Abstract
We present a simple method for creating monodisperse emulsions with microfluidic devices. Unlike conventional approaches that require bulky pumps, control computers, and expertise with device physics to operate devices, our method requires only the microfluidic device and a hand-operated syringe. The fluids needed for the emulsion are loaded into the device inlets, while the syringe is used to create a vacuum at the device outlet; this sucks the fluids through the channels, generating the drops. By controlling the hydrodynamic resistances of the channels using hydrodynamic resistors and valves, we are able to control the properties of the drops. This provides a simple and highly portable method for creating monodisperse emulsions.
Droplet-based microfluidic devices use micron-scale drops as “test tubes” for biological reactions.1, 2, 3 With the devices, the drops are loaded with cells, incubated to stimulate cell growth, picoinjected to introduce additional reagents, and sorted to extract rare specimens.4, 5, 6 This allows biological reactions to be performed with greatly enhanced speed and efficiency over conventional approaches: by reducing the drop volume, only picoliters of reagent are needed per reaction, while through the use of microfluidics, the reactions can be executed at rates exceeding hundreds of kilohertz. This combination of incredible speed and efficient reagent usage is attractive for a variety of applications in biology, particularly those that require high-throughput processing of reactions, including cell screening, directed evolution, and nucleic acid analysis.7, 8 The same advantages of speed and efficiency would also be beneficial for applications in the field, in which the amount of material available for testing is limited, and results are needed with short turnaround. However, a challenge to using these techniques in field applications is that the control systems developed to operate the devices are intended for use in the laboratory: to inject fluids, mechanical pumps are needed, while computers must adjust flow rates to maintain optimal conditions in the device.9, 10, 11, 12 In addition to significantly limiting the portability of the system, these qualities make them impractical for use outside the laboratory. For droplet-based microfluidic techniques to be useful for applications in the field, a general, robust, and portable system for operating them is needed.
In this paper, we introduce a general, robust, and portable system for operating droplet-based microfluidic devices. In this system, which we call syringe-vacuum microfluidics (SVM), we load the reagents needed for the emulsion into the inlets of a microfluidic drop maker; using a standard plastic syringe, we generate a vacuum at the outlet of the drop maker,13 sucking the reagents through the channels, generating drops, and transporting them to different regions for visualization and analysis. By controlling the vacuum strength and channel resistances using hydrodynamic resistors14, 15, 16 and single-layer membrane valves,17, 18 we are able to specify the flow rates in different regions of the device and to adjust them in real time. No pumps, control computers, or electricity is needed for these operations, making the entire system portable and of potential use for field applications. To characterize the adjustability and precision of this system, we vary channel resistances and vacuum pressures while measuring the effects on drop size and production frequency. We also show how to use this to form drops of many distinct reagents simultaneously using only a single vacuum syringe.
Monodisperse drop formation is the central operation in droplet-based microfluidics but can be quite challenging due to the need for precise, steady pumping of reagents; forming monodisperse drops with controlled properties is thus a stringent demonstration of the effectiveness of a control system. While there are many geometries available for microfluidic drop formation,19 in this discussion we use a simple cross-junction for its proven ability to form uniform emulsions at high rates of speed,20, 21 a schematic of which is shown in Fig. 1. The devices are fabricated in poly(dimethylsiloxane) (PDMS) using soft lithography.22 The drop formation channels have dimensions of 25 μm in width and 25 μm in height. To enable production of aqueous drops in oil, which are the most useful for biological assays, we require hydrophobic devices, which we achieve using an Aquapel chemical treatment: we flow Aqualpel through the channels for a few seconds, flush with air, and then bake the devices for 20 min at 65 °C. After this treatment, the channels are permanently hydrophilic, as is needed for forming aqueous-in-oil emulsions. To introduce reagents into the device, we use 200 μl plastic pipette tips inserted into the channel inlets. To apply the suction, we use a 10 ml Bectin-Dickenson plastic syringe coupled to the device through a 16 G needle and PE∕5 tubing. The other end of the tubing is inserted into the outlet of the device.
Figure 1.
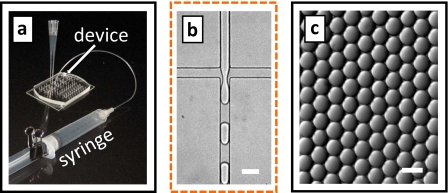
Schematic of the microfluidic drop maker for use with SVM. To form water drops in oil, the device must be hydrophobic, which we achieve by treating the channels with Aquapel. The water and surfactant-containing oil are loaded into pipette tips inserted into the device inlets at the locations indicated. To pump the fluids through the drop maker, a syringe applies a vacuum to the outlet; this sucks the fluids through the drop maker, forming drops. The drops are collected into the suction syringe, where they can be stored, incubated, and reintroduced into a microfluidic device for additional processing.
To begin forming drops, we fill the device with HFE-7500 fluorocarbon oil, displacing trapped air bubbles that could restrict flow and interfere with drop formation. Pipette tips containing reagents are then inserted into the device inlets, as shown in Fig. 1 and pictured in Fig. 2a; during this step, care must be taken to not trap air bubbles under the pipette tips, as they would restrict flow. For the fluids, we use distilled water for the droplet phase and HFE-7500 with the ammonium salt of Krytox 157 FSL at 1.8 wt % for the continuous phase. The suction syringe is then connected to the device outlet; to initiate drop formation, the piston is pulled outward and locked in place with a 1 in. binder clip, as shown in Fig. 2a. This expands the air in the syringe, generating a vacuum that is transferred to the device through tubing. Since the inlet reagents are open to the atmosphere and thus maintained at a pressure of 1 atm, this creates a pressure differential through the device that pumps the fluids. As the fluids flow through the cross-channel, forces are generated that create drops, as shown in Fig. 2b (enhanced online). Due to the very steady flow, the drops are highly monodisperse, as shown in Fig. 2c. After they are formed, the drops flow out of the device through the suction tube and are collected into the syringe. Depending on the emulsion formulation, drops may coalesce on the metal needle of the syringe; if so, an Upchurch fitting should be used to couple the tubing instead. The collected drops can be stored in the syringe, incubated, and reintroduced into additional microfluidic devices, as needed for the assay.
Figure 2.
Photograph of the microfluidic drop formation device with pipette tips containing emulsion reagents and vacuum syringe for pumping (a). Distilled water is used for the droplet phase and HFE-7500 fluorocarbon oil with fluorinated surfactant for the continuous phase. The vacuum applies a pressure differential through the device that pumps the fluids through the drop maker (b) forming drops. The drops are monodisperse, due to the controlled properties of drop formation in microfluidics (c). The scale bars denote 50 μm (enhanced online).
In many biological applications, drop size must be precisely controlled. This is essential, for example, when encapsulating molecules or cells in the drops, in which the number encapsulated depends on the drop size.3, 23, 24 With SVM, the drop size can be precisely controlled. Our strategy to accomplish this is motivated by the physics of microfluidic drop formation. In microfluidic devices, the capillary number of the flow is normally small, Ca<0.1; as a consequence, the drop formation physics follows a plugging∕squeezing mechanism, in which the drop size depends on the flow rate ratio of the dispersed-to-continuous phase.20, 25 By adjusting this ratio, we can thus control the drop size. To adjust this ratio, we use hydrodynamic resistor channels.14, 15, 16 These channels are analogous to electronic resistors in that for a fixed pressure drop (voltage) the flow rate through them (current) is inversely proportional to their resistance. By making the resistors longer or shorter, we adjust their resistance, thereby controlling the flow rate.
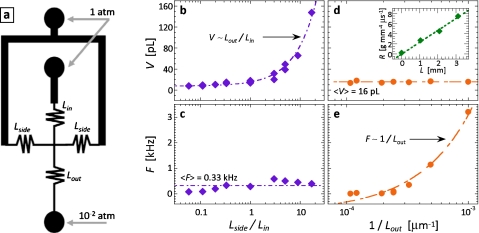
To use resistors to control the drop size, we place three on the inlets of the cross-junction, at the locations indicated in Fig. 3a. In this configuration, the flow rate ratio depends on the resistances of the central and side resistors: shortening the side resistors increases the continuous phase flow rate with respect to the dispersed phase, thereby reducing the ratio and, consequently, the drop size, whereas lengthening it increases the drop size. By varying the ratio, we produce drops over a range of sizes, as shown in Fig. 3b (enhanced online). The drop size is linear in the resistance ratio, indicating that it is linear in the flow rate ratio, as is expected for plugging∕squeezing drop formation [Fig. 3b].20, 25 This behavior is identical to that of pump-driven fluidics, demonstrating that SVM affords similar control.
Figure 3.
Drop properties can be controlled using resistor channels. The resistors are placed on the inlets of the drop maker at the locations indicated in (a). The resistors enable the flow rates of the inner and continuous phases to be controlled. By varying the length ratio of the inlet resistors, we control the flow rate ratio in the drop maker. This allows the drop volume to be controlled, as shown by drop volume plotted as a function of inlet resistor length ratio in (b); varying this ratio does not significantly affect the drop formation frequency, as shown in (c). By varying the length of the outlet resistor, we control the total flow rate through the device; this allows us to form drops of constant volume, but at a different formation frequency, as shown by the plots of volume and frequency as a function of the inverse of the outlet resistor length in (d) and (e), respectively. The measured hydrodynamic resistance of a resistor channel with water as a function of length is shown as inset into (d) (enhanced online).
We can also control the frequency of the drop formation using resistor channels. We place a resistor on the outlet of the device; this sets the total flow rate through the device, thereby adjusting drop frequency, as shown in Fig. 3e (enhanced online). To confirm that the size and frequency control are independent, we plot size as a function of the outlet resistance and frequency as a function of the resistance ratio [Figs. 3c, 3d]; both are constant as a function of these parameters, again demonstrating independent control. Frequency can also be adjusted by changing the strength of the vacuum, which can be accomplished by loading a prescribed volume of air into the syringe before expansion. In this case, the vacuum pressure applied is Pfin=Vin∕Vfin×Pin, where Vin is the initial volume of air in the syringe, Vfin is the volume after expansion, and Pin is the initial pressure, which is 1 atm. By loading a prescribed volume of air into the syringe before connecting it to the device and pulling the piston, the expansion factor can be reduced, thereby lowering the vacuum strength.
The flow rates through the microfluidic device depend on the applied pressure differential, which, in turn, depends on the value of the ambient pressure. Since ambient pressure may vary due to differences in altitude, the drop formation may also vary. However, since ambient pressure variations affect the inner and outer phase flows equally, this should alter the total flow rate but not the flow rate ratio. Consequently, we expect it to alter drop formation frequency but not drop size because while the frequency depends on absolute flow rate [as illustrated by Fig. 3e], drop size depends on the flow rate ratio [as illustrated in Fig. 3b]. Based on normal variations in atmospheric pressure on the surface of the Earth, we expect this to produce differences in the drop formation frequency of ∼25%, for example, when operating a device at sea level compared to at the top of a moderately sized mountain.
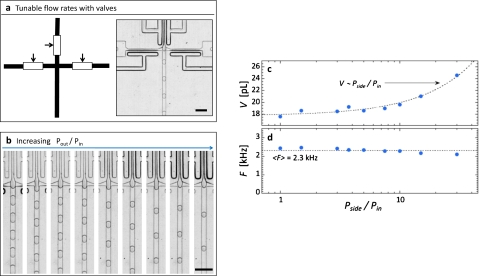
Resistor channels allow drop properties to be controlled, equivalent to what is possible with pump-driven flow; however, they do not allow real-time control because their dimensions are fixed during the fabrication. Real-time control is often needed, for example, as it is when performing reactions in drops for the first time, in which the optimal drop size is not known. To enable real-time control, we must adjust flow rates, which can be achieved using the fluidic analog of electronic potentiometers. Single-layer membrane valves are analogous fluidic components, consisting of a control channel that abuts a flow channel.17, 18 By pressurizing the control channel, the thin PDMS membrane between these channels is deflected laterally, constricting the flow channel, thereby increasing its hydrodynamic resistance and reducing its flow rate.18 To use these membrane valves to vary drop size, we replace the inlet resistors with inlet valves, as shown in Fig. 4a. To set the flow rate through a path, we actuate the valve with a defined pressure. To actuate the valves, we use air-filled syringes: a 1 ml syringe is filled with air and connected to the valve control channel through tubing; an additional component, a three-way stopcock is inserted between the syringe and needle, allowing the pressure to be locked in after optimal actuation conditions are obtained. We use one syringe to control the dispersed phase valves and another to control the continuous phase valves. The valves are pressurized by compressing the air in the syringes to a defined degree using the marked graduations; this is achieved by pressing the piston to a defined graduation mark, compressing the air contained within it, thus increasing pressure. The stopcock is then switched to the off position, locking in the actuation. This simple scheme allows precise actuation of the valves, for accurate, defined flow rates in the drop maker, and controlled drop size, as shown in Figs. 4b, 4c (enhanced online). The drop size can be varied at a rate of several hertz without noticeable loss of control; moreover, changing the drop size does not affect the frequency, indicating that, again, these properties are independent, as shown by the constant drop frequency with varying pressure ratio in Fig. 4d.
Figure 4.
Single-layer membrane valves allow the drop size to be varied in real time to screen for optimal reaction conditions. The valves are positioned on the inner and side inlets, as indicated in (a). By adjusting the actuation pressures of the valves, we vary the flow rates in the drop maker, thereby changing the drop size (b), as shown by the plot of drop volume as a function of the actuation pressure ratio in (c). Varying the inlet resistance ratio does not significantly alter drop formation frequency, as shown by frequency as a function of the pressure ratio in (d). A movie of drop formation during actuation of the valves are available in the supplemental material (Ref. 29). The scale bars denote 100 μm (enhanced online).
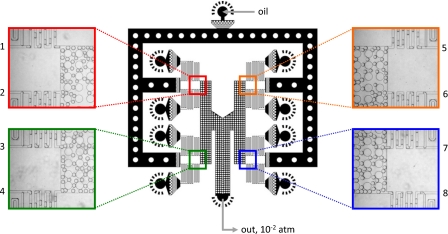
Another useful attribute of SVM is that it readily lends itself to parallel drop formation26 because the pressure that pumps the fluids through the channels is supplied by the atmosphere and is applied evenly over the whole outer surface of the device. This allows fluids to be introduced at equal pressures from different inlets, for forming drops with identical properties in different drop makers. To illustrate this, we use a parallel drop formation device to emulsify eight distinct reagents simultaneously; the product of this is an emulsion library, consisting of drops of identical size in which different drops encapsulate distinct reagents, useful for certain biological applications of droplet-based microfluidics.7 The microfluidic device consists of eight T-junction drop makers.25 The drop makers share one oil inlet and outlet but each has its own inner-phase inlet, as shown in Fig. 5. The oil and outlet channels are wide, ensuring negligible pressure drop through them, so that all T-junctions are operated under the same flow conditions. A distinct reagent fluid is introduced into the inner phase of each T-junction, for which we use eight concentrations of the dye Alexa Fluor 680 in water. After loading these solutions into the device through pipette tips, a syringe applies the vacuum to the outlet, sucking the reagents through the T-junctions, forming drops, as shown by the magnified images of the T-junctions during drop formation in Fig. 5. Since the drop makers are identical and operated under the same flow conditions, the drops formed are of the same size, as shown in the magnified images in Fig. 5 and in a movie available in the supplemental material.29
Figure 5.
Parallel drop formation device consisting of eight T-junction drop makers. The drop makers share a common oil inlet and outlet, both of which are wide to ensure even pressure distribution to all drop makers; support posts prevent these channels from collapsing under the suction. Each drop maker has its own inner-phase inlet, allowing emulsification of a distinct reagent. Since the drop maker dimensions and pressure differentials are constant through all drop makers, the drops formed are of the same size, as shown in the magnified images. The drops are ∼35 μm in diameter.
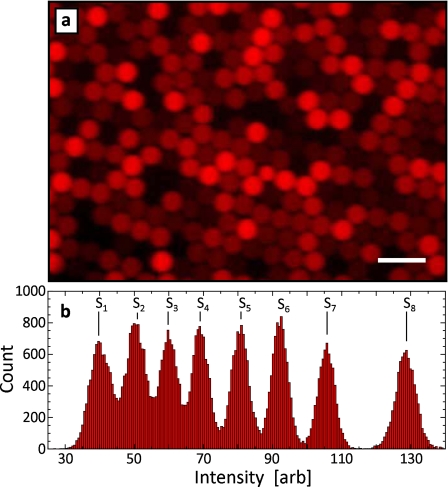
To verify that the dye solutions are successfully encapsulated, we image a sample of the collected drops with a fluorescent microscope. The drops are confined in a monolayer between two glass plates so they can be individually imaged. They are of the same size but have distinct fluorescence intensities, as shown in Fig. 6a. To quantify these differences, we measure the intensity of each drop and plot the results as a histogram [see Fig. 6b]. There are eight peaks in the histogram, corresponding to the eight dye concentrations, demonstrating that all dyes are encapsulated successfully. The peak areas are also similar, demonstrating that drops of different types are formed in equal amounts due to the uniformity of the parallel drop formation.
Figure 6.
Fluorescent microscope image of emulsion library created with parallel T-junction device (a). In this demonstration, eight concentrations of Alexa Fluor 680 dye are emulsified simultaneously, producing an emulsion library of eight elements. The drops are of the same size but encapsulate distinct concentrations of the dye solution, as demonstrated by the eight peaks in the intensity histograms in (b). The scale bar denotes 100 μm.
SVM is a simple, accessible, and highly controlled way to form monodisperse emulsions for biological assays. It allows controlled amounts of different reagents to be encapsulated in individual drops, drop size to be precisely controlled, and the ability to form drops of different reagents at the same time, in a parallel drop formation device. These properties should make SVM useful for biological applications of monodisperse emulsions;1, 2, 3 the portability of SVM should also make it useful for applications in the field, particularly when no electrical power source is available. The parallel emulsification technique should also be useful for particle templating from drops, in which the particles must be of the same size but composed of distinct materials.26, 27, 28, 29
ACKNOWLEDGMENTS
We thank Ralph Sperling, Jeremy Agresti, and Jess Porter Abate for the helpful discussions. This work was supported by the NSF (Grant No. DMR-1006546), the Harvard MRSEC (Grant No. DMR-0820484), and the Massachusetts Life Sciences Center.
References
- Kelly B. T., Baret J. C., Taly V. and Griffiths A. D., Chem. Commun. (Cambridge) 2007, 1773. [DOI] [PubMed] [Google Scholar]
- Taly V., Kelly B. T., and Griffiths A. D., ChemBioChem 8, 263 (2007). 10.1002/cbic.200600425 [DOI] [PubMed] [Google Scholar]
- Clausell-Tormos J., Lieber D., Baret J. C., El-Harrak A., Miller O. J., Frenz L., Blouwolff J., Humphry K. J., Koster S., Duan H., Holtze C., Weitz D. A., Griffiths A. D., and Merten C. A., Chem. Biol. 15, 875 (2008). 10.1016/j.chembiol.2008.08.004 [DOI] [PubMed] [Google Scholar]
- Baret J. C., Miller O. J., Taly V., Ryckelynck M., El-Harrak A., Frenz L., Rick C., Samuels M. L., Hutchison J. B., Agresti J. J., Link D. R., Weitz D. A., and Griffiths A. D., Lab Chip 9, 1850 (2009). 10.1039/b902504a [DOI] [PubMed] [Google Scholar]
- Frenz L., Blank K., Brouzes E., and Griffiths A. D., Lab Chip 9, 1344 (2009). 10.1039/b816049j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abate A. R., Hung T., Mary P., Agresti J. J., and Weitz D. A., Proc. Natl. Acad. Sci. U.S.A. 107, 19163 (2010). 10.1073/pnas.1006888107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouzes E., Medkova M., Savenelli N., Marran D., Twardowski M., Hutchison J. B., Rothberg J. M., Link D. R., Perrimon N., and Samuels M. L., Proc. Natl. Acad. Sci. U.S.A. 106, 14195 (2009). 10.1073/pnas.0903542106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agresti J. J., Antipov E., Abate A. R., Ahn K., Rowat A. C., Baret J. C., Marquez M., Klibanov A. M., Griffiths A. D., and Weitz D. A., Proc. Natl. Acad. Sci. U.S.A. 107, 4004 (2010). 10.1073/pnas.0910781107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Hansen C., and Quake S. R., Anal. Chem. 75, 4718 (2003). 10.1021/ac0346407 [DOI] [PubMed] [Google Scholar]
- Fredrickson C. K. and Fan Z. H., Lab Chip 4, 526 (2004). 10.1039/b410720a [DOI] [PubMed] [Google Scholar]
- Linder V., Sia S. K., and Whitesides G. M., Anal. Chem. 77, 64 (2005). 10.1021/ac049071x [DOI] [PubMed] [Google Scholar]
- Melin J. and Quake S. R., Annu. Rev. Biophys. Biomol. Struct. 36, 213 (2007). 10.1146/annurev.biophys.36.040306.132646 [DOI] [PubMed] [Google Scholar]
- Garstecki P., Fuerstman M. J., Fischbach M. A., Sia S. K., and Whitesides G. M., Lab Chip 6, 207 (2006). 10.1039/b510843h [DOI] [PubMed] [Google Scholar]
- Groisman A., Enzelberger M., and Quake S. R., Science 300, 955 (2003). 10.1126/science.1083694 [DOI] [PubMed] [Google Scholar]
- Kim L., Vahey M. D., Lee H. Y., and Voldman J., Lab Chip 6, 394 (2006). 10.1039/b511718f [DOI] [PubMed] [Google Scholar]
- Fuerstman M. J., Garstecki P., and Whitesides G. M., Science 315, 828 (2007). 10.1126/science.1134514 [DOI] [PubMed] [Google Scholar]
- Sundararajan N., Kim D., and Berlin A. A., Lab Chip 5, 350 (2005). 10.1039/b500792p [DOI] [PubMed] [Google Scholar]
- Abate A. R. and Weitz D. A., Appl. Phys. Lett. 92, 243509 (2008). 10.1063/1.2945797 [DOI] [Google Scholar]
- Abate A. R., Poitzsch A., Hwang Y., Lee J., Czerwinska J., and Weitz D. A., Phys. Rev. E 80, 026310 (2009). 10.1103/PhysRevE.80.026310 [DOI] [PubMed] [Google Scholar]
- Anna S. L., Bontoux N., and Stone H. A., Appl. Phys. Lett. 82, 364 (2003). 10.1063/1.1537519 [DOI] [Google Scholar]
- Ward T., Faivre M., Abkarian M., and Stone H. A., Electrophoresis 26, 3716 (2005). 10.1002/elps.200500173 [DOI] [PubMed] [Google Scholar]
- Xia Y. and Whitesides G. M., Angew. Chem., Int. Ed. 37, 550 (1998). [DOI] [PubMed] [Google Scholar]
- Edd J. F., Di Carlo D., Humphry K. J., Koster S., Irimia D., Weitz D. A., and Toner M., Lab Chip 8, 1262 (2008). 10.1039/b805456h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abate A. R., Chen C. H., Agresti J. J., and Weitz D. A., Lab Chip 9, 2628 (2009). 10.1039/b909386a [DOI] [PubMed] [Google Scholar]
- Garstecki P., Fuerstman M. J., Stone H. A., and Whitesides G. M., Lab Chip 6, 693 (2006). 10.1039/b605553m [DOI] [PubMed] [Google Scholar]
- Nisisako T., Torii T., Takahashi T., and Takizawa Y., Adv. Mater. (Weinheim, Ger.) 18, 1152 (2006). 10.1002/adma.200502431 [DOI] [Google Scholar]
- Nie Z. H., Xu S. Q., Seo M., Lewis P. C., and Kumacheva E., J. Am. Chem. Soc. 127, 8058 (2005). 10.1021/ja042494w [DOI] [PubMed] [Google Scholar]
- Xu S., Nie Z., Seo M., Lewis P., Kumacheva E., Stone H. A., Garstecki P., Weibel D. B., Gitlin I., and Whitesides G. M., Angew. Chem., Int. Ed. 44, 3799 (2005). 10.1002/anie.200590085 [DOI] [PubMed] [Google Scholar]
- See supplementary material at http://dx.doi.org/10.1063/1.3567093 for fast camera videos of drop makers being operated with syringe-vacuum microfluidics.