Abstract
Lateral fluid percussion injury (LFPI) is the most commonly used experimental model of human traumatic brain injury (TBI). To date, investigators using this model have produced injury using a pendulum-and-piston-based device (PPBD) to drive fluid against an intact dural surface. Two disadvantages of this method, however, are (1) the necessary reliance on operator skill to position and release the pendulum, and (2) reductions in reproducibility due to variable friction between the piston’s o-rings and the cylinder. To counteract these disadvantages, we designed a low-priced, novel, fluid percussion apparatus that delivers a pressure pulse of air to a standing column of fluid, forcing it against the intact dural surface. The pressure waveforms generated by this apparatus are similar to those reported in the LFPI/PPBD literature and had little variation in appearance between trials. In addition, our apparatus produced an acute and chronic TBI syndrome similar to that in the LFPI/PPBD literature, as quantified by histological changes, MRI structural changes and chronic behavioral sequelae.
Keywords: Lateral fluid percussion injury, Rat, Experimental TBI, Picospritzer
1. Introduction
Fluid percussion produces brain injury by creating pressure transients that are applied to an intact dural surface through a small craniotomy, resulting in both focal and diffuse cerebral injury (Laurer and McIntosh, 1999; McIntosh et al., 1989). Lateral fluid percussion injury (LFPI) is currently the most commonly used experimental model of traumatic brain injury (TBI; greater than 200 PubMed publications) and has been shown to reliably replicate many of the acute and chronic features of human TBI (Laurer and McIntosh, 1999; McIntosh et al., 1989; Morales et al., 2005; Pierce et al., 1998; Statler et al., 2001; Thompson et al., 2005). In the published literature using this model, the fluid pressure pulse has traditionally been created using a device consisting of a Plexiglas cylinder with a Plexiglas piston fitted to one end (a pendulum-and-piston-based device, PPBD). The entire system is filled with fluid. When the metal pendulum strikes the piston of the injury device, it creates a pressure wave that then travels through the cylinder and through a small plastic tube that fits into a syringe hub previously implanted in the animal’s skull. The primary disadvantages of this method are (1) the necessary reliance on operator skill to position and release the pendulum in order to obtain a similar, smooth pressure pulse wave with every injury, and (2) reductions in reproducibility due to variable friction between the piston’s o-rings and the cylinder (data not shown). We sought to design and build an apparatus for lateral fluid percussion injury without the piston or pendulum to counteract these potential disadvantages of the PPBD.
2. Materials and methods
2.1. Establishment of dural access
Dural access was achieved using methodology previously reported by Toth et al. (1997). This method has been well-validated (Santhakumar et al., 2001, 2003, 2005) and represents an adaptation of the original description of the LFPI/PPBD model in the rat (McIntosh et al., 1989). Briefly, adult male Sprague–Dawley rats (250–300 g) were anesthetized with isoflurane (Isosol, VEDCO, Inc., St. Joseph, MO) via nose cone and placed in a stereotaxic head frame. Anesthesia was maintained with 3–3.5% isoflurane during the entire procedure. After scalp incision, a 3 mm diameter craniotomy was created and centered at −3 mm from bregma and 3.5 mm left of the sagittal suture using a Dremel drill with a #105 bit. The dural surface was left intact. For support, two steel screws were placed in the skull in the following locations (1) 1 mm caudal to lambda and (2) in the right parietal bone opposite the craniotomy site. A female Luer-Loc hub (inside diameter of 3.5 mm) was centered over the craniotomy site and bonded to the skull with cyanoacrylate adhesive. Dental acrylic (Snap, Parkell, Inc., Edge-wood, NJ) was poured around the Luer hub and support screws. After the acrylic hardened, antibiotic ointment was placed around the injury cap and the animal was removed from the stereotaxic frame and returned to his cage to recover.
2.2. Apparatus design
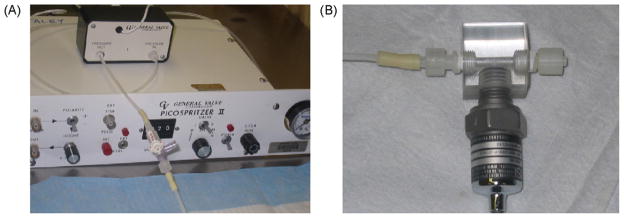
The Picospritzer-FPI (PFPI) apparatus utilized a Picospritzer II TM (Parker Hannifin, Pneutronics Division, Pine Brook, NJ) to deliver a standardized pressure pulse of air to a standing column of fluid. The Picospritzer TM uses a high-speed valve to deliver standardized pulses of compressed air. Pulses are triggered either manually or electronically via a transistor-transistor-logic-level gate. This device was originally designed to allow for precisely controlled picoliter ejections of fluid through micropipettes and has since been used successfully in a range of scientific applications (McCaman et al., 1977) (http://www.parker.com). The Picospritzer TM can deliver 10–100 psi impact forces of 2–999 ms pulse durations (1 ms intervals). For these experiments the Picospritzer TM was connected as detailed in Fig. 1 and as described below and then the system was tested and calibrated to ensure that no air or fluid leaks were present.
Fig. 1.
Picospritzer-generated fluid percussion injury (PFPI) apparatus. (A) The Picospritzer II TM, solenoid and the rigid Teflon tubing with stopcock port for fluid-loading; (B) the small acrylic hub at the end of the Teflon tubing in A contains a transducer port and a male locking Luer designed to connect to the female Luer previously implanted onto the animal’s skull. The transducer output is amplified, displayed on a digital storage oscilloscope and then stored on a computer for offline analysis.
The picospritzer solenoid was connected to the animal via a short length of rigid Teflon tubing (4 mm outside diameter, 2 mm inside diameter) and a small acrylic hub containing a transducer port and a male locking Luer hub designed to connect to the female Luer hub previously cemented over the animal’s craniotomy for dural access. A polypropylene stopcock (Medex, Dublin, OH, product number MX5341L) was inserted in the length of the rigid tubing to allow for a fluid-loading port (37 °C isotonic saline (McIntosh et al., 1989)). Before each injury, the fluid column was examined and all air bubbles were removed.
With injury, the transducer signal (MSP-300-200-P2-N1, Measurement Specialities, Hampton, VA) was collected using a Transbridge 4-channel transducer amplifier (WPI, Sarasota, FL) and displayed and stored on a digital storage oscilloscope (Tektronix TDS1001B, Allied Electronics, Fort Worth, TX). Each waveform was carefully inspected to ensure that the pressure was delivered smoothly. The stored data can then be transferred from the oscilloscope using NI Signal Express™ Interactive Measurement Software (National Instruments, Austin, TX, purchased with oscilloscope) for offline analysis.
2.3. PFPI procedure
Fifteen to twenty hours after craniotomy and Luer hub implantation, the animals were re-anesthetized with isoflurane in a 2:1 chamber (Santhakumar et al., 2001, 2003, 2005; Toth et al., 1997). After anesthesia was ensured by the absence of both the righting and the toe-pinch reflexes, the animal was removed from the chamber and connected to the PFPI apparatus. On return of a hind limb pinch response, the operator activated the PFPI apparatus via manual push-button trigger on the picospritzer base, administering the PFPI impact force. Although the animals were not actively receiving isoflurane at the time of impact, they were still anesthetized. After disconnection from the PFPI apparatus, the animals were returned to their cages to recover. Sham-injured animals underwent establishment of dural access and were anesthetized and connected to the PFPI apparatus, but the injury pulse was not triggered. All procedures as described were approved by the University of Colorado Institutional Animal Care and Use Committee.
2.4. MRI
Six injured and six sham-injured animals underwent MRI imaging at 48 h and 1 week after injury, using T2-weighted; gadolinium-enhanced (0.2 mmol/kg Omniscan® IV) T1-weighted; and gradient echo (GE) sequences. For all MRIs, the rats were anesthetized with a combination of ketamine and xylazine (75 mg/kg and 12.5 mg/kg, respectively in sterile saline, intraperitoneally). Scans were done using a 4.7 T Bruker PharmaScan. A quadrature birdcage coil (inner diameter 38 mm, so-called “rat-brain-coil”), tuned to the 1H frequency of 200.27 MHz, was used for RF transmission and reception. T2-weighted axial MR scans were acquired using a RARE (rapid acquisition with relaxation enhancement) sequence with the following parameters: field of view = 6.4 cm; slice thickness = 1.20 mm; interslice distance = 1.20 mm; number of slices = 24; TE/TR = 32/5000 ms; number of averages = 4 per phase encode step; matrix size 128 × 256. T1-weighted MR images were acquired using a MSME (multi-slice multi-echo) sequence (TE/TR of 11.9/700 ms).
For each set of T2-weighted images, the perimeter of each hemisphere was outlined on contiguous slices using manual segmentation and the intra-perimeter areas calculated (Image J, NIH). Using these areas and the known slice thickness of 1.2 mm, we were able to estimate the volume of each brain hemisphere. As a measure of injury-related brain swelling, the difference between left (injured, or inj) and right (contralateral, or CL) brain hemisphere volumes was calculated and normalized to the volume of the right hemisphere, using the following formula:
The mean% brain swelling in injured animals was compared to that in sham-injured animals at each time point using a two-tailed t-test for independent samples assuming unequal variances.
2.5. Test of forelimb use for vertical-lateral exploration (“Cylinder test”)
Using a previously published and validated protocol (Schallert, 2006), 10 PFPI-injured and 6 sham-injured animals were individually placed in a specially designed Plexiglass cylinder 30 cm high and 20 cm in diameter. When the animal reared to explore the wall of the cylinder, the number of times the right, left or both fore-limbs were used in the initial vertical exploratory placement was noted and video-recorded for later review and confirmation. After an animal made 20 vertical exploratory movements (or 20 min had passed), the test was concluded and the animal returned to its cage. Each animal underwent testing at five different time points: pre-injury, 24 h after injury, 1 week after injury, 3 weeks after injury and 6 weeks after injury. Limb use percent for each side was calculated using the formula:
where “ipsilateral placement” refers to the forelimb of interest. The percent change of right forelimb use from an individual animal’s pretest performance was calculated, and the mean change in right forelimb use among injured rats was compared to that of sham animals at each time point using a two-tailed t-test for independent samples assuming unequal variance.
2.6. Histology
Sixty-four to 78 days after injury (and after completion of the cylinder test protocol), five animals were deeply anesthetized with isoflurane (4%) and decapitated. Their brains were rapidly removed and post-fixed in 10% formalin and processed for paraffin embedding. To quantify hippocampal cell loss, three non-contiguous 6-μm coronal sections were taken between −3.36 and −4.00 from bregma (Lowenstein et al., 1992) and stained using a 0.3% solution of cresyl violet. Digital images of the ipsilateral and contralateral CA1, CA3 and hilar hippocampal subregions were captured at 100× magnification. In Adobe Photoshop, an area 580 microns in length and 190 microns in width was delineated in each CA1 and CA3 subregion. A 580 μm × 220 μm area was delineated in the hilal subregion (Rangel et al., 2005). At higher magnification (400×), a single, blinded investigator counted neurons with round cell bodies and prominent nucleoli (Zhang et al., 1998) in each delineated region. The subregion-specific neuronal counts were then summed to give a total cell count across the three sections. The mean total cell counts for the left (injured) hippocampal subregions were then compared to the mean total cell counts for the right (contralateral) hippocampal subregions using a two-tailed t-test for paired samples, assuming unequal variances.
3. Results
Initial testing of the PFPI apparatus focused on ensuring good waveform reproducibility, and was then followed by characterization of the resultant injury using (1) biological measures of injury severity (mortality and post-injury apnea duration); (2) magnetic resonance imaging (MRI) and histopathology to assess structural disruption and cell loss; and (3) a measure of gross motor function.
3.1. Waveform reproducibility using PFPI
The pressure waveforms generated by our apparatus are morphologically similar to those reported in the early LFPI/PPBD literature ((Sullivan et al., 1976), for example later papers have not contained figures of actual waveforms). The three important criteria for these waveforms are (1) a smooth, rapid upstroke followed by a smooth downstroke, (2) the peak of the curve should be within 20 ms of pulse onset and (3) the curves should be similar in appearance across trials. To demonstrate that our apparatus meets these three criteria, Fig. 2 contains a representative waveform from a single injury (Fig. 2A shows waveform shape and duration), as well as an average waveform from a total of 24 different trials, including the single waveform shown in Fig. 2A (Fig. 2B). Fig. 2B also demonstrates the small amount of variability at each point in the series of injury waveforms.
Fig. 2.
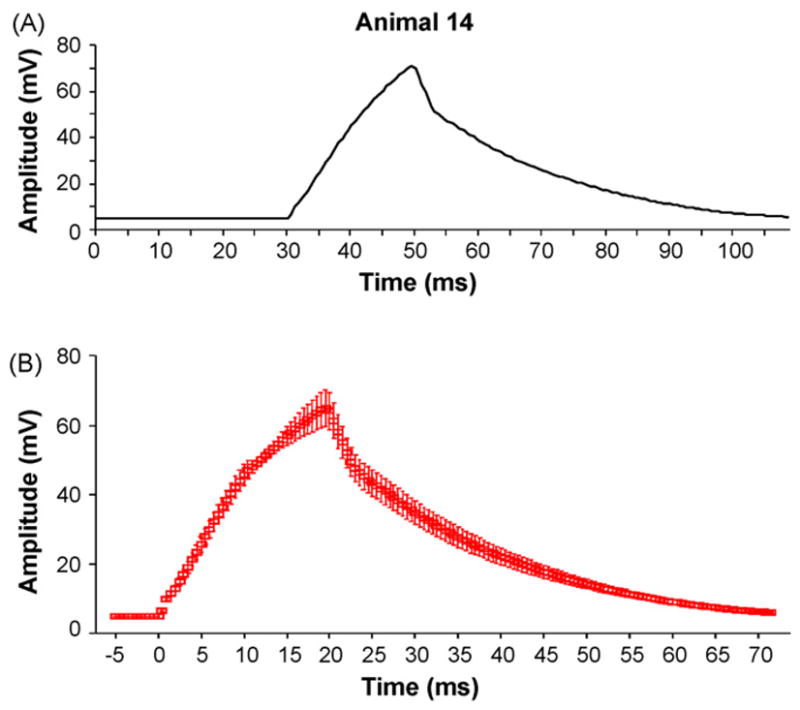
Sample traces generated by the PFPI. (A) The single PFPI-generated waveform demonstrates a smooth, rapid upstroke followed by a smooth downstroke with a peak amplitude within 20 ms of pulse initiation. X-axis, time in ms; Y-axis, amplitude in mv. Time 30 is the time of pulse initiation. (B) Average waveforms generated from 24 different animals. Note the small amount of variability at each epoch along the averaged curve. Error bars at each time point represent the mean ± SEM. X-axis, time in ms; Y-axis, amplitude in mv. Time 0 is the time of pulse initiation.
3.2. Biological measures of injury severity
During our apparatus development and testing, a total of 40 animals underwent moderate to severe PFPI (45–50 pounds/square inch, psi). Six animals did not survive injury, despite aggressive resuscitation with subcutaneous (SQ) fluids, rescue breaths and chest compressions (mortality rate = 15%). Post-injury apnea duration (another potential measure of injury severity) ranged from 2 to 25 s (mean = 17.33 ± 1.29 s, rescue breathing automatically initiated after 25 s of apnea).
3.3. PFPI-induced hemorrhage and tissue edema
Six injured and six sham-injured animals underwent MRI imaging at 48 h and 1 week after injury. Fig. 3 contains sample images done at 48 h after injury using the three different imaging sequences (gadolinium-enhanced T1-weighted, T2-weighted and gradient echo) and demonstrates the presence of injury-related tissue edema, blood–brain barrier disruption and subdural, intra-parenchymal and intraventricular hemorrhage. As shown in Table 1, the mean percent volume difference between hemispheres at 48 h (maximal tissue edema) in the injured animals was 7.37% (±1.31%), compared to a mean difference of 0.82% (±0.39%) in the sham-injured control animals (p = 0.005). At 1 week, the comparison of the injured and sham-injured animals is no longer significant, as might be expected as tissue edema resolves after injury.
Fig. 3.
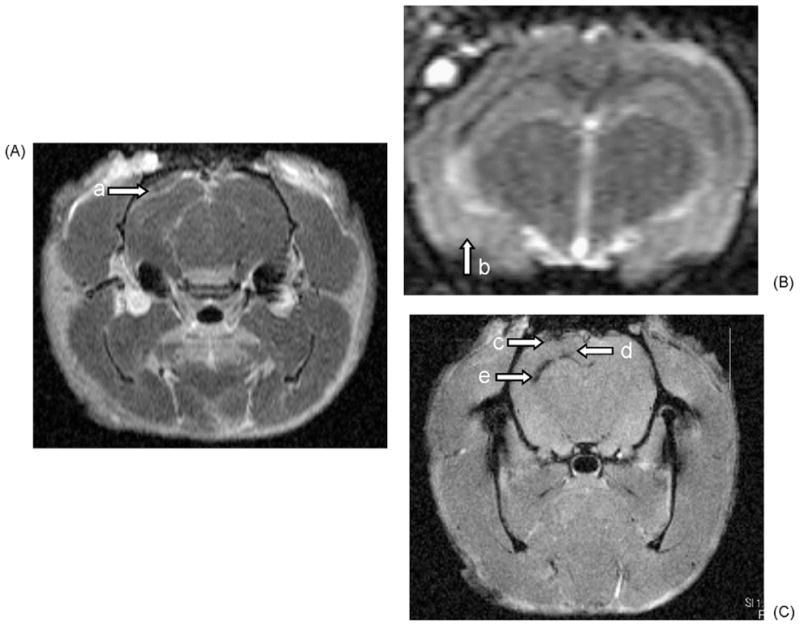
Sample MRI images 48 h after moderate to severe PFPI injury. Representative gadolinium-enhanced T1-weighted (A), T2-weighted (B), and gradient echo (C) MRI images from a single animal 48 h after PFPI injury. Extravasation of intravascular gadolinium can be seen on the gadolinium-enhanced T1-weighted images, documenting the presence of injury-related blood–brain barrier breakdown (arrow a). PFPI-related focal tissue edema appears as areas of increased signal in the cortex (arrow b). At 48 h after injury, areas of subdural (arrow c), intraparenchymal (arrow d) and intraventricular hemorrhage (arrow e) appear as dark signal on gradient echo images. All images oriented with superior up and left side to the left as viewed.
Table 1.
Percent difference in hemisphere volumes 48 h and 1 week after PFPI.
| 48 h post-injury |
1 week post-injury |
||||
|---|---|---|---|---|---|
| Animal ID | Injury severity | % Difference in hemisphere volumes (L-R) | Animal ID | Injury severity | % Difference in hemisphere volumes (L-R) |
| 410826 | Injured | 8.35 | 410826 | Injured | −0.77 |
| 410827 | Injured | 8.71 | 410827 | Injured | 6.37 |
| 426476 | Injured | 3.91 | 426476 | Injured | 0.70 |
| 426481 | Injured | 4.89 | 426480 | Injured | 1.10 |
| 426482 | Injured | 11.03 | 426481 | Injured | −1.96 |
| 410824 | Sham | 0.59 | 426482 | Injured | −1.86 |
| 410825 | Sham | 0.02 | 410824 | Sham | 0.21 |
| 426475 | Sham | 1.03 | 410825 | Sham | −0.39 |
| 426477 | Sham | 2.65 | 426475 | Sham | −1.16 |
| 426478 | Sham | 0.46 | 426477 | Sham | 2.52 |
| 426479 | Sham | 0.19 | 426478 | Sham | 0.27 |
| 426479 | Sham | −2.51 | |||
| Means | Injured | 7.37 (±1.31) | Means | Injured | 0.60 (±1.27) |
| Sham | 0.82 (±0.39) | Sham | −0.18 (±0.69) | ||
Images from Animal 426480 at 48 h could not be used due to a significant amount of slice angulation that captured non-homologous areas in the left and right hemispheres.
3.4. PFPI-induced hippocampal cell loss
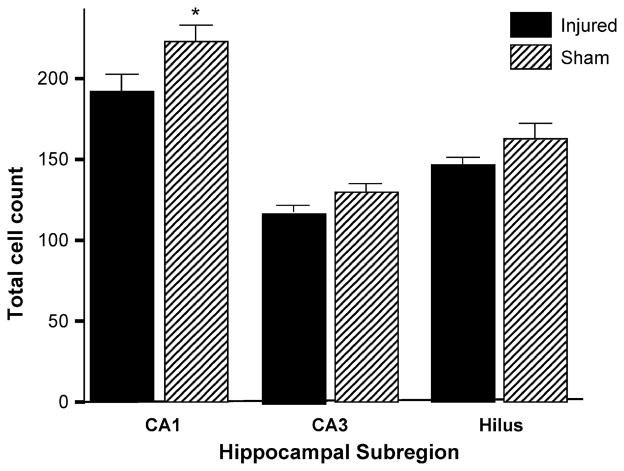
Cell counts were performed in each of three hippocampal subregions (CA1, CA3 and hilus) in five animals at 64–78 days post-injury (Fig. 4). In each of the hippocampal subregions tested, PFPI resulted in cell loss in the injured (left) hemisphere, when compared to the contralateral (right) hemisphere (Thompson et al., 2005). This difference was significant in the CA1 subregion (p = 0.004).
Fig. 4.
Hippocampal cell counts. At 64–78 days after injury, total cell counts show PFPI-related cell loss in the ipsilateral hippocampus in all subregions analyzed (n = 5). This difference was significant in the CA1 region (p = 0.004). X-axis, region and injury group; Y-axis, total cell count.
3.5. PFPI-induced persistent neurological dysfunction
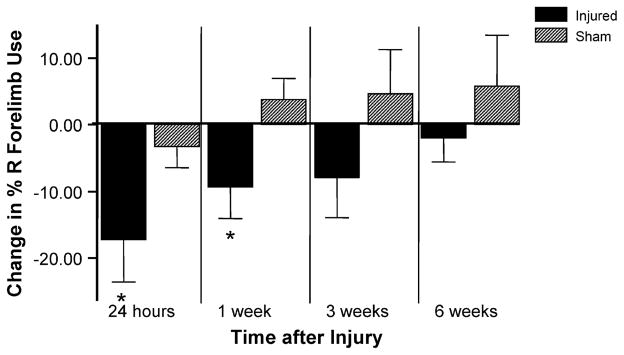
Ten injured and five sham-injured animals underwent neurological testing using the cylinder test at four different time points after injury (24 h, 1 week, 3 weeks and 6 weeks) and their performance compared to that obtained during pre-injury testing. No animals had limb use asymmetries greater than 60:40 in naive, pre-injury testing. PFPI-injured animals showed a gross motor deficit involving vertical exploratory behavior with the right forelimb for up to a week post-injury. At 24 h and 1 week after injury, the mean change in percent of right forelimb use from pre-injury testing in injured animals was significantly larger than that of sham-injured animals (p = 0.004 for 24 h post-injury comparison and p = 0.02 for 1 week post-injury comparison). This significant difference was no longer seen at the 3 and 6 weeks testing points, although a similar, but non-significant, trend was present at 3 weeks post-injury.
4. Discussion
LFPI using a pendulum-and-piston-based device (PPBD) is currently the most commonly used experimental model of human TBI. In an attempt to counteract some of the potential disadvantages of the design of the PPBD, we have developed and tested a new picospritzer-based apparatus (PFPI) for the delivery of fluid percussion injury. The pressure waveforms generated by the PFPI apparatus are similar to those reported in the LFPI/PPBD literature and, in addition, had little variation in appearance between trials. Our mortality rate, the main indicator of injury severity (Kharatishvili et al., 2006), is comparable to rates reported in the literature for moderately–severely injured LFPI-PPBD animals (McIntosh et al., 1989).
MRI imaging of PFPI-injured animals showed tissue edema, blood–brain barrier disruption and subdural, intraventricular and intraparenchymal hemorrhage, all of which have been reported with the LFPI-PPBD and are common findings after moderate and severe human TBI. In addition, intracranial hemorrhage is a well-known risk factor for some complications of TBI, such as post-traumatic epilepsy (Frey, 2003). Quantification of injury-related cerebral swelling on MRI showed a reproducible and significant amount of left hemisphere swelling (compared to the right hemisphere) at 48 h across all animals tested.
Detailed histopathological studies have shown that neuronal loss occurs after a single episode of mild, moderate or severe FPI using the PPBD (McIntosh et al., 1989; Ratzliff et al., 2002; Lowenstein et al., 1992; Toth et al., 1997; Coulter et al., 1996). Evidence of cell injury is immediately apparent (Lowenstein et al., 1992; Toth et al., 1997; Gallyas et al., 2002), with neuronal cell loss occurring maximally within the first week after injury (Toth et al., 1997). After PFPI, hippocampal cell counts were lower in all hippocampal subregions tested (CA1, CA3, hilus) in the ipsilateral (left) hemispheres, as compared to those in the contralateral hemispheres, with significant differences seen in the CA1 subregion, as has also been reported with LFPI using the PPBD (Pitkanen and McIntosh, 2006).
Lastly, this novel device also produced an acute and chronic behavioral syndrome as manifest by an emergent limb preference in vertical exploratory movements. The test of forelimb use for vertical–lateral exploration, or the cylinder test, has been shown to be a reliable measure of primary motor forelimb function after unilateral cerebral ischemia or hemorrhage in rats and mice (Schallert, 2006). Measuring the asymmetry in forepaw placing during vertical exploratory movements provides an index of lateralized brain injury. The cylinder test has been widely used in the experimental stroke and Parkinson’s disease literature (Schallert, 2006). As seen in Fig. 5, PFPI-injured animals showed a significant decrease in contralateral (right) forelimb use during vertical exploratory behavior for at least a week post-injury, a length of injury effect similar to many commonly used motor function measures after LFPI via PPBD (Fujimoto et al., 2004). To our knowledge, this is the first time that this behavioral test has been shown to successfully quantify the changes in behavior seen after experimental TBI.
Fig. 5.
Cylinder test results. At 24 h and 1 week after injury, the mean change in percent of right forelimb use from pre-injury testing in injured animals was significantly different than that of sham-injured animals (p = 0.004 for 24 h post-injury comparison and p = 0.02 for 1 week post-injury comparison). This significant difference was no longer seen at the 3 and 6 weeks testing points. X-axis, time and injury group; Y-axis, change in percent of right forelimb use.
The potential sources of variation in creating LFPI using a piston-and-pendulum-based device can be categorized as non-apparatus-specific or apparatus specific. The non-apparatus-specific sources of variability include variations in animal susceptibility (inter-strain and inter-animal variability), as well as variability in craniotomy location (Floyd et al., 2002) and cannot be addressed by changes in apparatus design. However, the apparatus-specific sources of variability can be addressed by changes in device design. Simply due to its design, the PFPI device can eliminate two major sources of apparatus-specific variability inherent to the pendulum-and-piston-based device (1) the necessary reliance on operator skill to position and release the pendulum and (2) reductions in reproducibility due to variable friction between the piston’s o-rings and the cylinder.
The main source of potential variability of the PFPI apparatus is the compressibility of the air at the top of the fluid column. Variation in the volume of air will change the upstroke of the pressure wave at the dura, with longer columns of air taking longer to be compressed and reducing the rate of rise of the pressure wave. In addition, the peak pressure could be reduced if the column of air were so large that it did not come to equilibrium with the pressure source (picospritzer), and therefore was not completely compressed during the pressure pulse. However, we have not found wide variability in the slope of the upstroke or the peak amplitudes of the PFPI-generated waveform (Fig. 2), as might be expected if the compressibility of air in the PFPI column was widely variable.
Recent studies suggest that the use of isoflurane anesthesia in the peri-injury period may confer a degree of neuroprotection after severe controlled cortical impact injury, a related experimental TBI model in the rat (Statler et al., 2000, 2006a,b). Isoflurane anesthesia is commonly used in LFPI using the PPBD, both in the procedure to establish dural access and in the injury itself. To minimize the potential impact of this drug on the creation of the PFPI injuries, we standardized our procedure to administer the PFPI impact immediately after recovery to toe-pinch. This timing has been suggested to minimize potential confounding from residual anesthetic effect, but to retain enough effect for humane treatment of the experimental animals. Although the PFPI method does not reduce the need for anesthetic/sedative use, it does allow for exact measurement of duration of anesthetic exposure (exposure time during craniotomy and establishment of dural access and time to complete anesthesia just prior to injury). Any residual isoflurane effect would be evenly expressed amongst treatment groups, and would still allow valid comparisons.
In addition to being less expensive, simpler to house and simpler to operate than the PPBD, our apparatus has little variability in waveform appearance, suggesting a marked homogeneity of injury force delivery. We have also found that PFPI-induced cerebral injury results in intraparenchymal hemorrhage, parenchymal ultrastructural disruption, hippocampal cell loss and persistent functional motor deficits after injury, all cardinal findings in human TBI. We expect that these findings, combined with the technical advantages of the PFPI apparatus, will drive needed additional investigation to fully characterize the PFPI-induced injury syndrome and its potential use in experimental TBI research.
Acknowledgments
This work was supported by the Colorado Injury Control Research Center and the National Institutes of Health (NS-35915, I.S.).
References
- Coulter DA, Rafiq A, Shumate M, Gong QZ, DeLorenzo RJ, Lyeth BG. Brain injury-induced enhanced limbic epileptogenesis: anatomical and physiological parallels to an animal model of temporal lobe epilepsy. Epilepsy Research. 1996;26:81–91. doi: 10.1016/s0920-1211(96)00044-7. [DOI] [PubMed] [Google Scholar]
- Floyd CL, Golden KM, Black RT, Hamm RJ, Lyeth BG. Craniectomy position affects morris water maze performance and hippocampal cell loss after parasagittal fluid percussion. Journal of Neurotrauma. 2002;19:303–16. doi: 10.1089/089771502753594873. [DOI] [PubMed] [Google Scholar]
- Frey LC. Epidemiology of posttraumatic epilepsy: a critical review. Epilepsia. 2003;44(Suppl 10):11–7. doi: 10.1046/j.1528-1157.44.s10.4.x. [DOI] [PubMed] [Google Scholar]
- Fujimoto ST, Longhi L, Saatman KE, McIntosh TK. Motor and cognitive function evaluation following experimental traumatic brain injury. Neuroscience and Biobehavioral Reviews. 2004;28:365–78. doi: 10.1016/j.neubiorev.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gallyas F, Farkas O, Mazlo M. Traumatic compaction of the axonal cytoskeleton induces argyrophilia: histological and theorectical importance. Acta Neuropathologica (Berl) 2002;103:36–42. doi: 10.1007/s004010100424. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I, Nissinen JP, McIntosh TK, Pitkanen A. A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience. 2006;140:685–97. doi: 10.1016/j.neuroscience.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Laurer HL, McIntosh TK. Experimental models of brain trauma. Current Opinions in Neurology. 1999:715–21. doi: 10.1097/00019052-199912000-00010. [DOI] [PubMed] [Google Scholar]
- Lowenstein DH, Thomas MJ, Smith DH, McIntosh TK. Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. Journal of Neuroscience. 1992;12:4846–53. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaman RE, Mc Kenna DG, Ono JK. A pressure system for intracellular and extracellular ejections of picoliter volumes. Brain Research. 1977;136:141. doi: 10.1016/0006-8993(77)90138-x. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, et al. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–44. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- Morales DM, Marklund N, Lebold D, Thompson HJ, Pitkanen A, Maxwell WL, et al. Experimental models of traumatic brain injury: do we really need to build a better mousetrap? Neuroscience. 2005;136:971–89. doi: 10.1016/j.neuroscience.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Pierce JES, Smith DH, Trojanowski JQ, McIntosh TK. Enduring cognitive, neurobehavioral, and histopathological changes persist for up to one year following experimental brain injury in rats. Neuroscience. 1998;87:359–69. doi: 10.1016/s0306-4522(98)00142-0. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, McIntosh TK. Animal models of post-traumatic epilepsy. Journal of Neurotrauma. 2006;23:241–61. doi: 10.1089/neu.2006.23.241. [DOI] [PubMed] [Google Scholar]
- Rangel P, Cysneiros RM, Arida RM, de Albuquerque M, Colugnati DB, Scorza CA, et al. Lovastatin reduces neuronal cell death in hippocampal CA1 subfield after pilocarpine-induced status epilepticus: preliminary results. Arquivos de Neuro-psiquiatria. 2005;63:972–6. doi: 10.1590/s0004-282x2005000600013. [DOI] [PubMed] [Google Scholar]
- Ratzliff AH, Santhakumar V, Howard A, Soltesz I. Mossy cells in epilepsy: rigor mortis or vigor mortis? Trends in Neurosciences. 2002;25:140–4. doi: 10.1016/s0166-2236(00)02122-6. [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Aradi I, Soltesz I. Role of mossy fiber sprouting and mossy cell loss in hyperexcitability: a network model of the dentate gyrus incorporating cell types and axonal topography. Journal of Neurophysiology. 2005;93:437–53. doi: 10.1152/jn.00777.2004. [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Ratzliff AD, Jeng J, Toth Z, Soltesz I. Long-term hyperexcitability in the hippocampus after experimental head trauma. Annals of Neurology. 2001;50:708–17. doi: 10.1002/ana.1230. [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Voipio J, Kaila K, Soltesz I. Post-traumatic hyperexcitability is not caused by impaired buffering of extracellular potassium. Journal of Neuroscience. 2003;23:5865–76. doi: 10.1523/JNEUROSCI.23-13-05865.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T. Behavioral tests for preclinical intervention assessment. NeuroRx. 2006;3:497–504. doi: 10.1016/j.nurx.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statler KD, Alexander H, Vagni V, Dixon CE, Clark RS, Jenkins L, et al. Comparison of seven anesthetic agents on outcome after experimental traumatic brain injury in adult, male rats. Journal of Neurotrauma. 2006a;23:97–108. doi: 10.1089/neu.2006.23.97. [DOI] [PubMed] [Google Scholar]
- Statler KD, Alexander H, Vagni V, Holubkov R, Dixon CE, Clark RS, et al. Isoflurane exerts neuroprotective actions at or near the time of severe traumatic brain injury. Brain Research. 2006b;1076:216–24. doi: 10.1016/j.brainres.2005.12.106. [DOI] [PubMed] [Google Scholar]
- Statler KD, Jenkins LW, Dixon CE, Clark RS, Marion DW, Kochanek PM. The simple model versus the super model: translating experimental traumatic brain injury research to the bedside. Journal of Neurotrauma. 2001;18:1195–206. doi: 10.1089/089771501317095232. [DOI] [PubMed] [Google Scholar]
- Statler KD, Kochanek PM, Dixon CE, Alexander HL, Warner DS, Clark RS, et al. Isoflurane improves long-term neurologic outcome versus fentanyl after traumatic brain injury in rats. Journal of Neurotrauma. 2000;17:1179–89. doi: 10.1089/neu.2000.17.1179. [DOI] [PubMed] [Google Scholar]
- Sullivan HG, Martinez J, Becker DP, Miller JD, Griffith R, Wist AO. Fluid-percussion model of mechanical brain injury in the cat. Journal of Neurosurgery. 1976;45:521–34. [PubMed] [Google Scholar]
- Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, Hovda DA, et al. Lateral fluid percussion brain injury: a 15-year review and evaluation. Journal of Neurotrauma. 2005;22:42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- Toth Z, Hollrigel GS, Gorcs T, Soltesz I. Instantaneous perturbation of dentate interneuronal networks by a pressure wave-transient delivered to the neocortex. Journal of Neuroscience. 1997;17:8106–17. doi: 10.1523/JNEUROSCI.17-21-08106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Raghupathi R, Saatman KE, Smith DH, Stutzmann JM, Wahl F, et al. Riluzole attenuates cortical lesion size, but not hippocampal neuronal loss, following traumatic brain injury in the rat. Journal of Neuroscience Research. 1998;52:342–9. doi: 10.1002/(SICI)1097-4547(19980501)52:3<342::AID-JNR10>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]