Abstract
The vesicular glutamate transporters (VGLUTs) regulate storage and release of glutamate in the brain. In adult animals, the VGLUT1 and VGLUT2 isoforms are widely expressed and differentially distributed, suggesting that neural circuits exhibit distinct modes of glutamate regulation. Studies in rodents suggest that VGLUT1 and VGLUT2 mRNA expression patterns are partly complementary, with VGLUT1 expressed at higher levels in cortex and VGLUT2 prominent subcortically, but with overlapping distributions in some nuclei. In primates, VGLUT gene expression has not been previously studied in any part of the brain. The purposes of the present study were to document the regional expression of VGLUT1 and VGLUT2 mRNA in the auditory pathway through A1 in cortex, and to determine whether their distributions are comparable to rodents. In situ hybridization with antisense riboprobes revealed that VGLUT2 was strongly expressed by neurons in the cerebellum and most major auditory nuclei, including the dorsal and ventral cochlear nuclei, medial and lateral superior olivary nuclei, central nucleus of the inferior colliculus, sagulum, and all divisions of the medial geniculate. VGLUT1 was densely expressed in the hippocampus and ventral cochlear nuclei, and at reduced levels in other auditory nuclei. In auditory cortex, neurons expressing VGLUT1 were widely distributed in layers II – VI of the core, belt and parabelt regions. VGLUT2 was most strongly expressed by neurons in layers IIIb and IV, weakly by neurons in layers II – IIIa, and at very low levels in layers V – VI. The findings indicate that VGLUT2 is strongly expressed by neurons at all levels of the subcortical auditory pathway, and by neurons in the middle layers of cortex, whereas VGLUT1 is strongly expressed by most if not all glutamatergic neurons in auditory cortex and at variable levels among auditory subcortical nuclei. These patterns imply that VGLUT2 is the main vesicular glutamate transporter in subcortical and thalamocortical (TC) circuits, whereas VGLUT1 is dominant in cortico-cortical (CC) and cortico-thalamic (CT) systems of projections. The results also suggest that VGLUT mRNA expression patterns in primates are similar to rodents, and establishes a baseline for detailed studies of these transporters in selected circuits of the auditory system.
Introduction
The storage and release of glutamate in excitatory circuits in the brain is regulated in part by the vesicular glutamate transporters (VGLUTs)(Fremeau et al., 2004a; Fremeau et al., 2004b; Fremeau et al., 2001; Gras et al., 2002; Herzog et al., 2001; Kaneko et al., 2002a; Kaneko et al., 2002b; Takamori, 2006; Takamori et al., 2000; Takamori et al., 2001). Of the three known transporters, the VGLUT1 and VGLUT2 isoforms are the most densely expressed in sensory pathways. VGLUT1 appears to be the main isoform expressed by neurons in cortex, while VGLUT2 appears to be dominant in the thalamus and brainstem. The regional differences in expression of these transporters are of interest, as there is evidence that they may be localized in synapses with different release probabilities and trafficking mechanisms, and therefore represent functionally distinct circuits (De Gois et al., 2005; Kaneko et al., 2002a; Mohrmann et al., 2008; Santos et al., 2009; Varoqui et al., 2002). For example, in the cortex of adult animals, including auditory cortex, VGLUT1 mRNA is strongly expressed by most neurons in layers II – VI, whereas VGLUT2 is expressed by a subset of neurons, mainly in the middle layers (De Gois et al., 2005; Fremeau et al., 2001; Graziano et al., 2008; Herzog et al., 2001). During postnatal development, VGLUT1 levels increase and VGLUT2 levels decrease to adult levels over similar periods, and remain co-expressed by subsets of neurons in adults, especially in the middle layers (De Gois et al., 2005). Similarly, the laminar distributions of VGLUT1 and VGLUT2 immunoreactive (-ir) terminals are partly complementary in that VGLUT1-ir terminals are concentrated in layers I – III and VGLUT2-ir is most dense in layer IV. (Fremeau et al., 2001; Fujiyama et al., 2004; Graziano et al., 2008; Kaneko et al., 2002b). The concentration of VGLUT2-ir in layer IV terminals suggests that these inputs are thalamic in origin (Hur et al., 2005). Yet, some terminals in layer IV contain both proteins (Graziano et al., 2008), suggesting that a subpopulation of neurons projecting to layer IV expresses both transporters. These findings are consistent with the observation that VGLUT1 and VGLUT2 mRNA are co-expressed by most neurons in the primary sensory relay nuclei of the rat thalamus, including the medial geniculate, lateral geniculate, and ventroposterior nuclei (Barroso-Chinea et al., 2008; Barroso-Chinea et al., 2007; Herzog et al., 2001). Otherwise, VGLUT2 mRNA expression in the thalamus is generally stronger and more broadly distributed compared to VGLUT1. Thus, it can be concluded that the two transporters are co-expressed in some thalamic and cortical circuits and complementary in others.
As in the thalamus, VGLUT2 mRNA expression in the brainstem is also strong among glutamatergic neurons in most nuclei (Berube-Carriere et al., 2009; Fremeau et al., 2001; Geisler et al., 2007; Graziano et al., 2008; Herzog et al., 2001; Hisano et al., 2002; Islam et al., 2008; Nair-Roberts et al., 2008; Stornetta et al., 2002; Wang et al., 2009), whereas VGLUT1 is strong in only a few (e.g., vestibular and cochlear nuclei, lateral reticular, external cuneate). The transcripts of neither transporter appear to be expressed in GABAergic nor monoaminergic populations (e.g., Purkinje cells, substantia nigra, locus coeruleus, raphe nuclei). Comparable findings are available for the mouse in the Allen Brain Atlas database (Lein et al., 2007) (http://mouse.brain-map.org). Detailed studies of gene expression in auditory nuclei are lacking, but gleaning from the sources listed above, VGLUT2 expression is strong in excitatory neurons in the principal auditory nuclei. VGLUT1 expression is weaker or absent in many of these nuclei, but appears to be strong in the dorsal and ventral cochlear nuclei. In contrast to gene expression patterns, VGLUT1 and VGLUT2 protein expression overlaps spatially in most nuclei, but is contained within circuits that are largely segregated, and therefore likely to subserve different functional roles (Altschuler et al., 2008; Ito et al., 2009; Kaneko et al., 2002b; Zhou et al., 2007).
GIven high sequence homology between humans and mice for these genes, it is reasonable to expect similar expression patterns in primates. To date, however, exploration of the VGLUT expression in primates has been limited to VGLUT1 and VGLUT2 immunoreactivity (-ir). Rubio et al (2008) studied VGLUT1-ir in the dorsal cochlear nucleus of the rhesus monkey, and found that the laminar and subregional distribution of VGLUT1-ir terminals were comparable to patterns in rats and mice (Kaneko et al., 2002b; Zhou et al., 2007). In macaque auditory cortex, the regional and laminar distribution of VGLUT2-ir terminals was related to established architectonic markers of the core, belt and parabelt regions (Hackett et al., 2009). Across laminae, VGLUT2-ir terminals were concentrated in the thalamorecipient layers (IIIb, IV), coextensive with elevated expression of parvalbumin (PV), acetylcholinesterase (AChE) and cytochrome oxidase (CO). A lesser band of immunoreactive terminals was located in layer VI. Regionally, VGLUT2-ir was highest in the primary, or core, region, intermediate in the surrounding belt areas, and very sparse in the parabelt, matching systematic reductions in the expression of AChE and CO along the core-belt-parabelt hierarchy (Hackett, 2010). The conspicuous concentration of VGLUT2-ir in the core, and significant reductions in the belt and parabelt suggested that subcortically, VGLUT2 mRNA might be preferentially expressed by neurons in the primary (lemniscal) pathway, which includes the ventral division of the medial geniculate complex (MGv), consistent with findings in rodents (Barroso-Chinea et al., 2007; Herzog et al., 2001). Although it would be convenient if VGLUT1 mRNA were preferentially expressed by neurons in non-primary auditory structures, the rodent data suggest that VGLUT1 mRNA will be expressed at reduced levels in the subcortical nuclei of primates and strong in cortical neurons.
To address these questions and better understand the distributions of neurons expressing VGLUT1 and VGLUT2 in the auditory pathway of primates, we employed in situ hybridization to study mRNA expression in owl monkeys from the cochlear nucleus to A1. Accordingly, it follows that VGLUT2 is the glutamate transporter utilized in the subcortical and thalamocortical (TC) auditory pathways, while VGLUT1 is prominent in corticocortical (CC) and corticothalamic (CT) circuits. A secondary purpose of this study was to provide much needed data on basic architectonic features of the primate auditory brainstem nuclei, which remain poorly studied. The combined results indicated that the distributions of VGLUT1 and VGLUT2 mRNA in the auditory pathway are largely complementary, but overlapping in some structures, in line with predictions based on previous studies in other species. The similarity to patterns of expression in mice and rats suggests that the expression of these genes is highly conserved in rodents and primates.
Methods
Animals
Three adult owl monkeys (Aotus trivirgatus) were used for the present studies. All surgical procedures were carried out according to the NIH Guidelines for the care and use of laboratory animals (NIH publication 86-23) under approved protocols from the Vanderbilt Animal Care and Use Committee.
Tissue preparation for histology
Animals were deeply anesthetized with a lethal dose of sodium pentobarbital (80mg/kg) and perfused transcardially with 0.9% saline in 0.1M phosphate buffer (PB) followed by 4% paraformaldehyde (PFA) in 0.1M PB. The brain was removed and postfixed for 3–6 hours in 4% PFA in 0.1M PB. The thalamus and brainstem were separated from the cerebral hemispheres, blocked, and cryoprotected in 30% sucrose in 0.1M PB for 3–5 days at 4°C. The block was cut into 40um frozen coronal sections on a sliding microtome and sections were stored at −20°C in cryoprotectant solution (30% glycerol, 30% ethylene glycol, 40% 0.1M phosphate-buffered saline). Brain sections were saved in 12 series, with alternating series of sections processed for Nissl using thionin, cytochrome oxidase (CO) (Wong-Riley, 1979), in situ hybridization for vesiclular glutamate transporter 1 (VGLUT1) and vesicular glutamate transporter 2 (VGLUT2) mRNA. Selected sections from auditory cortex were counterstained with green fluorescent Nissl stain (Neurotrace 500/525, 1% in DH20, Invitrogen Corp, Carlsbad, CA) to show laminar details.
In Situ Hybridization (ISH)
Two adjacent series in each animal were processed for localization of VGLUT1 and VGLUT2 mRNA. Digoxigenin (DIG)-labeled sense and antisense riboprobes for VGLUT1 and VGLUT2 were prepared from a macaque cDNA library through RT-PCR and conventional TA cloning techniques, and labeled using a DIG-dUTP labeling kit (Roche Diagnostics, Indianapolis, IN). The macaque VGLUT2 riboprobe was also used in the previous study (Takahata et al., 2010). Another VGLUT2 riboprobe was newly prepared from a galago cDNA library for the same position of VGLUT2 mRNA as macaques and used for one owl monkey case. The coding sequences of these genes are well conserved and the homology is high among species (approximately 90% between human and mouse). On the other hand, the homology between VGLUT1 and VGLUT2 mRNA is around 70%. Therefore, our preparation can reliably detect specific signals for each gene. The forward and reverse primers used for VGLUT1 were ccgctacattatcgccatca and cgatgggcacgatgatggtc respectively, which targeted position 204-1093 of human VGLUT1 (AB032436). The forward and reverse primers for VGLUT2 were gccatygtggacatggtcaa (y indicates c or t) and atractccaccatagtggac (r indicates a or g) respectively, targeting position 693-1888 of human VGLUT2 (NM_020346). The sense probes served as a negative control, and detected no signals stronger than the background reactivity. ISH was carried out as previously described (Takahata et al., 2010; Takahata et al., 2006). Briefly, free-floating sections were soaked in 4% PFA/0.1 M PB (pH 7.4) overnight at 4°C and treated with 10 μg/ml proteinase K for 30 min at 37°C. After acetylation with 0.25% acetic anyhydride in 0.9% triethanolamine and 0.12% hydrochloric acid, the sections were incubated in hybridization buffer (pH 7.5) containing 5X standard saline citrate (SSC; 150mM sodium chloride, 15 mM sodium citrate, pH 7.0), 50% formamide (FA), 2% blocking reagent (Roche Diagnostics), 0.1% N-lauroylsarcosine (NLS), 0.1% sodium dodecyl sulphate (SDS), 20mM maleic acid buffer, and 1ug/ml of the appropriate DIG-labeled riboprobe. Sections were hybridized overnight at 60°C and washed twice by successive immersion in 2X SSC, 50% FA, 0.1% NLS for 20 minutes at 60°C. Nonspecific mRNA was removed with 20ug/ml RNase A in RNase A buffer (10 mM Tris-HCl, 10 mM ethylenediamine-N,N,N′,N′-tetraacetic acid (EDTA), 500 mM NaCl; pH 8.0) for 15 minutes at 37°C and sections were washed again in 2X SSC, 0.1% NLS, followed by 0.2X SSC, 50% FA, 0.1% NLS, for 20 min each. Hybridized mRNA signals were visualized by alkaline phosphatase (AP) immunohistochemical staining using a DIG detection kit (Roche Diagnostics). Sections were mounted onto gelatin-subbed glass slides and dehydrated through a graded ethanol series (70% for 5 min, 90% for 10 min, 100% for 10 min), cleared in xylene (5 min), and then coverslipped with Permount.
Results
The distribution of VGLUT1 and VGLUT2 mRNA varied regionally in the brainstem and thalamus, and generally co-varied with patterns of CO expression. The lower power images of coronal sections at different rostral-caudal levels (Fig. 1) show that VGLUT2 mRNA was most densely expressed by neurons in the auditory nuclei (i.e., cochlear nucleus, inferior colliculus, medial geniculate), as well as the inferior olive, cerebellum, central gray, lateral geniculate, and pulvinar nuclei. VGLUT1 mRNA levels within neurons were present at reduced levels in most of the auditory nuclei, but remained strong in the ventral cochlear nuclei, cerebellum, and motor tract. The higher power images (Fig. 2) show VGLUT1 and VGLUT2 signals within neurons of the medial superior olive, inferior colliculus, and medial geniculate complex. In these subcortical structures, VGLUT1 was typically expressed at lower levels within neurons and exhibited an incomplete subcellular distribution of the signal, compared to VGLUT2. At higher magnification, additional differences in expression were found among the major auditory nuclei and their subdivisions. These results are discussed below.
Fig. 1.
Low magnification images of the owl monkey brainstem and thalamus at the level of the cochlear nucleus (left), inferior colliculus and superior olivary complex (middle), and medial geniculate (right). At each level, VGLUT1 and VGLUT2 mRNA expression are illustrated in adjacent sections to show their respective distribution patterns. Scale bar = 1mm.
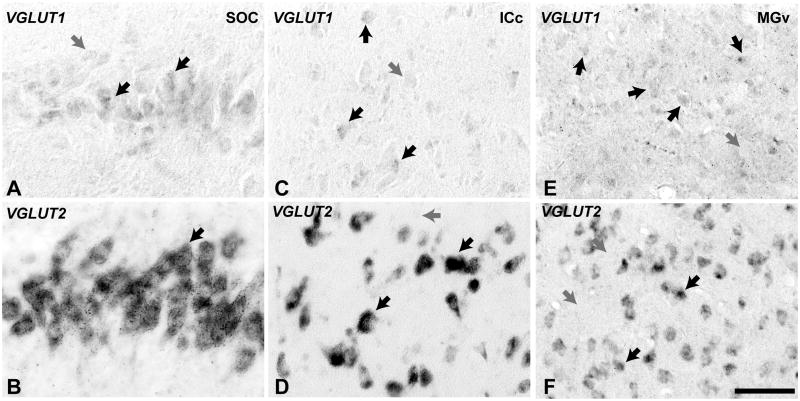
Fig. 2.
High magnification images of cells containing VGLUT1 and VGLUT2 mRNA. VGLUT1 signals (A, C, E) were found at reduced levels in these subcortical auditory structures, and its subcellular distribution was partial compared to VGLUT2 (B, D, F). Medial superior olivary nucleus (A, B); central nucleus of the inferior colliculus (C, D); ventral division of the medial geniculate (E, F). Scale bar = 50 um.
Cochlear Nucleus (CN)
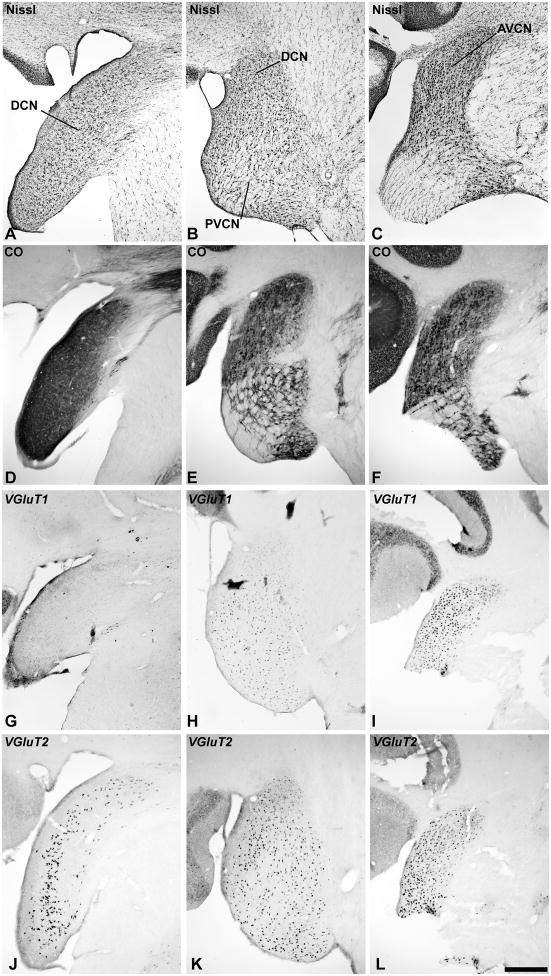
In the dorsal cochlear nucleus (DCN), laminar divisions were somewhat difficult to resolve, as noted by Moore (1980), but the combined architectonic markers used in this study suggested the presence of at least 3 layers (Fig. 3). In Nissl sections, the outermost (molecular) layer contained axons and small cells similar to that observed in other species (Fig. 3A). This layer could be divided into two domains based on CO expression (Fig. 3D). The deeper domain contained larger neurons and corresponds most closely to the fusiform cell layer. Interestingly, VGLUT2 mRNA expression in the DCN was largely confined to cells in this layer (Fig. 3J). In contrast, VGLUT1 mRNA was rather weakly expressed in this zone and elsewhere in the DCN, with expression at levels just above background (Fig. 3G). In the deepest third layer, was a thin line of cells that expressed VGLUT2 mRNA.
Fig. 3.
VGLUT mRNA expression in the cochlear nucleus (CN). Adjacent series of sections of the cochlear nucleus at the level of the DCN (left columns); PVCN (middle columns), and AVCN (right columns). Sections illustrated at each level were processed for Nissl (A – C), cytochrome oxidase (CO) (D – F), VGLUT1 mRNA (G – I), and VGLUT2 mRNA (J – L) by in situ hybridization. Scale bars = 500 um.
In the anteroventral (AVCN) and posteroventral (PVCN) divisions, VGLUT1 and VGLUT2 mRNA were densely expressed by the large neurons that were distributed rather evenly throughout both divisions (Fig. 3H – L). Their distribution matched that of neurons stained for Nissl (Fig. 3B – C). Although dual-label ISH was not performed in this study, the similarity in the distributions of neurons containing VGLUT1 and VGLUT2 mRNA suggests that most of these neurons in both nuclei express the mRNA for at least one or possibly both transporters (see Discussion).
Superior Olivary Complex (SOC)
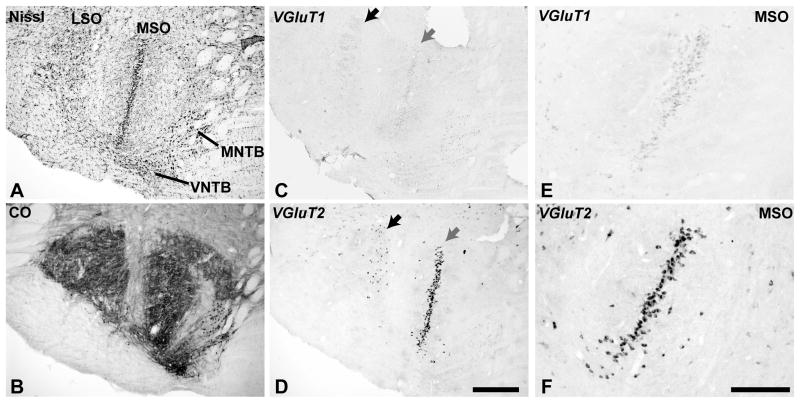
The SOC of the owl monkey contained nuclei identified herein as the medial (MSO), lateral (LSO), medial (MNTB) and ventral (VNTB) nuclei of the trapezoid body, as well as a several periolivary nuclei, which were not defined (Fig. 4). For example, the regions labeled as MNTB and VNTB did not have homogeneous architecture, and so may also include other divisions, such as the dorsomedial (DMPO) and ventromedial (VMPO) periolivary nuclei. In the MSO, the central line of principal cells stained darkly for Nissl substance and CO (Fig. 4A, B). CO reactivity was dense within soma and proximal dendrites (Fig. 4B). Cells in the central column densely expressed VGLUT2 mRNA (Fig. 4D, F). VGLUT1 mRNA was also expressed in these cells, but at greatly reduced levels compared to VGLUT2 in these cells and also to VGLUT1 in the AVCN and PVCN (Fig. 4C, E).
Fig. 4.
VGLUT mRNA expression in the superior olivary complex (SOC). Adjacent sections processed for Nissl (A), CO (B), VGLUT1 mRNA (C, D) and VGLUT2 mRNA (E, F). MSO, medial superior olive; LSO, lateral superior olive; MNTB and LNTB, medial and lateral nuclei of the trapezoid body. Scale bars = 500 um.
Based on cell distribution patterns in the Nissl preparations (Fig. 4A), it appears that VGLUT1 and VGLUT2 mRNA were also expressed in a subpopulation of neurons in the LSO (Fig. 4C, D). The arrows denote a location where the labeled neurons expressing VGLUT2 mRNA were contained in a cluster in the LSO along the lateral boundary of the MSO. VGLUT1 expression was rather weak in these neurons.
In the MNTB region, the somata of the principal neurons expressed CO, but only a subpopulation of neurons in the presumptive VNTB or other periolivary nuclei expressed CO (Fig. 4B). Scattered neurons in these periolivary nuclei expressed VGLUT1 or VGLUT2 mRNA. Generally, mRNA expression in these neurons was weaker compared to the MSO and LSO neurons.
Lateral Lemniscus and Sagulum
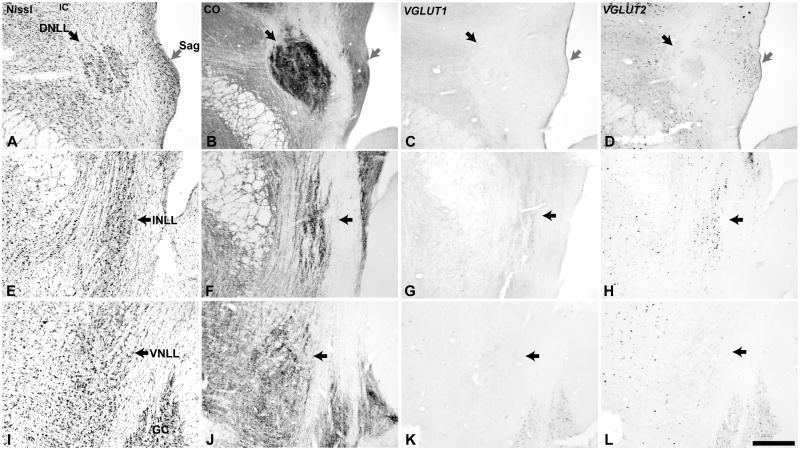
The three major divisions of the lateral lemniscus (LL) most commonly identified were well delineated in the owl monkey. Due to the plane of section, it was necessary to obtain a series of images to view each division. The dorsal nucleus (DNLL) was most prominent in rostral sections (Fig. 5). Its neurons stained darkly for Nissl substance and CO, and the neuropil was also darkly stained by CO. The intermediate (INLL) and ventral (VNLL) nuclei had similar features, and overall the architectonic appearance of the LL was comparable to the New world squirrel monkey (Emmers et al., 1963). The cells of the DNLL and VNLL generally did not express either VGLUT1 or VGLUT2 mRNA (Fig. 5C – L), although one or two cells in the DNLL were usually found in each section that contained moderate levels of VGLUT2, and less often VGLUT1. The relative absence of VGLUT mRNA labeling in the DNLL and VNLL is consistent with the dominance of inhibitory neurons in those nuclei, and matches findings in the rat (Ito et al., 2010). In contrast, numerous cells in the INLL and nearby sagulum strongly expressed VGLUT2, and frequently VGLUT1 at reduced levels.
Fig. 5.
VGLUT mRNA expression in the lateral lemniscus (LL) and sagulum (Sag). Adjacent sections through the dorsal nucleus (DNLL, black arrow) and sagulum (gray arrow)(A – D), intermediate nucleus (INLL, black arrow)(E – H), and ventral nucleus (VNLL, black arrow)(I – L). Sections illustrated at each level were processed for Nissl (A, E, I), cytochrome oxidase (CO) (B, F, J), VGLUT1 mRNA (C, G, K), and VGLUT2 mRNA (D, H, L) by in situ hybridization. GC, substantia griseum centralis. Scale bars = 500 um.
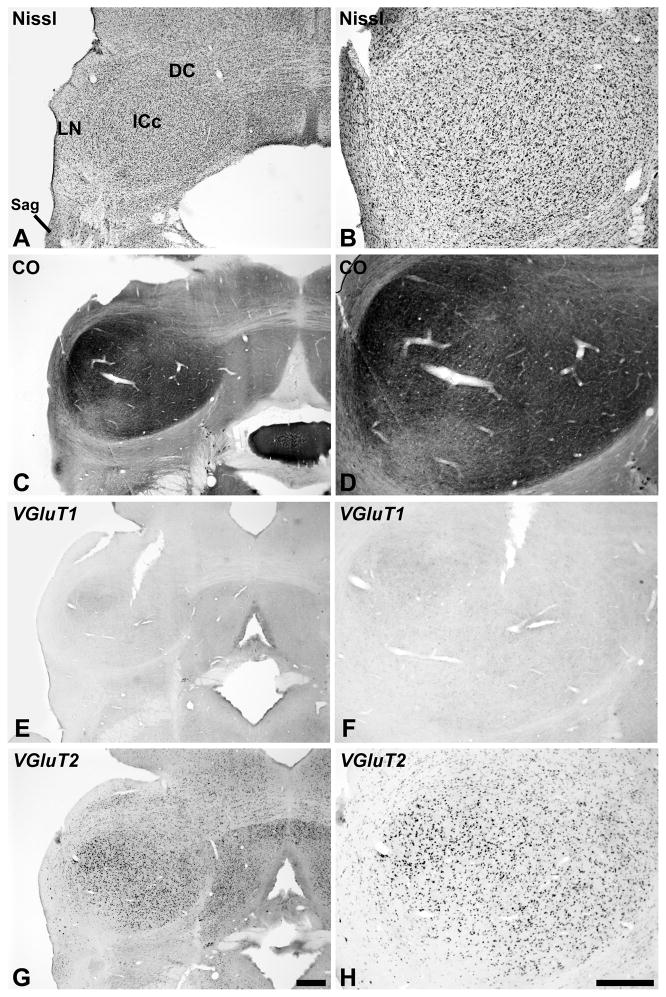
Inferior Colliculus
On the basis of Nissl and CO staining (Cant et al., 2006; Morest et al., 1984), we identified three major divisions of the inferior colliculus in the present study: central (ICc), dorsal cortex (DC), and lateral nucleus (LN)(Fig. 6). Although the architectonic appearance of each division was also heterogeneous, we made no attempt to further subdivide them. The central nucleus of the inferior colliculus (ICc) stood out from the surrounding structures with dense cell packing and generally elevated CO expression (Fig. 6A – D). Neurons in the ICc that strongly expressed VGLUT2 mRNA were broadly distributed throughout (Fig. 6G – H). VGLUT1 mRNA was expressed weakly by cells distributed in a pattern similar to that of VGLUT2 in the ICc (Fig. 6E – F). Neurons that were the most intensely reactive for either signal tended to be clustered in a smaller dorsolateral zone that was darkly labeled by CO.
Fig. 6.
VGLUT mRNA expression in the inferior colliculus (IC). The general locations of the central (ICc), lateral (LN), dorsal cortex (DC) divisions are indicted in panel A. Sections processed for Nissl (A, B), CO (C, D), VGLUT1 mRNA (E, F) and VGLUT2 mRNA (G, H) are illustrated at two different magnifications to show detail. Scale bars = 500 um.
In the DC and cell sparse region corresponding to the LN, CO expression levels were reduced compared to the ICc (Fig. 6C – D). We observed no clear indication of periodicities in LN in the distributions of CO or other markers used in this study, as found in rodents (Chernock et al., 2004). VGLUT1 mRNA expression was very weak in the LN and DC (Fig. 6E – F). In contrast, VGLUT2 mRNA was expressed in neurons distributed rather evenly throughout (Fig. 6G – H), except rostrally in the DC which was broken up by commissural axons. In general, VGLUT2 expression levels within LN and DC neurons were moderate compared to neurons in the ICc, although a few large cells in the LN were darkly stained.
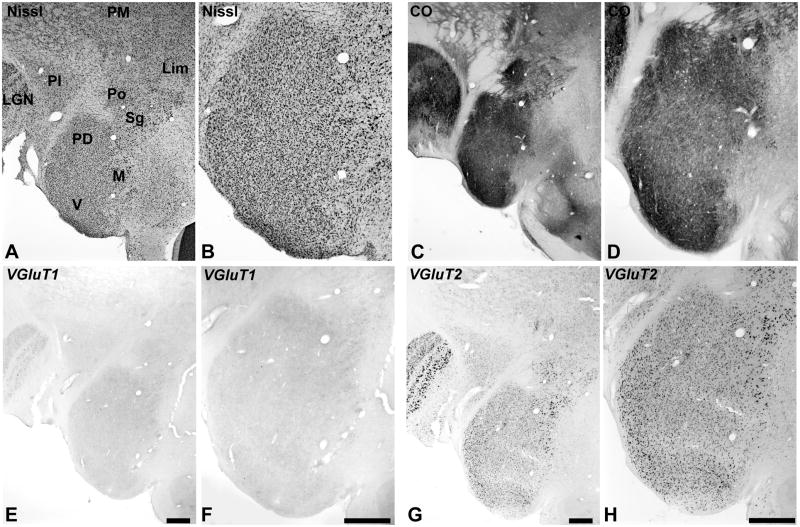
Medial Geniculate Complex
As shown in Fig. 7, the medial geniculate complex (MGC) was divided into three major divisions, as delineated in sections stained for Nissl (Fig. 6A). The criteria used to characterize them have been described in previous studies of New World primates (de la Mothe et al., 2006b; Morel et al., 1992). The three divisions recognized in this study are the ventral (V, MGv), medial or magnocellular (M, MGm), and posterodorsal (PD, MGpd). The anterodorsal division (MGad) is not illustrated. The MGC is flanked dorsally and medially by the lateral geniculate nucleus (LGN), inferior pulvinar (PI), medial pulvinar (PM), posterior (Po), suprageniculate (Sg), and limitans (Lim) nuclei. By comparing with the distribution of cells in the Nissl preparation, it appears that most of these neurons express VGLUT1 and VGLUT2 mRNA, but as in other nuclei, expression levels were different (Fig. 6E – H; see also Fig. 2). VGLUT2 mRNA was broadly expressed by neurons in all divisions, with slightly stronger expression in the MGv and dorsal MGm and extending into Po and Sg. VGLUT1 mRNA expression was distributed in a similar manner across divisions, but expression levels were much lower than for VGLUT2, due in part to a restricted subcellular distribution (Fig. 2e). Thus, it appears that most glutamatergic cells probably express both transcripts, but expression density is greater for VGLUT2 and varies between divisions. Note that VGLUT1 and VGLUT2 mRNA were also contained neurons of the LGN, but VGLUT1 levels were greater than in the MGC (data not shown).
Fig. 7.
VGLUT mRNA expression in the medial geniculate complex (MGC) and adjoining nuclei of the posterior thalamus. The posterodorsal (PD), medial or magnocellular (M), and ventral (V) divisions of the MGC are indicated in panel A, stained for Nissl. Also indicated are the locations of the lateral geniculate (LGN), inferior pulvinar (PI), medial pulvinar (PM), posterior (Po), suprageniculate (Sg), and limitans (Lim) nuclei. CO expression illustrated in panels C and D. VGLUT1 mRNA (E, F). VGLUT2 mRNA (G, H), shown at different magnifications. Scale bars = 500 um.
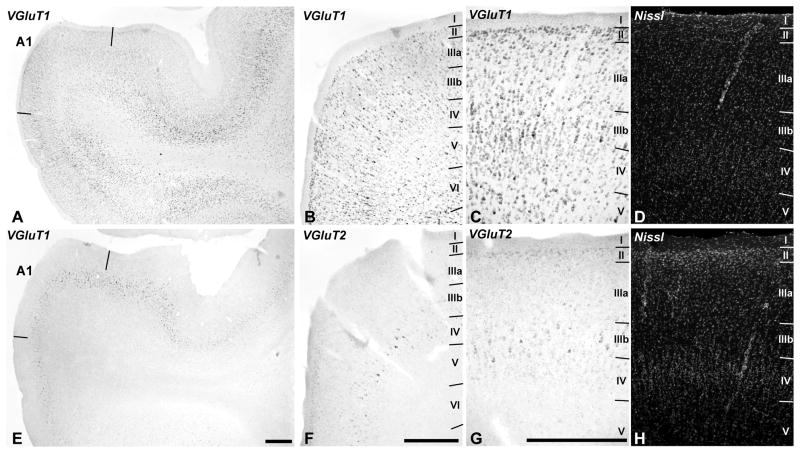
Auditory Cortex (Area A1)
In the auditory cortex (Fig. 8), VGLUT1 mRNA was strongly expressed by neurons from layers II to VI, including the smaller cells of layer IV. In contrast, VGLUT2 mRNA was most densely expressed in pyramidal neurons of layer IIIb, and at lower levels in layers II, IIIa, and IV. Expression in layers V and VI was very low for VGLUT2 mRNA. These laminar patterns were similar across core, belt and parabelt regions, suggesting that there were no major differences between regions of auditory cortex with respect to the expression of these genes.
Fig. 8.
VGLUT1 and VGLUT2 mRNA expression in auditory cortex. VGLUT1 mRNA (A – C) is expressed in neurons spanning layers II – VI. VGLUT2 mRNA (E – G) expression is strongest in layer IIIb, weaker in layers II, IIIa, and IV, and very low or absent in layers V and VI. Paired panels (C – D) and (G – H) are images of the same sections through A1, dual-labeled for mRNA by in situ hybridization (C, G) and fluorescent Nissl stain (D, H) to show laminar details. Scale bars = 500 um.
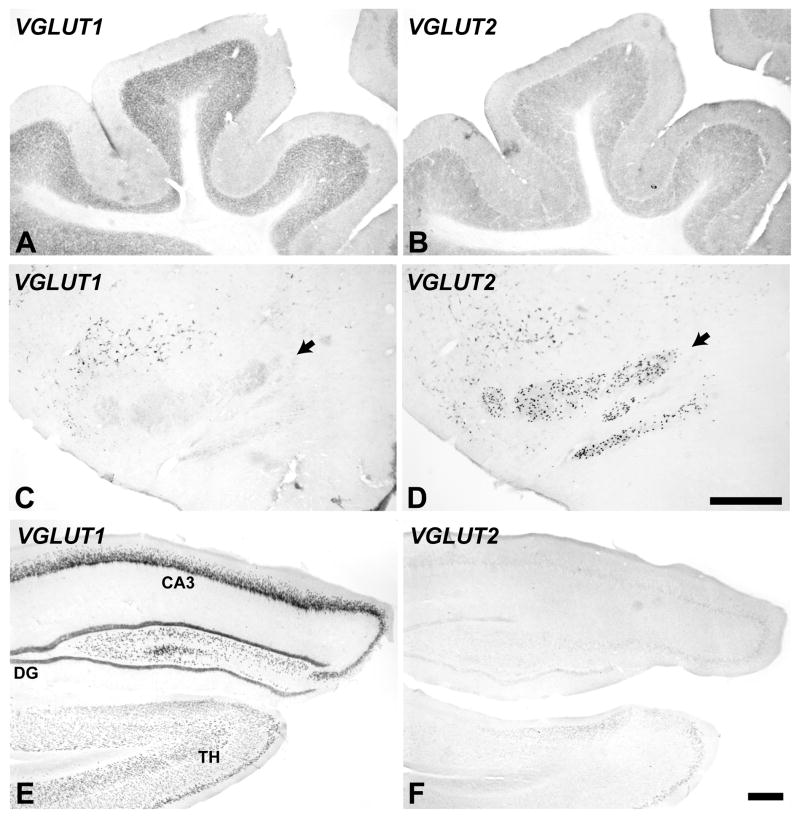
Other areas
To facilitate comparison with studies in other species, VGLUT mRNA expression was also illustrated for portions of the cerebellum, inferior olivary complex, and hippocampus (Fig. 9). Overall, expression of VGLUT1 and VGLUT2 in these structures was complementary. In the cerebellum, VGLUT1 expression was strong in the granular layer and relatively weak in the molecular layer. VGLUT2 was moderate in those layers, which contrasts from studies in most mammals where VGLUT2 levels are typically very low or absent. An exception is the study by Danik et al (2005) in which about 60% of neurons coexpressed both transcripts in adult animals. No cells expressed signals for either VGLUT1 or VGLUT2 mRNA in the pyramidal cell layer of the cerebellum. In the inferior olivary complex, VGLUT2 signals were strong in most neurons and VGLUT1 expression was very low. In the hippocampus, VGLUT1 mRNA is strongly expressed by pyramidal neurons and granule cells across divisions, whereas VGLUT2 mRNA expression is very low. These results compare well with those in rodents (Danik et al., 2005; Fremeau et al., 2001; Herzog et al., 2001; Hioki et al., 2003; Hisano et al., 2002).
Fig. 9.
VGLUT mRNA expression in the cerebellum (A, B), inferior olivary complex (C, D black arrows), and hippocampus (E, F). VGLUT1 mRNA (left panels). VGLUT2 mRNA (right panels). Scale bars = 500 um.
Discussion
The main purposes of this study were to determine the distribution of VGLUT1 and VGLUT2 mRNA expressing neurons in cortex and subcortical nuclei of the primate auditory pathway and compare those results to those derived from species that have been previously studied. In both mice and rats, previous studies have repeatedly found that their expression was complementary in some domains, and overlapping in others (Barroso-Chinea et al., 2008; Barroso-Chinea et al., 2007; Fremeau et al., 2004a; Fremeau et al., 2001; Graziano et al., 2008; Herzog et al., 2001; Ito et al., 2010). Perhaps the most widely cited difference has been that VGLUT1 is expressed at higher levels in structures including the cortex, cerebellum and hippocampus, whereas VGLUT2 expression is strongest in thalamus, brainstem and deep cerebellar nuclei. A less emphasized finding from the same studies, however, is that VGLUT1 is not absent from the thalamus and brainstem, but variably present at lower levels compared to VGLUT2, and even overlapping in some nuclei (e.g., MGC, LGN, VP). Similarly, VGLUT2 is not absent in cortex, but expressed mainly by neurons in the middle cortical layers.
These patterns are generally consistent with the findings of the present study. VGLUT2 mRNA was most intensely expressed by neurons located in nuclei of the primary (lemniscal) pathways and layers IIIb – IV in auditory cortex. VGLUT1 mRNA expression was widely and strongly expressed in layers II – VI in cortex and ventral cochlear nuclei, at moderate levels in the medial geniculate, and at low levels compared to VGLUT2 in the other primary auditory nuclei. Thus, with respect to our principal hypothesis, we can conclude that VGLUT2 is the main vesicular glutamate transporter in subcortical and thalamocortical (TC) circuits, whereas VGLUT1 is dominant in cortico-cortical (CC) and cortico-thalamic (CT) systems of projections.
Yet, the overlapping distributions of neurons expressing VGLUT1 and VGLUT2 in the primary nuclei and middle layers of cortex suggest that both transporters are used in some circuits. In early postnatal development in rats, the co-expression of VGLUT1 and VGLUT2 transcripts occurs in the majority of neurons in the cortex, cerebellum and hippocampus, then decrease in numbers over time (Danik et al., 2005; De Gois et al., 2005). In adult animals, co-expression is found at variable levels in several structures, including the hippocampus, thalamus, brainstem and cortex (Danik et al., 2005; De Gois et al., 2005; Herzog et al., 2001; Ito et al., 2010). Of particular relevance to the present study is a recent study of the auditory brainstem nuclei of the rat by Ito and Oliver (2010). They found that VGLUT2 expression was prominent in nuclei containing glutamatergic neurons, but also found that many of these neurons also co-expressed VGLUT1. In thalamus, co-expression has been observed in the primary sensory and association nuclei, but is limited to VGLUT2 in the midline and intralaminar nuclei (Barroso-Chinea et al., 2007). In cortex, most of the neurons in layer IV that express VGLUT2 mRNA also express VGLUT1 mRNA (De Gois et al., 2005). The functional significance of VGLUT co-expression is less clear, as relatively little is known about the ways in which VGLUT isoforms differ with respect to glutamate regulation, and how those factors may impact the systems of projections in which they are expressed. Among the more general observations is that VGLUT1 and VGLUT2 are associated with different release probabilities, but apparently not differences in neuronal firing rates in some brain regions (see Fremeau et al 2001 see Fremeau et al 2004). Hisano et al (2002) suggested that the VGLUTs may transport glutamate independently according to different kinetic properties, or perhaps are segregated into two different populations of synaptic vesicles that selectively transport glutamate, Whether these are properties that characterize all circuits remains to be determined, but given the known differences in their distributions throughout the brain, it does seem likely that glutamate is differentially regulated in circuits using these transporters. Therefore, we would expect their impact on neuronal activity to reflect those differences.
It is of special interest in this context that the VGLUT gene and protein expression are developmentally regulated (Blaesse et al., 2005; Danik et al., 2005; De Gois et al., 2005; Gillespie et al., 2005) and also modulated by activity (De Gois et al., 2005). Changes in levels of expression and co-expression at selected synapses may alter synaptic transmission and play a role in plasticity. The VGLUTs are critical for establishment of normal hearing (Seal et al., 2008), and not surprisingly, their expression is modified by hearing loss. For example, Zeng et al (2008) found that VGLUT1-ir is downregulated in the magnocellular (deep layers) of the DCN after unilateral deafening, whereas VGLUT2-ir was upregulated in the outer layers, which receive significant nonauditory inputs, suggesting compensatory enhancement of those inputs. Thus, given their roles in signaling, in general, and dynamic changes during development and various forms of synaptic plasticity, it is important that we establish more precisely the patterns of VGLUT expression and co-expression throughout the brain.
Implications for organization of the primate auditory pathways
Historically, the organization of the auditory pathways has emphasized and depended on studies conducted in species other than primates. Relatively few studies have focused on human or nonhuman primates, especially in the more caudal portions of the brainstem. The present study is one of very few in which the architectonic features of the entire pathway have been considered in a primate. As such, it may serve as a useful reference. Overall, our findings revealed no gross differences in organization of the subcortical pathways between that of primates and other species, especially as compared to the auditory cortex, where differences between species are more pronounced (or more obvious) (Hackett, 2010; Winer, 1992; Winer et al., 2007). Otherwise, the main contribution of this study was to document VGLUT1 and VGLUT2 mRNA expression in the auditory pathway and provide a baseline for future studies. Some of these results are briefly discussed below.
VGLUT1 and VGLUT2 expression in the auditory brainstem and thalamus
In the cochlear nuclei, VGLUT1 and VGLUT2 mRNA was densely expressed in principal neurons of the AVCN and PVCN, suggesting that ascending projections from these divisions of the CN utilize either or both transporters. In the DCN, VGLUT2 mRNA was prominent in what appears to correspond to the fusiform layer, but VGLUT1 expression was very weak overall. These findings corroborate findings in mice (Graziano et al., 2008; Lein et al., 2007). Since we did not perform dual ISH in these experiments, we could not assess whether the transporters were colocalized in the same neurons. However, comparison of the distributions of VGLUT and Nissl stained cells suggests that VGLUT1 and VGLUT2 coexpression in a majority of glutamatergic neurons in the ventral nuclei is likely, as found in the rat (Ito et al., 2010).
In the SOC, little is known about the architectonic organization or connections of the principal and especially periolivary nuclei in primates compared to some other species. The present results are in agreement with previous studies, however, in demonstrating that the SOC of the owl monkey has a well developed MSO and LSO, although the LSO does not appear to be as large as in other mammals, such as cats, and its internal organization not as elaborate (Bazwinsky et al., 2005; Hilbig et al., 2007; Kulesza, 2007; Moore, 1987; Moore, 2000; Moore et al., 1971). Given that the principal cells of the AVCN and PVCN expressed VGLUT1 and/or VGLUT2 mRNA, it can be assumed that some of their projections contribute to the glutamatergic terminations on neurons in the MSO and LSO (Blaesse et al., 2005; Cant et al., 1986; Cant et al., 2003; Suneja et al., 1995), and IC (Cant et al., 2003; Ito et al., 2010; Oliver, 1987). By comparison, the prominent expression of VGLUT2 by MSO and LSO neurons indicates that their targets mainly utilize the VGLUT2 transporter.
In the lateral lemniscus, VGLUT2 expression was primarily limited to a population of neurons in the INLL. The DNLL and VNLL contained few, if any, neurons that expressed either VGLUT1 or VGLUT2. This is consistent with findings in the rat, where very few neurons were found to express the mRNA of either transporter (Ito et al., 2010), as well as earlier observations that most neurons in the DNLL and VNLL are GABAergic or glycinergic (Adams et al., 1984; Aoki et al., 1988; Moore et al., 1987; Roberts et al., 1987; Winer et al., 1995).
In the inferior colliculus, we found that architectonic organization was rather similar to other species. Of particular interest was the structural heterogeneity within the central nucleus (ICc), which is well known from both anatomical and physiological work in other species (Cant, 2005; Oliver, 2005). For example, VGLUT2 mRNA and CO expression were strongest in the central nucleus (ICc) overall, but especially in the dorsolateral quadrant of the ICc, which is an ITD-sensitive low best frequency domain. By comparison, VGLUT1 mRNA was weakly expressed by neurons in the ICc, but distributed in a similar manner as VGLUT2. This gradient is complementary to that observed in the rhesus monkey IC for Wisteria fluoribunda agglutinin binding, which was more intense in dorsally and medially (Hilbig et al., 2007). Given these gradients, it may be worth noting that in a recent study of the IC in cats, injections of retrograde tracers in a corresponding dorsolateral zone labeled neurons clustered in the ipsilateral dorsal (low-frequency) MSO (93%) and dorsolateral LSO (7%) (Loftus et al., 2010). It was suggested that these projections represented two sources of excitatory input to this portion of the IC, and that ITD-sensitive MSO inputs contributed to binaural properties of neurons in this sector. This is consistent with recent anatomical work in the gerbil (Cant et al., 2006). Given that neurons in the MSO and LSO most strongly expressed VGLUT2 mRNA, it is therefore likely that most or all excitatory SOC projections to the IC mainly express the VGLUT2 protein, while weaker VGLUT1 co-expression appears to characterize a subset of these projections (Ito et al., 2010). Of related interest are recent studies of the IC in rats, where dual fluorescence immunohistochemistry was used to examine VGLUT1 and VGLUT2 protein expression in relation to cells labeled by specific markers (Altschuler et al., 2008; Ito et al., 2009). Although both proteins were densely expressed in the IC, their colocalization in terminals tended to be infrequent. Altschuler et al (2008) reported that VGLUT1 and VGLUT2 immunoreactivity was mainly expressed in different terminals throughout the IC. VGLUT2 terminals made somatic and dendritic contacts, and outnumbered those reactive for VGLUT1, which primarily contacted dendrites. The findings by Ito et al (2009) were comparable in those respects, adding that distinct patterns of VGLUT2 terminations characterized two types of GABAergic neurons in the IC: a larger-celled population with VGLUT2 axosomatic contacts, and a smaller-celled population without somatic contacts. Taken together, the collective findings are consistent with the established view that projections to the IC contribute distinct information to functional zones in the IC, which are passed on to other structures (e.g., MGC) (Cant et al., 2007), but also indicate that glutamate is differentially regulated by VGLUT1 and VGLUT2 in these circuits.
The architectonic features of the owl monkey medial geniculate complex were consistent with a previous study of this species (Morel et al., 1992), and similar to the organizational schemes adopted in other monkeys (de la Mothe et al., 2006b; Hackett et al., 2007; Jones, 2003; Molinari et al., 1995). VGLUT1 and VGLUT2 were expressed in all divisions of the MGC, but their patterns varied. As observed in mice and rats (Barroso-Chinea et al., 2007; Fremeau et al., 2001; Lein et al., 2007), VGLUT2 mRNA was strongly expressed by neurons in all of its subdivisions. VGLUT1 was also expressed across all divisions, but was much weaker overall. The broad distribution of VGLUT1 and VGLUT2 mRNA in neurons of all MGC divisions indicates that both transporters are used by TC projections to core, belt and parabelt fields of auditory cortex (Hackett et al., 2009), and may even be co-expressed by the majority of glutamatergic MGC neurons. In that case, we can expect that the majority of axon terminals in the thalamorecipient layers of auditory cortex will express both VGLUT1 and VGLUT2 proteins, as observed in somatosensory cortex of mice (Graziano et al., 2008).
VGLUT1 and VGLUT2 expression in auditory cortex
VGLUT1 and VGLUT2 gene expression in A1 showed a striking pattern in this study. VGLUT1 mRNA was expressed by the majority of pyramidal neurons in layers II – VI. In contrast, VGLUT2 mRNA was most strongly expressed by neurons in layers IIIb, weaker in layers II, IIIa and IV, and absent or at very low levels in layers V and VI. This differs from the typical pattern of VGLUT2 protein expression in auditory cortex, which is concentrated in presumptive TC terminals in layer IIIb, IV, and VI (Hackett et al., 2009). Of additional interest was that VGLUT1 and VGLUT2 mRNA expression did not appear to vary significantly between the core, belt, and parabelt regions of auditory cortex. This contrasts with the gradients typically observed with other functional markers (see Hackett, 2010). Overall, these expression patterns are very similar to those found in the sensory cortices of rats (Fremeau et al., 2001; Graziano et al., 2008; Ito et al., 2010), and have a number of important implications with respect to the organization of auditory pathways.
First, we can conclude that VGLUT1 is the main glutamate transporter used in the CC projections of auditory cortex neurons, and that VGLUT2 is utilized in a subset of those, perhaps favoring feedforward projections to higher areas and lateral type connections to adjoining areas (Fitzpatrick et al., 1980; Galaburda et al., 1983; Hackett, 2010). Evidence of VGLUT1 – VGLUT2 coexpression suggests that some neurons in the middle layers express both transporters (De Gois et al., 2005).
Second, the apparent absence of VGLUT2 mRNA in layers V and VI neurons suggests that VGLUT1 is the principal or only glutamate transporter utilized by the descending corticothalamic and corticotectal (CT) projections to MGC and IC (de la Mothe et al., 2006b; FitzPatrick et al., 1978; Luethke et al., 1989; Winer et al., 2002). A similar conclusion was reached by Ito and Oliver (2010), based on the absence of VGLUT2 expression in layer V neurons labeled by tracer injections in the IC. If it turns out that cortico-thalamo-cortical (CTC) circuits contribute to driven activity in higher-order areas of the auditory cortex (e.g., belt, parabelt), as recently reported in the somatosensory system (Theyel et al 2010), then the present findings indicate that VGLUT1 would mediate the descending CT projections, whereas mainly VGLUT2 (and possibly VGLUT1) would be utilized in the ascending TC projections. The very low levels of VGLUT2-ir terminals in the parabelt (Hackett et al., 2009), however, suggest that a VGLUT2-mediated CTC circuit would be unlikely to drive activity in that region of auditory cortex, but could perhaps facilitate the actions of feedforward projections to the parabelt from lower areas in the belt region.
Third, the CT projections, which arise from neurons that primarily or exclusively express VGLUT1, differ from the system of descending projections with neurons of origin in the thalamus or upper brainstem, because VGLUT2 is the dominant transporter in most nuclei, and VGLUT1 is more selectively or more weakly expressed (Barroso-Chinea et al., 2007). In the IC, for example, the LN and DC receive descending projections from the MGC and AC, and so there would be an overlapping distribution of terminals utilizing the VGLUT1 and/or VGLUT2 transporters. Studies combining anterograde tract tracing and immunohistochemistry for the VGLUTs and various recipient cell types will be needed to sort out these details.
Conclusions and future directions
In this study, VGLUT1 and VGLUT2 gene expression were surveyed in the auditory pathway of the owl monkey. Overall, it can be concluded that VGLUT1 and VGLUT2 mRNA are widely expressed throughout the auditory pathway. Upon closer inspection of their distributions, however, and from consideration of other studies, it appears that the VGLUT1 and VGLUT2 proteins are utilized by distinct systems of projections in most structures, and overlapping in others. VGLUT2 is most strongly expressed in the subcortical pathways, especially in the primary nuclei. VGLUT1 is more weakly and variably expressed in neurons of most subcortical nuclei with the exception of the ventral cochlear nuclei, and strongly expressed by most pyramidal neurons in cortex. Thus, as indexed by VGLUT expression, excitatory neurotransmission in the ascending and descending auditory pathways is mediated by at least two systems, but in a manner that varies between nuclei and neuronal subpopulations.
The present findings and those of related studies reviewed above suggest that much could be learned about normal and impaired auditory function by conducting studies that document and expand our understanding of VGLUT expression and regulation in auditory circuits. Among the studies that could be pursued include 1) co-labeling of cells and terminals in tracer studies of specific projections/circuits; 2) co-expression of VGLUT1, VGLUT2, VGLUT3 mRNA with markers of specific cell types to reveal neurochemically-distinct populations of neurons; 3) physiological characterization of neurons that exhibit different VGLUT expression profiles; 4) tracking up- or down-regulation of VGLUTs in development or after specific environmental manipulations.
Research Highlights.
Vesicular glutamate transporter 1 and 2 mRNA are expressed in partly overlapping circuits in the auditory pathway of primates.
Vesicular glutamate transporter 1 is most strongly expressed in auditory cortex and ventral cochlear nuclei.
Vesicular glutamate transporter 1 expression is dominant in the subcortical nuclei.
Patterns of vesicular glutamate transporter 1 and 2 mRNA expression are comparable to rodents.
Acknowledgments
The authors gratefully acknowledge the gift of the plasmid and VGLUT2 riboprobes from Drs. Tetsuo Yamamori and Akiya Watakabe (National Institute for Basic Biology, Japan). We also acknowledge the support of NIH/NIDCD grant RO1 DC04318 to T.A. Hackett, and Drs. Lisa de la Mothe and Corrie Camalier for helpful comments and discussions.
List of Abbreviations
- A1
Auditory area 1
- AVCN
Anteroventral cochlear nucleus
- CB
Calbindin
- CN
Cochlear nucleus
- CO
Cytochrome oxidase
- DC
Dorsal cortex of the inferior colliculus
- DCN
Dorsal cochlear nucleus
- GC
Substantia griseum centralis
- IC
Inferior colliculus
- ICc
Inferior colliculus, central nucleus
- LGN
Lateral geniculate nucleus, thalamus
- Lim
Limitans nucleus, thalamus
- LN
Lateral nucleus of the inferior colliculus
- LSO
Lateral superior olivary nucleus
- MGad
Medial geniculate complex, anterodorsal division
- MGC
Medial geniculate complex
- MGm
Medial geniculate complex, magnocellular division
- MGpd
Medial geniculate complex, posterodorsal division
- MGv
Medial geniculate complex, ventral division
- MNTB
Medial nucleus of the trapezoid body
- MSO
Medial superior olivary nucleus
- PL
Lateral pulvinar nucleus, thalamus
- PM
Medial pulvinar nucleus, thalamus
- Po
Posterior nucleus, thalamus
- PVCN
Posteroventral cochlear nucleus
- Sg
Suprageniculate nucleus, thalamus
- SOC
Superior olivary complex
- VGLUT
Vesicular glutamate transporter
- VNTB
Ventral nucleus of the trapezoid body
Footnotes
Comments about Dr. Jeffrey Winer
This manuscript is dedicated to the memory of Dr. Jeffrey Winer, a brilliant and creative scientist and friend, whose contributions to the auditory community will never be forgotten. As an anatomist, he had no equal. The attention to detail in the beautiful work that he so carefully prepared is unmatched. It has inspired me from the first paper I read and shall continue to serve as an example to follow and goal to be reached. As a friend and mentor, Jeff was patient and true. He encouraged me to continue on in the field in times of great doubt, and gave advice and support to see it through. I shall always be grateful for his life and friendship.
Publisher's Disclaimer: This a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Troy A. Hackett, Vanderbilt University School of Medicine, Dept. of Hearing and Speech Sciences, Dept. of Psychology
Toru Takahata, Vanderbilt University, Dept. of Psychology
Pooja Balaram, Vanderbilt University, Dept. of Psychology
References
- Adams JC, Mugnaini E. Dorsal nucleus of the lateral lemniscus: a nucleus of GABAergic projection neurons. Brain Res Bull. 1984;13:585–90. doi: 10.1016/0361-9230(84)90041-8. [DOI] [PubMed] [Google Scholar]
- Altschuler RA, Tong L, Holt AG, Oliver DL. Immunolocalization of vesicular glutamate transporters 1 and 2 in the rat inferior colliculus. Neuroscience. 2008;154:226–32. doi: 10.1016/j.neuroscience.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki E, Semba R, Keino H, Kato K, Kashiwamata S. Glycine-like immunoreactivity in the rat auditory pathway. Brain Res. 1988;442:63–71. doi: 10.1016/0006-8993(88)91432-1. [DOI] [PubMed] [Google Scholar]
- Barroso-Chinea P, Castle M, Aymerich MS, Lanciego JL. Expression of vesicular glutamate transporters 1 and 2 in the cells of origin of the rat thalamostriatal pathway. J Chem Neuroanat. 2008;35:101–7. doi: 10.1016/j.jchemneu.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Barroso-Chinea P, Castle M, Aymerich MS, Perez-Manso M, Erro E, Tunon T, Lanciego JL. Expression of the mRNAs encoding for the vesicular glutamate transporters 1 and 2 in the rat thalamus. J Comp Neurol. 2007;501:703–15. doi: 10.1002/cne.21265. [DOI] [PubMed] [Google Scholar]
- Bazwinsky I, Bidmon HJ, Zilles K, Hilbig H. Characterization of the rhesus monkey superior olivary complex by calcium binding proteins and synaptophysin. Journal of Anatomy. 2005;207:745–761. doi: 10.1111/j.1469-7580.2005.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berube-Carriere N, Riad M, Dal Bo G, Levesque D, Trudeau LE, Descarries L. The dual dopamine-glutamate phenotype of growing mesencephalic neurons regresses in mature rat brain. J Comp Neurol. 2009;517:873–91. doi: 10.1002/cne.22194. [DOI] [PubMed] [Google Scholar]
- Blaesse P, Ehrhardt S, Friauf E, Nothwang HG. Developmental pattern of three vesicular glutamate transporters in the rat superior olivary complex. Cell Tissue Res. 2005;320:33–50. doi: 10.1007/s00441-004-1054-8. [DOI] [PubMed] [Google Scholar]
- Cant NB. Projections from the cochlear nuclear complex to the inferior colliculus. In: Winer J, Schreiner CE, editors. The inferior colliculus. Springer; New York: 2005. pp. 115–131. [Google Scholar]
- Cant NB, Casseday JH. Projections from the anteroventral cochlear nucleus to the lateral and medial superior olivary nuclei. J Comp Neurol. 1986;247:457–76. doi: 10.1002/cne.902470406. [DOI] [PubMed] [Google Scholar]
- Cant NB, Benson CG. Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei. Brain Res Bull. 2003;60:457–74. doi: 10.1016/s0361-9230(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Cant NB, Benson CG. Organization of the inferior colliculus of the gerbil (Meriones unguiculatus): differences in distribution of projections from the cochlear nuclei and the superior olivary complex. J Comp Neurol. 2006;495:511–28. doi: 10.1002/cne.20888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant NB, Benson CG. Multiple topographically organized projections connect the central nucleus of the inferior colliculus to the ventral division of the medial geniculate nucleus in the gerbil, Meriones unguiculatus. J Comp Neurol. 2007;503:432–53. doi: 10.1002/cne.21391. [DOI] [PubMed] [Google Scholar]
- Chernock ML, Larue DT, Winer JA. A periodic network of neurochemical modules in the inferior colliculus. Hear Res. 2004;188:12–20. doi: 10.1016/S0378-5955(03)00340-X. [DOI] [PubMed] [Google Scholar]
- Danik M, Cassoly E, Manseau F, Sotty F, Mouginot D, Williams S. Frequent coexpression of the vesicular glutamate transporter 1 and 2 genes, as well as coexpression with genes for choline acetyltransferase or glutamic acid decarboxylase in neurons of rat brain. J Neurosci Res. 2005;81:506–21. doi: 10.1002/jnr.20500. [DOI] [PubMed] [Google Scholar]
- De Gois S, Schafer MK, Defamie N, Chen C, Ricci A, Weihe E, Varoqui H, Erickson JD. Homeostatic scaling of vesicular glutamate and GABA transporter expression in rat neocortical circuits. J Neurosci. 2005;25:7121–33. doi: 10.1523/JNEUROSCI.5221-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Mothe LA, Blumell S, Kajikawa Y, Hackett TA. Thalamic connections of auditory cortex in marmoset monkeys: core and medial belt regions. J Comp Neurol. 2006b;496:72–96. doi: 10.1002/cne.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmers R, Akert K. A stereotaxic atlast of the brain of the squirrel monkey (Saimiri Sciureus) University of Wisconsin Press; Madison: 1963. [Google Scholar]
- FitzPatrick KA, Imig TJ. Projections of auditory cortex upon the thalamus and midbrain in the owl monkey. J Comp Neurol. 1978;177:537–55. doi: 10.1002/cne.901770402. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick KA, Imig TJ. Auditory cortico-cortical connections in the owl monkey. J Comp Neurol. 1980;192:589–610. doi: 10.1002/cne.901920314. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004a;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science. 2004b;304:1815–9. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–60. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Kuramoto E, Okamoto K, Hioki H, Furuta T, Zhou L, Nomura S, Kaneko T. Presynaptic localization of an AMPA-type glutamate receptor in corticostriatal and thalamostriatal axon terminals. Eur J Neurosci. 2004;20:3322–30. doi: 10.1111/j.1460-9568.2004.03807.x. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Pandya DN. The intrinsic architectonic and connectional organization of the superior temporal region of the rhesus monkey. J Comp Neurol. 1983;221:169–84. doi: 10.1002/cne.902210206. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–43. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie DC, Kim G, Kandler K. Inhibitory synapses in the developing auditory system are glutamatergic. Nat Neurosci. 2005;8:332–8. doi: 10.1038/nn1397. [DOI] [PubMed] [Google Scholar]
- Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El Mestikawy S. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–51. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano A, Liu XB, Murray KD, Jones EG. Vesicular glutamate transporters define two sets of glutamatergic afferents to the somatosensory thalamus and two thalamocortical projections in the mouse. J Comp Neurol. 2008;507:1258–76. doi: 10.1002/cne.21592. [DOI] [PubMed] [Google Scholar]
- Hackett TA. Information flow in the auditory cortical network. Hear Res. 2010 doi: 10.1016/j.heares.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TA, de la Mothe LA. Regional and laminar distribution of the vesicular glutamate transporter, VGluT2, in the macaque monkey auditory cortex. J Chem Neuroanat. 2009;38:106–16. doi: 10.1016/j.jchemneu.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TA, De La Mothe LA, Ulbert I, Karmos G, Smiley J, Schroeder CE. Multisensory convergence in auditory cortex, II. Thalamocortical connections of the caudal superior temporal plane. J Comp Neurol. 2007;502:924–52. doi: 10.1002/cne.21326. [DOI] [PubMed] [Google Scholar]
- Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El Mestikawy S. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21:RC181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbig H, Nowack S, Boeckler K, Bidmon HJ, Zilles K. Characterization of neuronal subsets surrounded by perineuronal nets in the rhesus auditory brainstem. Journal of Anatomy. 2007;210:507–517. doi: 10.1111/j.1469-7580.2007.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hioki H, Fujiyama F, Taki K, Tomioka R, Furuta T, Tamamaki N, Kaneko T. Differential distribution of vesicular glutamate transporters in the rat cerebellar cortex. Neuroscience. 2003;117:1–6. doi: 10.1016/s0306-4522(02)00943-0. [DOI] [PubMed] [Google Scholar]
- Hisano S, Sawada K, Kawano M, Kanemoto M, Xiong G, Mogi K, Sakata-Haga H, Takeda J, Fukui Y, Nogami H. Expression of inorganic phosphate/vesicular glutamate transporters (BNPI/VGLUT1 and DNPI/VGLUT2) in the cerebellum and precerebellar nuclei of the rat. Brain Res Mol Brain Res. 2002;107:23–31. doi: 10.1016/s0169-328x(02)00442-4. [DOI] [PubMed] [Google Scholar]
- Hur EE, Zaborszky L. Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: a combined retrograde tracing in situ hybridization study [corrected] J Comp Neurol. 2005;483:351–73. doi: 10.1002/cne.20444. [DOI] [PubMed] [Google Scholar]
- Islam MR, Atoji Y. Distribution of vesicular glutamate transporter 2 and glutamate receptor 1 mRNA in the central nervous system of the pigeon (Columba livia) J Comp Neurol. 2008;511:658–77. doi: 10.1002/cne.21871. [DOI] [PubMed] [Google Scholar]
- Ito T, Oliver DL. Origins of glutamatergic terminals in the inferior colliculus identified by retrograde transport and expression of VGLUT1 and VGLUT2 genes. Frontiers in neuroanatomy. 2010;4:1–11. doi: 10.3389/fnana.2010.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Bishop DC, Oliver DL. Two classes of GABAergic neurons in the inferior colliculus. J Neurosci. 2009;29:13860–9. doi: 10.1523/JNEUROSCI.3454-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. Chemically defined parallel pathways in the monkey auditory system. Ann N Y Acad Sci. 2003;999:218–33. doi: 10.1196/annals.1284.033. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F. Complementary distribution of vesicular glutamate transporters in the central nervous system. Neurosci Res. 2002a;42:243–50. doi: 10.1016/s0168-0102(02)00009-3. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F, Hioki H. Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J Comp Neurol. 2002b;444:39–62. doi: 10.1002/cne.10129. [DOI] [PubMed] [Google Scholar]
- Kulesza J, Randy J. Cytoarchitecture of the human superior olivary complex: Medial and lateral superior olive. Hearing Research. 2007;225:80–90. doi: 10.1016/j.heares.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–76. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Loftus WC, Bishop DC, Oliver DL. Differential patterns of inputs create functional zones in central nucleus of inferior colliculus. J Neurosci. 2010;30:13396–408. doi: 10.1523/JNEUROSCI.0338-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luethke LE, Krubitzer LA, Kaas JH. Connections of primary auditory cortex in the New World monkey, Saguinus. J Comp Neurol. 1989;285:487–513. doi: 10.1002/cne.902850406. [DOI] [PubMed] [Google Scholar]
- Mohrmann R, Matthies HJ, Woodruff E, 3rd, Broadie K. Stoned B mediates sorting of integral synaptic vesicle proteins. Neuroscience. 2008;153:1048–63. doi: 10.1016/j.neuroscience.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari M, Dell’Anna ME, Rausell E, Leggio MG, Hashikawa T, Jones EG. Auditory thalamocortical pathways defined in monkeys by calcium-binding protein immunoreactivity. J Comp Neurol. 1995;362:171–94. doi: 10.1002/cne.903620203. [DOI] [PubMed] [Google Scholar]
- Moore JK. The human auditory brain stem: a comparative view. Hear Res. 1987;29:1–32. doi: 10.1016/0378-5955(87)90202-4. [DOI] [PubMed] [Google Scholar]
- Moore JK. Organization of the human superior olivary complex. Microscopy Research and Technique. 2000;51:403–412. doi: 10.1002/1097-0029(20001115)51:4<403::AID-JEMT8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Moore JK, Moore RY. A comparative study of the superior olivary complex in the primate brain. Folia Primatol (Basel) 1971;16:35–51. doi: 10.1159/000155390. [DOI] [PubMed] [Google Scholar]
- Moore JK, Moore RY. Glutamic acid decarboxylase-like immunoreactivity in brainstem auditory nuclei of the rat. J Comp Neurol. 1987;260:157–74. doi: 10.1002/cne.902600202. [DOI] [PubMed] [Google Scholar]
- Morel A, Kaas JH. Subdivisions and connections of auditory cortex in owl monkeys. J Comp Neurol. 1992;318:27–63. doi: 10.1002/cne.903180104. [DOI] [PubMed] [Google Scholar]
- Morest DK, Oliver DL. The neuronal architecture of the inferior colliculus in the cat: defining the functional anatomy of the auditory midbrain. J Comp Neurol. 1984;222:209–36. doi: 10.1002/cne.902220206. [DOI] [PubMed] [Google Scholar]
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152:1024–31. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver DL. Projections to the inferior colliculus from the anteroventral cochlear nucleus in the cat: possible substrates for binaural interaction. J Comp Neurol. 1987;264:24–46. doi: 10.1002/cne.902640104. [DOI] [PubMed] [Google Scholar]
- Oliver DL. Neuronal organization in the inferior colliculus. In: Winer J, CES, editors. The inferior colliculus. Springer; New York: 2005. pp. 69–114. [Google Scholar]
- Roberts RC, Ribak CE. GABAergic neurons and axon terminals in the brainstem auditory nuclei of the gerbil. J Comp Neurol. 1987;258:267–80. doi: 10.1002/cne.902580207. [DOI] [PubMed] [Google Scholar]
- Santos MS, Li H, Voglmaier SM. Synaptic vesicle protein trafficking at the glutamate synapse. Neuroscience. 2009;158:189–203. doi: 10.1016/j.neuroscience.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Akil O, Yi E, Weber CM, Grant L, Yoo J, Clause A, Kandler K, Noebels JL, Glowatzki E, Lustig LR, Edwards RH. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron. 2008;57:263–75. doi: 10.1016/j.neuron.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Sevigny CP, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 mRNA is present in C1 and several other groups of brainstem catecholaminergic neurons. J Comp Neurol. 2002;444:191–206. doi: 10.1002/cne.10141. [DOI] [PubMed] [Google Scholar]
- Suneja SK, Benson CG, Gross J, Potashner SJ. Evidence for glutamatergic projections from the cochlear nucleus to the superior olive and the ventral nucleus of the lateral lemniscus. J Neurochem. 1995;64:161–71. doi: 10.1046/j.1471-4159.1995.64010161.x. [DOI] [PubMed] [Google Scholar]
- Takahata T, Hashikawa T, Tochitani S, Yamamori T. Differential expression patterns of OCC1-related, extracellular matrix proteins in the lateral geniculate nucleus of macaque monkeys. J Chem Neuroanat. 2010;40:112–22. doi: 10.1016/j.jchemneu.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Takahata T, Komatsu Y, Watakabe A, Hashikawa T, Tochitani S, Yamamori T. Activity-dependent expression of occ1 in excitatory neurons is a characteristic feature of the primate visual cortex. Cereb Cortex. 2006;16:929–40. doi: 10.1093/cercor/bhj034. [DOI] [PubMed] [Google Scholar]
- Takamori S. VGLUTs: ‘exciting’ times for glutamatergic research? Neurosci Res. 2006;55:343–51. doi: 10.1016/j.neures.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–94. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of differentiation-associated brain-specific phosphate transporter as a second vesicular glutamate transporter (VGLUT2) J Neurosci. 2001;21:RC182. doi: 10.1523/JNEUROSCI.21-22-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqui H, Schafer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci. 2002;22:142–55. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29:340–58. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer J. The functional architecture of the medial geniculate body and the primary auditory cortex. In: Webster D, Popper A, Fay R, editors. The Mammalian Auditory Pathways. Springer-Verlag; New York: 1992. pp. 222–409. [Google Scholar]
- Winer JA, Lee CC. The distributed auditory cortex. Hear Res. 2007;229:3–13. doi: 10.1016/j.heares.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Larue DT, Pollak GD. GABA and glycine in the central auditory system of the mustache bat: structural substrates for inhibitory neuronal organization. J Comp Neurol. 1995;355:317–53. doi: 10.1002/cne.903550302. [DOI] [PubMed] [Google Scholar]
- Winer JA, Chernock ML, Larue DT, Cheung SW. Descending projections to the inferior colliculus from the posterior thalamus and the auditory cortex in rat, cat, and monkey. Hear Res. 2002;168:181–95. doi: 10.1016/s0378-5955(02)00489-6. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Zhou J, Nannapaneni N, Shore S. Vesicular glutamate transporters 1 and 2 are differentially associated with auditory nerve and spinal trigeminal inputs to the cochlear nucleus. J Comp Neurol. 2007;500:777–87. doi: 10.1002/cne.21208. [DOI] [PubMed] [Google Scholar]