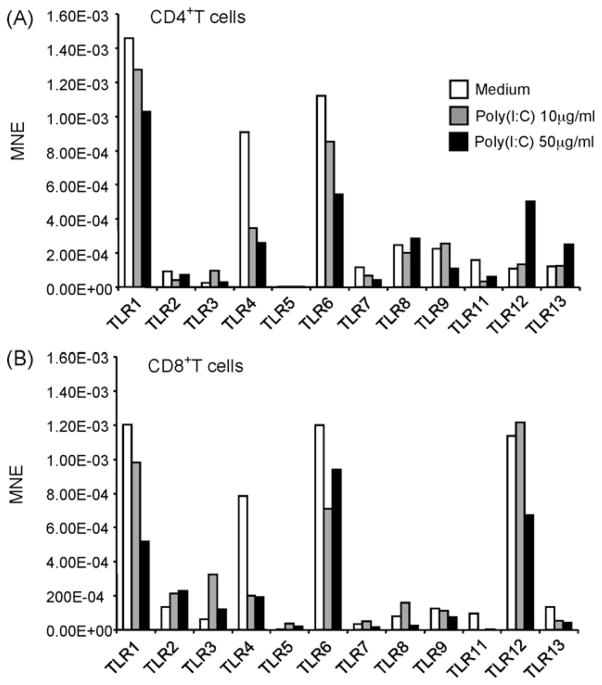
Poly(I:C) treatment of T cells alters their expression of TLRs. Purified CD4+ (A) or CD8+ (B) T cells from C57BL/6 mice were treated with either 10 μg/ml or 50 μg/ml of poly(I:C) and the expression of TLRs was determined by real Time RT-PCR. TLR expression is shown as mean normalized expression (MNE) relative to the expression of β-actin.
3.3. Poly(I:C) treatment alters the expression of TLRs
To better understand the physiological role of TLR expression in T cells, CD4+ or CD8+ T cells were purified from spleen of C57BL/6 mice and treated with poly(I:C). The effect of poly(I:C) on TLR expression was determined by real time RT-PCR (Fig. 3A). Treatment of CD4+ cells with 10 μg/ml and 50 μg/ml of poly(I:C) resulted in a dose dependent decrease in the expression of TLR1, TLR4, TLR6, and TLR7. TLR12 and TLR13 and to a lesser extent TLR8 were upregulated after treatment with 50 μg/ml of poly(I:C). Although the baseline expression levels of TLR3 were significantly lower than the other TLRs analyzed, treatment with 10 μg/ml of poly(I:C) resulted in an almost a three fold increase in its expression (Fig. 3A). Similarly, treatment of purified CD8+ T cells with the same doses of poly(I:C) resulted in a dose dependent decrease in the expression levels of TLR1, TLR4, and TLR6 (Fig. 3B). Of note, the base line expression of TLR5 in CD4+ and CD8+ T cells was very low; treatment with poly(I:C) had no effect. Therefore, in both purified CD4+ and CD8+ cells over expression of TLRs with poly(I:C) treatment was seen in TLR3, its cognate receptor. This suggests that CD4+ and to a larger extent CD8+ T cells can directly respond to poly(I:C) stimulation by up-regulating the expression of its receptor and by down-regulating expression of other TLRs. Modulation of TLR2, TLR4 and TLR8 after poly(I:C) treatment was also observed in vivo after systemic treatment of C57BL/6 mice with 200 μg/mouse poly(I:C) (data not shown), analyzed by flow cytometry.
3.4. Polyclonal activation of CD4+ and CD8+ T cells alters their TLR expression
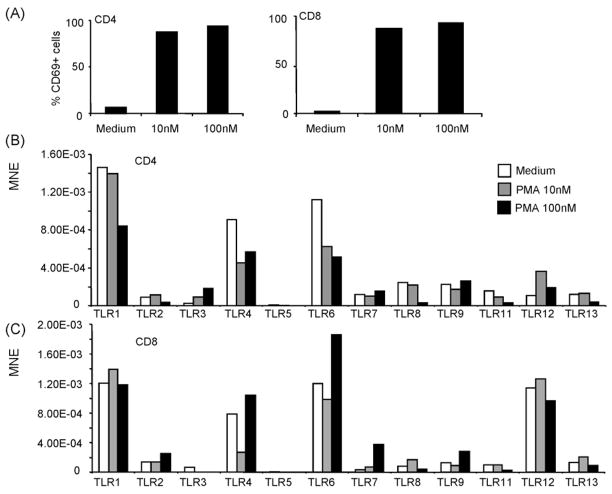
To determine whether TLR expression in T cells can be altered after polyclonal activation of these cells, CD4+ or CD8+ T cells purified from spleen of C57BL/6 mice were treated with either 10 nM or 100 nM PMA, which is a potent mitogen to T cells. PMA treatment resulted in cell activation as noted by the substantial up-regulation of the activation marker CD69 (Fig. 4A). PMA-induced activation of CD4+ T cells resulted in the down-regulation of TLR1, TLR2, TLR4, TLR6, TLR8, TLR11 and TLR13, while it induced dose dependent up-regulation of TLR3 (Fig. 4B). Thus, treatment with 10 nM of PMA resulted in the up-regulation of TLR2 and TLR12, however at the higher dose of 100 nM it decreased the levels of TLR expression. Treatment of CD8+ T cells with 10 nM PMA resulted in up-regulation of TLR8 and TLR13, whilst their treatment with 100 nM PMA resulted in down-regulation in the expression of TLR8, TLR11 and TLR13 and substantial up-regulation in the expression of TLR2, TLR4, TLR6, TLR7 and TLR9 (Fig. 4C). These data indicate that the profile of TLR expression in T cells can be modulated during activation.
Fig. 4.
Polyclonal activation of CD4+ and CD8+ T cells modulate their TLR expression. Purified CD4+ or CD8+ T cells were activated with either 10 nM or 100 nM of PMA. T cell activation was confirmed by CD69 expression (A). After activation, TLR expression was determined by real Time RT-PCR (B). TLR expression is shown as mean normalized expression (MNE) relative to the expression of β-actin.
3.5. Antigen-specific stimulation of CD8+ T cells alters their expression of TLRs
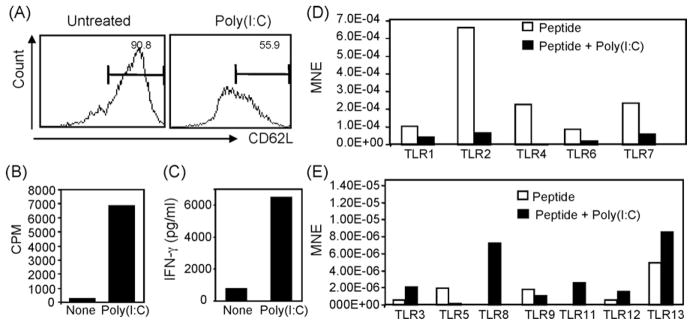
To determine the direct impact of this TLR3L on T cell responses, experiments were designed to determine whether poly(I:C) can augments T cell responses in vitro. To this end, OT-1 cells harvested from spleen of naïve C57BL/6 mice were stimulated with OVAp-pulsed syngenic splenocytes for three days in the presence or absence of 50 μg/ml of poly(I:C) and then the activation and proliferation of OT-1 cells were determined by flow cytometry. Under this setting both T cells and non-T cells were exposed to poly(I:C). It was found that stimulation with OVAp alone induced about 50% down-regulation of CD62L expression (Fig. 1, left panel) associated with very low proliferation (Fig. 5B). Addition of poly(I:C) sustained CD62L expression on OT-1 cells (Fig. 1A, right panel) and significantly augmented their proliferation (Fig. 6B). The production levels of IFN-γ by OT-1 cells in response to OVAp alone was minimal, while addition of poly(I:C) enhanced both the production of this cytokine (Fig. 5C).
Fig. 5.
Antigen-specific activation of CD8+ T cells alters their expression of TLRs. Un-fractionated spleen cell suspension (1 × 106 cells/ml) from C57BL/6 mice. OT-1 mice were primed with 1 μg/ml OVAp for 18 h in the presence or absence of 50 μg/ml of the TLR3L poly(I:C). Cells were harvested and stained with mAbs against Vα2, CD8, and CD62L. (A) Ag-specific OT-1 cells (Vα2+CD8+) were assayed for CD62L expression. (B) Shows assessment of the Ag-specific T cell proliferation. OT-1 cells were cultured and stimulated with 1 μg/ml OVAp for three days in the presence or absence of 50 μg/ml of the TLR3L poly(I:C), and the level of their proliferation was then assessed by thymidine uptake. (C) Supernatants were harvested from the cells cultured in (B), and the levels of IFN-γ production were measured. (D) CD8+ T cells were sorted from the culture in (A) and TLR expression was determined in the sorted cells by real Time RT-PCR (B). TLR expression is shown as mean normalized expression (MNE) relative to the expression of β-actin.
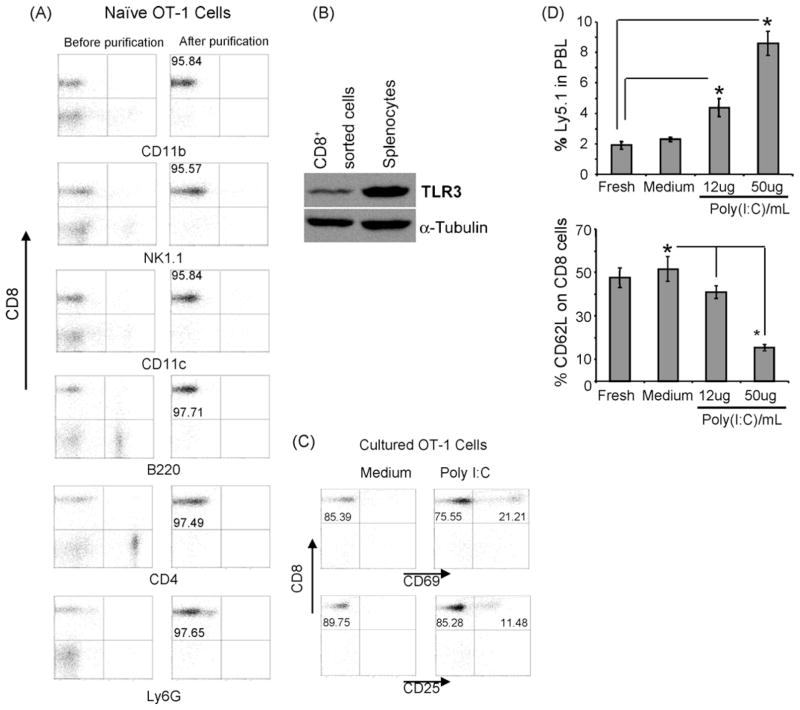
Fig. 6.
In vitro treatment of purified CD8+ T cells with poly(I:C) results in increases in their activation and expansion upon their adoptive transfer. (A) Naïve CD8+ T cells from OT-1 from C57BL/6 mice were purified by cell sorting. Cell purity was confirmed by flow cytometry analyses of specific markers for other cell subsets before and after purification. (B) Protein expression of TLR3 was measured by Western blot analysis in both un-fractionated splenocytes and purified CD8+ T cells from naïve OT-1 mice. (C) Treatment of highly purified CD8+ T cells with 50 μg/ml poly(I:C) for 16 h resulted in their activation as evidenced by the increase in expression of CD69 and CD25, for flow cytometry analysis, the same number of events of CD8+ cells from all culture conditions were collected. (D) Fresh, untreated or poly(I:C) treated naïve highly purified OT-1 cells (from Ly5.1 donor) were adoptively transferred to naïve wild type host (Ly5.2). Mice were vaccinated with OVAp 24 h after transfer. Peripheral blood was collected three days after vaccination and the fraction of Ly5.1+ was determined by flow cytometry. CD8+ activation of the donor population was determined by assaying the expression of CD62L on the Ly5.1+Vα2+ fraction. *P < 0.05 as compared to their control counterparts.
To further determine whether induction of Ag-specific T cell proliferation can alter their TLR expression profile, purified naïve OT-1 cells were activated in vitro with OVAp-pulsed splenocytes for 1 day in the presence or absence of poly(I:C). Then, CD8+ T cells were sorted by flow cytometry and their expression levels of TLRs was determined compared to untreated OT-1 cells. Activation in response to antigen priming was demonstrated by a decrease in the expression of CD62L (Fig. 5A). Addition of 50 μg/ml poly(I:C) induced up-regulation of TLR3, TLR8, TLR11, TLR12 and TLR13 (Fig. 5E), suggesting that Ag-specific stimulation of T cells modulate their expression of TLR, in particular in the presence of a TLR agonist such as poly(I:C).
3.6. CD8+ T cells respond directly to the TLR3L poly(I:C)
To directly corroborate the direct effect of poly(I:C) on CD8+ T cells, naïve OT-1 cells highly purified from spleen of naïve OT-1 (C57BL/6) mice were generated by automated cell sorting. Other cells, such as NK (NK1.1+) cells, DCs (CD11c+), B (B220+) cells, and macrophages (CD11b+), known to respond to poly(I:C) were excluded as evidenced by flow cytometry analyses of the sorted fraction (Fig. 6A). Of note, these purified CD8+ T cells expressed TLR3 at the protein level; un-fractionated spleen cells were used as positive control for the expression of TLR3 in CD8+ T cells (Fig. 6B). Treatment of the highly purified CD8+ fraction with 50 μg/ml of poly(I:C) in vitro for overnight (16 h) resulted in their activation as evidenced by the increased expression of CD69 and CD25 (Fig. 6C). Interestingly, when these poly(I:C)-conditioned OT-1 cells were adoptively transferred into naïve recipient followed by OVAp vaccination, they showed a marked increase in their expansion compared with fresh or untreated cultured OT-1 cells (Fig. 6D). A correlation between the cell expansion and activation was also observed, expression of CD62L was inversely correlated with frequency of donor OT-1 cells (Fig. 6E), suggesting that pre-treatment of naïve CD8+ T cells with poly(I:C) results in their activation which in turns results in their increased expansion and activation after in vivo priming.
4. Discussion
In the present study, the expression of TLRs among purified CD4+ and CD8+ T cells was demonstrated. Importantly, evidence for a physiological function of TLR on T cells is shown. Treatment of purified CD4+ or CD8+ T cells with poly(I:C) results in changes in the expression of certain TLRs. Additionally, activation with the T cell mitogen PMA also modulated the expression of TLRs. Furthermore, addition of poly(I:C) at the time of antigen priming of CD8+ T cells in vitro increased expression of its cognate receptor TLR3 in these cells. Importantly, treatment of purified naïve OT-1 cells with poly(I:C) induced activation of these cells, and drove them to mount higher expansion in vivo upon their adoptive transfer followed by vaccination. These results demonstrate a direct effect of poly(I:C) on CD8+ T cells and support the notion of direct involvement of TLRs in adaptive immune responses.
Given that addition of certain TLR agonists to different vaccine regimens results in enhanced T cell responses, a better understanding of the functionality of TLRs on CD8+ T cells would improve the rationale application of TLR-based immunotherapy. Until recently, most investigations on TLR have focused on cells of the innate immune system and on the role of TLRs in the initiation of antigen-specific responses following recognition of microbial products by antigen presenting cells. We have recently reported that poly(I:C) is a potent adjuvant for CD8+ T cell responses through stimulation of innate immune responses, including the rapid release of inflammatory cytokines and chemokines in serum, as well as the rapid activation of NK cells, macrophages, and DCs [36,38]. The present study further extends the potent adjuvant effects of poly(I:C) to the Ag-specific responses of T cells both in vitro and in vivo. It was found herein, however, that conditioning of DCs in vitro with poly(I:C) is not sufficient to optimize the OT-1 T cell responses in vivo unless the recipient host was conditioned again with poly(I:C) (Fig. 1). Therefore, it could be hypothesized that other cellular components such as NK cells or T cells in the host microenvironment are involved in the adjuvant effects mediated by triggering TLR3 signaling pathway. In our previous studies we explored the roles mediated by NK cells and the cytokines induced by these cells in mediating the adjuvant effects of poly(I:C) [38]. Therefore, our studies were focused herein to explore whether T cells also respond directly to poly(I:C). We found clearly that both CD4+ and CD8+ T cells express different TLRs. Expression of TLR1, TLR2, TLR6, TLR7 and TLR9 mRNA in murine, feline, and human T cells have also been reported [24,34,39,40]. Some studies performed on CD4+ cells in BALB/c mice [24], however, showed no expression of TLR2 in these cells. In contrast, our data showed that expression of TLR2 on CD4+ cells is the highest compared to the expression of other TLRs. This discrepancy in TLR2 expression could be attributed to a possible strain-dependent quantitative and qualitative differences in TLR expression, or because we used real-time RT-PCR analysis which is more accurate and quantitative than the conventional RT-PCR. This led us to compare the expression of TLRs in purified subsets of CD4+ or CD8+ T cells from C57BL/6 or BALB/c mice. Our detailed analysis showed that splenic CD4+ T cells purified from BALB/c and C57BL/6 mice express different levels of TLRs with the magnitude of TLR2 > TLR4 > TLR6 > TLR7 > TLR5 > TLR3 > TLR9 for CD4+ in C57BL/6 mice, and TLR4 > TLR6 > TLR2 > TLR9 > TLR7 > TLR3 > TLR5 for CD4+ in BALB/c mice. Of note, the TLR expression was comparable in CD4+ T cells of BALB/c and C57BL except for TLR2 which was two-fold higher in C57BL/6 mice (Fig. 2). In the case of CD8+ T cells, their TLR expression showed TLR2 > TLR6 > TLR7 > TLR4 > TLR3 > TLR9 > TLR5 in C57BL/6 mice and TLR6 > TLR4 > TLR9 > TLR2 > TLR7 > TLR3 > TLR5 in BALB/c mice. Of note, expression of TLR4, TLR6, and TLR9 was higher in BALB/c mice than in C57BL/6 mice. Overall, the magnitude of TLR expression in CD8+ T cells was higher than in CD4+ T cells. Together, these data suggest a marked difference in TLR expression is dependent on both the animal strain and the cell type. Alteration of TLR expression in T cells after their activation has also been reported in preclinical and clinical studies under different activation settings. For instance, anti-CD3 activated CD4+ T cells showed increases in TLR3, TLR5, and TLR9 and decreases in TLR2 and TLR4 expression levels [24]. TLR expression showed significant increase in memory (CD44+) CD4 and CD8 T cells after 14 days of burn injury [41]. Virally-infected T cell lines [40], as well as CD8+ T cells and B cells [42] from virally-infected individuals also showed altered TLR expression levels. Thus, altogether, these data suggest that modulation of TLR expression in cells of adaptive immunity upon their activation might be a possible mechanism to regulate the ongoing T cell-mediated immunity.
In addition to the difference in TLR expression based on the H2 background or T cell type, our data also showed that antigen-dependent activation of T cells induced alteration in the expression profile of TLR with a tendency to increase the expression levels of TLR3. When OT-1 cells were conditioned with OVAp and poly(I:C) in vitro, the cells showed higher activation phenotype (CD62Lhigh)), higher proliferation, and higher capability of producing large amount of IFN-γ (Fig. 5A–C). These in vitro adjuvant effects of poly(I:C) on OT-1 cells could be attributed to its direct effects on CD8+ T cells, since these cells showed appreciated levels of TLR expression (Fig. 5D and E), hypothesizing that CD8+ T cells respond directly to the TLR3L poly(I:C). By testing this hypothesis directly, it was found that triggering TLR3 signaling pathway in CD8+ T cells instructed these cells to express better Ag-specific functionality upon their adoptive transfer in vivo (Fig. 6). Similar to our results, the TLR1/2L Pam3 was reported to co-stimulate Ag-activated T cells in vitro, which was associated with increases in the cell proliferation, survival and functions [43], and that ligation of TLR3 in effector CD8+ T cells, but neither naïve nor central memory cells, in vitro increased their IFN-γsecretion [44]. Furthermore, activation of naïve CD4+ T cells led to increases in the expression levels of TLR3, TLR5, and TLR9 and decreases in the expression of TLR2 and TLR4, explaining why treatment of these activated CD4+ T cells only with the TLR3L poly(I:C) and the TLR9L CpG, but not with the TLR2L peptidoglycan or the TLR4L LPS, directly enhanced their survival in vitro and in vivo [24]. Of note, this study showed that this TLR3 and TLR9-induced CD4+ T cell activation required NF-αB activation and was associated with Bcl-xL up-regulation. Recent studies have also shown direct effect of CpG on CD4+ T cells [24,45] through a MyD88 and PI3K-dependent mechanism [46]. The direct effects of TLRLs on T cells discussed above would explain some of their potent adjuvant effects and also explain why T cells stimulated by antigenic peptide in vivo divide well, but fail to accumulate efficiently unless TLR agonists are present [47]. Taken with our results, it can be suggested that TLRLs can directly target cellular components of adaptive immunity including CD4+ and CD8+ T cells.
Expression of functional TLRs on T cells has been found to be extended to regulatory cells since recent observations also suggest that TLRLs have the capacity to directly regulate T cell responses and modulate the suppressive activity of CD4+CD25+ regulatory T (Treg) cells. For instance, it has been found that Treg cells express TLR5 at levels comparable to those on monocytes and DCs [48], and that the TLR9L CpG synergizes with anti-CD3 to induce partial abrogation in the suppressive activity of Treg cells [48]. By contrast, costimulation of Treg cells with the TLR5L flagellin potently increased their suppressive capacity and enhanced expression of FOXP3, the surrogate marker for Treg cells [49,50], by inducing the regulatory molecule SOCS-1 (suppressor of cytokine signalling-1) [51]. These data indicate that the quality of the T cell response depends on what type of TLR is triggered. Therefore, the nature of TLR/TLRL signaling pathway should be carefully considered during application of TLR-based stimulation of innate or adaptive immunity. Large-scale analysis of immune cell TLR expression in the mouse revealed that cells of the innate immune system express a broader number of TLR than cells of the adaptive immune system [29]. It remains, however, to perform quantitative analysis at the level of each TLR expression by cells of innate and adaptive immunity, since the former might express much higher message of TLR than the latter. These studies might explore whether the quantity of TLR expression impacts on the quality of the cell responses to TLRLs.
The current concept on the mechanisms underlying the adjuvant effects of TLRLs is the direct targeting and stimulation of innate immune cells. Our data presented in this study support the concept that TLRLs can also directly target the adaptive immune cells. Considering this concept during TLRL-based treatments would improve the application of these adjuvants to active vaccination. Furthermore, this direct responsiveness of T lymphocytes to TLRLs offers new perspectives for the immunotherapeutic manipulation of T cell responses in the adoptive immunotherapy setting. Ultimately, TLRLs can condition T cells during their stimulation with cognate Ag in vitro and can also condition the host microenvironment upon adoptive transfer of in vitro TLRL-conditioned T cells. Such dual conditioning system would lead to robust T cell responses.
Acknowledgments
This work was supported by the National Institutes of Health Grant 1 R01 CA94856-01.
References
- 1.Palucka K, Banchereau J. Linking innate and adaptive immunity. Nat Med. 1999;5(8):868–70. doi: 10.1038/11303. [DOI] [PubMed] [Google Scholar]
- 2.Palucka K, Banchereau J. Dendritic cells: a link between innate and adaptive immunity. J Clin Immunol. 1999;19(1):12–25. doi: 10.1023/a:1020558317162. [DOI] [PubMed] [Google Scholar]
- 3.Belardelli F, Ferrantini M. Cytokines as a link between innate and adaptive antitumor immunity. Trends Immunol. 2002;23(4):201–8. doi: 10.1016/s1471-4906(02)02195-6. [DOI] [PubMed] [Google Scholar]
- 4.Ishii KJ, Uematsu S, Akira S. ‘Toll’ gates for future immunotherapy. Curr Pharm Des. 2006;12(32):4135–42. doi: 10.2174/138161206778743484. [DOI] [PubMed] [Google Scholar]
- 5.Bowie A, O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal generators for pro-inflammatory interleukins and microbial products. J Leukoc Biol. 2000;67(4):508–14. doi: 10.1002/jlb.67.4.508. [DOI] [PubMed] [Google Scholar]
- 6.Honda K, Sakaguchi S, Nakajima C, Watanabe A, Yanai H, Matsumoto M, et al. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc Natl Acad Sci USA. 2003;100(19):10872–7. doi: 10.1073/pnas.1934678100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, et al. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420(6913):324–9. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, et al. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol. 2002;169(12):6668–72. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413(6851):78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- 10.Horng T, Barton GM, Flavell RA, Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature. 2002;420(6913):329–33. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- 11.Horng T, Barton GM, Medzhitov R. TIRAP: an adapter molecule in the Toll signaling pathway. Nat Immunol. 2001;2(9):835–41. doi: 10.1038/ni0901-835. [DOI] [PubMed] [Google Scholar]
- 12.O’Neill LA. How Toll-like receptors signal: what we know and what we don’t know. Curr Opin Immunol. 2006;18(1):3–9. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, et al. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol. 2003;33(4):827–33. doi: 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- 14.Matsushima H, Yamada N, Matsue H, Shimada S. TLR3-, TLR7-, and TLR9-mediated production of proinflammatory cytokines and chemokines from murine connective tissue type skin-derived mast cells but not from bone marrow-derived mast cells. J Immunol. 2004;173(1):531–41. doi: 10.4049/jimmunol.173.1.531. [DOI] [PubMed] [Google Scholar]
- 15.Hoebe K, Janssen EM, Kim SO, Alexopoulou L, Flavell RA, Han J, et al. Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trif-independent pathways. Nat Immunol. 2003;4(12):1223–9. doi: 10.1038/ni1010. [DOI] [PubMed] [Google Scholar]
- 16.de Jong EC, Vieira PL, Kalinski P, Schuitemaker JH, Tanaka Y, Wierenga EA, et al. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse th cell-polarizing signals. J Immunol. 2002;168(4):1704–9. doi: 10.4049/jimmunol.168.4.1704. [DOI] [PubMed] [Google Scholar]
- 17.Davidson B, Reich R, Lazarovici P, Nesland JM, Skrede M, Risberg B, et al. Expression and activation of the nerve growth factor receptor TrkA in serous ovarian carcinoma. Clin Cancer Res. 2003;9(6):2248–59. [PubMed] [Google Scholar]
- 18.Schwarz K, Storni T, Manolova V, Didierlaurent A, Sirard JC, Rothlisberger P, et al. Role of Toll-like receptors in costimulating cytotoxic T cell responses. Eur J Immunol. 2003;33(6):1465–70. doi: 10.1002/eji.200323919. [DOI] [PubMed] [Google Scholar]
- 19.Arico E, Robertson K, Allen D, Ferrantini M, Belardelli F, Nash AA. Humoral immune response and protection from viral infection in mice vaccinated with inactivated MHV-68: effects of type I interferon. J Interferon Cytokine Res. 2002;22(11):1081–8. doi: 10.1089/10799900260442502. [DOI] [PubMed] [Google Scholar]
- 20.Sivori S, Falco M, Della Chiesa M, Carlomagno S, Vitale M, Moretta L, et al. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci USA. 2004 July 6;101(27):10116–21. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salem ML, El-Naggar SA, Kadima A, Gillanders WE, Cole DJ. The adjuvant effects of the toll-like receptor 3 ligand polyinosinic-cytidylic acid poly (I:C) on antigen-specific CD8+ T cell responses are partially dependent on NK cells with the induction of a beneficial cytokine milieu. Vaccine. 2006;24(24):5119–32. doi: 10.1016/j.vaccine.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Salem ML, Kadima AN, Cole DJ, Gillanders WE. Defining the antigen-specific T-cell response to vaccination and poly(I:C)/TLR3 signaling: evidence of enhanced primary and memory CD8 T-cell responses and antitumor immunity. J Immunother. 2005;28(3):220–8. doi: 10.1097/01.cji.0000156828.75196.0d. [DOI] [PubMed] [Google Scholar]
- 23.Salem ML, Kadima AN, El-Naggar SA, Rubinstein MP, Chen Y, Gillanders WE, et al. Defining the ability of cyclophosphamide preconditioning to enhance the antigen-specific CD8+ T-cell response to peptide vaccination: creation of a beneficial host microenvironment involving type I IFNs and myeloid cells. J Immunother. 2007;30(1):40–53. doi: 10.1097/01.cji.0000211311.28739.e3. [DOI] [PubMed] [Google Scholar]
- 24.Gelman AE, Zhang J, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J Immunol. 2004;172(10):6065–73. doi: 10.4049/jimmunol.172.10.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lore K, Betts MR, Brenchley JM, Kuruppu J, Khojasteh S, Perfetto S, et al. Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J Immunol. 2003;171(8):4320–8. doi: 10.4049/jimmunol.171.8.4320. [DOI] [PubMed] [Google Scholar]
- 26.Maurer T, Heit A, Hochrein H, Ampenberger F, O’Keeffe M, Bauer S, et al. CpG-DNA aided cross-presentation of soluble antigens by dendritic cells. Eur J Immunol. 2002;32(8):2356–64. doi: 10.1002/1521-4141(200208)32:8<2356::AID-IMMU2356>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 27.Thompson KA, Strayer DR, Salvato PD, Thompson CE, Klimas N, Molavi A, et al. Results of a double-blind placebo-controlled study of the double- stranded RNA drug polyI:polyC12U in the treatment of HIV infection. Eur J Clin Microbiol Infect Dis. 1996;15(7):580–7. doi: 10.1007/BF01709367. [DOI] [PubMed] [Google Scholar]
- 28.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, et al. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168(9):4531–7. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 29.Applequist SE, Wallin RP, Ljunggren HG. Variable expression of Toll-like receptor in murine innate and adaptive immune cell lines. Int Immunol. 2002;14(9):1065–74. doi: 10.1093/intimm/dxf069. [DOI] [PubMed] [Google Scholar]
- 30.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168(2):554–61. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 31.Guillot L, Le Goffic R, Bloch S, Escriou N, Akira S, Chignard M, et al. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem. 2005;280(7):5571–80. doi: 10.1074/jbc.M410592200. [DOI] [PubMed] [Google Scholar]
- 32.Xu D, Komai-Koma M, Liew FY. Expression and function of Toll-like receptor on T cells. Cell Immunol. 2005;233(2):85–9. doi: 10.1016/j.cellimm.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 33.Brentano F, Schorr O, Gay RE, Gay S, Kyburz D. RNA released from necrotic synovial fluid cells activates rheumatoid arthritis synovial fibroblasts via Toll-like receptor 3. Arthritis Rheum. 2005;52(9):2656–65. doi: 10.1002/art.21273. [DOI] [PubMed] [Google Scholar]
- 34.Mansson A, Adner M, Cardell LO. Toll-like receptors in cellular subsets of human tonsil T cells: altered expression during recurrent tonsillitis. Respir Res. 2006;7(1):36. doi: 10.1186/1465-9921-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabinovich GA, Rubinstein N, Matar P, Rozados V, Gervasoni S, Scharovsky GO. The antimetastatic effect of a single low dose of cyclophosphamide involves modulation of galectin-1 and Bcl-2 expression. Cancer Immunol Immunother. 2002;50(11):597–603. doi: 10.1007/s00262-001-0238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salem ML, Kadima AN, Cole DJ, Gillanders WE. Defining the antigen-specific T-cell response to vaccination and poly(I:C)/TLR3 signaling: evidence of enhanced primary and memory CD8 T-cell responses and antitumor immunity. J Immunother. 2005;28(3):220–8. doi: 10.1097/01.cji.0000156828.75196.0d. [DOI] [PubMed] [Google Scholar]
- 37.Salem ML. Estrogen, a double-edged sword: modulation of TH1- and TH2-mediated inflammations by differential regulation of TH1/TH2 cytokine production. Curr Drug Targets Inflamm Allergy. 2004;3(1):97–104. doi: 10.2174/1568010043483944. [DOI] [PubMed] [Google Scholar]
- 38.Salem ML, Kadima A, EL-Naggar S, Rubinstein MM, Gillanders WE, Cole DJ. Defining the ability of cyclophosphamide preconditioning to enhance the antigen-specific CD8 T cell response to peptide vaccination: creation of a beneficial host microenvironment involving type I IFNs and myeloid cells. J Immunother. 2007;30:40–53. doi: 10.1097/01.cji.0000211311.28739.e3. [DOI] [PubMed] [Google Scholar]
- 39.Ferigo N, Cottalorda J, Allard D, Gentil-Perret A, Fessy M, Berger C, et al. Successful treatment via chemotherapy and surgical resection of a femoral hemangiopericytoma with pulmonary metastasis. J Pediatr Hematol Oncol. 2006;28(4):237–40. doi: 10.1097/01.mph.0000212903.61276.4b. [DOI] [PubMed] [Google Scholar]
- 40.Ignacio G, Nordone S, Howard KE, Dean GA. Toll-like receptor expression in feline lymphoid tissues. Vet Immunol Immunopathol. 2005;106(3–4):229–37. doi: 10.1016/j.vetimm.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 41.Cairns B, Maile R, Barnes CM, Frelinger JA, Meyer AA. Increased Toll-like receptor 4 expression on T cells may be a mechanism for enhanced T cell response late after burn injury. J Trauma. 2006;61(2):293–8. doi: 10.1097/01.ta.0000228969.46633.bb. discussion: 8–9. [DOI] [PubMed] [Google Scholar]
- 42.Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Diminished expression and function of TLR in lymphatic filariasis: a novel mechanism of immune dysregulation. J Immunol. 2005;175(2):1170–6. doi: 10.4049/jimmunol.175.2.1170. [DOI] [PubMed] [Google Scholar]
- 43.Cottalorda A, Verschelde C, Marcais A, Tomkowiak M, Musette P, Uematsu S, et al. TLR2 engagement on CD8 T cells lowers the threshold for optimal antigen-induced T cell activation. Eur J Immunol. 2006;36(7):1684–93. doi: 10.1002/eji.200636181. [DOI] [PubMed] [Google Scholar]
- 44.Tabiasco J, Devevre E, Rufer N, Salaun B, Cerottini JC, Speiser D, et al. Human effector CD8+ T lymphocytes express TLR3 as a functional coreceptor. J Immunol. 2006;177(12):8708–13. doi: 10.4049/jimmunol.177.12.8708. [DOI] [PubMed] [Google Scholar]
- 45.Bendigs S, Salzer U, Lipford GB, Wagner H, Heeg K. CpG-oligodeoxynucleotides co-stimulate primary T cells in the absence of antigen-presenting cells. Eur J Immunol. 1999;29(4):1209–18. doi: 10.1002/(SICI)1521-4141(199904)29:04<1209::AID-IMMU1209>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 46.Gelman AE, LaRosa DF, Zhang J, Walsh PT, Choi Y, Sunyer JO, et al. The adaptor molecule MyD88 activates PI-3 kinase signaling in CD4+ T cells and enables CpG oligodeoxynucleotide-mediated costimulation. Immunity. 2006;25(5):783–93. doi: 10.1016/j.immuni.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202(5):637–50. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiffoleau E, Heslan JM, Heslan M, Louvet C, Condamine T, Cuturi MC. TLR9 ligand enhances proliferation of rat CD4+ T cell and modulates suppressive activity mediated by CD4+ CD25+ T cell. Int Immunol. 2007;19(2):193–201. doi: 10.1093/intimm/dxl136. [DOI] [PubMed] [Google Scholar]
- 49.Crellin NK, Garcia RV, Hadisfar O, Allan SE, Steiner TS, Levings MK. Human CD4+ T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 in CD4+CD25+ T regulatory cells. J Immunol. 2005;175(12):8051–9. doi: 10.4049/jimmunol.175.12.8051. [DOI] [PubMed] [Google Scholar]
- 50.Kabelitz D. Expression and function of Toll-like receptors in T lymphocytes. Curr Opin Immunol. 2007;19(1):39–45. doi: 10.1016/j.coi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 51.Okugawa S, Yanagimoto S, Tsukada K, Kitazawa T, Koike K, Kimura S, et al. Bacterial flagellin inhibits T cell receptor-mediated activation of T cells by inducing suppressor of cytokine signalling-1 (SOCS-1) Cell Microbiol. 2006;8(10):1571–80. doi: 10.1111/j.1462-5822.2006.00731.x. [DOI] [PubMed] [Google Scholar]