Abstract
Objective
Lead exposure in children can lead to neuropsychological impairment. This study tested whether primary prevention interventions in the newborn period prevent elevated blood lead levels (BLLs).
Methods
The Philadelphia Lead Safe Homes (LSH) Study offered parental education, home evaluation, and lead remediation to the families of urban newborns. Households were randomized to a standard lead education group or maintenance education group. We conducted home visits at baseline, six months, and 12 months. To compare BLLs, we identified a matched comparison group.
Results
We enrolled and randomized 314 newborns in the intervention component; 110 completed the study. There were few significant differences between the randomized groups. In the combined intervention groups, positive results on visual inspection declined from baseline to 12 months (97.0% to 90.6%, p=0.007). At baseline, 36.9% of homes were above the U.S. Environmental Protection Agency's lead dust standard, compared with 26.9% at 12 months (p=0.032), mainly due to a drop in windowsill dust levels. Both groups showed a significant increase in parental scores on a lead education test. Children in the intervention and matched control groups had similar geometric mean initial BLLs (2.6 vs. 2.7, p=0.477), but a significantly higher percentage of children in the intervention group had an initial blood lead screening compared with those in the matched group (88.9% vs. 84.4%, p=0.032).
Conclusions
A study of primary prevention of lead exposure showed a higher blood lead screening rate for the combined intervention groups and mean BLLs at one year of age not statistically different from the comparison group. Most homes had lead hazards. Lead education significantly increased knowledge.
Poor housing conditions have been associated with adverse health outcomes for many years. This relationship has been well documented for lead poisoning.1–5 An emphasis on prevention of lead exposure and elevated blood lead levels (BLLs) (level ≥10 micrograms per deciliter [μg/dL]) has been driven by studies documenting the adverse effects, including neuropsychological impairment, in U.S. children with lower BLLs, including levels <10 μg/dL.6–13 Contact with deteriorating lead-based paint (LBP) and lead-contaminated dust and soil is currently the primary source of lead exposure for U.S. children.14
Primary prevention of lead exposure and lead poisoning has been a policy priority for both the Centers for Disease Control and Prevention (CDC) and the U.S. Department of Housing and Urban Development (HUD) for several years. One CDC publication focused entirely on strategies for a housing-based approach to primary prevention of lead poisoning1 recommended screening of high-risk housing (by home evaluation) and children (through blood lead testing). A more recent publication recommended primary prevention of both housing-based lead hazards and other sources of lead.9,15
Primary prevention interventions, such as lead hazard control (LHC) of a property before a child is poisoned, cleaning, or educational interventions, could be simple ways to reduce or prevent lead exposure. A few studies have examined this question but were unable to show the benefits. For example, Lanphear16 et al. undertook a randomized controlled trial of lead dust control in 275 urban children, followed from six months until 24 months of age, to evaluate the effectiveness of dust control in preventing or limiting elevation of BLLs. The authors found no differences in BLL or dust levels between the intervention and control groups. There was also no difference in BLLs in a follow-up study at 48 months.17 Another study of primary prevention by Dugbatey et al.18 randomized low-income inner-city pregnant women into full case management, partial case management, and control groups. The children's BLLs were collected every six months. In the analysis, there were no significant differences in the BLLs of children in the three groups, refuting the hypothesis that the full case management group would have lower levels. Dugbatey et al. also reported on the difficulty of obtaining data in this population.18
A Cochrane Collaborative review19 examined 12 studies to determine the efficacy of household interventions in preventing or decreasing subject children's exposure to lead. These studies, including those utilizing primary and secondary prevention, were broken down into the following categories of intervention: educational only, environmental (interior dust control or soil abatement) only, and a combination of the two. Several studies16,20 began when the children were younger than one year of age and showed mean baseline BLLs of <10 μg/dL. The analysis found that neither educational nor environmental interventions alone effectively reduced BLLs or floor dust levels; however, the authors noted that, with a follow-up period of <12 months, the relatively long half-life of lead in blood might have biased the change in BLL that was observed toward a null effect. Three of the 12 studies used a combination of education and dust control, similar to the Lead Safe Homes (LSH) Study described in this article, but could not be analyzed through meta-analysis due to differences in data collection.
The Philadelphia LSH Study was designed to test the efficacy of educational and environmental interventions in a cohort of urban newborns and their families during the first year of life. It was modeled on the Philadelphia Lead Safe Babies program, a primary prevention program for children younger than one year of age.21 Relatively few studies have looked at intensive interventions during the newborn age, when, presumably, a child's BLLs have not yet been elevated from postnatal lead exposure. The study intended to prevent the typical peaking of BLLs that occurs between 12 and 36 months of age, due to persistence of hand-to-mouth activity, when more mobile children have greater access to LBP hazards.22 Our study provided environmental evaluation and remediation twice (at baseline and 12 months), as well as detailed lead exposure prevention education. The study also examined the effect of education regarding proper home maintenance, which addressed recommendations of the HUD Task Force report that parents, rental property owners, and homeowners become educated about LBP hazards and lead safe work practices to avoid lead hazards in their homes.23
METHODS
Study population
The Philadelphia LSH Study was a randomized trial that offered environmental education, evaluation, and remediation, as needed, to the families and homes of high-risk newborn children. It was conducted by workers from the Philadelphia Department of Public Health, the Children's Hospital of Philadelphia, the National Nursing Centers Consortium, St. Christopher's Hospital for Children, and Drexel University, whose institutional review boards approved the study, which was funded through a HUD Lead Technical Studies grant.
We recruited study children from urban outpatient practices located in low-income neighborhoods of Philadelphia, where the prevalence of children with elevated BLLs is higher than average. After the outreach workers explained the study and obtained informed consent from the child's caregiver, the study coordinator selected the next card in the random sequence to randomize that family to receive either standard lead-poisoning prevention education (standard education group; hereafter, SEG) or standard education with additional extensive education regarding essential maintenance practices for keeping a home in lead safe condition (maintenance education group; hereafter, MEG). The outreach workers reviewed the MEG educational points with the families at each study visit. The additional education was compiled into a 22-page handbook, which included information on the problems regarding older homes and LBP hazards and a series of tips for families, such as preventing damage to paint, looking out for peeling or chipping paint, completing the maintenance diary each month, reporting problems with LBP hazards in their homes, working safely with LBP (this involved a list of explicit actions to take to maintain safety), and a list of “dos” and “don'ts” for household cleaning. The handbook was created using information from booklets published by the U.S. Environmental Protection Agency (EPA), HUD, CDC, and the Maine Department of Environmental Protection.
We randomized blocks using computer-generated random numbers. The outreach workers educated participating families during the baseline, six-month, and 12-month home visits. Parents were given cleaning materials and supplies, and workers reinforced the prevention education (including specific cleaning instructions) during study visits. (Workers conducted the six-month educational intervention by phone for study families unable to arrange a home visit.) The study staff and health department staff (arranging LHC work) followed a detailed protocol for attempting to reach families lost to follow-up, including multiple phone and mail contacts, visits to the last known address, and contact with the subject's primary care provider.
We recruited the study children from outpatient practices of The Children's Hospital of Philadelphia, St. Christopher's Hospital for Children, and several nurse-managed health centers participating in the National Nursing Centers Consortium. A comparison group, with a 2:1 match, was identified from The Children's Hospital of Philadelphia clinical database, and controls were matched by age, census tract, racial/ethnic background, and gender. Eight children could not be matched on racial/ethnic background but were matched for the other characteristics. We created the comparison group to compare the BLLs of children receiving one of the study interventions with those who had received the community standard for prevention of elevated BLLs, such as information from the child's health-care provider during clinical visits. We utilized an electronic recruitment tool that was managed by the Pediatric Research Consortium for The Children's Hospital of Philadelphia practices. Families were asked if someone could call them about the study, and a list of interested parents was regularly relayed to study staff. Other children were identified through wall posters and direct referral from health-care providers. Eligible children resided in Philadelphia County, spoke either English or Spanish, had a home that was judged to be in a condition enabling remediation (in stable condition), and did not have a history of elevated BLLs. The outreach worker team included a bilingual worker, and all documents were translated into Spanish. We excluded families if they had participated in the Lead Safe Babies program or received services from the Childhood Lead Poisoning Prevention Program of the Philadelphia Department of Public Health for other children in the family.
The study team formed a community advisory board, which comprised representatives from the targeted community, and met regularly following recommendations detailed in the National Research Council's 2005 report on housing-related health hazards involving children.24
Parental knowledge assessment
All families were administered pre- and posttests with both standard lead and maintenance questions on the first home visit (before and after the education was given), and the standard lead test was repeated at six and 12 months. The maintenance posttest was also administered to MEG families at these visits. The study utilized the shortened version of the Chicago Lead Knowledge Test,25 modified by Hans Kersten.26 The test evaluates parental knowledge regarding lead exposure prevention. Test-retest reliability of the full Chicago Lead Knowledge Test was 0.96 but has not been assessed for the shortened version. The SEG test had 14 questions, and possible scores ranged from 1 to 14, with one point scored for each correctly answered question. The MEG test was similar, except it had only 10 questions, with a possible score ranging from 1 to 10.
Quality control measures
Per HUD specifications, we formulated a quality assurance plan detailing the protocols and quality assurance procedures to be used for specimen (lead dust) and data collection. BLLs were drawn by each subject's primary care provider, and results were reported to study staff.
Collection of study data
Data verification procedures were in place to ensure accuracy of data collection and data entry. The study manager entered data into a Microsoft® Excel database. The study manager and principal investigator routinely performed quality assurance evaluations on selected study charts to identify errors in data entry. Following completion of data cleaning and quality assurance procedures, the data was imported into a statistical software program, SPSS® version 18,27 for analysis.
Home evaluation
The outreach workers systematically evaluated each subject's residence by looking at the condition of paint (intact, fair, or poor) and testing for lead dust levels at baseline and at 12 months. Areas judged to be fair had a paint defect or visible dust on ≤10 square feet (sq ft) of exterior surfaces, ≤2 sq ft of interior surfaces, or less than 10% of small surfaces. Areas were labeled as poor if these limits were exceeded. Homes were reassessed whenever a subject moved.
Collection of lead dust specimens
Study personnel trained by a certified risk assessor, in accordance with EPA and HUD protocols, uniformly collected the dust wipe specimens for home evaluation. Field audits were performed. They took samples from two floor areas and one windowsill area, where the infant was likely to spend the most time during the first year. Measured areas of the floor (1 sq ft) and windowsill (variable area) were sampled with a standard pre-wetted towelette.
Field blank and spiked specimens were submitted regularly. Specimens were initially analyzed by the International Asbestos Testing Laboratory of Mount Laurel, New Jersey, and then by EMSL of Westmont, New Jersey, both of which are National Lead Laboratory Accreditation Program-certified laboratories for dust wipe analysis. Clearance dust wipes, including a full set of 13 wipes, were collected after environmental remediation, per HUD protocol, with homes re-cleaned until all clearance levels were below EPA standards.
Home remediation/LHC work
We offered LHC work for homes that had either an elevation of any of the three lead dust levels above EPA standards (>40 μg/sq ft for floors and >250 μg/sq ft for windowsills) or visual evaluation results showing at least one area in poor or at least two areas in fair condition at either the baseline or 12-month home evaluation. A small number of homes meeting criteria for referral were not referred, due to minimal areas of concern on visual inspection and the high numbers of homes meeting criteria for this work. Once referred, health department abatement staff evaluated the property and specified the LHC work, including paint stabilization and replacement of deteriorated building components when needed, repainting, and specialized lead dust cleaning (known as a Superclean). Services were rendered by Pennsylvania-certified Phildelphia Public Health Department abatement staff members or lead abatement contractors, per HUD guidelines.28 All homes receiving remediation also received a Superclean. The MEG families were also asked to assess their homes monthly for needed repair or maintenance work, such as deterioration of the paint or presence of water leaks, by use of a special diary, and these families were referred to Childhood Lead Poisoning Prevention Program staff in the same manner. During contacts with the families by phone at one, three, and nine months from enrollment and in person during home visits at six and 12 months, the outreach workers questioned MEG families about identification of new areas noted to need repair or maintenance work.
The results of the environmental evaluation and dust wipe testing were reported within several weeks by letter to the parent/guardian and the property owner. For houses with an identified lead problem, the letter indicated that the home would need to be further checked and some type of remediation work would be required, which could be provided by study staff at no charge.
BLLs
Blood lead testing (in most cases, venous) was carried out by the subject's primary care provider and employed the services of five different laboratories, all of which participated in at least one proficiency program for BLL analysis. All of the study children's providers were encouraged to do blood lead screening of their patients using the high-risk protocol recommended by the Philadelphia Department of Public Health at 9–12 months, 15–18 months, 2 years, and 3 years of age. The Children's Hospital of Philadelphia clinics caring for the control children had access to general information and posted recommendations, but did not receive a specific screening protocol.
Periodic cleaning summary
Outreach workers assessed cleaning activity by questioning the parent at the baseline, six-month, and 12-month home visits. We tabulated results for wet dusting and mopping of the subject child's bedroom, living room, dining room, bathroom, and hallway areas and broke them into the following categories of cleaning frequency: low (quarterly), moderate (bimonthly or monthly), or high (daily or weekly).
Statistical analysis
Study Question 1 hypothesized that study interventions would result in lower BLLs in the LSH Study cohort when compared with a group of children whose parents did not receive these interventions. We compared geometric mean BLLs (due to non-normal distribution) of the study children and a comparison group using a two-tailed t-test. Sample size calculations estimated, with an average (pooled) standard deviation of 2.5, that 256 children per group would provide 80% power to detect a mean difference of 0.6 μg/dL in BLLs.
Study Question 2 hypothesized that at least 15% fewer MEG homes would have home evaluation results meeting criteria for remediation at 12 months, compared with SEG homes, using Chi-square comparison. This analysis required a sample size of 128 per group (for both the SEG and MEG) to compare remediation rates of 30% vs. 45%, to achieve 80% power in a one-tailed test.
Study Question 3 involved calculating descriptive statistics of multiple housing variables over time. Outcomes included whether the households met criteria for home evaluation failure, referral for remediation work, cleaning frequency, and cost of remediation. We performed two different analyses to compare percentages regarding housing characteristics. A Chi-square test compared the percentages for all children with data at each visit, as if they were two independent groups. This approach maximized the sample size, since only one-third of the sample had data at 12 months. We performed a McNemar's test on the study children with data at both visits. This approach compares subjects to themselves, providing a cleaner comparison, but excludes most of the baseline data.
Study Question 4 determined significant predictors of BLL by using bivariate tests such as Mann-Whitney U tests and t-tests (for dichotomous variables), analysis of variance (multiple groups), and correlation coefficients (numeric variables). Key child, family and household, home assessment, study arm, and test-score characteristics were evaluated.
Study Question 5 hypothesized that parents receiving lead education would increase their general lead knowledge at baseline and retain this over time, and the MEG would score higher on the standard lead test than the SEG. We used two-tailed Wilcoxon tests to determine changes from baseline within groups and Mann-Whitney U tests to compare groups at each visit. A Spearman correlation was used to test for association (hypothesized to be negative) between parental knowledge at 12 months and 12-month BLLs. This correlation was assessed separately for the two groups. With 128 children per group, the study had adequate power (80%) to detect correlations as small as 0.3.
RESULTS
Cohort characteristics
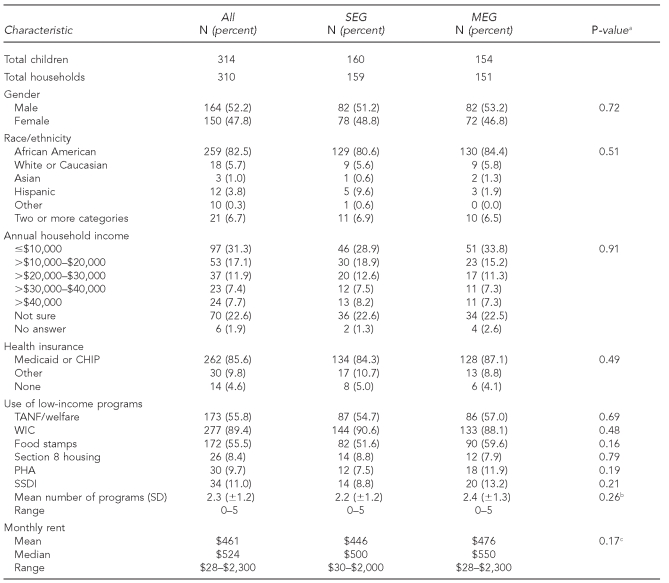
A total of 314 newborn children were enrolled and randomized to the SEG (n=160) and MEG (n=154) arms; 110 (SEG: n=51; MEG: n=59) completed the 12-month study. Twenty newborns were formally withdrawn from the study. Demographic characteristics of the group are displayed in Table 1 for each study arm and the entire cohort. The cohort was predominantly African American (82.5%) and low-income; 85.6% received either Medicaid or state Children's Health Insurance Program (CHIP) insurance. Families utilized an average of 2.3 poverty assistance programs, from a list of six programs, with no significant differences between groups. Very few reported lead exposure from hobbies (4.8%), while 39.8% of parents had ever worked in construction, auto mechanics, battery plants, or other jobs potentially associated with exposure. Fourteen homes (4.5%) reported a non-study child being diagnosed with an elevated BLL, and 10 homes (3.2%) reported a history of chelation therapy for the parent, his/her partner, or a non-study child. Twenty-seven study mothers (8.6%) were born outside of the United States, compared with 36 study fathers (11.5%); they were predominantly from Latin American, Caribbean, and African countries (data not shown).
Table 1.
Demographic and socioeconomic characteristics of the Philadelphia Lead Safe Homes Study cohort
aP-values reported are two-tailed and refer to significance of Chi-square tests comparing SEG and MEG study arms.
bTwo-tailed p-value for parametric t-test comparing SEG and MEG study arm means (skewness statistic: –0.15)
cTwo-tailed p-value for Mann-Whitney U test comparing SEG and MEG study arm means
SEG = standard education group
MEG = maintenance education group
CHIP = Children's Health Insurance Program
TANF = Temporary Assistance for Needy Families
WIC = Special Supplemental Nutrition Program for Women, Infants, and Children
PHA = Philadelphia Housing Authority
SSDI = Social Security Disability Insurance
SD = standard deviation
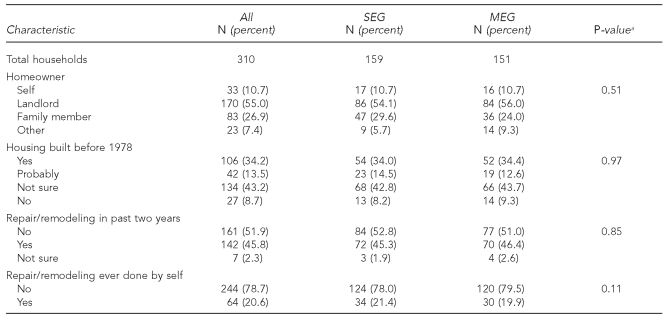
Table 2 displays characteristics of the study children's housing, also without significant differences between the two groups. Most of the cohort consisted of renters (55.0%), with 10.7% owning their home and 26.9% living in a home owned by a family member. Regarding housing age, 34.2% said their home was built prior to 1978, and 13.5% said it probably was; only 8.7% reported a home built after 1978. There were no significant differences for any of the characteristics displayed in Tables 1 and 2, when families that completed the 12-month visit were compared with those who did not.
Table 2.
Home characteristics of the Philadelphia Lead Safe Homes Study cohort
aP-values refer to significance of Chi-square tests comparing SEG and MEG study arms.
SEG = standard education group
MEG = maintenance education group
BLL results
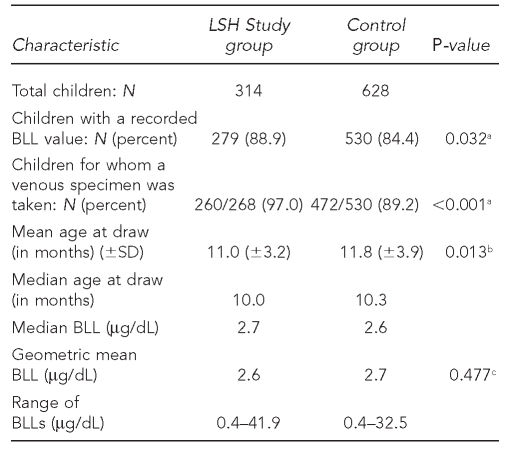
The first study question hypothesized that study interventions would result in lower BLLs in study children when compared with a group whose parents did not receive the LSH interventions. The initial BLLs (drawn around one year of age) are displayed in Table 3. (BLLs drawn around 2 years of age will be compared at a later date.) Geometric mean BLLs were 2.6 and 2.7 for the LSH cohort and comparison group and not significantly different (p=0.477). With the final number of BLL results for each group, we did not have the power to detect any differences smaller than 0.6 between the group means. The control group was significantly older than the LSH cohort: 11.8 months of age (standard deviation [SD] = 3.9) vs. 11.0 months of age (SD=3.2), p=0.013. Lead screening rates were high: 279 (88.9%) of LSH children had a first BLL taken by around one year of age compared with 530 (84.4%) in the matched comparison group (p=0.032). BLLs by study arm were not significantly different, with geometric means of 2.6 for the SEG and 2.7 for the MEG (p=0.680) (data not shown).
Table 3.
Comparison of the study cohort and control group BLL values: Philadelphia Lead Safe Homes Study
aP-values are one-tailed significance for Chi-square test.
bP-values are two-tailed significance for Mann-Whitney U test.
cP-values are two-tailed significance for unpaired t-test.
BLL = blood lead level
LSH = Lead Safe Homes
SD = standard deviation
μg/dL = microgram per deciliter
We explored predictors of higher BLLs through bivariate analyses. Independent variables with significant correlations included older age at first blood draw (rs=0.257, p=0.010); dust wipe levels at baseline and 12 months (p=0.003 for both time periods by unpaired t-test); and needing a referral for LHC work at 12 months (p=0.002 by unpaired t-test). As none of these variables was surprising as a predictor, we determined that a multivariate analysis would be of limited value.
Housing results
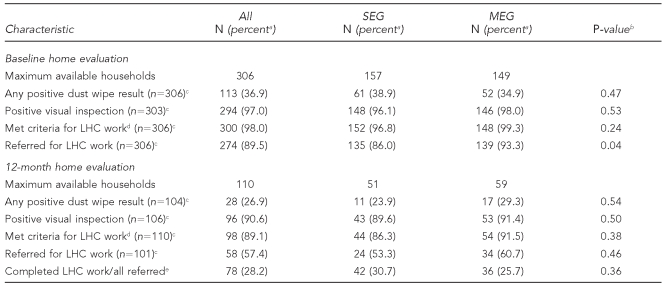
Housing results are summarized in Table 4 and Figure 1.
Table 4.
Home evaluation data summary: Philadelphia Lead Safe Homes Study
aPercentages are based on the number of households for which data were available for each variable.
bP-values refer to significance of Chi-square tests comparing SEG and MEG study arms.
cNumber of households for which data were available for this variable
dA home evaluation result was interpreted as positive if either of the floor dust wipe samples was at 40 micrograms/square foot (μg/sq ft) or higher; the window sample was at 250 μg/sq ft or higher; and/or the visual inspection showed two or more areas rated fair or one or more areas rated poor.
eAll referred homes: n=277; all referred SEG homes: n=137; all referred MEG homes: n=140
SEG = standard education group
MEG = maintenance education group
LHC = lead hazard control
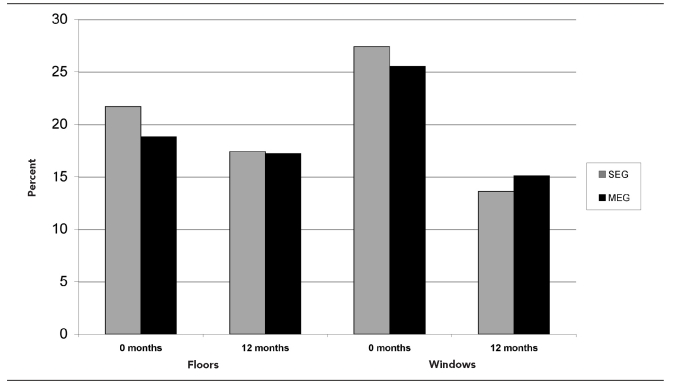
Figure 1.
Percentage of homes with a positive dust wipe samplea at baseline and 12 months, by study arm: Philadelphia Lead Safe Homes Study
aPositive floor dust wipe sample ≥40 micrograms/square foot (μg/sq ft); positive window dust wipe sample ≥250 μg/sq ft
SEG = standard education group
MEG = maintenance education group
Visual inspection results.
Ninety-seven percent (n= 294/303) of homes had positive results on visual inspection at baseline vs. 90.6% (n=96/106) at 12 months (p=0.007 by Chi-square test). For the 105 homes with evaluations at both time points, a higher percentage had positive results on home evaluation at baseline than at 12 months (n=103, 98.1% vs. n=95, 90.5%; p=0.021 by McNemar's test). The percentages for each study arm were not significantly different.
Lead dust results.
For lead dust results, 36.9% of homes (n=113) were positive (above the EPA standard) at baseline, compared with 26.9% (n=28) at 12 months (p=0.032 by Chi-square test). The floor dust wipe results for the LSH cohort changed from 20.3% positive (n=62) to 17.3% positive (n=18) (p=0.26 by Chi-square test), whereas the window dust results decreased from 26.5% positive (n=81) to 15.1% positive (n=16) (p=0.011 by Chi-square test). There were no significant differences between the two study arms (Figure 1). For houses with data at both times (n=106), there was a significant change in the percent with any dust wipe level above EPA standards from 39.4% (n=41) at baseline to 26.9% (n=28) at 12 months (p=0.031 by McNemar's test). For houses with data at both times, there were no significant changes in percent for floor or sill dust levels above EPA standards.
Met criteria for remediation.
Ninety-eight percent (n=300) of homes at baseline met criteria for remediation vs. 89% (n=98) at 12 months (p<0.001 by Chi-square test). A significantly higher proportion of homes having complete housing data at both time points met criteria for remediation at baseline (n=108/110, 98.2%) compared with 12 months (n=98/110, 89.1%) (p=0.006 by McNemar's test). Study arms did not differ in proportions of homes meeting criteria for remediation at baseline and at 12 months, but we did not have an adequate number in each group at 12 months (n=128 per group) to detect a difference of 15% in remediation needs. As discussed previously, not all homes that fit the criteria were referred for remediation.
Referred for remediation.
We referred 89.5% (n=274) of homes for LHC work at baseline, compared with 57.4% (n=58) at 12 months (p<0.001 by Chi-square test). Of 101 homes with data at baseline and 12 months, 93 (92.1%) were referred for LHC work at baseline vs. 58 (57.4%) at 12 months (p<0.001 by McNemar's test). At baseline, a significantly higher percentage of MEG families (93.3%, n=139) than SEG families (86.0%, n=135) (p=0.04) were referred for LHC work; the difference was not significant at 12 months.
Home condition improved and the need for remediation work decreased at 12 months. A total of 78 homes (28.2%) were remediated over the course of the study; with a mean of five months, a median of four months, and a range of two to 15 months from the time of referral to the work completion date. Of those receiving LHC work before the 12-month visit, half passed the home evaluation at 12 months. BLLs at one year of age for children having LHC work prior to the first blood test did not differ significantly from those of children whose homes were referred for work but did not receive it. The mean cost of remediation work was $4,971.80 (range of $1,886.60 to $10,973.00) for the SEG and $5,918.37 (range of $1,000.00 to $12,224.00) for the MEG (p=0.174); median costs were $4,656.00 for the SEG and $5,512.10 for the MEG. There were no significant differences in cleaning levels between the SEG and MEG, except that a higher percentage of SEG families (44%, n=31) mopped their dining rooms, compared with the MEG families (23%, n=13) (p=0.04). The entire cohort improved their cleaning activity over the course of the study. Higher posttest scores were associated with increased dusting activity of kitchens and basements at six and 12 months in both groups. Twenty of the MEG families identified a need for maintenance work. Of these, eight had the work completed; it was not completed in situations where the family was not cooperative with these attempts.
Parental knowledge acquisition
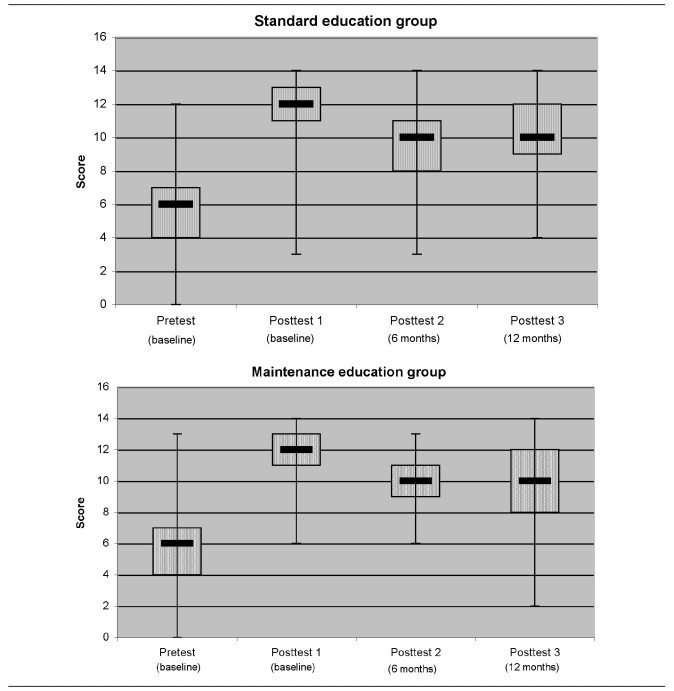
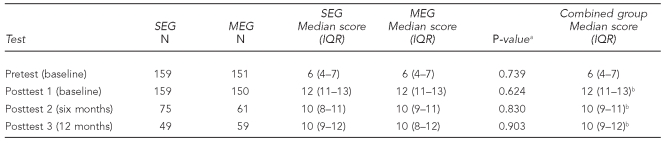
The combined groups' standard education test scores rose during the baseline visit from a pretest mean of 6 to a posttest mean of 12 (p<0.001) (Figure 2 and Table 5). Subsequent comparisons indicated retention of most of the material. A higher percentage performed well on questions about lead exposure pathways, but not as well on questions about good nutrition. Median scores were not significantly different between arms (Table 5). The MEG parents achieved significant gains in knowledge on a separate maintenance test over the study course, with median scores and interquartile ranges of 8 (7–9) for the pretest; 9 (8–10) for posttest 1; 9 (8–9) for -posttest 2; and 9 (8–9) for posttest 3 (all were significant at p<0.05 when compared with the pretest by Wilcoxon test) (data not shown). A negative association between increased parental knowledge score and a child's BLL at one year of age was hypothesized, with 80% power to detect a correlation of 0.35, with 50 children per group. Study results showed non-significant, but positive, Spearman correlation coefficients for the SEG (rs=0.181, p=0.223, n=47) and MEG (rs=0.103, p=0.454, n=55). Therefore, the study did not demonstrate an impact of parental knowledge on the children's first BLLs at one year of age.
Figure 2.
Box-and-whisker plots of Standard Education Test median scores, by study arm: Philadelphia Lead Safe Homes Study
Table 5.
Standard Education Test median scores, by study arm and for entire cohort: Philadelphia Lead Safe Homes Study
aTwo-tailed p-value by Mann-Whitney U test comparing MEG and SEG study arms
bp<0.001 compared with baseline by Wilcoxon test
SEG = standard education group
MEG = maintenance education group
IQR = interquartile range
DISCUSSION
The Philadelphia LSH Study was a randomized controlled trial of primary prevention interventions during the first year of life for 314 urban newborns. We did not find significant differences in initial BLLs (drawn around one year of age) between study children and a matched comparison group of children, nor were there significant differences in BLLs of children in the SEG vs. those in the MEG. Although both the study and comparison groups had high blood-lead screening rates around one year of age (88.9% and 84.4%), a significantly higher percentage of LSH Study children were screened. We found that most study homes had lead hazards at baseline, with some decrease in lead dust levels for floors (non-significant) and windowsills (significant) by the end of the study. The study documented parental acquisition of knowledge about lead exposure prevention, which was retained during the year-long study. The MEG did not improve on most measures, when compared with the SEG.
In comparing results with those of previous studies, results were similar to the Lanphear et al. 1999 study,16 with no significant differences in BLLs between intervention and control groups; however, our study demonstrated significant decreases in lead dust levels. Our geometric mean BLLs were very low (2.6 μg/dL for the LSH cohort and 2.7 μg/dL for the comparison group), and the mean ages of draw were 11.0 months for the study cohort and 11.8 months for the comparison group. This young age might account for lower levels, as the typical pattern in children's BLLs is an increase around one year of age, with a peak between 18 and 36 months of age.22 These low values may reflect the decrease in geometric mean BLLs nationally (1.9 μg/dL for children aged 1–5 years with data from the 1991–2004 National Health and Nutrition Examination Survey)29 and in Philadelphia (3.0 μg/dL in 2008) (Personal communication, Peter Palermo, Philadelphia Department of Public Health, Childhood Lead Poisoning Prevention Program, August 2009).
As mean levels decline, differences in population means will be harder to demonstrate. Differences at 2 years of age may be greater due to the factors described previously. We intend to collect, analyze, and report on the 24-month BLL data once it becomes available.
Dugbatey et al. experienced problems similar to our study regarding retention and follow-up, such as difficulty in finding clients due to changes in address and phone numbers and non-compliance with study visits.18 Some declined study participation due to concerns about lack of approval by family members or eviction by landlords. Dugbatey et al. concluded that the competing needs of survival in poverty made concern about a child's lead exposure less compelling for these mothers, especially without obvious signs of disease. Our study experienced a similar difficulty in retention, and other articles have discussed the challenges of conducting inner-city health research.30
Limitations and strengths
Our study had several limitations. As noted previously, a significant part of the cohort was lost to follow-up for the six- and 12-month visits, although only 20 participants formally withdrew. Study outreach workers had difficulty reaching families due to frequent phone number changes or service suspension, as well as moves to another address. The use of disposable phones may have contributed to this intermittent phone access. Another factor affecting attrition was that the baseline visit was held when family members were home more often, during the baby's first months. It became much harder to reach parents when they were back at work or school. We arranged to do the six-month visit over the phone, increasing the number of participating families. We also had difficulty accessing families' homes to arrange for and complete the LHC work. It seems the length of time children were followed and the number of encounters scheduled led to challenges in maintaining participation for this cohort. This may explain why relatively few studies have evaluated these types of primary prevention interventions. A shorter study period might increase response rates but would restrict testing of the interventional impact on the household to a shorter time. BLLs could be followed for several years, even if the main study period was abbreviated.
Despite multiple calls and visits to homes by study and health department staff, many families were unresponsive to getting their homes remediated, even though remediation work was free. Unfortunately, according to the health code in Philadelphia, the health department can only order and enforce (through Lead Court) remediation of properties with identified lead hazards if they house children with known elevated BLLs. So, we could not order property owners to accept the remediation work, although we strongly recommended it. Reasons cited by Dugbatey et al.18 for lack of participation, such as the difficulties of living in poverty, lack of approval by family members (especially those either owning or signing the lease on the property), or fear of eviction by landlords, may have explained the poor remediation rate, which was only 28% for the enrolled families.
We experienced some problems with consistency of the study protocol and quality control. Some of the homes receiving a positive result for the home evaluation were not referred for LHC work due to small areas with paint deterioration. Not all SEG families received the maintenance pre- and posttests during the baseline visit, so results were tabulated only for the MEG group. Results from the first laboratory that analyzed lead dust levels of spike specimens were not optimal; however, results from the second laboratory were considerably improved.
A study strength was enrollment of a large number of children soon after birth and follow-up of some of them during their first year of life. The electronic recruitment tool built into The Children's Hospital of Philadelphia patients' charts was very helpful in identifying interested families; approximately half of those contacted enrolled in the study. Additionally, a BLL was obtained for most children by one year of age.
Recommendations for future research could build upon the successes of the current study. Utilizing a larger control group might capture a larger number of children with BLLs for comparison. Electronic recruiting would also be recommended. As the provision of additional maintenance education was not effective, it would not be recommended. As discussed previously, the difficulties of a longer-term intervention need to be weighed against benefits for future studies. Replication of primary prevention interventions in other metropolitan areas should be considered.
CONCLUSIONS
A year-long study of educational and environmental interventions for primary prevention of lead exposure resulted in significantly more lead screening for the study group, although mean BLLs around one year of age were not statistically different between the study group and a control group. Most of the homes had lead hazards; a small percentage of these households cooperated for LHC work, and a significantly lower percentage of the group had a positive home evaluation at 12 months. The provision of standard lead education was associated with significant increases in knowledge, which were retained. The MEG did not differ significantly from the SEG on most parameters examined, indicating that the additional education was not effective.
Acknowledgments
The authors thank the staffs of the Lead Safe Homes Study, The Children's Hospital of Philadelphia (including the Pediatric Research Consortium and the Center for Biomedical Informatics), St. Christopher's Hospital for Children, and the National Nursing Centers Consortium for their excellent work.
Footnotes
This study was funded by a Lead Technical Studies grant from the U.S. Department of Housing and Urban Development (HUD).
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of HUD.
REFERENCES
- 1.Centers for Disease Control and Prevention (US). Preventing lead exposure in young children: a housing-based approach to primary prevention of lead poisoning. Atlanta: CDC; 2004. [Google Scholar]
- 2.Dixon SL, Gaitens JM, Jacobs DE, Strauss W, Nagaraja J, Pivetz T, et al. Exposure of U.S. children to residential dust lead, 1999–2004: II. The contribution of lead-contaminated dust to children's blood lead levels. Environ Health Perspect. 2009;117:468–74. doi: 10.1289/ehp.11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krieger J, Higgins DL. Housing and health: time again for public health action. Am J Public Health. 2002;92:758–68. doi: 10.2105/ajph.92.5.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lanphear BP, Hornung R, Ho M. Screening housing to prevent lead toxicity in children. Public Health Rep. 2005;120:305–10. doi: 10.1177/003335490512000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandel M, Phelan K, Wright R, Hynes HP, Lanphear BP. The effects of housing interventions on child health. Pediatric Ann. 2004;33:475–81. doi: 10.3928/0090-4481-20040701-14. [DOI] [PubMed] [Google Scholar]
- 6.Braun JM, Kahn RS, Froehlich T, Auinger P, Lanphear BP. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U.S. children. Environ Health Perspect. 2006;114:1904–9. doi: 10.1289/ehp.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canfield RL, Kreher DA, Cornwell C, Henderson CR., Jr Low-level lead exposure, executive functioning, and learning in early childhood. Child Neuropsychol. 2003;9:35–53. doi: 10.1076/chin.9.1.35.14496. [DOI] [PubMed] [Google Scholar]
- 8.Canfield RL, Henderson CR, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med. 2003;348:1517–26. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (US). Preventing lead poisoning in young children. Atlanta: CDC; 2005. [Google Scholar]
- 10.Chiodo LM, Jacobson SW, Jacobson JL. Neurodevelopmental effects of postnatal lead exposure at very low levels. Neurotoxicol Teratol. 2004;26:359–71. doi: 10.1016/j.ntt.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Jusko TA, Henderson CR, Lanphear BP, Cory-Slechta DA, Parsons PJ, Canfield RL. Blood lead concentrations <10 microg/dL and child intelligence at 6 years of age. Environ Health Perspect. 2008;116:243–8. doi: 10.1289/ehp.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, et al. Low-level environmental lead exposure and children's intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–9. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tellez-Rojo MM, Bellinger DC, Arroyo-Quiroz C, Lamadrid-Figueroa H, Mercado-Garcia A, Schnaas-Arrieta L, et al. Longitudinal associations between blood lead concentrations lower than 10 microg/dL and neurobehavioral development in environmentally exposed children in Mexico City. Pediatrics. 2006;118:e323–30. doi: 10.1542/peds.2005-3123. [DOI] [PubMed] [Google Scholar]
- 14.Lanphear BP, Matte TD, Rogers J, Clickner RP, Dietz B, Bornschein RL, et al. The contribution of lead-contaminated house dust and residential soil to children's blood lead levels. A pooled analysis of 12 epidemiologic studies. Environ Res. 1998;79:51–68. doi: 10.1006/enrs.1998.3859. [DOI] [PubMed] [Google Scholar]
- 15.Levin R, Brown MJ, Kashtock ME, Jacobs DE, Whelan EA, Rodman J, et al. Lead exposures in U.S. children, 2008: implications for prevention. Environ Health Perspect. 2008;116:1285–93. doi: 10.1289/ehp.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lanphear BP, Howard C, Eberly S, Auinger P, Kolassa J, Weitzman M, et al. Primary prevention of childhood lead exposure: a randomized trial of dust control. Pediatrics. 1999;103(4 Pt 1):772–7. doi: 10.1542/peds.103.4.772. [DOI] [PubMed] [Google Scholar]
- 17.Lanphear BP, Eberly S, Howard CR. Long-term effect of dust control on blood lead concentrations. Pediatrics. 2000;106:E48. doi: 10.1542/peds.106.4.e48. [DOI] [PubMed] [Google Scholar]
- 18.Dugbatey K, Croskey V, Evans RG, Narayan G, Osamudiamen OE. Lessons from a primary-prevention program for lead poisoning among inner-city children. J Environ Health. 2005;68(15-20):26. [PubMed] [Google Scholar]
- 19.Yeoh B, Woolfenden S, Wheeler D, Alperstein G, Lanphear BP. Household interventions for prevention of domestic lead exposure in children. Cochrane Database Syst Rev. 2008;(2):CD006047. doi: 10.1002/14651858.CD006047.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Jordan CM, Yust BL, Robison LL, Hannan P, Deinard AS. A randomized trial of education to prevent lead burden in children at high risk for lead exposure: efficacy as measured by blood lead monitoring. Environ Health Perspect. 2003;111:1947–51. doi: 10.1289/ehp.6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothman NL, Lourie R, Gaughan J Lead Awareness: North Philly Style Grant Team. Lead awareness: North Philly style. Am J Public Health. 2002;92:739–41. doi: 10.2105/ajph.92.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Binns HJ, Campbell C, Brown MJ Centers for Disease Control and Prevention Advisory Committee on Childhood Lead Poisoning Prevention. Interpreting and managing blood lead levels of less than 10 microg/dL in children and reducing childhood exposure to lead: recommendations of the Centers for Disease Control and Prevention Advisory Committee on Childhood Lead Poisoning Prevention. Pediatrics. 2007;120:e1285–98. doi: 10.1542/peds.2005-1770. [DOI] [PubMed] [Google Scholar]
- 23.Department of Housing and Urban Development (US), Lead-Based Paint Hazard Reduction and Financing Task Force. Putting the pieces together: controlling lead hazards in the nation's housing. Washington: HUD; 1995. [Google Scholar]
- 24.Lo B, O'Connell ME, editors. National Research Council and Institute of Medicine, Committee on Ethical Issues in Housing-Related Health Hazard Research Involving Children, Youth and Families. Ethical considerations for research on housing-related health hazards involving children. Washington: National Academies Press; 2005. [Google Scholar]
- 25.Mehta S, Binns HJ. What do parents know about lead poisoning? The Chicago Lead Knowledge Test. Pediatric Research Group. Arch Pedriatr Adolesc Med. 1998;152:1213–8. doi: 10.1001/archpedi.152.12.1213. [DOI] [PubMed] [Google Scholar]
- 26.Kersten HB, Moughan B, Moran MM, Spector ND, Smals LE, DeLago CW. A videotape to improve parental knowledge of lead poisoning. Ambul Pediatr. 2004;4:344–7. doi: 10.1367/A03-032R.1. [DOI] [PubMed] [Google Scholar]
- 27.SPSS, Inc. SPSS: Version 18. Chicago: SPSS, Inc; 2009. [Google Scholar]
- 28.Department of Housing and Urban Development (US). Guidelines for the evaluation and control of lead-based paint hazards in housing. Washington: HUD; 1995. [Google Scholar]
- 29.Jones RL, Homa DM, Meyer PA, Brody DJ, Caldwell KL, Pirkle JL, et al. Trends in blood lead levels and blood lead testing among U.S. children aged 1 to 5 years, 1988–2004. Pediatrics. 2009;123:e376–85. doi: 10.1542/peds.2007-3608. [DOI] [PubMed] [Google Scholar]
- 30.Bayoumi AM, Hwang SW. Methodological, practical, and ethical challenges to inner-city health research. J Urban Health. 2002;79(4) Suppl 1:S35–42. doi: 10.1093/jurban/79.suppl_1.S35. [DOI] [PMC free article] [PubMed] [Google Scholar]