Abstract
The pathogenic mechanisms whereby the Thr104Ile and Tyr108Cys mutations in the gonadotropin-releasing hormone receptor (GnRHR) gene cause hypogonadotropic hypogonadism in humans are unknown. Transient expression of Thr104Ile and Tyr108Cys mutants in COS-7 cells revealed that both GnRHR mutants neither bind nor respond to agonist. Removal of Lys191 rescued function of both mutants, while addition of a carboxyl-terminal targeting sequence only rescued function of the Thr104Ile mutant. Exposure to the pharmacoperone In3 rescued almost completely Thr104Ile mutant function to wild-type levels, whereas rescue was partial for the Tyr108Cys GnRHR. Additional mutations that block formation of bridges involving Cys108 showed that a Cys108-Cys200 disulfide bridge is the predominant moiety formed in the Tyr108Cys mutant. Thr104Ile and Tyr108Cys GnRHRs are misfolded structures whose function is rescuable by genetic and/or pharmacological strategies. The Tyr108Cys mutant forms an aberrant disulfide bridge that prevents formation of the required Cys14-Cys200 bridge essential for GnRHR plasma membrane expression.
Keywords: Gonadotropin-releasing hormone (receptor), hypogonadotropic hypogonadism, molecular basis of disease, mutation, pharmacoperone, protein misfolding
1.1 Introduction
Gonadotropin-releasing hormone is a key regulator of reproductive function. Hypothalamic GnRH interacts with a membrane receptor in the pituitary gonadotrope leading to synthesis and release of gonadotropins, which directly regulate gonadal function (Knobil, 1974; Santen and Bardin, 1973). The mammalian GnRH receptor (GnRHR) type I belongs to Family A of G protein-coupled receptors (GPCRs) (Millar et al., 2004; Ulloa-Aguirre and Conn, 1998). Unlike related receptors (Millar, 2003), the GnRHR exhibits several unique structural characteristics, including the lack of the carboxyl-terminal extension (C-tail) into the cytosol (McArdle et al., 1999; Millar, 2003; Millar et al., 2004), whose presence is associated with differential physiological receptor regulation of other receptors (Blomenrohr et al., 1999; Heding et al., 1998; Lin et al., 1998) (Fig. 1). Another feature of the human GnRHR is the presence of the amino acid residue Lys at position 191 in the extracellular loop (EL) 2, which is frequently Glu or Gly in non-primate mammals (Janovick et al., 2006; Ulloa-Aguirre et al., 2006); in rat and mice GnRHRs the orthologous amino acid is absent, resulting in a GnRHR structure that is one residue smaller and conferring the rodent receptor with an increased expression at the cell plasma membrane (PM) (Arora et al., 1999).
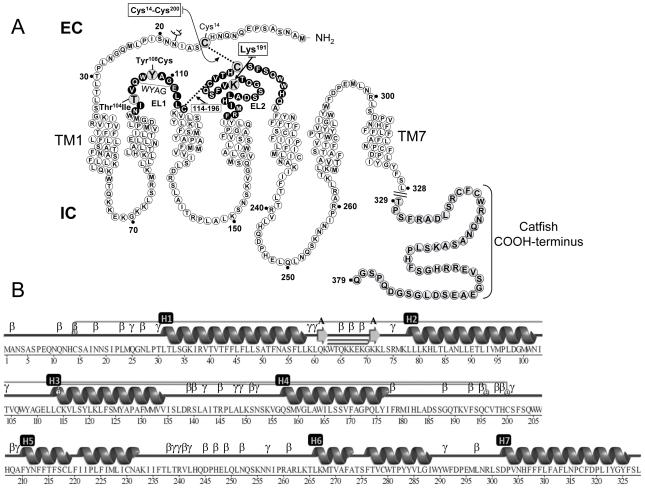
Figure 1.
A: Schematic representation of the hGnRHR amino acid sequence. Residues shown in black circles are in the EL1 and EL2 regions; large grey circles show the amino acid residues mutated or deleted in the present study. Also shown is the sequence of the catfish COOH-terminus (residues 329-379) added to the WT hGnRHR and receptor mutants. EC: intracellular space; IC: intracellular space. B: Schematic representation of the primary and secondary structure of the hGnRHR based on a model recently published by Jardon-Valadez and colleagues (Jardon-Valadez et al., 2008b). Structures were obtained using PDBsum (Laskowski, 2009). Linear representation of secondary structure is represented as cartoons, TM helices are labeled as H1-7, and β and γ labels in B indicate β and γ turns, respectively. Disulfide bridges are represented by gray lines joining the corresponding Cys residues.
In human, hypogonadotropic hypogonadism (HH) has several etiologies including lossof-function mutations in the human (h) GNRHR gene that abolishes the ability to respond to GnRH, leading to decreased or apulsatile gonadotropin release and reproductive failure (Ulloa-Aguirre et al., 2004b). To date, 21 inactivating mutations in the hGNRHR gene have been described as a cause of partial or complete forms of HH; the majority (~90%) of mutant hGnRHR proteins whose function has been examined to date (19 mutants) are trafficking-defective receptors (Janovick et al., 2002; Maya-Nunez et al., 2002; Ulloa-Aguirre et al., 2004b). With the exception of Ser168Arg and Ser217Arg mutants (which cause irreparable misfolding because of the large degree of thermodynamic distortion that prevents formation of a critical Cys14-Cys200 disulfide bridge), the function of the majority of misfolded hGnRHRs can be restored in vitro by genetic approaches or pharmacoperone drugs (i.e. cell permeant antagonists that promote correct folding and trafficking of misfolded mutants to the PM) (Janovick et al., 2003; Leanos-Miranda et al., 2002; Topaloglu et al., 2006; Ulloa-Aguirre et al., 2004a; Ulloa-Aguirre et al., 2006). In this regard, two particular genetic modifications, deletion of Lys191 (ΔLys191) and/or addition of the catfish GnRHR C-tail sequence, have shown to increase PM expression of the wild-type (WT) receptor and to rescue function of misfolded mutant hGnRHRs (Maya-Nunez et al., 2000; Maya-Nunez et al., 2002; Ulloa-Aguirre et al., 2004a). The first modification eliminates a residue (Lys191) that appears to destabilize the protein structure required for an efficient formation of the Cys14-Cys200 disulfide bridge (Arora et al., 1999; Janovick et al., 2006; Jardon-Valadez et al., 2009; Ulloa-Aguirre et al., 2006) during the folding process, whereas the second allows for posttranslational modifications that favor both trafficking and anchoring of the receptor to the PM (Blomenrohr et al., 1999; Lin et al., 1998; Maya-Nunez et al., 2000). Although these strategies efficiently correct routing of misfolded hGnRHRs, restoration of function occurs only when the mutations do not compromise domains involved in agonist binding, receptor activation or G protein coupling (Conn and Ulloa-Aguirre, 2010; Ulloa-Aguirre et al., 2004b).
The EL1 of the GnRHR plays an important role in receptor function, particularly in ligand binding with at least three residues at the EL1-transmembrane domain (TM) 2 junction (Asp98, Phe101 and Asn102) being involved. Its amino acid sequence (particularly the first 5-7 amino acid residues) is highly conserved among several mammalian and non-mammalian species as well as between type I and type II GnRHRs (Millar et al., 2004). This loop also presents the conserved sequence Trp-Tyr-Ala-Gly (WYAG) (Millar et al., 2004). In other rhodopsin-like GPCRs for peptides and biogenic amines, this sequence corresponds to the Trp-Xaa-Phe-Gly motif [(W/F)XΦG, in which Φ is a hydrophobic residue], which has been shown to be important for agonist-mediated receptor activation (Klco et al., 2006). The importance of the EL1 in the hGnRHR is further emphasized by the naturally occurring loss-of-function mutations Thr104Ile, Gln106Arg and Tyr108Cys (within the WYAG sequence) which lead to distinct forms of HH (Antelli et al., 2006; de Roux et al., 1999; de Roux et al., 1997).
In the present study, we employed a combined strategy (mutagenesis, biochemical studies, and pharmacological approaches) to determine the biochemical basis of HH due to the Thr104Ile and Tyr108Cys mutations in the hGnRHR (Antelli et al., 2006).
1.2 Material and Methods
1.2.1 Construction of hGnRHR mutants
Thr104Ile and Tyr108Cys hGnRHR mutants were constructed using the full-length WT hGnRHR cloned into pcDNA3.1 at KpnI and XbaI restriction enzyme sites as template. Site-directed mutagenesis was performed using the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). Forward and reverse mutagenic oligonucleotide primers (Life Technologies, Grand Island, NY) were designed according to the hGnRHR cDNA sequence reported by Chi et. al.(Chi et al., 1993) (GenBank accession No. L07949). Mutants lacking Lys191 (at the EL2) (Thr104Ile/ΔLys191 and Tyr108Cys/ΔLys191 hGnRHRs) or containing the catfish GnRHR C-tail (Thr104Ile/C-tail and Tyr108Cys/C-tail hGnRHRs) were constructed employing a hGnRHR/ΔLys191 or a hGnRHR/C-tail DNA construct (Maya-Nunez et al., 2002) as templates, respectively. Human GnRHR mutants with further replacements in Tyr108 (Tyr108Phe and Tyr108Thr hGnRHRs) were also constructed employing the same mutagenesis procedure described above. Double (Y108C/C200A and Y108C/C14A) and triple (Y108C/C200A/ΔLys191, Y108C/C200A/C-tail, Y108C/C14A/ΔLys191, and Y108C/C14A/C-tail) mutants were prepared by overlap extension PCR, using hGnRHR WT, hGnRHR/ΔLys191, and hGnRHR/C-tail as templates. The PCR products were digested with KpnI and XbaI restriction enzymes and ligated into the same sites in pcDNA3.1 (Janovick et al., 2002). The identities of all constructs were verified by automated sequencing employing the BigDye Terminator Cycle Sequence kit (Applied Biosystems, Foster City, CA).
1.2.2 Transient transfection of COS-7 cells
Wild-type and mutant hGnRHRs were transiently expressed in COS-7 cells as previously described (Maya-Nunez et al., 2002). Either fifty thousand or 1 × 105 cells per well were plated in 48-well plates [for assessing agonist-stimulated inositol phosphates (IP) production] or 24-well plates (for binding experiments) (Costar, Cambridge, MA), respectively, and 20 h later the cells were transfected with 50 ng (25 ng when mutant pairs were cotransfected) or 200 ng (100 ng for cotransfections) (for IP production and binding studies, respectively) of each hGnRHR DNA construct per well using lipofectamine (Leanos-Miranda et al., 2005). Where indicated, 1μg/ml In3 (Merck and Company, Rahway, NJ) in 1% DMSO was added for 4 h, and then removed 18 h before agonist exposure (Janovick et al., 2002).
1.2.3 Measurement of IP production
Fifty-one hours after the start of transfection, the cells were preloaded with 4 μCi/ml [3H]-myo-inositol. Inositol phosphates (IP) production was measured after exposure of the cells to the GnRH agonist, Buserelin (Sigma, St. Louis, MO) for 2 h. Quantification of IP production by Dowex anion exchange chromatography and liquid scintillation spectroscopy was performed as described previously (Huckle and Conn, 1987).
1.2.4 Receptor binding assay
COS-7 cells were transiently transfected as described above. Twenty hours later, the cells were washed twice with DMEM/0.1% BSA and cultured in DMEM for 18 h before addition of [125I]-Buserelin (specific activity 700 μCi/μg). Cells were incubated at room temperature for 90 min in the presence or absence of excess (10μM) unlabeled Buserelin plus [125I]-Buserelin. Thereafter, the medium was removed, the plates containing the cells were placed on ice, washed twice with ice-cold PBS, and then the cells were solubilized by adding 0.2 M NaOH/0.1% SDS. Aliquots of samples were then transferred to glass tubes to determine radioactivity content. Specific binding was calculated by subtracting non-specific binding (binding in the presence of 10μM Buserelin) from total binding (no unlabeled Buserelin agonist added).
For the radioreceptor assay, COS-7 cells were transfected as described above and incubated at room temperature for 90 min in the presence or absence of excess cold GnRH agonist (Buserelin) plus [125I]-Buserelin or [125I]-Buserelin plus increasing concentrations (10−11 to 10−6 M) of GnRH agonist. Thereafter, the medium was removed and the plates containing the cells were placed on ice and washed with ice-cold PBS. The cells were then solubilized and aliquots of samples were transferred to glass tubes and analyzed for radioactivity content.
1.2.5 Computational modeling
The WT GnRHR model was generated using the crystal structure of bovine rhodopsin as template for the TM domains; the structure was refined by molecular dynamics simulations in a fully hydrated phospholipid bilayer. Hydrogen bonds between residues in EL1 and EL2 were identified using a donor-acceptor atom cutoff distance of 3.5 Å and a donor-hydrogen-acceptor angle of less than 30°. The analysis was performed using the last 40 ns of the simulation trajectory of the WT GnRHR refined model (Jardon-Valadez et al., 2009). Visual inspection of EL1-EL2 interactions were performed using Pymol 0.99 (De Lano, 2002) and VMD (Humphrey et al., 1996).
1.2.6 Statistical analysis
Data were analyzed with one-way ANOVA and then with the Tukey test for pairwise, post hoc comparisons. Binding parameters were calculated from the dose-response displacement curves using the software GraphPad Prism 4.0 (GraphPad Software Inc., La Jolla, CA).
1.3 Results
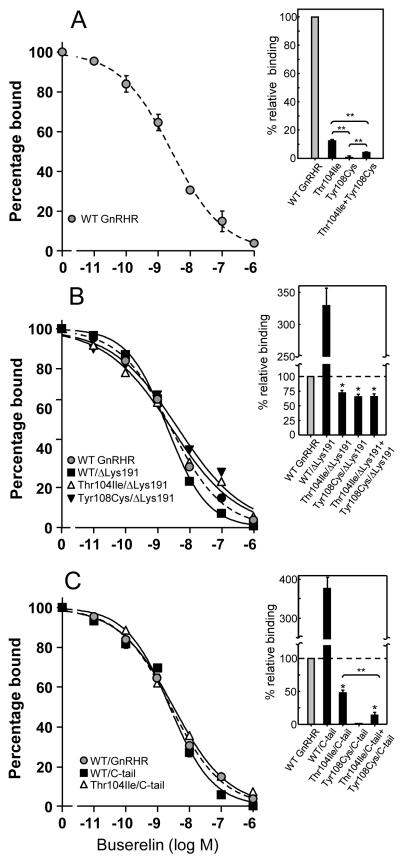
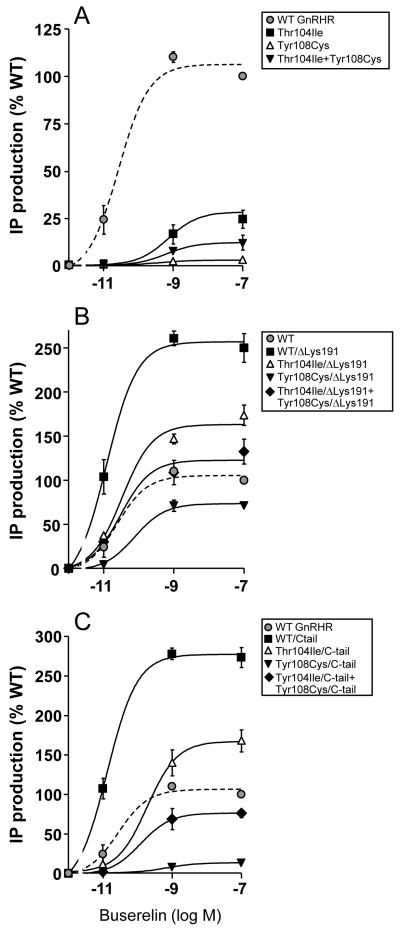
Specific 125I-Buserelin binding to cells expressing the Thr104Ile or Tyr108Cys hGnRHRs was considerably reduced or absent, respectively (Fig. 2A). As previously observed (Jardon-Valadez et al., 2009; Maya-Nunez et al., 2000; Maya-Nunez et al., 2002), removal of Lys191 from the WT GnRHR led to a significant increase in 125I-Buserelin binding, which was most probably due to increased PM expression of the modified receptor (Arora et al., 1999; Maya-Nunez et al., 2002) (Fig. 2B). Deletion of Lys191 from both Thr104Ile and Tyr108Cys mutants, resulted in a substantial increase in maximal GnRH agonist binding to levels 72 ± 3% and 64 ± 7%, respectively, from that exhibited by the WT receptor (Fig. 2B). The binding affinity of 125I-Buserelin at the hGnRHRs lacking Lys191 was comparable to that shown by the WT receptor, although in the case of the Tyr108Cys/ΔLys191 mutant, a modest (~2.5-fold) decrease was detected (Table 1). Despite the markedly reduced 125I-Buserelin-binding capacity of the hGnRHR Thr104Ile, this mutation did not completely abolish Buserelin-stimulated activation of the receptor (Fig. 3A) as the mutant receptor displayed ~30% of the maximal Buserelin-stimulated IP production exhibited by the WT receptor. This was not the case of the Tyr108Cys mutant, in which Buserelin-stimulated IP production was virtually absent. Deletion of Lys191 from the mutant receptors increased Buserelin-stimulated maximal IP production to levels below (Tyr108Cys mutant) or above (Thr104Ile mutant) that shown by the WT receptor, but markedly lower than that presented by the WT hGnRHR/ΔLys191 (Fig. 3B). Thus, whereas removal of Lys191 from the Thr104Ile mutant produced a phenotype for agonist binding and agonist-stimulated IP production comparable to the WT receptor, this modification did not completely restore WT functional features to the Tyr108Cys mutant.
Figure 2.
Displacement of [125I]-Buserelin by increasing concentrations of the unlabeled GnRH agonist (Buserelin) in COS-7 cells transfected with the WT hGnRHR and mutant receptors (A), hGnRHRs lacking Lys191 (B) or hGnRHRs bearing the catfish C-tail (C). Insets: Specific labeled agonist binding to cultured COS-7 cells transiently expressing the hGnRHRs with or without the genetic modifications imposed. Insets also include labeled GnRH agonist binding after cotransfection of the Thr104Ile and Tyr108Cys mutants with or without the genetic modifications. Results are the means ± SEM of 3-4 experiments in triplicate incubations. *, p<0.01 vs WT, WT/ΔLys191 and WT/C-tail receptors; **, p<0.01.
Table 1.
Binding parameters, maximal Buserelin-stimulated inositol phosphate (IP) production, and ED50 of the WT and mutant hGnRHRs (means ± SEM from three or more independent experiments)
| GnRHR | IC50 (nM) |
Cell surface receptor number (fmol × 105 cells) |
Max IP production (% GnRHR WT) |
ED50 (pM) |
|---|---|---|---|---|
| WT | 2.7 ± 0.2 a | 4.6 ± 0.9 a | 100 a | 22.1 ± 5.4 a |
| T104I | - | - | 25 ± 3 b | - |
| Y108C | - | - | 3 ± 0.5 c | - |
|
| ||||
| WT ΔK191 | 2.2 ± 0.2 a | 11.5 ± 2.2 b | 258 ± 8 d | 13.5 ± 3.4 a |
| T104I ΔK191 | 3.4 ± 0.6 a,b | 3.8 ± 0.6 a,c | 166 ± 4 e | 24.0 ± 3.1 a |
| Y108C ΔK191 | 6.7 ± 0.9 b | 4.4 ± 0.6 a | 73 ± 9 f | 76 ± 10 b |
|
| ||||
| WT/C-tail | 2.5 ± 0.2 a | 16.0 ± 1.4 b | 277 ± 5 d | 13.5 ± 1.4 a |
| T104I/C-tail | 4.3 ± 0.9 a,b | 2.1 ±0.6 a | 167 ± 8 e | 72.1 ± 8.0 b |
| Y108C/C-tail | - | - | 13 ± 3 g | - |
Different superscript letters indicate statistically significant differences between means in the same column (p<0.05 for IC50 and cell surface receptor number; p≤ 0.01 for maximal IP production; p<0.01 for ED50).
Figure 3.
Inositol phosphate (IP) dose-response curves for Buserelin in cultured COS-7 cells transiently expressing the WT and hGnRH mutant receptors (A), or hGnRHRs lacking Lys191 (B) or bearing the catfish C-tail (C). The graphs also include IP production in cells cotransfected with the Thr104Ile and Tyr108Cys mutants with or without the genetic modifications imposed. Results are the means ± SEM of 3 or more experiments in triplicate incubations.
Addition of the C-tail to the WT hGnRHR substantially increased its PM expression (Fig. 2C and Table 1). Therefore, we assumed that introduction of this distant domain to the EL1 hGnRHR mutants would better allow monitoring the functional features of the mutant receptors without altering the spatial and biochemical relationships between the EL1 and EL2 (Figs. 4 and S1) that may be altered by the change in the configuration of the EL2 provoked by removing Lys191. Addition of the catfish C-tail to the Thr104Ile mutant receptor increased 125I-Buserelin binding modestly (Fig. 2C); nevertheless, the functional recovery (IP production) of this particular Thr104Ile/C-tail chimera was similar to that exhibited by its Thr104Ile/ΔLys191 counterpart and above that presented by the unmodified WT hGnRHR. Interesting, preincubation of cells expressing the Thr104Ile mutant with In3 allowed nearly complete functional recovery of the mutant receptor (to 70.3 ± 8.4% Buserelin-stimulated WT levels in the absence of In3), whereas in the case of the Tyr108Cys mutant rescue was 39.3 ± 5.0% and it was necessary to delete Lys191 in order to reach complete functional recovery in response to the pharmacoperone (see below). In contrast to the effects provoked by deleting Lys191, insertion of the C-tail to the Tyr108Cys mutant was unable to promote measurable 125I-Buserelin binding or functional rescue of this mutant (Fig. 2C). Thus, addition of the C-tail to the Thr104Ile hGnRHR allowed us to confirm that the primary defect of the mutant lies in misfolding of the receptor molecule. In the case of the Tyr108Cys mutant, this modification unveiled the presence of a more severe defect that most probably involved changes in the intermolecular interactions between the EL1 and EL2 (Fig. 4) that may potentially lead to severe conformational defects incompatible with cell surface membrane expression and/or binding to agonist.
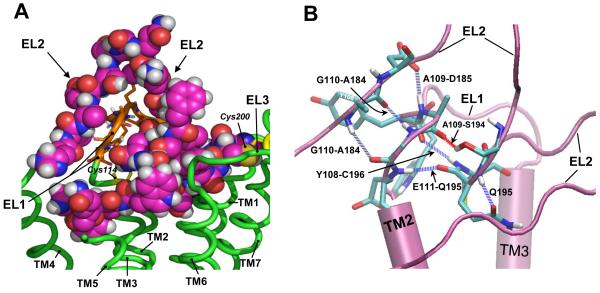
Figure 4.
A: Conformation of the EL1 (orange ribbons) and EL2 (spheres) in the WT hGnRHR (green ribbons). The EL2 is embracing the EL1 favoring direct interactions between their corresponding side chains as well as backbone atoms. B: Representative snapshot of interactions between EL1 (residues Tyr108, Ala109, Gly110, and Glu111) and EL2 (residues Ala184, Asp185, Ser194, and Gln195) forming a H-bond network (blue and red dashed lines). Also shown in this configuration is a H-bond between Gln195 side-chain and its backbone. Only fragments of TM2, TM3, and EL2 are shown for clarity.
Coexpression of both hGnRHR mutants (with or without further genetic modifications) resulted in intermediate responses (agonist binding and maximal Buserelin-stimulated IP production) between those exhibited by the Thr104Ile and Tyr108Cys mutants when expressed alone (Figs. 2B and C and 3A-C).
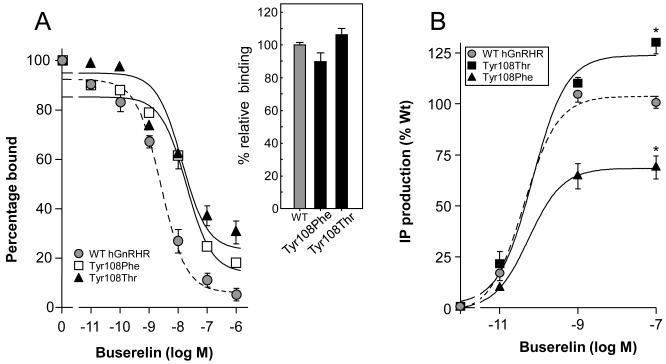
Tyrosine at position 108 is located within the highly conserved GnRHR WYAG sequence present in ~90% of mammalian GnRH type I receptors cloned to date (Millar et al., 2004). Replacement of this residue with phenylalanine (GnRHR Tyr108Phe) (an aromatic, highly hydrophobic residue that lacks the reactive hydroxyl group present in Tyr) or with Thr (a residue that restricts the conformations the main-chain can adopt) yielded mutant receptors that were expressed at similar levels than the WT GnRHR (Fig. 5A). Nevertheless, the displacement curves of both mutant receptors exhibited a shift to the right, yielding IC50 values nearly 5- to 10-fold greater (10.9 ± 0.4 and 23.0 ± 0.6 nM for the Tyr108Thr and Tyr108Phe hGnRHR mutants, respectively; p<0.01 vs WT) than the WT receptor (Fig. 5A). A modest, but significant impairment in maximal Buserelin-stimulated IP production was detected in the Tyr108Phe mutant (relative maximal IP production, 71.5 ± 3.4%; p<0.01 vs WT), whereas in the case of the Tyr108Thr hGnRHR the substitution favored effector activation as disclosed by a 30% increase in Buserelin-stimulated IP production (relative maximal IP production, 130 ± 5.0%; p<0.01 vs WT) (Fig. 5B). These data indicated that the Tyr108 in the hGnRHR may be replaceable by distinctly different amino acid residues without considerably affecting PM expression and function of the receptor, suggesting that the Tyr→Cys substitution provoked formation of an aberrant disulfide bridge either with Cys14 at the amino-terminus or Cys200 at the EL2.
Figure 5.
Displacement of [125I]-Buserelin by increasing concentrations of unlabeled GnRH agonist (Buserelin) in COS-7 cells transfected with the WT hGnRHR and Tyr108Phe or Tyr108Thr mutant receptors (A). Inset: Specific labeled-agonist binding to cultured COS-7 cells transiently expressing the WT and mutant hGnRHRs. No statistically significant differences were detected among the three hGnRHRs. (B) Inositol phosphate (IP) dose-response curves for Buserelin in cultured COS-7 cells transiently transfected with the WT human GnRHR and Tyr108Phe or Tyr108Thr mutant receptors. *, p<0.01 vs WT hGnRHR
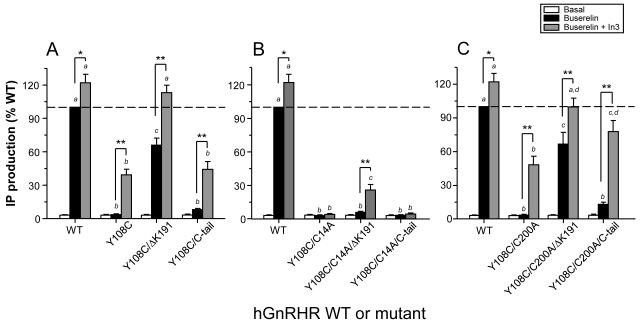
In order to further study the foregoing issue, a series of additional experiments that employed pharmacological and further mutational strategies were performed. Formation of the critical Cys14-Cys200 disulfide bridge can be prevented by making the Cys14Ala or Cys200Ala mutants, which results in hGnRHRs that are retained in the endoplasmic reticulum, but can be rescued either by pharmacoperones (i.e. In3) or by deletion of Lys191. We reasoned that if a Cys108-Cys14 bridge forms in the Tyr108Cys mutant, hGnRHRs bearing additional substitutions (eg. Cys14Ala or Cys200Ala) should behave differently. The Cys14Ala substitution will block formation of the Cys108-Cys14 disulfide bond and lead to a structure that is rescuable by pharmacoperones or by deleting Lys191. In contrast, if the Cys108-Cys14 bridge forms, the Cys200Ala mutant will not block Cys108-Cys14 bridge formation and the resulting mutant will be unrescuable by these same means. If Cys108-Cys200 bridge forms, the reverse is true. As shown in Fig. 6A mutant Tyr108Cys was partially rescued by the pharmacoperone In3 or by deleting Lys191, whereas both exposure to the pharmacoperone and deletion of Lys 191 led to an additive effect as disclosed by complete functional rescue of the mutant receptor, corroborating previous studies showing that these rescuing strategies have different sites of action (Janovick et al., 2009; Maya-Nunez et al., 2002). Addition of the C-tail combined with In3 exposure only increased maximal Buserelin-stimulated IP production up to ~40 of WT levels, reflecting more the effect of the pharmacoperone than the addition of the C-tail (see Fig. 3C). The results obtained from the functional experiments with the double (Tyr108Cys/Cys14Ala and Tyr108Cys/Cys200Ala) and triple (Tyr108Cys/Cys14Ala/ΔLys191 and Tyr108Cys/Cys200Ala/ΔLys191) Tyr108 mutants are shown in Figs. 6B and C. Only the triple Tyr108Cys/Cys14Ala/ΔLys191 mutant exhibited some detectable Buserelin-stimulated response upon exposure to In3, while in the case of mutants bearing the Cys200Ala substitution, the behavior was similar to that exhibited by the Tyr108Cys mutant with or without Lys191 or the C-tail.
Figure 6.
Basal, Buserelin-stimulated IP production, and IP response to In3 exposure in cultured COS-7 cells transiently expressing the WT hGnRHR or the Tyr108Cys mutant with or without Lys191 or the catfish C-tail (A), and the Tyr108Cys/Cys14Ala (B) or the Tyr108Cys/Cys200Ala (C) double mutants with or without further genetic modifications (removal of Lys191 or addition of the catfish C-tail). Cells were transfected with the hGnRHRs cDNAs as described in Materials and Methods and incubated in medium alone (white bars), 0.1 □M Buserelin (black bars), or 1 μg/ml In3 followed by 0.1 μM Buserelin (grey bars). Results are the means ± SEM of four independent experiments in quadruplicate incubations. *, p<0.05; **, p<0.01. Different letters above bars indicate statistically significant (p<0.01) differences between bars of the same color.
1.4 Discussion
In the present study, we carried out functional studies to assess the biochemical mechanism by which hGnRH receptor mutants Thr104Ile and Tyr108Cys lead to HH when expressed in vivo (Antelli et al., 2006); both mutations are located in the EL1 of the receptor, a highly conserved domain among several mammalian and non-mammalian GnRHRs (Millar et al., 2004; Pfleger et al., 2008). Threonine 104 is close to residues involved in ligand-receptor interaction and is a highly conserved residue in type I, type II and type III GnRHRs, with only three exceptions (Bull frog II, Rana dII, and Xenopus I GnRHRs) in which threonine is replaced with methionine (Millar et al., 2004). On the other hand, Tyr108 belongs to the WXFG motif, which in many GPCRs is involved in agonist-mediated receptor activation (Klco et al., 2006); although Tyr108 is also a highly conserved residue among GnRHRs, it may occasionally be replaced with His or Leu in different species, but Cys does not appear in the WT of any species (Millar et al., 2004), likely since Cys108 may result in inappropriate disulfide bonds that severely modify the configuration of the receptor (Sitia and Braakman, 2003).
Deletion of Lys191 from the Thr104Ile and Tyr108Cys hGnRHRs restored function of both receptors to near WT levels. Since, in the absence of the destabilizing effect of Lys191 agonist binding by the Thr104Ile and Tyr108Cys receptors increased from undetectable levels to ~70% and ~60% respectively, compared to that observed for the WT receptor, it is reasonable to assume that both mutations led to misfolded receptors that trafficked inefficiently from the site of synthesis in the endoplasmic reticulum to the PM. Similar results were found when the catfish C-tail was added to the Thr104Ile mutant sequence [a modification that presumably does not alter the conformation of the extracellular domains of the receptor, but which behaves as a targeting sequence increasing the movement to the plasma membrane (Blomenrohr et al., 1999)] or when Thr104Ile-transfected cells were exposed to In3, findings that support the above assumption on the effect of this mutation in folding and intracellular trafficking of the hGnRHR molecule. The observation that PM expression of all Thr104Ile modified receptors (Thr104Ile/ΔLys191 and Thr104Ile/C-tail) was well below that exhibited by the WT/ΔLys191 receptor, suggests that a significant portion of the mutant receptors still remained as inefficiently processed molecules by the cell as a consequence of the Thr→Ile substitution. The finding that In3 led to almost complete functional rescue of the Thr104Ile mutant, confirmed that the primary defect of this mutant lies in misfolding of the receptor molecule, which apparently does not compromise domains involved in binding to agonist, receptor activation or coupling to G proteins. The defect in misfolding probably results from the replacement of the polar threonine with the hydrophobic isoleucine at position 104, which, in turn, may modify the hydrophobicity and configuration of the EL1 and secondarily the TM2-TM3 intermolecular interactions(Humphrey et al., 1996; Jardon-Valadez et al., 2008a), which are amenable for correction with pharmacoperones (Conn and Ulloa-Aguirre, 2010; Janovick et al., 2009). These findings emphasize that even slight deviations from the WT conformation may profoundly alter expression of the receptor protein at the PM, which is consonant with the observation that many mutations that cause HH are charge changes in single residues (Conn et al., 2006).
Addition of the catfish C-tail to the Tyr108Cys hGnRHR led to a different scenario: neither agonist binding nor function of the receptor could be restored, suggesting that the conformational change provoked by the Tyr→Cys replacement severely affected the ability of the receptor to bind agonist and evoke receptor activation or, alternatively, that the primary defect is so severe that it prevented the misfolded protein to pass the quality control system of the endoplasmic reticulum, leading to complete blockage of outward trafficking to the PM. The fact that the amino acid residue in the second position of the WXAG motif may be highly variable (i.e. there is no consensus residue in the second position of this motif), but never involves a cysteine residue at least in the 100 human rhodopsin-like GPCRs studied to date (Klco et al., 2006), strongly suggests that the substitution with cysteine at this particular position profoundly altered receptor function by affecting formation of EL1-EL2 connecting disulfide bridges. Formation of Cys108-Cys114 or Cys108-Cys196 disulfide bonds seemed unlikely, particularly considering that the Cys114-Cys196 bridge, which favors the formation of H-bonds between these loops (Fig. 4B), is a structural feature associated with the fundamental stability of the GnRHR (Cook and Eidne, 1997) and that its breaking leads to pharmacoperone-recalcitrant complete loss of function (Conn and Janovick, 2009). On the other hand, the mechanism of pharmacoperone and deletion of Lys191 is to remove the requirement (from the hGnRHR) for the Cys14-Cys200 bridge (Janovick et al., 2006; Ulloa-Aguirre et al., 2006). Using these latter strategies, we found that functional recovery of the Tyr108Cys/Cys200Ala mutant was possible indicating that blocking formation of the Cys108-Cys200 bridge resulted in a mutant that could be rescued. The observation that the Tyr108Cys/Cys14Ala mutant could not be rescued or was only marginally rescued when both approaches were simultaneously applied, may be interpreted as meaning that the Ala replacement at position 14 did not protect against formation of the bridge that prevents rescue. Accordingly, it can be concluded that the hGnRHR bearing a Cys108-Cys200 disulfide bridge is the predominant moiety formed in the Tyr108Cys mutant.
It was interesting to find that coexpression of both mutant hGnRHRs (Thr104Ile and Tyr108Cys hGnRHRs, emulating what actually is found in compound heterozygous HH patients) resulted in IP responses and agonist binding that were intermediate between those exhibited by the individual mutants. Since the functional activity of the Tyr108Cys mutant was virtually null when expressed alone, these partial responses may be attributed to the marginal (albeit clearly detectable) expression level of the Thr104Ile hGnRHR. Thus, it is possible that residual expression (and function) of the Thr104Ile receptor in vivo may account for the responses to GnRH analog administration shown by compound Thr104Ile/Tyr108Cys heterozygous HH patients (Antelli et al., 2006).
In summary, this study provides in vitro evidence on the functional and structural abnormalities provoked by the Thr104Ile and Tyr108Cys naturally occurring hGnRHR mutations. Apparently, both substitutions lead to misfolding and deficient outward trafficking of the mutant receptors. In both defective receptors, function can be restored by introducing additional genetic modifications, mainly removal of Lys191, which promote cell surface delivery of the misfolded receptor from the endoplasmic reticulum (Conn and Janovick, 2009; Janovick et al., 2006; Janovick et al., 2009; Ulloa-Aguirre et al., 2006) or by treatment with a pharmacoperone drug. The conformational alteration provoked by the Tyr108Cys replacement is more severe than that resulting from the Thr104Ile substitution due to formation of an aberrant Cys108-Cys200 disulfide bond. The residual function shown by the Thr104Ile mutant may explain the partial HH phenotype found in compound heterozygous patients bearing the Thr104Ile and Tyr108Cys hGnRHR alleles (Antelli et al., 2006).
Supplementary Material
Acknowledgements
This work was supported by NIH grants: TW/HD-00668, DK85040, RR030229 and P51RR000163 (P.M.C.), grants 45991-M and 86881 (A.U-A), and 83142 (A.A-R) from CONACyT, México and grant FIS/IMSS/PROT/132 from the IMSS, Mexico (G.M.-N). A.U-A. and A.L-M. are recipients of Research Career Development Awards from the Fundación IMSS, Mexico.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antelli A, Baldazzi L, Balsamo A, Pirazzoli P, Nicoletti A, Gennari M, Cicognani A. Two novel GnRHR gene mutations in two siblings with hypogonadotropic hypogonadism. Eur J Endocrinol. 2006;155:201–205. doi: 10.1530/eje.1.02198. [DOI] [PubMed] [Google Scholar]
- Arora KK, Chung HO, Catt KJ. Influence of a species-specific extracellular amino acid on expression and function of the human gonadotropin-releasing hormone receptor. Mol Endocrinol. 1999;13:890–896. doi: 10.1210/mend.13.6.0291. [DOI] [PubMed] [Google Scholar]
- Blomenrohr M, Heding A, Sellar R, Leurs R, Bogerd J, Eidne KA, Willars GB. Pivotal role for the cytoplasmic carboxyl-terminal tail of a nonmammalian gonadotropin-releasing hormone receptor in cell surface expression, ligand binding, and receptor phosphorylation and internalization. Mol Pharmacol. 1999;56:1229–1237. doi: 10.1124/mol.56.6.1229. [DOI] [PubMed] [Google Scholar]
- Chi L, Zhou W, Prikhozhan A, Flanagan C, Davidson JS, Golembo M, Illing N, Millar RP, Sealfon SC. Cloning and characterization of the human GnRH receptor. Mol Cell Endocrinol. 1993;91:R1–6. doi: 10.1016/0303-7207(93)90278-r. [DOI] [PubMed] [Google Scholar]
- Conn PM, Janovick JA. Trafficking and quality control of the gonadotropin releasing hormone receptor in health and disease. Mol Cell Endocrinol. 2009;299:137–145. doi: 10.1016/j.mce.2008.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PM, Knollman PE, Brothers SP, Janovick JA. Protein folding as posttranslational regulation: evolution of a mechanism for controlled plasma membrane expression of a G protein-coupled receptor. Mol Endocrinol. 2006;20:3035–3041. doi: 10.1210/me.2006-0066. [DOI] [PubMed] [Google Scholar]
- Conn PM, Ulloa-Aguirre A. Trafficking of G-protein-coupled receptors to the plasma membrane: insights for pharmacoperone drugs. Trends Endocrinol Metab. 2010;21:190–197. doi: 10.1016/j.tem.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JV, Eidne KA. An intramolecular disulfide bond between conserved extracellular cysteines in the gonadotropin-releasing hormone receptor is essential for binding and activation. Endocrinology. 1997;138:2800–2806. doi: 10.1210/endo.138.7.5233. [DOI] [PubMed] [Google Scholar]
- De Lano WL. The PyMOL Moleculr Graphics System. San Carlos, CA: 2002. [Google Scholar]
- de Roux N, Young J, Brailly-Tabard S, Misrahi M, Milgrom E, Schaison G. The same molecular defects of the gonadotropin-releasing hormone receptor determine a variable degree of hypogonadism in affected kindred. J Clin Endocrinol Metab. 1999;84:567–572. doi: 10.1210/jcem.84.2.5449. [DOI] [PubMed] [Google Scholar]
- de Roux N, Young J, Misrahi M, Genet R, Chanson P, Schaison G, Milgrom E. A family with hypogonadotropic hypogonadism and mutations in the gonadotropin-releasing hormone receptor. N Engl J Med. 1997;337:1597–1602. doi: 10.1056/NEJM199711273372205. [DOI] [PubMed] [Google Scholar]
- Heding A, Vrecl M, Bogerd J, McGregor A, Sellar R, Taylor PL, Eidne KA. Gonadotropin-releasing hormone receptors with intracellular carboxyl-terminal tails undergo acute desensitization of total inositol phosphate production and exhibit accelerated internalization kinetics. J Biol Chem. 1998;273:11472–11477. doi: 10.1074/jbc.273.19.11472. [DOI] [PubMed] [Google Scholar]
- Huckle WR, Conn PM. Use of lithium ion in measurement of stimulated pituitary inositol phospholipid turnover. Methods Enzymol. 1987;141:149–155. doi: 10.1016/0076-6879(87)41063-x. [DOI] [PubMed] [Google Scholar]
- Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14:33–38. 27–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Janovick JA, Goulet M, Bush E, Greer J, Wettlaufer DG, Conn PM. Structure-activity relations of successful pharmacologic chaperones for rescue of naturally occurring and manufactured mutants of the gonadotropin-releasing hormone receptor. J Pharmacol Exp Ther. 2003;305:608–614. doi: 10.1124/jpet.102.048454. [DOI] [PubMed] [Google Scholar]
- Janovick JA, Knollman PE, Brothers SP, Ayala-Yanez R, Aziz AS, Conn PM. Regulation of G protein-coupled receptor trafficking by inefficient plasma membrane expression: molecular basis of an evolved strategy. J Biol Chem. 2006;281:8417–8425. doi: 10.1074/jbc.M510601200. [DOI] [PubMed] [Google Scholar]
- Janovick JA, Maya-Nunez G, Conn PM. Rescue of hypogonadotropic hypogonadism-causing and manufactured GnRH receptor mutants by a specific protein-folding template: misrouted proteins as a novel disease etiology and therapeutic target. J Clin Endocrinol Metab. 2002;87:3255–3262. doi: 10.1210/jcem.87.7.8582. [DOI] [PubMed] [Google Scholar]
- Janovick JA, Patny A, Mosley R, Goulet MT, Altman MD, Rush TS, 3rd, Cornea A, Conn PM. Molecular mechanism of action of pharmacoperone rescue of misrouted GPCR mutants: the GnRH receptor. Mol Endocrinol. 2009;23:157–168. doi: 10.1210/me.2008-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardon-Valadez E, Aguilar-Rojas A, Maya-Nunez G, Leanos-Miranda A, Pineiro A, Conn PM, Ulloa-Aguirre A. Conformational effects of Lys191 in the human GnRHR: mutagenesis and molecular dynamics simulations studies. J Endocrinol. 2009;201:297–307. doi: 10.1677/JOE-08-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardon-Valadez E, Ulloa-Aguirre A, Pineiro A. Modeling and molecular dynamics simulation of the human gonadotropin-releasing hormone receptor in a lipid bilayer. J Phys Chem B. 2008a;112 doi: 10.1021/jp800544x. [DOI] [PubMed] [Google Scholar]
- Jardon-Valadez E, Ulloa-Aguirre A, Pineiro A. Modeling and Molecular Dynamics Simulation of the Human Gonadotropin-Releasing Hormone Receptor in a Lipid Bilayer. J. Phys. Chem. B. 2008b;112:10704–10713. doi: 10.1021/jp800544x. [DOI] [PubMed] [Google Scholar]
- Klco JM, Nikiforovich GV, Baranski TJ. Genetic analysis of the first and third extracellular loops of the C5a receptor reveals an essential WXFG motif in the first loop. J Biol Chem. 2006;281:12010–12019. doi: 10.1074/jbc.M600548200. [DOI] [PubMed] [Google Scholar]
- Knobil E. On the control of gonadotropin secretion in the rhesus monkey. Recent Prog Horm Res. 1974;30:1–46. doi: 10.1016/b978-0-12-571130-2.50005-5. [DOI] [PubMed] [Google Scholar]
- Laskowski RA. PDBsum new things. Nucleic Acids Res. 2009;37:D355–359. doi: 10.1093/nar/gkn860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leanos-Miranda A, Janovick JA, Conn PM. Receptor-misrouting: an unexpectedly prevalent and rescuable etiology in gonadotropin-releasing hormone receptor-mediated hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2002;87:4825–4828. doi: 10.1210/jc.2002-020961. [DOI] [PubMed] [Google Scholar]
- Leanos-Miranda A, Ulloa-Aguirre A, Janovick JA, Conn PM. In vitro coexpression and pharmacological rescue of mutant gonadotropin-releasing hormone receptors causing hypogonadotropic hypogonadism in humans expressing compound heterozygous alleles. J Clin Endocrinol Metab. 2005;90:3001–3008. doi: 10.1210/jc.2004-2071. [DOI] [PubMed] [Google Scholar]
- Lin X, Janovick JA, Brothers S, Blomenrohr M, Bogerd J, Conn PM. Addition of catfish gonadotropin-releasing hormone (GnRH) receptor intracellular carboxyl-terminal tail to rat GnRH receptor alters receptor expression and regulation. Mol Endocrinol. 1998;12:161–171. doi: 10.1210/mend.12.2.0056. [DOI] [PubMed] [Google Scholar]
- Maya-Nunez G, Janovick JA, Conn PM. Combined modification of intracellular and extracellular loci on human gonadotropin-releasing hormone receptor provides a mechanism for enhanced expression. Endocrine. 2000;13:401–407. doi: 10.1385/ENDO:13:3:401. [DOI] [PubMed] [Google Scholar]
- Maya-Nunez G, Janovick JA, Ulloa-Aguirre A, Soderlund D, Conn PM, Mendez JP. Molecular basis of hypogonadotropic hypogonadism: restoration of mutant (E(90)K) GnRH receptor function by a deletion at a distant site. J Clin Endocrinol Metab. 2002;87:2144–2149. doi: 10.1210/jcem.87.5.8386. [DOI] [PubMed] [Google Scholar]
- McArdle CA, Davidson JS, Willars GB. The tail of the gonadotrophin-releasing hormone receptor: desensitization at, and distal to, G protein-coupled receptors. Mol Cell Endocrinol. 1999;151:129–136. doi: 10.1016/s0303-7207(99)00024-6. [DOI] [PubMed] [Google Scholar]
- Millar RP. GnRH II and type II GnRH receptors. Trends Endocrinol Metab. 2003;14:35–43. doi: 10.1016/s1043-2760(02)00016-4. [DOI] [PubMed] [Google Scholar]
- Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin-releasing hormone receptors. Endocr Rev. 2004;25:235–275. doi: 10.1210/er.2003-0002. [DOI] [PubMed] [Google Scholar]
- Pfleger KD, Pawson AJ, Millar RP. Changes to gonadotropin-releasing hormone (GnRH) receptor extracellular loops differentially affect GnRH analog binding and activation: evidence for distinct ligand-stabilized receptor conformations. Endocrinology. 2008;149:3118–3129. doi: 10.1210/en.2008-0002. [DOI] [PubMed] [Google Scholar]
- Santen RJ, Bardin CW. Episodic luteinizing hormone secretion in man. Pulse analysis, clinical interpretation, physiologic mechanisms. J Clin Invest. 1973;52:2617–2628. doi: 10.1172/JCI107454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia R, Braakman I. Quality control in the endoplasmic reticulum protein factory. Nature. 2003;426:891–894. doi: 10.1038/nature02262. [DOI] [PubMed] [Google Scholar]
- Topaloglu AK, Lu ZL, Farooqi IS, Mungan NO, Yuksel B, O’Rahilly S, Millar RP. Molecular genetic analysis of normosmic hypogonadotropic hypogonadism in a Turkish population: identification and detailed functional characterization of a novel mutation in the gonadotropin-releasing hormone receptor gene. Neuroendocrinology. 2006;84:301–308. doi: 10.1159/000098147. [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Conn PM. G protein-coupled receptors and the G protein family. In: Conn PM, editor. Handbook of Physiology. Oxford University Press; New York: 1998. pp. 87–124. Section 7. [Google Scholar]
- Ulloa-Aguirre A, Janovick JA, Brothers SP, Conn PM. Pharmacologic rescue of conformationally-defective proteins: implications for the treatment of human disease. Traffic. 2004a;5:821–837. doi: 10.1111/j.1600-0854.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Janovick JA, Leanos-Miranda A, Conn PM. Misrouted cell surface GnRH receptors as a disease aetiology for congenital isolated hypogonadotrophic hypogonadism. Hum Reprod Update. 2004b;10:177–192. doi: 10.1093/humupd/dmh015. [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Janovick JA, Leanos-Miranda A, Conn PM. G-protein-coupled receptor trafficking: understanding the chemical basis of health and disease. ACS Chem Biol. 2006;1:631–638. doi: 10.1021/cb600360h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.