Abstract
Objective
We examined the impact of a combination of home environmental interventions and nurse case management services on total settled dust loadings and on allergen concentrations in the homes of asthmatic children.
Methods
Using a randomized longitudinal controlled trial study design, we randomly assigned homes of asthmatic children in Milwaukee to either a control (n=64) or an intervention (n=57) group. Control group homes received a visual assessment, education, bed/pillow dust mite encasings, and treatment of lead-based paint hazards. The intervention group received these same services plus nurse case management that included tailored, individual asthma action plans, provision of minor home repairs, home cleaning using special vacuuming and wet washing, and integrated pest management. Dust vacuum samples were collected from measured surface areas of floors in the TV room, kitchen, and child's bedroom at baseline and at three-, six-, and 12-month follow-up visits. Dust loading (mass per surface area) is a means of measuring total dust and the total amount of allergen present.
Results
For the intervention group, geometric mean dust loadings declined significantly from baseline (39 milligrams per square foot [mg/ft2]) to post-intervention (11 mg/ft2) (p<0.001). Baseline dust loading, treatment group, visit, and season were significant predictors of follow-up dust loadings. Mean post-intervention dust loadings were 72% higher in the control group. The total amount of allergen in settled house dust declined significantly following the intervention because total dust loading declined; the concentration of allergens in settled dust did not change significantly.
Conclusion
The combination of nurse case management and home environmental interventions promotes collaboration between health and housing professionals and is effective in reducing exposures to allergens in settled dust.
Asthma is an increasingly prevalent chronic illness, affecting low-income children at a disproportionately high rate.1 In a survey of 460 caregivers at two public schools and a local Women, Infants, and Children program office in Milwaukee, Wisconsin, more than 14% of caregivers reported that their child had physician-diagnosed asthma.2
Indoor allergens in the home have been linked to both the development and exacerbation of asthma.3 The Institute of Medicine determined that there is sufficient evidence of a causal relationship between exposure to cockroach allergens and exacerbation of asthma in sensitized individuals, and limited evidence linking exposure to cockroach allergens with the development of asthma.1 The National Cooperative Inner-City Asthma Study (NCICAS) showed a relationship between exposure and sensitivity to cockroach allergens and asthma morbidity in inner-city children with asthma.4 The NCICAS also found that mouse allergen exposure and atopy may contribute to mouse sensitization in inner-city children with asthma.5 Cockroach and mouse allergens are commonly found in inner-city housing.4,6–8
There is evidence that home environmental treatments may reduce allergen concentrations, especially those that incorporate several different types of housing intervention instead of just a single method.1,3 Extermination alone has not been found to eliminate pest allergen sources because significant allergen levels remain in settled dust, while cleaning alone in the absence of complete extermination does not eliminate the allergen sources.1 The efficacy of home-based multifaceted environmental interventions on asthma has been reviewed elsewhere.9–11 In homes receiving integrated pest management (IPM), mouse allergen levels significantly decreased on kitchen floors and in bedrooms (floors plus bedding) over a five-month period.12 A study in Seattle, Washington, found that reductions in dust allergen loadings on floors were significantly greater in homes that received a high-intensity intervention than in homes that received a low-intensity intervention.13 The high-intensity intervention consisted of seven home visits by community health workers during a year and a full set of asthma trigger controls, including education about dust mites, moisture, mold, and cockroaches, while low-intensity homes had a single home visit and more limited asthma trigger control resources.
Several studies have shown the benefits of education and case management in reducing indoor exposures and asthma morbidity in children with asthma. A home-based intervention that focused on reducing exposure to multiple indoor allergens and environmental tobacco smoke through education and behavioral change resulted in significant reductions in dust mite and cockroach allergens on various surfaces.14 Several studies have shown the benefits of case management for children with asthma. Pediatric asthma outreach programs by nurses have been effective.15–17 The NCICAS also determined interventions by social workers to reduce asthma morbidity among inner-city children to be cost-effective.18,19
This article presents the results of a randomized longitudinal control study designed to examine the impact of a combination of home environmental interventions and nurse case management services on total settled dust loadings and on allergen concentrations in the homes of asthmatic children.
METHODS
The Children's Hospital of Wisconsin's Human Research Review Board approved the study on September 3, 2003, before data collection began. Enrollment of children and homes occurred from October 2003 to April 2005 from four pediatric clinics serving low-income children in areas that have the highest asthma hospitalization rates in Milwaukee. A dwelling was eligible for enrollment if (1) at least one child resided at the dwelling who was younger than 17 years of age and clinically diagnosed with mild to persistent asthma during a clinic visit or reported an emergency department visit or hospital stay, and (2) the dwelling was located in one of 10 high-risk zip codes. After obtaining informed consent, we assigned the home to either the intervention or control group based on prior random number assignment.
Four data-collection visits were made to dwellings in both study groups: a baseline visit and three follow-up visits (at three months, six months, and 12 months after baseline). One additional visit, at immediate post-intervention, was made only to dwellings in the intervention group. The immediate post-intervention visit took place within 24 hours after the contractor completed all environmental interventions in the dwelling except IPM. Visual assessments and environmental sampling were conducted at each visit.
Visual assessments occurred at the baseline, immediate post-intervention (intervention group only), three-month follow-up, six-month follow-up, and 12-month follow-up visits. During the baseline visual assessment, data collectors documented general signs of housing deterioration inside and outside the building using a standardized protocol.20 Data collectors were blinded to the assigned treatment group when completing the assessments. A healthy homes hazard checklist -developed for this study summarized the types of specific interior hazards (excess moisture, evidence of pests, safety hazards, and housing cleanliness) observed. Use of this checklist also helped to ensure that the intervention was of high quality and comprehensive in addressing identified hazards.
Nurse case management services
At the baseline visit to both intervention and control group homes, the nurse case manager (NCM) provided bed encasings for the index child's bed, as well as basic education and informational tools regarding asthma self-management and environmental trigger control. This education was repeated at the 12-month visit to both intervention and control group homes. In control group dwellings, the nurse provided education, answered questions from the family, and, if needed, referred the family to their primary care provider for further assistance in accordance with normal community standards.
In addition to these services, the NCM provided home-based nurse case management interventions only for the intervention group homes, which represented a departure from the solely clinical focus of traditional nursing services. The NCM conducted up to six visits to intervention group homes. At the baseline visit, the NCM used a modification of a structured asthma trigger assessment21 to develop a household-specific asthma action plan to establish family goals with the residents. The NCM also used a standard education tool22 to educate the family about (1) understanding asthma symptoms, signs, and peak flows; (2) using an action plan and medications appropriately; (3) controlling environmental triggers; (4) quitting smoking (if indicated); and (5) visiting the child's physician for follow-up preventive care. The NCM referred intervention group caregivers to other community services as needed. At subsequent visits to intervention group dwellings, the NCM used the asthma trigger assessment information to encourage compliance with the action plan, provide additional education and support to the family, and refer them to other services as needed.
Home environmental interventions
In both control group and intervention group dwellings, if indicated based on a baseline visual assessment, lead-based paint hazards were eliminated in accordance with U.S. Department of Housing and Urban Development guidelines using primarily paint stabilization; the cost of lead paint stabilization varies, depending on the extent of the paint deterioration and the type of building component involved.23 Both study groups received a battery-operated smoke detector if no working smoke detector was present at the baseline visit. No other home environmental interventions (other than the bed encasings provided as part of the NCM interventions) were performed in the control group dwellings.
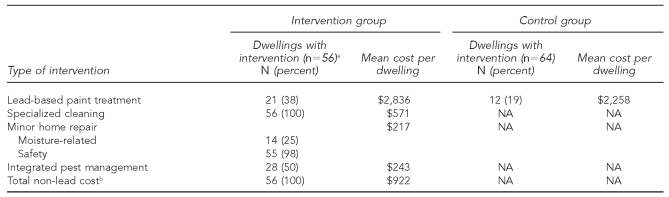
In intervention group homes, we completed minor home repair as a result of the referral from the NCM to home repair services. The types of minor repairs were determined using the baseline visual assessment and included downspout/gutter repair, repair of water damage, repair of cabinets, removal of deteriorated carpet, and installation of linoleum (Table 1). We provided all intervention group residents with battery-operated carbon monoxide (CO) detectors and installed cabinet locking devices and electric socket covers in dwellings housing children younger than 3 years of age. We performed specialized professional cleaning of all horizontal surfaces to remove dust and visible mold through high-efficiency particulate air filter vacuuming and wet washing of all horizontal surfaces. Finally, we implemented IPM if determined to be needed based on the visual assessment. Initially, IPM contractors placed traps in homes to confirm the presence or absence of cockroaches or rodents in each dwelling and to determine the extent of infestation and where the nests were located. Contractors used gel bait and/or boric acid to treat the cockroach infestation, while rodenticide blocks were used for rodents. Gel baits were placed in different rooms, mostly in cracks and crevices in walls and ceilings and in wall voids, depending on extent of infestation. The rodenticide blocks were placed in attics, basements, and kitchens in voids or areas that could not be reached by children or pets. If the infestation required the rodenticide blocks to be placed in areas accessible to children or pets, the blocks were first encased in tamper-resistant units. All major cracks and crevices were sealed or blocked after treatment. In multifamily buildings, the whole building was treated.
Table 1.
Housing environmental interventions and costs from a randomized controlled study of nurse case management and housing intervention effects on allergen exposures among asthmatic children in Milwaukee, 2003–2005
aData were not available for one of the 57 enrolled units.
bTotal does not include the cost of the nurse case management portion of the intervention.
NA = not applicable
We also collected dust samples from the floor of each of three rooms: the television (TV) room, kitchen, and child's bedroom. If multiple asthmatic children were enrolled from a single dwelling, the bedroom of the child with the worst asthma symptoms (self-reported at enrollment) was sampled. We collected samples from the predominant floor surface type (e.g., bare or carpeted) within each room in the highest traffic area of the interior entryway into the room. Each floor dust sample was collected using a Douglas ReadiVac™ vacuum cleaner (Douglas Quikut, Walnut Ridge, Arkansas). A dust collector (from Johns Hopkins DACI [Dermatology, Allergy and Clinical Immunology] Reference Laboratory, Baltimore, Maryland) was fitted onto the bottom of the vacuum hose. A clean crevice tool was attached to the hose over the folded-down plastic extender on the dust collector, fixing the collector in place inside the vacuum hose. A 2-foot by 3-foot marked floor area was vacuumed for approximately two minutes. On bare floors and on floors that appeared to be clean, one or more additional adjacent 2-foot by 3-foot areas were vacuumed as needed to obtain a sufficient quantity of dust inside the dust collector for analysis (at least 1 tablespoon). In kitchens, we sampled the entire bare floor area. After each sample was collected, it was sealed in a labeled plastic bag. Dust samples were held in a –20 degrees Celsius (°C) freezer until processed and analyzed by the laboratory.
Samples were prepared and analyzed by the City of Milwaukee Public Health Laboratories. Samples were desiccated for two hours, after which they were weighed. Desiccated samples were sieved through a 300-micron mesh sieve and weighed again. The quantity of dust was insufficient for the laboratory to determine allergen concentrations if the sieved sample weight was <30 milligrams (mg). Sieved dust samples were divided into 30- to 100-mg (±5 mg) aliquots, extracted in a phosphate-buffered saline solution overnight on a laboratory rocker, and centrifuged for 20 minutes. The supernatant was removed with a Pasteur pipette and stored at –20°C for up to six months. Enzyme-linked immunosorbent assays (Indoor Biotechnologies, Charlottesville, Virginia) were used to analyze the extracts for cockroach, dust mite, and mouse allergens.24–27 Results were reported in micrograms of allergen per gram of dust (μg/g) for all allergens except for bla g 1, a type of cockroach allergen, which was reported in units of allergen per gram of dust (U/g). Both study groups received copies of allergen sampling results. For all calculations, we used SAS® version 9.1.28
The average sieved dust loading (measured in milligrams per square foot [mg/ft2]) for the TV room, child's bedroom, and kitchen was calculated for each dwelling at each visit. The averages were natural log transformed for modeling and hypothesis testing. Paired t-tests were used to test for a change in the geometric mean (GM) dust loading from baseline to follow-up visits. For the intervention group, we used McNemar's test to test the hypothesis that the percent of allergen levels greater than the limit of detection (>LOD) was the same at baseline and immediate post-intervention. Two sample t-tests were used to determine if the mean (e.g., average square footage) was the same in the intervention and control groups. We used a Chi-square test to determine if a categorical variable (e.g., appeared clean at baseline or did not appear clean at baseline) was associated with the study group.
The total dust and sieved dust loadings were significantly correlated (Pearson correlation coefficient: r=0.976, p<0.001). On average, sieved dust loading constituted 68% of the total dust loading. A repeated-measures model was used to predict log average sieved dust loading at follow-up visits using a stepwise variable selection procedure. We developed repeated-measures logistic regression models to predict the log-odds of the probability that the selected allergens were >LOD at the follow-up visit. Only allergen, room type, and surface type combinations that had at least 40% of measurements >LOD at baseline were modeled.
RESULTS
We screened 290 dwellings for possible enrollment into the study. Of these, 57 (19.6%) were ineligible due to owner-related factors, such as no response, refusal from rental property owners, or delinquent tax status. Residents were determined to be ineligible in 46 dwellings (15.9%) due to resident refusal or no response and families moving before the enrollment visit. Housing-related factors led to the ineligibility of 36 dwellings (12.4%) due to multifamily apartment buildings, location outside the study target area, and existing building inspection orders. Thus, we enrolled 151 dwellings in the study. Due to missing data for some units, our statistical analysis included 121 dwellings (57 intervention, 64 control).
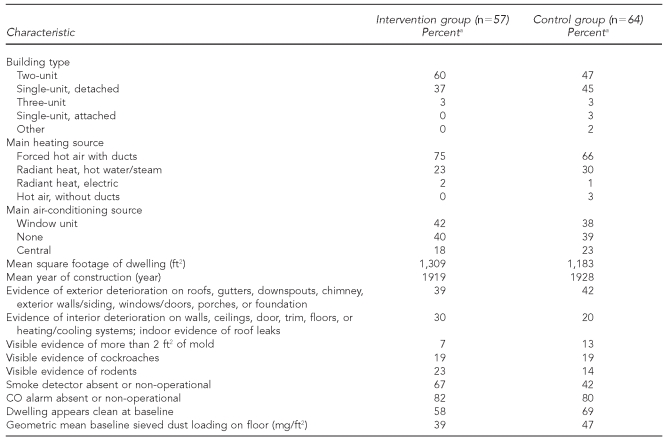
More than half of the study dwellings (52%) were located in two-unit buildings, and 41% were single-family dwellings (Table 2). We found no significant difference in baseline GM settled dust loadings in the two treatment groups (p=0.578).
Table 2.
Baseline housing characteristics from a randomized controlled study of nurse case management and housing intervention effects on allergen exposures among asthmatic children in Milwaukee, 2003–2005
aAll values are percentages except where indicated in parentheses next to characteristic description.
ft2 = square foot
CO = carbon monoxide
mg/ft2 = milligram per square foot
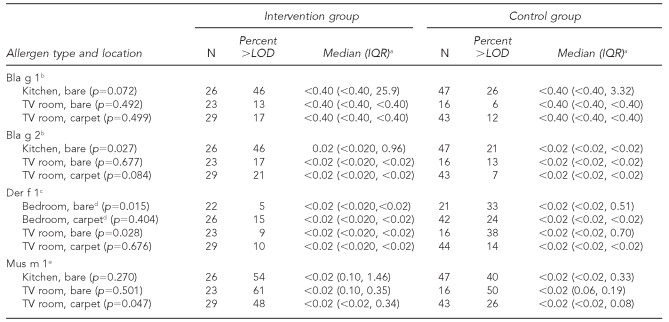
Mouse allergen (mus m 1) was the most frequently detected allergen at baseline, found in 54% of intervention and 40% of control group dwellings, followed by cockroach (bla g 1 and bla g 2) and dust mite (der f 1) allergens (Table 3).
Table 3.
Baseline allergen concentrations from a randomized controlled study of nurse case management and housing intervention effects on allergen exposures among asthmatic children in Milwaukee, 2003–2005
aMedians for Bla g 1 are in units of allergen per gram of dust; all other medians are in micrograms of allergen per gram of dust.
bCockroach allergen type
cDust mite allergen type
dAffected child's bedroom
eMouse allergen type
LOD = limit of detection
IQR = interquartile range
TV = television
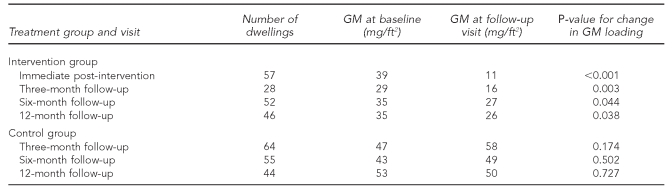
As noted previously, a one-time specialized cleaning was performed in all intervention group dwellings, and one or more safety devices (e.g., CO alarms, smoke detectors, cabinet locks, and electric socket covers) were provided to almost every intervention group dwelling (Table 1). For the intervention group, a significant reduction occurred in the GM sieved dust loading from baseline to immediate post-intervention (p<0.001). The GM loading dropped 72% from 39 mg/ft2 to 11 mg/ft2 (Table 4). The loadings began to rise from the immediate post-intervention visit to the 12-month visit, but still remained less than the baseline loadings at the 12-month follow-up visit (p=0.038). For the control group, there was no significant change in the GM sieved dust loading from baseline to the three-month (p=0.174), six-month (p=0.502) or 12-month (p=0.727) follow-up visit (Table 4).
Table 4.
Geometric mean sieved dust loadings (mg/ft2) across follow-up visits from a randomized controlled study of nurse case management and housing intervention effects on allergen exposures among asthmatic children in Milwaukee, 2003–2005
mg/ft2 = milligrams per square foot
GM = geometric mean
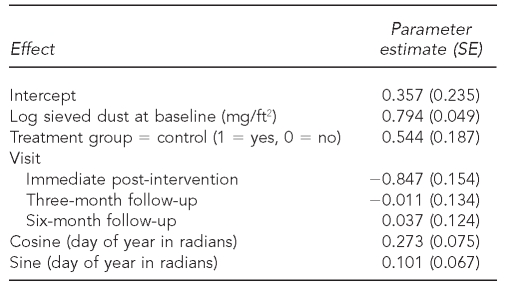
The model included 346 sieved dust loadings from 121 dwellings (57 intervention, 64 control). Baseline dust loading, treatment group, visit, and season were significant variables in the model (Table 5). Follow-up sieved dust loadings were 80% higher in winter than in summer. Dust loadings were 72% higher in control group dwellings compared with intervention group dwellings. Higher baseline dust loadings were associated with higher follow-up dust loadings. There was no significant difference between dust loadings at the three-month, six-month, or 12-month follow-up visit (p=0.916). Dust loadings at immediate post-intervention were significantly different from loadings at the three-month, six-month, and 12-month follow-up visits (p<0.001 for all). Dust loadings were about 40% higher at the follow-up visits than at immediate post-intervention.
Table 5.
Dust loading model results from a randomized controlled study of nurse case management and housing intervention effects on allergen exposures among asthmatic children in Milwaukee, 2003-2005
SE = standard error
mg/ft2 = milligrams per square foot
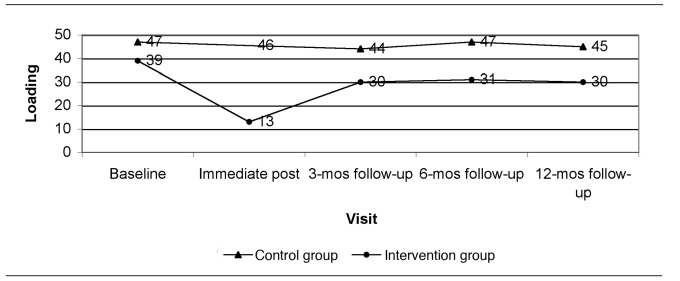
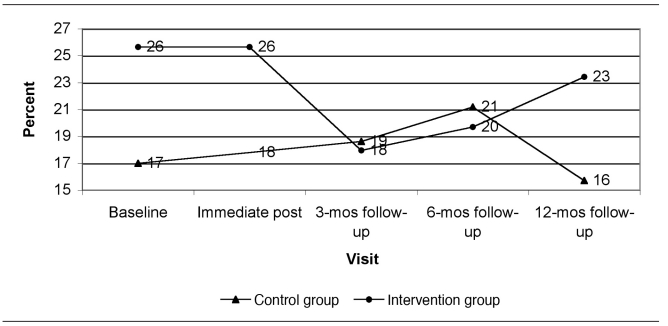
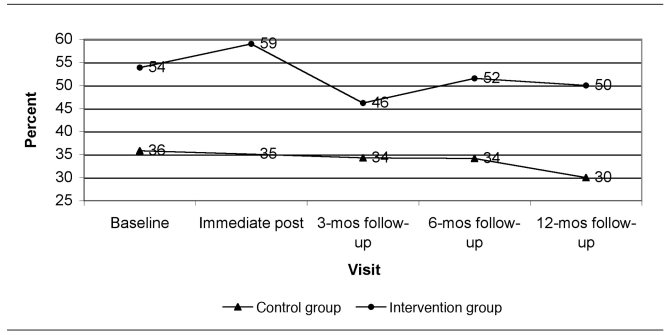
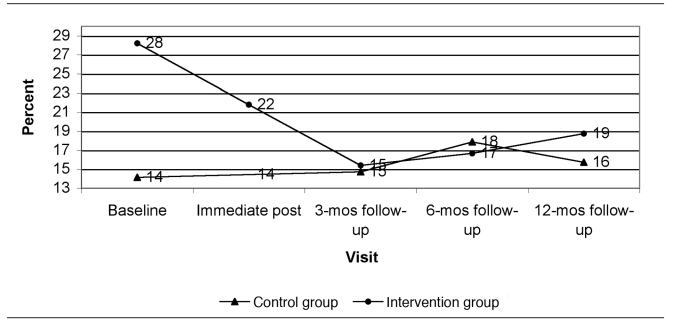
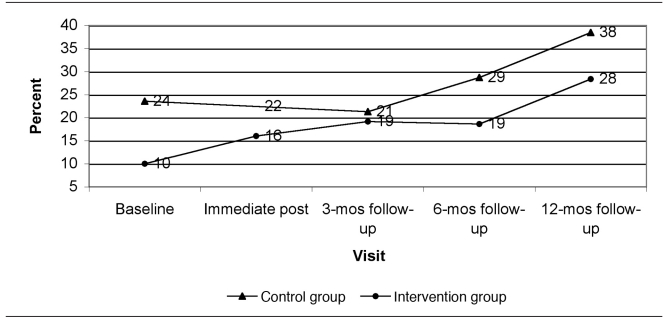
Figure 1 presents the predicted dust loadings from immediate post-intervention to the 12-month follow-up visit at the time of year with average seasonal effect (end of April or October) and at the GM baseline dust loading for each group. Figures 2 through 5 show the percent of allergen measurements >LOD by treatment group and visit for bla g 1, bla g 2, der f 1, and mus m 1, respectively. For the cockroach allergen models, the odds of being >LOD at follow-up visits were 56 to 135 times higher if the level was >LOD at baseline. For mus m 1 on bare floors in the kitchen and TV room, the odds were seven times higher, but in the TV room on carpeted floors the odds were 25 times higher. The only model that showed any change in allergen levels over the follow-up time period was mus m 1 on bare kitchen floors.
Figure 1.
Sieved dust loading (mg/ft2): actual at baseline and model predicted at follow-up, with the average seasonal effect, from a randomized controlled study of nurse case management and housing intervention effects on allergen exposures among asthmatic children in Milwaukee, 2003–2005
mg/ft2 = milligrams per square foot
mos = months
Figure 2.
Percent of bla g 1 (cockroach allergen) concentrations greater than the limit of detection by visit and study group (all rooms and surface types combined): results from a randomized controlled study of nurse case management and housing intervention effects on allergen exposures among asthmatic children in Milwaukee, 2003–2005
mos = months
Figure 5.
Percent of mus m 1 (mouse allergen) concentrations greater than the limit of detection by visit and study group (all rooms and surface types combined): results from a randomized controlled study of nurse case management and housing intervention effects on allergen exposures among asthmatic children in Milwaukee, 2003–2005
mos = months
The season was significant only for mus m 1 on carpeted floors in the TV room and for bla g 1 on bare floors in the kitchen. The odds of being >LOD were 20 times higher in winter (end of January) than in summer (end of July) for bla g 1 and 34 times higher for mus m 1.
DISCUSSION
Although allergen concentrations were not significantly reduced, the combination of nurse case management and home environmental interventions yielded a -significant decrease in settled dust loadings in treated dwellings compared with control group dwellings. If allergen concentration remains constant, but dust loadings decline, then the total amount of allergen available to the child is also significantly reduced. Dust loadings were reduced by 72% from baseline to immediate post-intervention. Even without additional professional interventions, dust loadings remained significantly below baseline loadings up to the 12-month follow-up visit, reducing the exposure burden for children residing in treated homes. The reductions in dust loading were most likely due to the specialized cleaning interventions performed in each intervention group dwelling. Continued cleaning by residents using the supplies provided also may have contributed to the relatively low dust loadings over time.
Modeling also indicated that the season influences dust loadings (i.e., highest in winter, lowest in summer). Other studies of dust lead loadings (not the total dust loadings measured in this study) have found the highest levels in the summer and the lowest ones in the winter.29 The reasons for this discrepancy are not known, although in our study, sieved dust loading was not significantly correlated with dust lead loading on floors (Pearson correlation coefficient of log-transformed values: r= –0.139, p=0.133). It is possible that lead dust behaves differently from total dust. While the contribution of outdoor dust lead to indoor dust lead loading may be larger in the summer when the temperature is hot and the soil moisture content is low, household dust is composed of more than particles from outdoor soil.29 These other components (e.g., shed skin particles, carpet fibers, and lint) may contribute to higher dust loadings in the winter when house conditions are drier and people spend more time indoors. The relationship between dust loading and season should be included in future studies of cleaning-related interventions.
Although allergen concentrations were not significantly reduced, there was a substantial reduction in dust loading, indicating a benefit to the asthmatic children residing in intervention homes. The lack of significant reductions in allergen concentrations may be related, at least in part, to the low frequencies of detection at baseline. The levels of allergens were generally less than those found in other inner-city studies. For example, in a study of homes of asthmatic children in Baltimore, Maryland, 85% of bla g 1 samples were >LOD on kitchen floors of intervention group homes (median concentration = 22 U/g); in contrast, our study found that only 46% of bla g 1 samples were >LOD (median concentration <LOD of 0.4 U/g).7 In the NCICAS study, 87% of baseline samples from kitchen floors were >LOD for mouse allergen (median = 1.6 μg/g), while our study found only 54% >LOD (median <LOD of 0.02 μg/g).6 The baseline results for dust mite allergen in this study tended to be less than those in other studies, but a direct comparison is problematic because our study collected allergen samples only from floors in specific rooms, and other studies collected samples that combined dust from different surfaces (e.g., floor and bedding) and/or different rooms (e.g., living rooms and bedrooms).7,14,30–32
Limitations
One limitation in this study is the absence of a visit to the control group homes immediately following the intervention in the experimental group, although the effect in later months remains quite pronounced, suggesting that this difference is unlikely to change the results. Cleanliness and the relatively stable baseline characteristics of the study homes may have been a factor contributing to the low frequencies of allergen detections at baseline. For example, the exclusion of dwellings with substantial structural problems (i.e., those with outstanding building inspection orders) may have prevented the inclusion of homes with more serious housing-related hazards.
Interestingly, the allergen detected most frequently in settled dust in this study was mouse allergen mus m 1, even though mouse allergen is carried on very small particles that become airborne easily and remain airborne.8 This finding is similar to the NCICAS study, showing that mouse allergen may be at least as important as cockroach or dust mite allergens.5 Subsequent studies should include rodent eradication as part of an IPM program and the collection of mus m 1 data.
IPM, when conducted appropriately, involves a combination of tools to eliminate pests. Other studies have shown the effectiveness of IPM in reducing mouse and cockroach allergen concentrations; however, IPM had no effect on these allergens in this study.12,33 The implementation of the multipronged IPM approach proved difficult in Milwaukee due to inadequate training/oversight of pest management contractors and too few IPM visits (mean = 2). It was often difficult to change resident behavior regarding food storage and disposal of food debris.
CONCLUSION
Although allergen concentrations were not significantly reduced, the combination of nurse case management and home environmental interventions yielded a significant decrease in settled dust loadings in treated dwellings compared with control group dwellings. If allergen concentration remains constant, but dust loadings decline, then the total amount of allergen available to the child is also significantly reduced. Even without additional professional interventions, dust loadings remained significantly below baseline loadings up to the 12-month follow-up visit, reducing the exposure burden for children residing in treated homes. The combination of nurse case management and home environmental interventions promotes collaboration between health and housing professionals and is effective in reducing exposures to allergens in settled dust.
Figure 3.
Percent of bla g 2 (cockroach allergen) concentrations greater than the limit of detection by visit and study group (all rooms and surface types combined): results from a randomized controlled study of nurse case management and housing intervention effects on allergen exposures among asthmatic children in Milwaukee, 2003–2005
mos = months
Figure 4.
Percent of der f 1 (dust mite allergen) concentrations greater than the limit of detection by visit and study group (all rooms and surface types combined): results from a randomized controlled study of nurse case management and housing intervention effects on allergen exposures among asthmatic children in Milwaukee, 2003–2005
mos = months
Footnotes
This study was funded under a U.S. Department of Housing and Urban Development Healthy Homes Demonstration Grant to the Milwaukee Health Department (grant #WILHH0108-02). The findings and conclusions in this article are those of the authors and do not represent the official views of the federal government.
REFERENCES
- 1.Institute of Medicine, Division of Health Promotion and Disease Prevention, Committee on the Assessment of Asthma and Indoor Air. Clearing the air: asthma and indoor air exposures. Washington: National Academy Press; 2000. [Google Scholar]
- 2.Meurer JR, Cohn JH, Kuhn EM, Bolton M, Fiore BJ. Asthma surveillance in an urban WIC office; Presented at the Pediatric Academic Societies Annual Meeting of the Ambulatory Pediatric Association; 2003 May 3–6; Seattle. [Google Scholar]
- 3.Eggleston PA, Bush RK American Academy of Asthma, Allergy and Immunology. Environmental allergen avoidance: an overview. J Allergy Clin Immunol. 2001;107(3) Suppl:S403–5. doi: 10.1067/mai.2001.113673. [DOI] [PubMed] [Google Scholar]
- 4.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–63. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 5.Phipatanakul W, Eggleston PA, Wright EC, Wood RA National Cooperative Inner-City Asthma Study. Mouse allergen. II. The relationship of mouse allergen exposure to mouse sensitization and asthma morbidity in inner-city children with asthma. J Allergy Clin Immunol. 2000;106:1075–80. [Google Scholar]
- 6.Phipatanakul W, Eggleston PA, Wright EC, Wood RA The National Cooperative Inner-City Asthma Study. Mouse allergen. I. The prevalence of mouse allergen in inner-city homes. J Allergy Clin Immunol. 2000;106:1070–4. doi: 10.1067/mai.2000.110796. [DOI] [PubMed] [Google Scholar]
- 7.Breysse PN, Buckley TJ, Williams D, Beck CM, Jo SJ, Merriman B, et al. Indoor exposures to air pollutants and allergens in the homes of asthmatic children in inner-city Baltimore. Environ Res. 2005;98:167–76. doi: 10.1016/j.envres.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Matsui EC, Simons E, Rand C, Butz A, Buckley TJ, Breysse P, et al. Airborne mouse allergen in the homes of inner-city children with asthma. J Allergy Clin Immunol. 2005;115:358–63. doi: 10.1016/j.jaci.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Crocker D, Hopkins D, Kinyota S, Dumitru G, Ligon C, Lawrence B. Home-based interventions to reduce asthma morbidity and mortality; Presentation to the Task Force on Community Preventive Services; 2008 Jun 26; Atlanta. [Google Scholar]
- 10.Krieger J, Takaro TK, Song L, Beaudet N, Edwards K. A randomized controlled trial of asthma self-management support comparing clinic-based nurses and in-home community health workers: the Seattle-King County Healthy Homes II Project. Arch Pediatr Adolesc Med. 2009;163:141–9. doi: 10.1001/archpediatrics.2008.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker EA, Israel BA, Robins TG, Mentz G, Lin XH, Brakefield-Caldwell W, et al. Evaluation of Community Action Against Asthma: a community health worker intervention to improve children's asthma-related health by reducing household environmental triggers for asthma. Health Educ Behav. 2008;35:376–95. doi: 10.1177/1090198106290622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phipatanakul W, Cronin B, Wood RA, Eggleston PA, Shih MC, Song L, et al. Effect of environmental intervention on mouse allergen levels in homes of inner-city Boston children with asthma. Ann Allergy Asthma Immunol. 2004;92:420–5. doi: 10.1016/S1081-1206(10)61777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krieger JW, Takaro TK, Song L, Weaver M. The Seattle King-County Healthy Homes Project: a randomized controlled trial of a community healthy worker intervention to decrease exposure to indoor asthma triggers. Am J Public Health. 2005;95:652–9. doi: 10.2105/AJPH.2004.042994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan WJ, Crain EF, Gruchalla RS, O'Connor GT, Kattan M, Evans R, 3rd, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351:1068–80. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 15.Wissow L, Warshow M, Box J, Baker D. Case management and quality assurance to improve care of inner-city children with asthma. Amer J Diseases in Children. 1988;142:748–52. doi: 10.1001/archpedi.1988.02150070062026. [DOI] [PubMed] [Google Scholar]
- 16.Stout JW, White LC, Rogers LT, McRorie T, Morray B, Miller-Ratcliffe M, et al. The Asthma Outreach Project: a promising approach to comprehensive asthma management. J Asthma. 1998;35:119–27. doi: 10.3109/02770909809055413. [DOI] [PubMed] [Google Scholar]
- 17.Greineder D, Loane K, Parks P. A randomized controlled trial of a pediatric asthma outreach program. J Allergy Clin Immunol. 1999;103:436–40. doi: 10.1016/s0091-6749(99)70468-9. [DOI] [PubMed] [Google Scholar]
- 18.Evans R, 3rd, Gergen PJ, Mitchell H, Kattan M, Kercsmar C, Crain E, et al. A randomized clinical trial to reduce asthma morbidity among inner-city children: results of the National Cooperative Inner-City Asthma Study. J Pediatr. 1999;135:332–8. doi: 10.1016/s0022-3476(99)70130-7. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan SD, Weiss KB, Lynn H, Mitchell H, Kattan M, Gergen PJ, et al. The cost effectiveness of an inner-city asthma intervention for children. J Allergy Clin Immunol. 2002;110:576–81. doi: 10.1067/mai.2002.128009. [DOI] [PubMed] [Google Scholar]
- 20.Galke W, Clark S, McLaine P, Bornschein R, Wilson J, Succop P, et al. National evaluation of the US Department of Housing and Urban Development Lead-Based Paint Hazard Control Grant Program: study methods. Environ Res. 2005;98:315–28. doi: 10.1016/j.envres.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Custovic A, Murray CS, Gore RB, Woodcock A. Controlling indoor allergens. Ann Allergy Asthma Immunol. 2002;88:432–41. doi: 10.1016/S1081-1206(10)62378-2. [DOI] [PubMed] [Google Scholar]
- 22.University of Wisconsin, Healthy Homes Partnership. Help yourself to a healthy home: protect your children's health. Madison (WI): Regents of the University of Wisconsin System; 2002. [cited 2010 Aug 11]. Also available from: URL: http://www.uwex.edu/healthyhome/book.html. [Google Scholar]
- 23.Department of Housing and Urban Development (US). Guidelines for the evaluation and control of lead-based paint hazards in housing. Washington: HUD, Office of Lead-Based Paint Abatement and Poisoning Prevention; 1995. [Google Scholar]
- 24.Chapman MD, Heymann PW, Wilkins SR, Brown MJ, Platts-Mills TA. Monoclonal immunoassays for major dust mite (Dermatophagoides) allergens, Der p I and Der f I, and quantitative analysis of the allergen content of mite and house dust extracts. J Allergy Clin Immunol. 1987;80:184–94. doi: 10.1016/0091-6749(87)90128-x. [DOI] [PubMed] [Google Scholar]
- 25.Chapman MD, Aalberse RC, Brown MJ, Platts-Mills TA. Monoclonal antibodies to the major feline allergen Fel d I. II. Single step affinity purification of Fel d I, N-terminal sequence analysis, and development of a sensitive two-site immunoassay to assess Fel d I exposure. J Immunol. 1988;140:812–8. [PubMed] [Google Scholar]
- 26.Pollart SM, Smith TF, Morris EC, Gelber LE, Platts-Mills TA, Chapman MD. Environmental exposure to cockroach allergens: analysis with monoclonal antibody-based enzyme immunoassays. J Allergy Clin Immunol. 1991;87:505–10. doi: 10.1016/0091-6749(91)90009-d. [DOI] [PubMed] [Google Scholar]
- 27.Ohman JL, Jr, Hagberg K, MacDonald MR, Jones RR, Paigen BJ, Kacergis JB. Distribution of airborne mouse allergen in a major mouse breeding facility. J Allergy Clin Immunol. 1994;94:810–7. doi: 10.1016/0091-6749(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 28.SAS Institute, Inc. SAS: Version 9.1. Cary (NC): SAS Institute, Inc; 2002–2003. [Google Scholar]
- 29.Yiin LM, Rhoads GG, Lioy PJ. Seasonal influences on childhood lead exposure. Environ Health Perspect. 2000;108:177–82. doi: 10.1289/ehp.00108177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arbes SJ, Jr, Sever M, Mehta J, Gore JC, Schal C, Vaughn B, et al. Abatement of cockroach allergens (Bla g 1 and Bla g 2) in low-income urban housing: month 12 continuation results. J Allergy Clin Immunol. 2004;113:109–14. doi: 10.1016/j.jaci.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 31.Kitch BT, Chew G, Burge HA, Muilenberg ML, Weiss ST, Platts-Mills TA, et al. Socioeconomic predictors of high allergen levels in homes in the greater Boston area. Environ Health Perspect. 2000;108:301–7. doi: 10.1289/ehp.00108301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leaderer BP, Belanger K, Triche E, Holford T, Gold DR, Kim Y, et al. Dust mite, cockroach, cat, and dog allergen concentrations in homes of asthmatic children in the northeastern United States: impact of socioeconomic factors and population density. Environ Health Perspect. 2002;110:419–25. doi: 10.1289/ehp.02110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eggleston PA, Butz A, Rand C, Curtin-Brosnan J, Kanchanaraksa S, Swartz L, et al. Home environmental intervention in inner-city asthma: a randomized controlled clinical trial. Ann Allergy Asthma Immunol. 2005;95:518–24. doi: 10.1016/S1081-1206(10)61012-5. [DOI] [PubMed] [Google Scholar]