Abstract
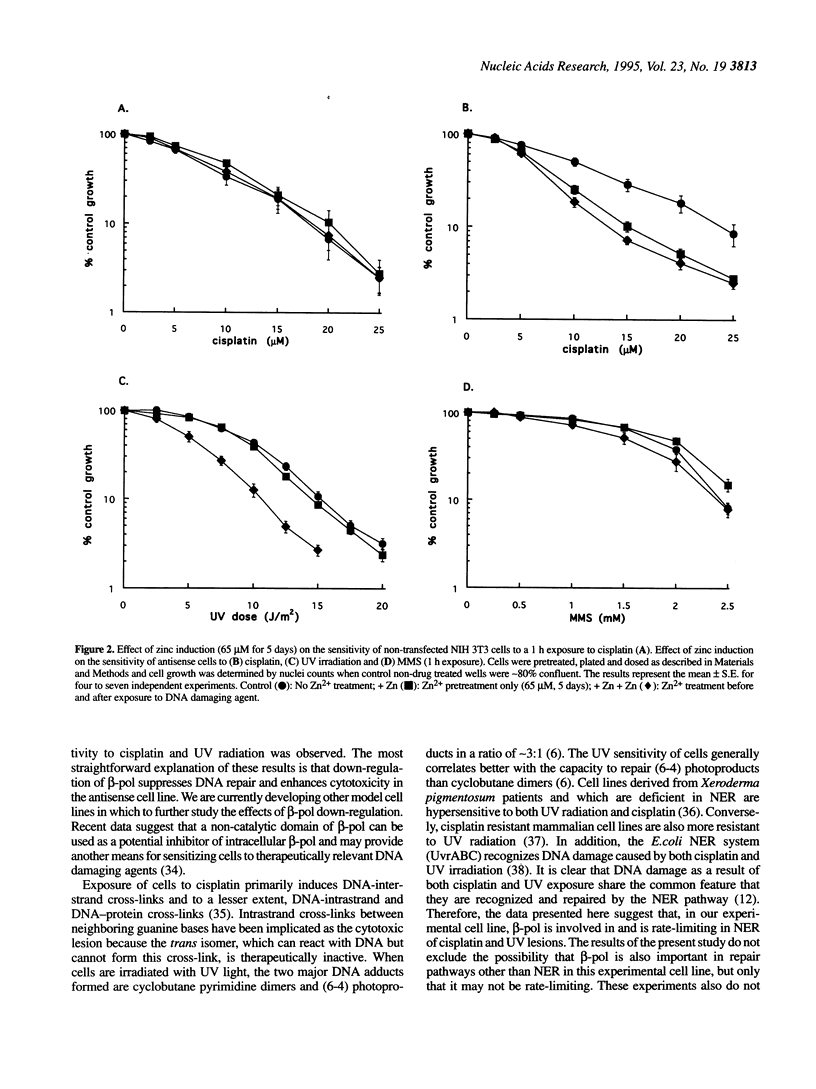
Previously, mouse NIH 3T3 cells were stably transfected with human DNA polymerase beta (beta-pol) cDNA in the antisense orientation and under the control of a metallothionein promoter [Zmudzka, B.Z. and Wilson, S.H. (1990) Som. Cell Mol. Gen., 16, 311-320]. To assess the feasibility of enhancing the efficacy of chemotherapy by an antisense approach and to confirm a role for beta-pol in cellular DNA repair, we looked for increased sensitivity to DNA damaging agents under conditions where beta-pol is down-regulated in the antisense cell line. Such a sensitization is anticipated only where beta-pol is rate-limiting in a DNA repair pathway. A number of agents were tested: cis-diamminedichloroplatinum II (cisplatin); 1,3-bis(2-chloroethyl)-1- nitrosourea (BCNU); ionizing radiation and the radio-mimetic drug bleomycin; the bifunctional alkylating agents nitrogen mustard and L-phenylalanine mustard (melphalan); the monofunctional alkylating agent methyl methane sulfonate (MMS) and ultraviolet (UV) radiation. In the cases of cisplatin and UV radiation, a significant enhancement of cytotoxicity was observed. Damage as a result of both of these agents is thought to be repaired by the nucleotide excision repair (NER) pathway. The results suggest that, in this cell line, beta-pol is involved in and is rate-limiting in NER. We propose that down-regulation of beta-pol by antisense approaches might be used to enhance the cytotoxic effects of cisplatin and other DNA damaging chemotherapeutic agents.
Full text
PDF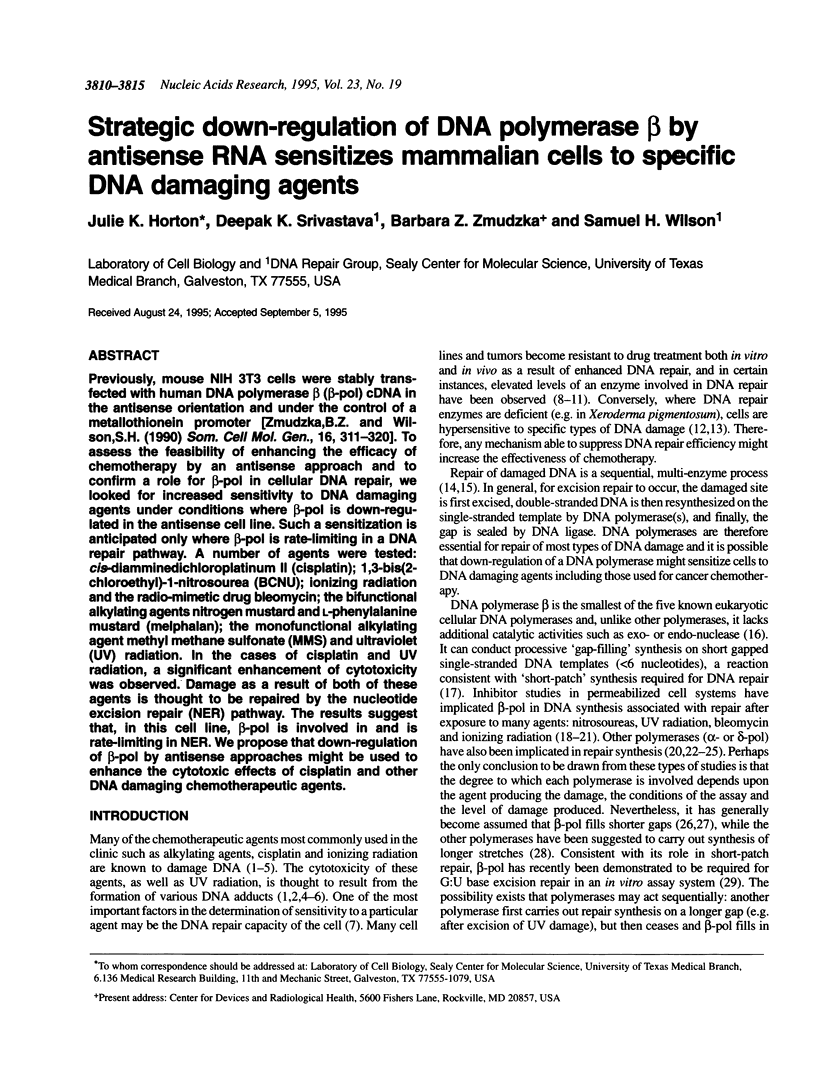



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler W. B. Preparing nuclei from cells in monolayer cultures suitable for counting and for following synchronized cells through the cell cycle. Anal Biochem. 1984 Aug 15;141(1):70–73. doi: 10.1016/0003-2697(84)90426-3. [DOI] [PubMed] [Google Scholar]
- Byrnes R. W., Templin J., Sem D., Lyman S., Petering D. H. Intracellular DNA strand scission and growth inhibition of Ehrlich ascites tumor cells by bleomycins. Cancer Res. 1990 Sep 1;50(17):5275–5286. [PubMed] [Google Scholar]
- Carmichael J., Hickson I. D. Keynote address: mechanisms of cellular resistance to cytotoxic drugs and X-irradiation. Int J Radiat Oncol Biol Phys. 1991 Feb;20(2):197–202. doi: 10.1016/0360-3016(91)90089-m. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E., Gruenert D. C. Repair of psoralen adducts in human DNA: differences among xeroderma pigmentosum complementation groups. J Invest Dermatol. 1984 Apr;82(4):311–315. doi: 10.1111/1523-1747.ep12260596. [DOI] [PubMed] [Google Scholar]
- Dijt F. J., Fichtinger-Schepman A. M., Berends F., Reedijk J. Formation and repair of cisplatin-induced adducts to DNA in cultured normal and repair-deficient human fibroblasts. Cancer Res. 1988 Nov 1;48(21):6058–6062. [PubMed] [Google Scholar]
- Dresler S. L., Frattini M. G. DNA replication and UV-induced DNA repair synthesis in human fibroblasts are much less sensitive than DNA polymerase alpha to inhibition by butylphenyl-deoxyguanosine triphosphate. Nucleic Acids Res. 1986 Sep 11;14(17):7093–7102. doi: 10.1093/nar/14.17.7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresler S. L., Lieberman M. W. Identification of DNA polymerases involved in DNA excision repair in diploid human fibroblasts. J Biol Chem. 1983 Aug 25;258(16):9990–9994. [PubMed] [Google Scholar]
- Eastman A., Schulte N. Enhanced DNA repair as a mechanism of resistance to cis-diamminedichloroplatinum(II). Biochemistry. 1988 Jun 28;27(13):4730–4734. doi: 10.1021/bi00413a022. [DOI] [PubMed] [Google Scholar]
- Ehmann U. K., Cook K. H., Friedberg E. C. The kinetics of thymine dimer excision in ultraviolet-irradiated human cells. Biophys J. 1978 May;22(2):249–264. doi: 10.1016/S0006-3495(78)85487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenberg-Schwager M. Review of repair kinetics for DNA damage induced in eukaryotic cells in vitro by ionizing radiation. Radiother Oncol. 1989 Apr;14(4):307–320. doi: 10.1016/0167-8140(89)90143-6. [DOI] [PubMed] [Google Scholar]
- Fritz G., Tano K., Mitra S., Kaina B. Inducibility of the DNA repair gene encoding O6-methylguanine-DNA methyltransferase in mammalian cells by DNA-damaging treatments. Mol Cell Biol. 1991 Sep;11(9):4660–4668. doi: 10.1128/mcb.11.9.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geleziunas R., McQuillan A., Malapetsa A., Hutchinson M., Kopriva D., Wainberg M. A., Hiscott J., Bramson J., Panasci L. Increased DNA synthesis and repair-enzyme expression in lymphocytes from patients with chronic lymphocytic leukemia resistant to nitrogen mustards. J Natl Cancer Inst. 1991 Apr 17;83(8):557–564. doi: 10.1093/jnci/83.8.557. [DOI] [PubMed] [Google Scholar]
- Gorbacheva L. B., Kukushkina G. V., Durdeva A. D., Ponomarenko N. A. In vivo DNA damage and resistance to 1-methyl-1-nitrosourea and 1,3-bis(2-chloroethyl)-1-nitrosourea in L1210 leukemia cells. Neoplasma. 1988;35(1):3–14. [PubMed] [Google Scholar]
- Hoffmann J. S., Pillaire M. J., Maga G., Podust V., Hübscher U., Villani G. DNA polymerase beta bypasses in vitro a single d(GpG)-cisplatin adduct placed on codon 13 of the HRAS gene. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5356–5360. doi: 10.1073/pnas.92.12.5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain I., Chaney S. G., Sancar A. Repair of cis-platinum-DNA adducts by ABC excinuclease in vivo and in vitro. J Bacteriol. 1985 Sep;163(3):817–823. doi: 10.1128/jb.163.3.817-823.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain I., Morton B. S., Beard W. A., Singhal R. K., Prasad R., Wilson S. H., Besterman J. M. Specific inhibition of DNA polymerase beta by its 14 kDa domain: role of single- and double-stranded DNA binding and 5'-phosphate recognition. Nucleic Acids Res. 1995 May 11;23(9):1597–1603. doi: 10.1093/nar/23.9.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübscher U., Kuenzle C. C., Spadari S. Functional roles of DNA polymerases beta and gamma. Proc Natl Acad Sci U S A. 1979 May;76(5):2316–2320. doi: 10.1073/pnas.76.5.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennerwein M. M., Eastman A., Khokhar A. R. The role of DNA repair in resistance of L1210 cells to isomeric 1,2-diaminocyclohexaneplatinum complexes and ultraviolet irradiation. Mutat Res. 1991 Jan;254(1):89–96. doi: 10.1016/0921-8777(91)90044-p. [DOI] [PubMed] [Google Scholar]
- Katz E. J., Andrews P. A., Howell S. B. The effect of DNA polymerase inhibitors on the cytotoxicity of cisplatin in human ovarian carcinoma cells. Cancer Commun. 1990;2(4):159–164. doi: 10.3727/095535490820874515. [DOI] [PubMed] [Google Scholar]
- Keyse S. M., Tyrrell R. M. Excision repair in permeable arrested human skin fibroblasts damaged by UV (254 nm) radiation: evidence that alpha- and beta-polymerases act sequentially at the repolymerisation step. Mutat Res. 1985 Jul;146(1):109–119. doi: 10.1016/0167-8817(85)90061-6. [DOI] [PubMed] [Google Scholar]
- Kraker A. J., Moore C. W. Elevated DNA polymerase beta activity in a cis-diamminedichloroplatinum(II) resistant P388 murine leukemia cell line. Cancer Lett. 1988 Jan;38(3):307–314. doi: 10.1016/0304-3835(88)90022-5. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Haipek C. A., Clarkson J. M. (6-4)Photoproducts are removed from the DNA of UV-irradiated mammalian cells more efficiently than cyclobutane pyrimidine dimers. Mutat Res. 1985 Jul;143(3):109–112. doi: 10.1016/s0165-7992(85)80018-x. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L. The relative cytotoxicity of (6-4) photoproducts and cyclobutane dimers in mammalian cells. Photochem Photobiol. 1988 Jul;48(1):51–57. doi: 10.1111/j.1751-1097.1988.tb02785.x. [DOI] [PubMed] [Google Scholar]
- Mosbaugh D. W., Linn S. Gap-filling DNA synthesis by HeLa DNA polymerase alpha in an in vitro base excision DNA repair scheme. J Biol Chem. 1984 Aug 25;259(16):10247–10251. [PubMed] [Google Scholar]
- Nishida C., Reinhard P., Linn S. DNA repair synthesis in human fibroblasts requires DNA polymerase delta. J Biol Chem. 1988 Jan 5;263(1):501–510. [PubMed] [Google Scholar]
- Plooy A. C., van Dijk M., Berends F., Lohman P. H. Formation and repair of DNA interstrand cross-links in relation to cytotoxicity and unscheduled DNA synthesis induced in control and mutant human cells treated with cis-diamminedichloroplatinum(II). Cancer Res. 1985 Sep;45(9):4178–4184. [PubMed] [Google Scholar]
- Prasad R., Beard W. A., Wilson S. H. Studies of gapped DNA substrate binding by mammalian DNA polymerase beta. Dependence on 5'-phosphate group. J Biol Chem. 1994 Jul 8;269(27):18096–18101. [PubMed] [Google Scholar]
- Raaphorst G. P., Azzam E. I., Feeley M., Sargent M. D. Inhibitors of repair of potentially lethal damage and DNA polymerases also influence the recovery of potentially neoplastic transforming damage in C3H10T-1/2 cells. Radiat Res. 1990 Jul;123(1):49–54. [PubMed] [Google Scholar]
- Roberts J. J., Thomson A. J. The mechanism of action of antitumor platinum compounds. Prog Nucleic Acid Res Mol Biol. 1979;22:71–133. doi: 10.1016/s0079-6603(08)60799-0. [DOI] [PubMed] [Google Scholar]
- Robins P., Harris A. L., Goldsmith I., Lindahl T. Cross-linking of DNA induced by chloroethylnitrosourea is presented by O6-methylguanine-DNA methyltransferase. Nucleic Acids Res. 1983 Nov 25;11(22):7743–7758. doi: 10.1093/nar/11.22.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W. E., Ewig R. A., Kohn K. W. Differences between melphalan and nitrogen mustard in the formation and removal of DNA cross-links. Cancer Res. 1978 Jun;38(6):1502–1506. [PubMed] [Google Scholar]
- Sakumi K., Sekiguchi M. Structures and functions of DNA glycosylases. Mutat Res. 1990 Sep-Nov;236(2-3):161–172. doi: 10.1016/0921-8777(90)90003-n. [DOI] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. DNA repair enzymes. Annu Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Scanlon K. J., Kashani-Sabet M., Miyachi H. Differential gene expression in human cancer cells resistant to cisplatin. Cancer Invest. 1989;7(6):581–587. doi: 10.3109/07357908909017533. [DOI] [PubMed] [Google Scholar]
- Singhal R. K., Prasad R., Wilson S. H. DNA polymerase beta conducts the gap-filling step in uracil-initiated base excision repair in a bovine testis nuclear extract. J Biol Chem. 1995 Jan 13;270(2):949–957. doi: 10.1074/jbc.270.2.949. [DOI] [PubMed] [Google Scholar]
- Smith D. G., Brent T. P. Response of cultured human cell lines from rhabdomyosarcoma xenografts to treatment with chloroethylnitrosoureas. Cancer Res. 1989 Feb 15;49(4):883–886. [PubMed] [Google Scholar]
- Sweasy J. B., Loeb L. A. Mammalian DNA polymerase beta can substitute for DNA polymerase I during DNA replication in Escherichia coli. J Biol Chem. 1992 Jan 25;267(3):1407–1410. [PubMed] [Google Scholar]
- Wiebauer K., Jiricny J. Mismatch-specific thymine DNA glycosylase and DNA polymerase beta mediate the correction of G.T mispairs in nuclear extracts from human cells. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5842–5845. doi: 10.1073/pnas.87.15.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmudzka B. Z., Wilson S. H. Deregulation of DNA polymerase beta by sense and antisense RNA expression in mouse 3T3 cells alters cell growth. Somat Cell Mol Genet. 1990 Jul;16(4):311–320. doi: 10.1007/BF01232459. [DOI] [PubMed] [Google Scholar]
- Zwelling L. A., Michaels S., Schwartz H., Dobson P. P., Kohn K. W. DNA cross-linking as an indicator of sensitivity and resistance of mouse L1210 leukemia to cis-diamminedichloroplatinum(II) and L-phenylalanine mustard. Cancer Res. 1981 Feb;41(2):640–649. [PubMed] [Google Scholar]




