Abstract
Chemoradiation is the treatment of choice for locally advanced head and neck squamous cell carcinoma (HNSCC). However, radioresistance, which contributes to local recurrence, remains a significant therapeutic problem. In this study, we characterized SM-164, a small SMAC mimetic compound that promotes degradation of cIAP-1 (also known as BIRC2) and releases active caspases from XIAP inhibitory binding, as a radiosensitizing agent in HNSCC cells. We found that SM-164 at nanomolar concentrations induced radiosensitization in some HNSCC cell lines in a manner dependent on intrinsic sensitivity to caspase activation and apoptosis induction. Blockage of caspase activation via siRNA knockdown or a pan-caspase inhibitor, z-VAD-fmk largely abrogated SM-164 radiosensitization. On the other hand, the resistant lines with a high level of BCL-2 that blocks caspase activation and apoptosis induction became sensitive to radiation upon BCL-2 knockdown. Mechanistic studies revealed that SM-164 radiosensitization in sensitive cells was associated with NFκB activation and TNFα secretion, followed by activation of caspases-8 and -9, leading to enhanced apoptosis. Finally, SM-164 also radiosensitized human tumor xenograft, while causing minimal toxicity. Thus, SM-164 is a potent radiosensitizer via a mechanism involving caspase activation and holds promise for future clinical development as a novel class of radiosensitizer for the treatment of a subset of head and neck cancer patients.
Keywords: Apoptosis, NF-κB activation, TNFα secretion, Caspase activation, cIAP-1 degradation, Radiosensitization
Introduction
Radiotherapy is the treatment of choice for locally advanced head and neck squamous cell carcinomas (HNSCC), but intrinsic tumor radioresistance contributes to the poor 5-year relapse-free survival (1). Both laboratory studies and clinical investigations have suggested that multiple factors contribute to radioresistance of HNSCC. A major factor is abnormal activation of EGFR signaling pathways (2). Other contributing factors include BCL-2 expression and TP53 mutation (3), expression of survivin and BCL-xL (4), disruption of the FAS-mediated apoptotic pathway (5), and NF-κB activation (6). A potential role of cIAP-1 in HNSCC carcinogenesis and radioresistance was implicated by the amplification at the chromosome 11q22 region in some HNSCC tumors, where the cIAP-1/BIRC2 gene resides (7–9), and by the association of lymph node metastasis and poor survival of patients with cIAP-1 nuclear expression (10).
cIAP-1, cIAP-2 (also known as BIRC3), and XIAP are three well-known family members of IAP (Inhibitor of Apoptosis) (11). While the main function of XIAP is to suppress apoptosis via binding to and thus inhibiting active caspases 3/7 and 9 (12), both cIAP-1 and cIAP-2 are implicated in NF-κB activation (13) and suppression of caspase-8 activation during TNFα signaling (14). Upon binding to TNFR1 and TNFR2, TNFα can signal both cell survival and cell death (15) through two separate protein complexes (16). The prosurvival complex (complex I) contains TNFα/TNFR1, TRADD, TRAF2, RIP1 and cIAP-1/2. This complex recruits and activates IKK, leading to the activation of NFκB (13, 17). The death-inducing signaling complex (DISC, complex II) is also assembled following internalization of the TNFR1 and consists of TRADD and RIP1, which then recruits FADD and caspase-8 to form DISC (16). Although DISC can be formed, it may be unable to induce cell death as long as there is a prosurvival signal being generated. Thus, disruption of a prosurvival complex would facilitate the activation of DISC to induce cell killing.
Survival function of IAPs is negatively regulated by SMAC (Second Mitochondria-derived Activator of Caspase), a mitochondrial protein that is released to the cytoplasm upon induction of apoptosis (18, 19). SMAC binds to XIAP, as well as to cIAP-1 and cIAP-2, via its N-terminal AVPI tetra-peptide binding motif to abrogate their inhibitory binding to both caspase 9 and caspases 3/7 (12). Small molecule SMAC mimetics have been designed and developed to mimic this AVPI binding motif of SMAC (20). Recently, SMAC mimetics were found to induce rapid autoubiquitination and degradation of cIAP-1, resulting in NF-κB activation and TNFα-dependent apoptosis (21–24). Thus, by eliminating cIAP-1 via SMAC mimetic-mediated autoubiquitination, prosurvival complex I is inactivated, which facilitates activation of complex II to induce apoptosis (25).
NF-κB (26) is activated by ionizing radiation via induced degradation of IκB (27). Activated NF-κB, on one hand, induces an adaptive resistance to ionizing radiation (28) as a cellular defensive mechanism, and on the other hand, increases the production of TNFα for apoptosis induction under certain circumstances (16, 29). Radiation is also well known to induce G2 arrest and apoptosis (30).
In this study, we investigated SM-164, a potent and well-characterized SMAC mimetic (31, 32) as a radiosensitizer in HNSCC cells. Our data showed that by eliminating cIAP-1, SM-164 acts as an effective radiosensitizer both in vitro and in vivo, in a subset of HNSCC lines, through NFκB activation that increases TNFα secretion, followed by activation of caspase-8 and -9 and induction of apoptosis. The present study lays the ground work for clinical development of SM-164 as a novel class of radiosensitizing agent for the treatment of a subset of head and neck cancers which are sensitive to caspase activation.
Materials and Methods
Cell Culture
Human HNSCC lines, including UMSCC-1, -12, -17B, and -74B were grown in DMEM with 10% fetal bovine serum. Human lung fibroblast MRC5 cells were a gift from Dr. A. Rehemtulla and cultured in RPMI1640 with 10% fetal calf serum. The cell line authentication is as follows: All four HNSCC cell lines were from Dr. T. Carey at the University of Michigan (33) and have been tested and authenticated by genetic profiling with various microsatellite loci, using Profiler Plus PCR Amplification Kit (Applied Biosystems, Foster City, CA) (34). The identities of the HNSCC cell lines were last tested and confirmed in September (for UMSCC-1, -12, -74B) and November (for UMSCC-17B) of 2010, respectively. SM-164 was synthesized by us as described (31, 32).
Radiation exposure and clonogenic assay
Cells were seeded in 6-well plates and exposed to different doses of radiation (Philips RT250, Kimtron Medical) after 2 hrs pre-treatment with SM-164, followed by incubation at 37 °C for 7 to 9 days. Survival curves were fitted using the linear-quadratic equation, and the mean inactivation dose was calculated (35).
siRNA silencing of caspases -8, -9, and BCL-2
The siRNA oligonucleotides for silencing caspase-8 (5’-GCCCAAACUUCACAGCAUU-3’), caspase-9 (5’-CGGUGAAAGGGAUUUAUAA-3’), scrambled control siRNA (siCONT: 5’-ATTGTATGCGATCGCAGACTT-3’), and SMARTpool siRNA targeting BCL-2 were from Dharmacon (USA). Cells were transfected with siRNA using Lipofectamine 2000 and split 48 hrs later. One portion was used for clonogenic assay and the other portion for immunoblotting (36).
Immunoblotting
The assay was performed as described (37) using antibodies against cIAP-1 (a gift from Dr. Silke) (24), Caspase-8, BCL-2, BCL-xL, MCL-1 (Santa Cruz, CA), β-Actin (Sigma, MO), XIAP and Caspase-9 (BD PharMingen, CA), cIAP-2 (Cell Signaling, MA).
ATPlite growth assay and IC50 determination
Cells were seeded in 96 well plates in triplicate and treated with SM-164 with various doses for 24 hrs. Cell viability was measured with ATPlite kit (Perkin Elmer) (37).
FACS analysis
Cells were treated with SM-164, or exposed to radiation alone or in combination. Cells were harvested 24 or 48 hours post radiation and analyzed by flow cytometry (37).
Luciferase reporter assay
Cells were seeded into a 96-well plate and transfected with a luciferase reporter driven by NFκB consensus sequence (pNifty plasmid, Invivogen, CA), along with Renilla construct. Cells were treated 24 hr later with SM-164, 4 Gy or the combination for 24 hr, followed by luciferase activity assay (Promega) with TNFα (10 ng/ml, BD biospheres) treatment as a positive control. The results are presented as the fold activation after normalization with Renilla.
Caspase activation assay
The activity of caspase-8, -9, -2 or -3 was analyzed using a fluorogenic caspase assay with Ac-IETD-AFC (BD PharMingen), Ac-LEHD-AFC or Z-VDVAD-AFC or Ac-DEVD-AFC (Calbiochem) as a substrate, respectively (37). The results are expressed as fold change compared to the control.
RT-PCR
Total RNA was isolated using TRIZol (Invitrogen), reversely transcribed using Superscript II (GibcoBRL) with oligo d(T) (Applied Biosystems). PCR was performed with the primer sets spanning at least two intron-exon junctions for TNFα and TRAIL (38).
TNFα ELISA
Cells were plated onto 6-well plates and irradiated with 4 Gy, treated with SM-164 (100 nM), or the combination. The conditioned medium (CM) was removed at 24, 48 and 72 hrs post treatment for ELISA assay using a TNFα ELISA kit (Cayman Chemicals Co.).
In vivo anti-tumor study
All animal studies were conducted in accordance with the guidelines established by the University Committee on Use and Care of Animals (UCUCA). Five million UMSCC-1 cells were inoculated s.c in both flanks of nude mice. The mice were randomized and the treatment started when the tumor size reached 70 mm3 at 18 days after inoculation. SM-164 (5 mg/kg, i.v.) and radiation (2 Gy) were given once a day, 5 days a week for two weeks. Radiation was delivered directly to the tumor with the rest of the animal shielded. For combination treatment, SM-164 was given 2–3 hrs prior to radiation exposure with the same schedule as for individual treatments. The tumor growth was measured three times a week, and average tumor volumes were calculated, as estimated from the formula (L×W2)/2, from at least 12 tumors in each group.
Statistical analysis
ANOVA was used with SPSS software for statistical comparisons involving multiple groups, followed by an SNK post hoc test to determine significance of each two groups (p < 0.05). Paired or unpaired two-tailed Student’s t test was used in comparisons between two groups.
Results
Sensitivity of HNSCC lines to radiation or SM-164 as a single agent
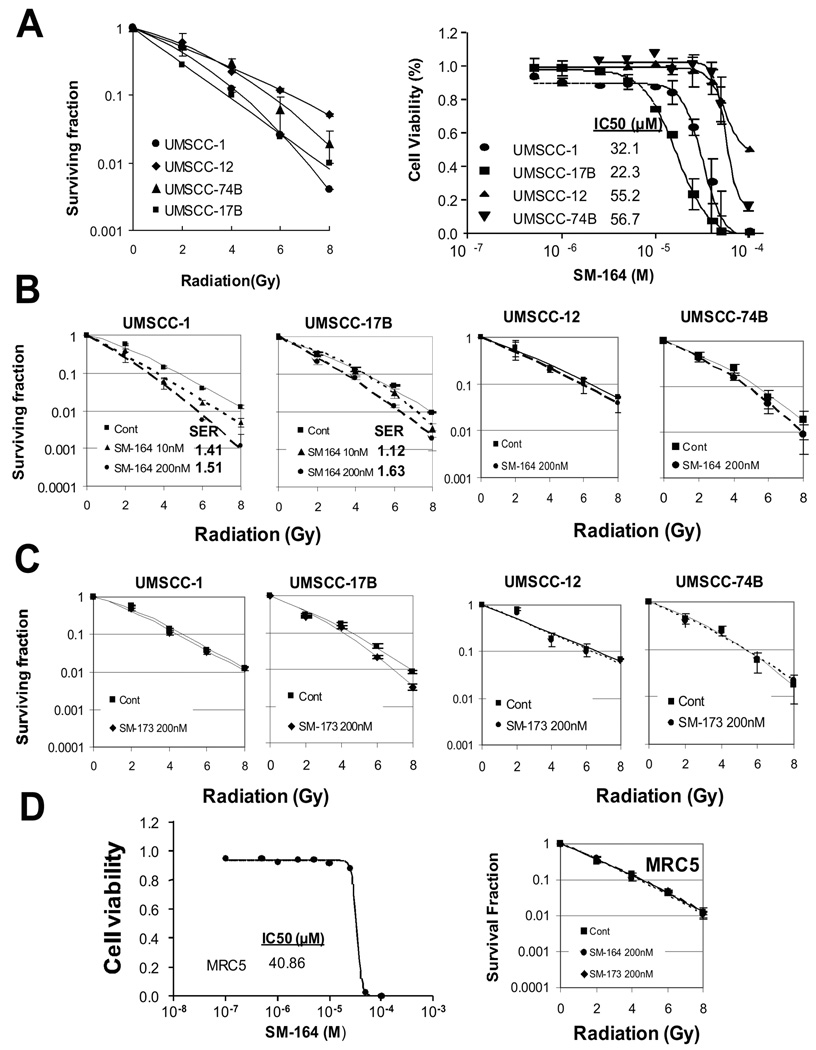
We first determined radiosensitivity among four HNSCC lines by classic clonogenic assay after exposure to various doses of radiation. As shown in Figure 1A (left), two lines (UMSCC-1 and UMSCC-17B) were relatively sensitive, whereas the other two lines (UMSCC-12 and UMSCC-74B) were relatively resistant to radiation. We then determined the sensitivity of these HNSCC lines to SM-164, a SMAC mimetic small molecule compound (chemical structure shown in Fig. S1A). Unlike MDA-MB-231 human breast cancer and SK-OV3 ovarian cancer cell lines, in which SM-164 was highly potent at low nanomolar concentrations (31, 32, 37), all four HNSCC lines were highly resistant to SM-164 as a single agent with the IC50 values ranging from 22 to 57 µM, although the two radiosensitive lines were relatively more sensitive to SM-164 (Fig. 1A, right).
Figure 1. SM-164 sensitizes a subset of HNSCC cell lines to radiation.
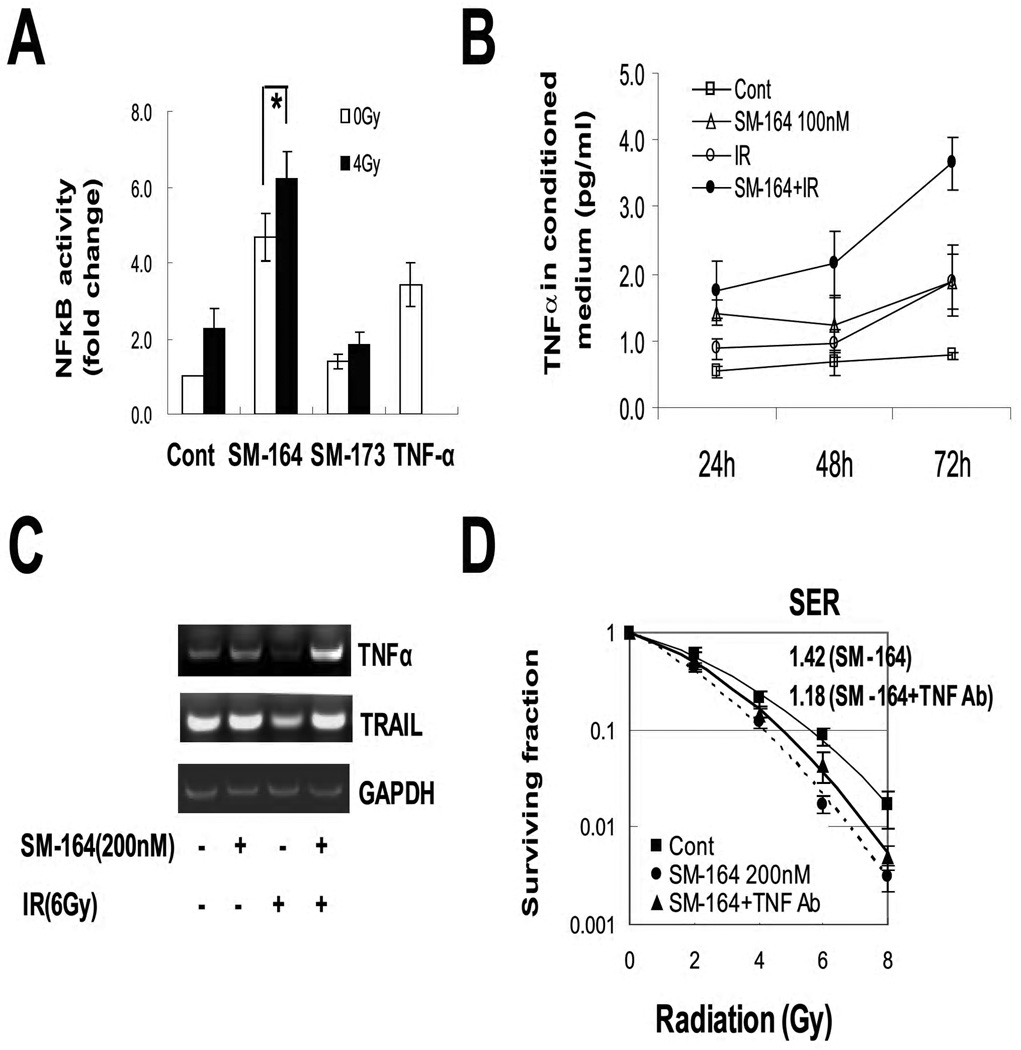
(A) Sensitivity of HNSCC lines to radiation and SM-164: Four lines of HNSCC cells were seeded in 6-well plates in triplicate and irradiated. The colonies with more than 50 cells were counted after 9 days. Surviving fraction was calculated as the proportion of seeded cells following irradiation to form colonies relative to that of untreated cells (mean ± SEM (n=3) (left panel). Cells were seeded in 96-well plate in triplicate and treated the following day with various concentrations of SM-164 for 24 hrs. Cells were then lysed for ATPlite assay (mean ± SEM (n=3) (right panel). (B&C) Radiosensitization by SM-164 (B), but not its inactive SM-173 (C) in HNSCC cells: Cells were seeded in 6-well plates and irradiated. SM-164 or SM-173 was added to culture media 2 hrs prior to radiation. SER was calculated as the ratio of the mean inactivation dose under untreated control conditions divided by the mean inactivation dose after SM-164 treatment (mean ± SEM, n=3). D) Normal fibroblasts are resistant to SM-164: MRC5 cells were treated with SM-164 for 24 hrs, followed by ATP-lite cell viability assay (left). MRC5 cells were pretreated with SM-164 or its inactive analog SM-173 for 2 hrs prior to radiation exposure, followed by standard clonogenic assay (right). Mean ± SEM (n=2).
A subset of HNSCC cell lines is sensitive to SM-164 induced radiosensitization
We next determined the potential radiosensitizing effect of SM-164 using drug concentrations much lower than the IC50 value in HNSCC cells. Cells were treated with SM-164 at 10 or 200 nM in combination with different doses of radiation. A SM-164 dose-dependent radiosensitization was observed in UMSCC-1 cells with an SER (Sensitivity Enhancement Ratio) of 1.4 or 1.5, respectively. In UMSCC-17B cells, only higher dose caused radiosensitization with an SER of 1.6 (Fig. 1B, two left panels). In UMSCC-12 and UMSCC-74B cells, SM-164 at 200 nM showed no or minimal radiosensitization (Fig. 1B, two right panels). An inactive analogue of SM-164, SM-173 (31) had no effect on either group of lines at 200 nM (Fig. 1C), indicating a specific effect of SM-164.
We also determined potential toxicity of SM-164 on human lung fibroblast MRC5 cells alone or in combination with radiation and found that MRC5 cells were resistant to SM-164 with an IC50 value of 41 µM, and are resistant to SM-164 radiosensitization with an SER of 1.05 (Fig. 1D).
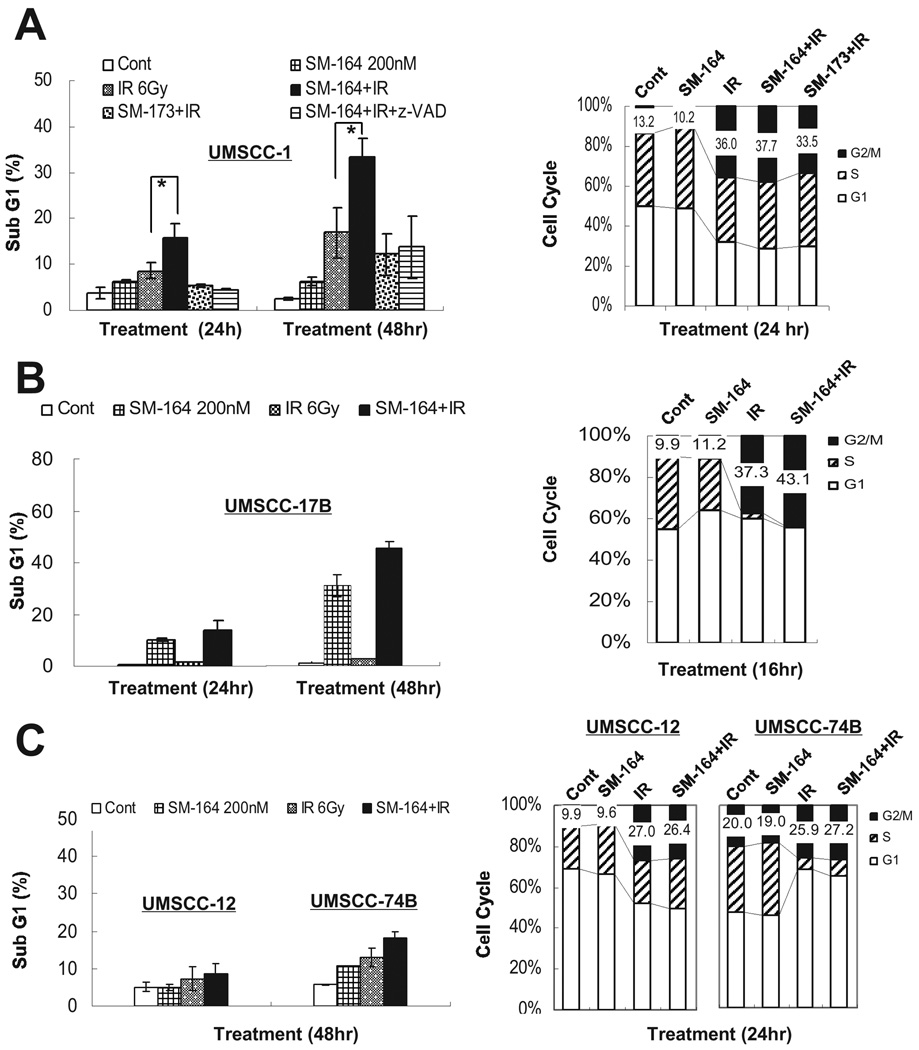
Radiosensitization by SM-164 is attributable to enhanced induction of apoptosis
To determine the nature of SM-164 radiosensitization, we performed FACS analysis of all four lines treated with SM-164 or radiation, alone or in combination. In sensitive UMSCC-1 cells, exposure to 200 nM SM-164 or irradiation with 6 Gy induced a moderate level of apoptosis (sub-G1 population). The combination of radiation and SM-164, but not its inactive analogue, SM-173, significantly enhanced radiation-induced apoptosis (Fig. 2A, left panel, p<0.05). Importantly, apoptosis induced by combination of SM-164 and radiation was completed blocked by z-VAD, a pan caspase inhibitor, indicating the involvement of caspase activation. Similar results were seen at 48 hr post-treatment with a higher degree of apoptosis induction (Fig. 2A, left panel). On the other hand, SM-164 had no effect on radiation-induced G2/M arrest (Fig. 2A, right panel). Consistently, in another sensitive UMSCC-17B line, SM-164 alone caused a time-dependent induction of apoptosis, which was enhanced by radiation (from 30% to 44% of the population at 48 hr), although radiation alone had a minimal effect on apoptosis induction (Fig. 2B, left panel). Again, SM-164 had a minimal effect on radiation-induced G2/M arrest (Fig. 2B, right panel). As expected, SM-164 had no or little radiation-enhancing activity for apoptosis induction and G2/M arrest in two resistant lines, UMSCC-12 and UMSCC-74B (Fig. 2C), consistent with the lack of SM-164 radiosensitization. Take together, these results suggest that SM-164-mediated radiosensitization in sensitive lines is associated with enhanced apoptosis.
Figure 2. FACS profiling to determine the effect of SM-164 on apoptosis or cell cycle progression.
UMSCC-1 (A), UMSCC-17B (B), and UMSCC-12 and -74B (C) cells were treated with indicated agents, alone or in combination, followed by FACS analysis for apoptosis (sub-G1 population) at 24 or 48 hr later (*, p<0.05) (n=3), or for cell cycle progression at the different phases of cell cycle at 16 hr or 24 hr after treatment (a representative result is shown).
SM-164 radiosensitization is independent of cIAP-1 degradation, but dependent on caspase activation
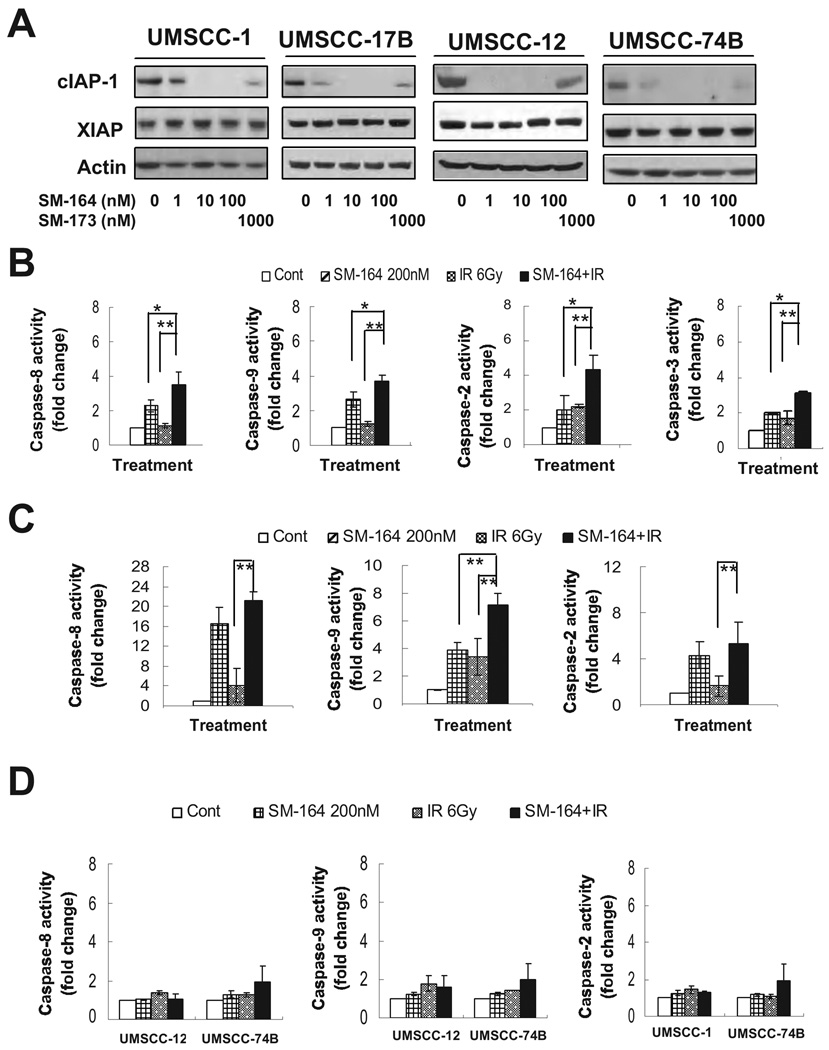
We next addressed why some HNSCC lines are sensitive, whereas others are resistant to SM-164 radiosensitization. Several groups have recently reported that SMAC mimetic compounds induced a rapid degradation of cIAP-1 (21–24, 31). We, therefore, determined if the difference in SM-164 sensitivity is attributable to different levels of cIAP-1 and/or XIAP or different degree of targeted cIAP-1 degradation by SM-164. As shown in Figure 3A, the levels of both cIAP-1 and XIAP varied slightly among the four lines but did not correlate with SM-164 sensitivity. Furthermore, in all four lines, SM-164 caused a dose-dependent degradation of cIAP-1 with 100% elimination at 10 nM, whereas an inactive analogue, SM-173, had a minimal effect on cIAP-1 at 1000 nM (Fig. 3A, top panels). Consistent with previous reports (21–24), SM-164 had no effect on XIAP levels at the concentrations used (Fig. 3A, middle panels). Moreover, SM-164-induced cIAP-1 degradation occurred rapidly with a complete elimination of cIAP-1 at 60 min post treatment, and again, no effect on XIAP was found (Fig. S1B). Thus, SM-164 radiosensitization is independent of the levels or degradation of cIAP-1 or XIAP.
Figure 3. SM164 promotes cIAP-1 degradation and caspase activation.
(A) Dose dependent degradation of cIAP-1: Cells were treated with SM-164 or SM-173 for 6 hrs, followed by immunoblotting. (B-D) Caspase activation: UMSCC-1 (B), UMSCC-17B (C), and UMSCC-12 and -74B (D) cells were treated with SM-164, radiation or both, and subjected to caspase activity assay for caspases-8, -9, -2, and -3 (for UMSCC-1). Mean ± SEM (n=3) (*, p<0.05; **, p<0.01).
Since the mechanism of SM-164 action is to promote cIAP-1 degradation and to disrupt XIAP binding to active caspases (31), we reasoned that removal of negative blockers of caspase activation by SM- 164 would be effective only in cells that undergo caspase activation upon external stimuli, but not in cells which are resistant to caspase activation. We, therefore, determined potential involvement of caspase activation in SM-164 radiosensitization. Cells were treated with SM-164, radiation alone, or the combination and the activity of caspases 8, 9 and 2 was measured. As shown in Figure 3B, in sensitive UMSCC-1 cells, SM-164 alone treatment caused a 3-, 2.5- and 2-fold activation of caspases 9, 8 or 2, respectively, whereas radiation alone had a minimal effect on caspase activation. Combinational treatment caused a significant increase in caspase activation with an up to 4-fold increases for caspases 9, 8 and 2, as well as a significant increase in caspase 3 activity. Similar results were observed in another sensitive line of UMSCC-17B cells with combination treatment causing maximal activation of caspases (Fig. 3C). Neither of the two resistant lines showed caspase activation after treatment with SM-164 alone or combined with radiation (Fig. 3D).
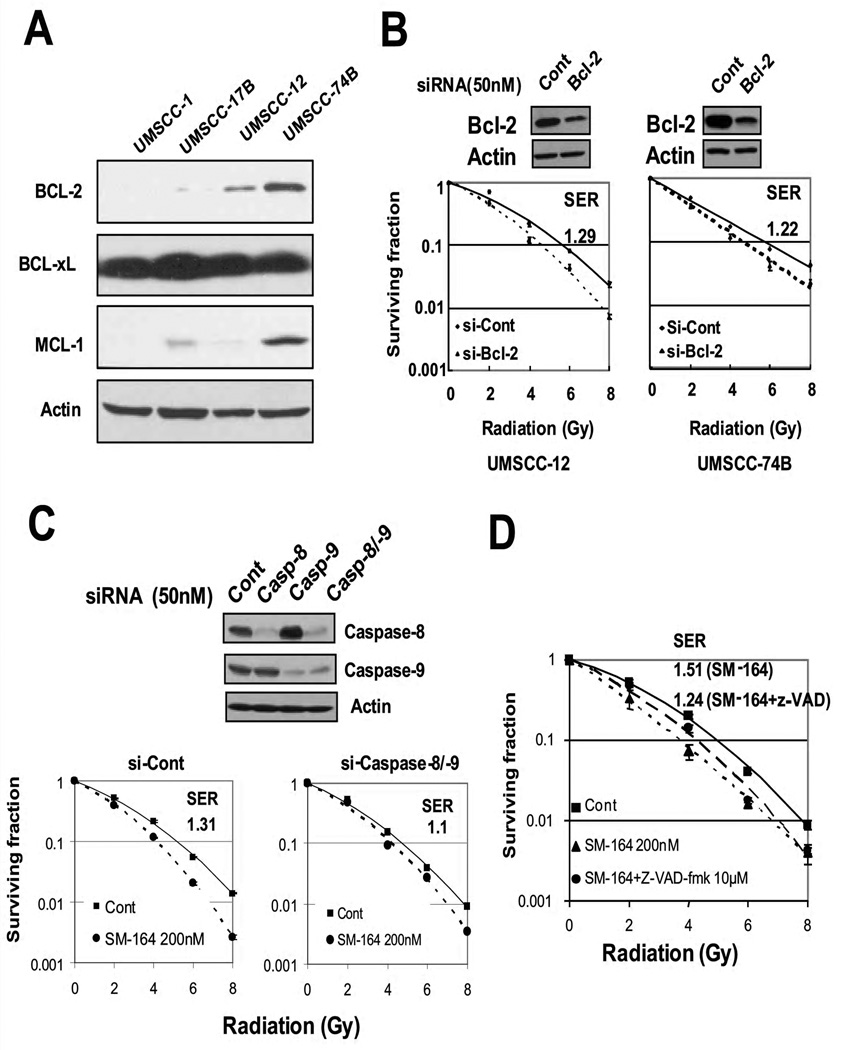
BCL-2 knockdown by siRNA sensitizes resistant HNSCC cells to radiation, whereas blockage of caspase activation largely abrogated SM-164 radiosensitization in sensitive cells
We further investigated the potential mechanism by which cells were intrinsically either sensitive or resistant to caspase activation by focusing on three anti-apoptotic BCL-2 family members. While the BCL-xL levels were very high in all four lines and MCL-1 levels varied among lines, the BCL-2 levels were inversely correlated to intrinsic sensitivity to caspase activation with undetectable or very low levels in the two sensitive lines, but a high level in two resistant lines (Fig. 4A). Since BCL-2 overexpression correlated with radioresistance in primary HNSCC tumor tissues (3), we tested if BCL-2 knockdown would sensitize these two BCL-2 highly-expressed lines to radiation. Indeed, BCL-2 knockdown to ~50% of the original level sensitized otherwise resistant UMSCC-12 and UMSCC-74 cells to radiation with an SER of 1.29 and 1.22, respectively (Fig. 4B). Thus, the BCL-2 levels correlated with radioresistance, whereas SM-164 radiosensitization is determined by intrinsic cellular sensitivity to caspase activation when BCL-2 level is very low or undetectable.
Figure 4. Radiosensitization by BCL-2 siRNA knockdown and blockage of SM-164 radiosensitization by caspase siRNA knockdown or caspase inhibitor.
(A) The levels of anti-apoptotic BCL-2 family members: Subconfluent cells were subjected to immnoblotting analysis using antiobodies against BCL-2, BCL-xL and MCL-1. (B). Radiosensitization by BCL-2 knockdown: Cells were transfected with SMARTpool siRNA targeting BCL-2. Forty-eight hrs later, one portion of cells was for immunoblotting (top) and other portion was plated for clonogenic assay (bottom, mean ± SEM, n=3). (C) Partial abrogation of SM-164 radiosensitization by siRNA knockdown of caspases: UMSCC-1 cells were transfected with siRNA, targeting caspases-8 and -9. Forty-eight hrs later, one portion of cells was for immunoblotting and other portion was plated for clonogenic assay (n=2). (D) Partial abrogation of SM-164 radiosensitization by a pan caspase inhibitor: UMSCC-1 cells were treated with SM-164 alone (2 hrs prior to radiation) or in combination, respectively, with z-VAD-fmk (10 µM, 3 hrs prior to radiation). Effect of z-VAD-fmk on SM-164 radiosensitization was assessed by clonogenic survival assay (mean ± SEM (n=2).
We then determined if caspase activation is causally related to SM-164 radiosensitization by blocking caspases via siRNA knockdown and inhibitor treatment. In UMSCC-1 cells, transfection of siRNAs against caspases 8 and 9 caused a greater than 70% reduction of their levels, and consequently decreased SM-164 radiosensitization with an SER reduction from 1.32 to 1.14 (Fig. 4C, p<0.05). SM-164 radiosensitization was also partially blocked by z-VAD-fmk, a pan-caspase inhibitor, with an SER reduction from 1.51 to 1.24 in UMSCC-1 cells (Fig. 3D, p<0.05). Thus, caspase activation plays a major role in SM-164 radiosensitization in sensitive HNSCC cells.
SM-164 increased radiation-induced NF-κB activation and TNFα secretion in UMSCC-1 cells
A number of recent studies have shown that sensitivity of cancer cells to apoptosis induced by a SMAC mimetic as a single agent directly correlates with TNFα levels (21, 22), and blockage of TNFα signals by TNFα antibody or TNFR siRNA, abolishes SMAC mimetic-induced apoptosis (22–24). Given the fact that ionizing radiation causes activation of NF-κB (26, 27), whereas TNFα is an NF-κB target (29), which induces apoptosis if cIAP-1 is removed (14), we determined if NF-κB activation, followed by TNFα secretion, is the major mechanism for apoptosis-induction and SM-164 radiosensitization in sensitive cells. As shown in Figure 5A, irradiation of UMSCC-1 cells with 4 Gy induced a 2-fold activation of NF-κB, whereas the treatment with SM-164 induced a 4.5-fold activation. An additive 6.5-fold induction was observed with the combination. The inactive analogue SM-173 had no effect alone or in combination with radiation. TNFα, a known inducer of NFκB used as a positive control in the assay, caused a 3-fold activation. Thus, it appears that by eliminating cIAP-1, SM-164 increased radiation-induced NF-κB activation in sensitive cells.
Figure 5. NFκB activation and TNFα secretion during SM-164 radiosensitization.
(A) NFκB activity: Cells were transfected with a NF-κB luciferase reporter (pNifty plasmid), along with Renilla, in 6-well plates, and split into a 96-well plate 24 hr later. Cells were then left untreated (control), or treated with TNFα (10 ng/ml) for 3 hrs as a positive control, or irradiated with 4 Gy. SM-164 or SM-173 (100 nM) were added immediately after radiation. Cells were lysed 24 hrs later for luciferase activity assay (Promega). Results (n=3) are presented as the fold activation after normalization with Renilla (*, p<0.05). (B) Increased TNFα secretion: TNFα levels in conditioned media were measured by ELISA. The values were normalized with protein concentrations of the cell lysates. Mean ± SEM (n=3). (C) Induction of TNFα mRNA: UMSCC-1 cells were treated with SM-164, or radiation (6 Gy) or the combination. Cells were harvested 24 hrs later for RT-PCR analysis to determine mRNA expressions of TNFα with TRAIL as a control. (D). Partial blockage of SM-164 radiosensitization by TNFα Ab: UMSCC-1 cells were left untreated or treated with SM-164 (200 nM) for 2 hr with or without TNFα antibody (50 ng/ml) before irradiation. Cells were grown in media containing SM-164±TNFα Ab for 9 days before the colonies were counted (mean ± SEM (n=2).
We next determined if the sensitivity to SM-164 radiosensitization correlated with increased production of TNFα by measuring TNFα levels in conditioned medium (CM) in UMSCC-1 cells. As shown in Figure 5B, a single treatment with SM-164 or radiation induced a minor increase in TNFα secretion into the CM. In contrast, the combined treatment induced a significant time-dependent increase in TNFα secretion, reaching the peak induction of 4-fold at 72 hrs. We further performed RT-PCR analysis of mRNA expressions of TNFα and TRAIL, a ligand for death receptor 5 (DR5) as a control. As shown in Figure 4C, TNFα mRNA, but not TRAIL mRNA, was induced only by combined treatment, indicating the TNFα increase occurred at the transcriptional level. Finally, we tested if blockage of TNFα by TNFα neutralizing antibody would abolish SM-164 radiosensitization. As shown in Figure 5D, inclusion of TNFα Ab reduced SM-164-induced SER from 1.42 to 1.18, indicating a partial rescue, which is statistically different (p<0.05). Thus, increased TNFα secretion, likely as a result of NFκB activation following the cIAP-1 elimination, contributes at least in part to the radiosensitizing effect of SM-164 in UMSCC-1 cells.
SM-164 degraded cIAP-1 and radiosensitized UMSCC-1 in vivo xenograft tumors
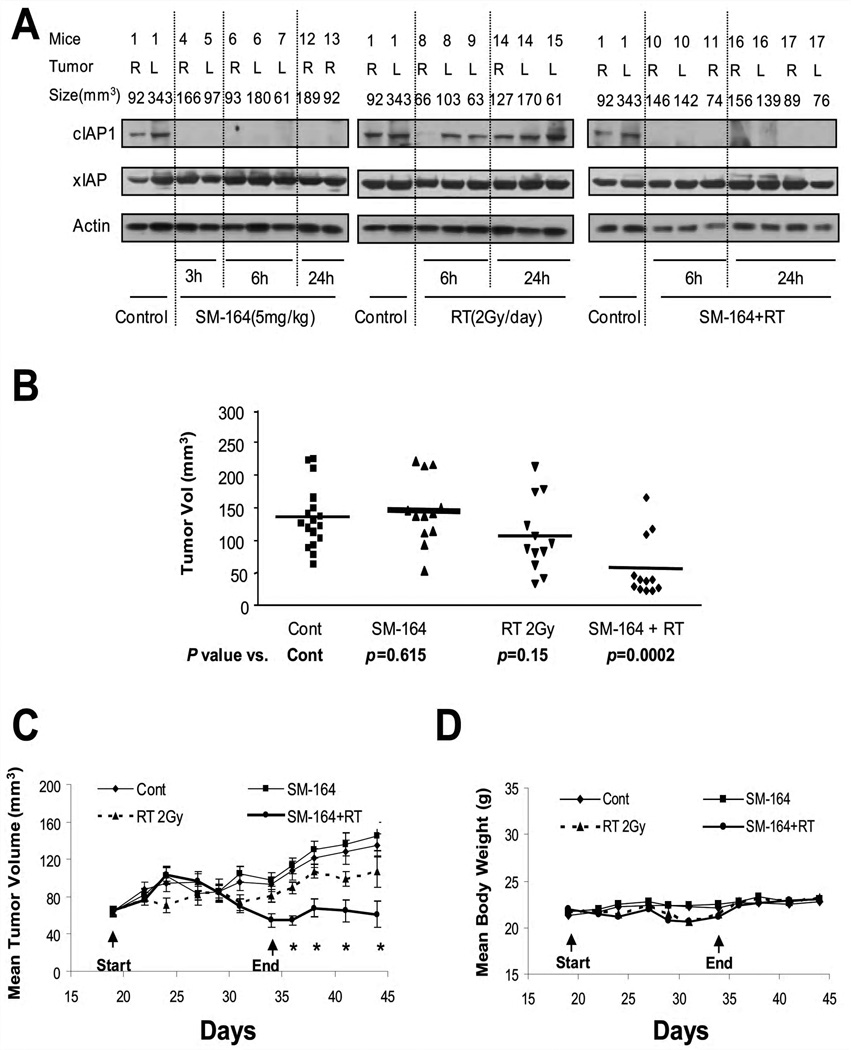
Finally, we determined SM-164 radiosensitization in vivo using the UMSCC-1 xenograft model. To ensure that SM-164 “hits” its target cIAP-1 in in vivo tumors and to determine when radiation should be delivered post the drug administration, we measured cIAP-1 levels among 4 groups of tumor samples at the indicated times after a single administration of SM-164 or a single dose of radiation or the drug-radiation combination. As shown in Figure 6A, cIAP-1 was detectable in control tumors. SM-164 administration eliminated cIAP-1 starting at 3 hrs post treatment and for up to 24 hrs. As expected, radiation alone had no effect on cIAP-1. The combination of SM-164 and radiation also eliminated cIAP-1 by 6 hr, and cIAP-1 had not returned to the basal level in any tumor by 24 hrs. Our results clearly demonstrated that SM-164 indeed “hits” the target and suggested that the radiation should be delivered 2–3 hrs post administration of SM-164, when cIAP-1 is undetectable.
Figure 6. SM-164 radiosensitization in UMSCC-1 in vivo xenograft tumor model.
(A) SM-164 eliminated cIAP-1 in tumor tissues: In experiment one, five million UMSCC-1 cells were inoculated s.c in both flank sides (R, right; L, left) of nude mice. The mice were randomized when tumor size reached 70 mm3 at 18 days after inoculation and were left untreated or for a single treatment with SM-164, radiation, or drug-radiation combination. Tumor tissues were harvested at indicated time points for immunoblotting. (B &C) In vivo radiosensitizing activity of SM-164 in the UMSCC-1 xenograft model: In experiment 2, five million UMSCC-1 cells were inoculated s.c in both flank sides of nude mice. The treatment started 18 days after inoculation when tumor size reached 70 mm3, followed by tumor growth measurement. Student’s t test was used to compare each treatment group with the control group. Shown are the results on day 44 (mean ± SEM, * indicates p<0.05) (B), and tumor growth curve (C). (D). SM-164 is well tolerated by mice: Body weight was measured during the treatment and thereafter and plotted (mean ± SEM).
We next determined the in vivo radiosensitizing activity of SM-164. As shown in Figure 6B&C, administration of SM-164 alone at a dose of 5 mg/kg i.v./day for 5 days/week for 2 weeks had no effect on tumor growth in nude mice. Radiation treatment at the clinically relevant dose of 2 Gy/day for 5 days/week for 2 weeks had a moderate, but not statistically significant anti-tumor activity. In contrast, the combination of SM-164 and radiation caused a remarkable suppression of tumor growth, which is statistically significantly greater than either treatment alone beginning at day 34 (Fig 6C). The combination treatment was well-tolerated by the animals with a minimal loss of body weight (Fig 6D). Taken together, our results indicate that SM-164 sensitizes UMSCC-1 head and neck cancer cells to radiation, as assayed in both in vitro cell culture and in vivo tumor xenograft models, and acts as a novel class of radiosensitizer.
Discussion
In this study, we determined the radiosensitizing activity of SM-164, a small molecule SMAC mimetic compound that promotes a rapid degradation of cIAP-1 and disrupts inhibitory binding of XIAP to caspases 9 and 3 (31) in HNSCC cells. We found that SM-164, at non-toxic sub-nanomolar concentrations, significantly sensitized a subset of HNSCC cells to radiation both in vitro cell culture and in vivo xenograft tumor models. Surprisingly, although cIAP-1 degradation contributes to SM-164 radiosensitization, sensitivity of HNSCC cells to SM-164 radiosensitization is determined neither by the degradation of cIAP-1, nor by cellular levels of cIAP-1 and/or XIAP. Rather, it is determined by intrinsic cellular sensitivity to caspase activation with an inverse relationship to endogenous BCL-2 levels. It is very likely that lack of BCL-2 expression in sensitive cells facilitates caspase activation, whereas by removal of negative blockers of cIAP-1 and XIAP, SM-164 confers full activation of caspases, leading to enhanced killing and increased sensitivity to radiation. Consistently, the high levels of BCL-2 rendered HNSCC cells resistant to radiation and knockdown of BCL-2 via siRNA silencing sensitized cells to radiation.
Our finding that SM-164 can induce apoptosis when combined with radiation is consistent with a recent study showing that overexpression of SMAC protein itself enhanced radiation-induced apoptosis in several lines of cancer cells, including neuroblastoma, glioblastoma, and pancreatic carcinoma (39). Our mechanistic study revealed that both sensitive lines are intrinsically sensitive to activation of a number of caspases, including -8, -9, and -2 by SM-164, or radiation, but to a lesser extent. The combination of SM-164 and radiation further activates these caspases, leading to an enhanced apoptosis. The causal effect of caspase activation in SM-164-induced radiosensitization was demonstrated by complete abrogation of apoptosis induction by z-VAD treatment (Fig. 2A), and significant blockage of SM-164 radiosensitization by caspase knockdown or z-VAD treatment (Fig. 4C&D). On the other hand, in UMSCC-12 and UMSCC-74B with very high levels of BCL-2, neither caspase 8, 9, nor 2 was significantly activated by either SM-164 or radiation, and consistently, neither cell line was radiosensitized by SM-164.
It is well established that ionizing radiation activates NF-κB (26) through degradation of IκB (27). Activated NF-κB, on one hand, induces survival proteins, TRAF1/2 and cIAP-1/2 to inhibit apoptosis via suppressing caspase-8 activation (14). On the other hand, activated NF-κB induces its downstream target, TNF-α to initiate the death receptor pathway for apoptosis induction if survival proteins, such as TRAF-2 or cIAP-1, are removed (21–24). This notion is further supported by our recent study that UMSCC-1 cells were sensitized to radiation by siRNA silencing of TRAF2 (36), as well as by siRNA silencing of cIAP-1 or cIAP-2 (Fig. S1C). Here, we showed that SM-164 further activated radiation-induced NF-κB, leading to increased TNFα transcription and secretion. The role of TNFα in SM-164 radiosensitization was clearly demonstrated by a substantial abrogation of radiosensitization in the presence of a TNFα neutralizing antibody. Thus, in UMSCC-1 cells, NF-κB activation, followed by TNFα secretion and caspase-8 activation upon cIAP-1 degradation, is the major mechanism of SM-164 radiosensitization.
In summary, we report here that SM-164 is a potent and novel class of radiosensitizer in a subset of HNSCC cells. SM-164 radiosensitization is determined by intrinsic sensitivity to caspase activation in cells with undetectable or low BCL-2 expression. Under these conditions, removal of negative blockers, such as cIAP-1 and XIAP by SM-164 leads to a full activation of caspases 8 and 9 via NF-κB/TNFα death receptor pathway. Although multiple factors contribute to radioresistance of HNSCC, including activation of EGFR, NFκB, and COX-2 signals (2, 6, 40, 41), our study does suggest that HNSCC patients, whose tumors lack the BCL-2 expression (seen in 76% of primary HNSCC tumors out of 85 cases) (3), and are intrinsically sensitive to caspase activation, might benefit from combinational therapy with SM-164 and radiation. Thus, SM-164 shows some promise for future development as a novel class of radiosensitizing drugs for a subset of HNSCC patients, although caution should be taken for potential side effects, given the role of IAPs in modulation of inflammatory signaling and immunity in addition to regulating caspases and apoptosis (42).
Supplementary Material
Acknowledgements
We would like to thank Dr. Thomas Carey at the University of Michigan for providing us head and neck squamous carcinoma cell lines and Mr. Martin Graham for his help in genotyping of UMSCC cell lines used in this study. We also thank Dr. John Silke at La Trobe University, Australia, for anti-cIAP-1 antibody and Dr. Philip Baker at McGill University, Canada, for the RT-PCR primer sequences.
This work is supported by the NCI grants (CA111554 and CA118762), the Ronald P. and Joan M. Nordgren Endowed Cancer Research Fund and a seed grant from Head and Neck Cancer SPORE from the University Michigan Comprehensive Cancer Center to YS.
Abbreviations
- BCL-2
B-cell leukemia/lymphoma 2
- BIRC-2
Baculoviral IAP Repeat-Containing protein 2
- BIRC-3
Baculoviral IAP Repeat-Containing protein 3
- cIAP-1
Cellular Inhibitor of Apoptosis-1
- cIAP-2
Cellular Inhibitor of Apoptosis-2
- DISC
Death-Inducing Signaling Complex
- FACS
Fluorescence Activated Cell Sorting
- FADD
Fas Associated Death Domain protein
- HNSCC
Head and Neck Squamous Cell Carcinomas
- IKK
Inhibitor of KappaB Kinase
- RIP
Receptor-Interacting Protein
- SER
Sensitivity Enhancement Ratio
- SMAC
Second Mitochondria-derived Activator of Caspase
- TRADD
TNFR1 Associated Death Domain protein
- TNF
Tumor Necrosis Factor
- TRAF-2
TNF Receptor-Associated Factor 2
- XIAP
X-linked Inhibitor of Apoptosis
Footnotes
Conflict of interest: Authors declare no conflict of interest.
References
- 1.Thariat J, Milas L, Ang KK. Integrating radiotherapy with epidermal growth factor receptor antagonists and other molecular therapeutics for the treatment of head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;69:974–984. doi: 10.1016/j.ijrobp.2007.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nyati MK, Morgan MA, Feng FY, Lawrence TS. Integration of EGFR inhibitors with radiochemotherapy. Nat Rev Cancer. 2006;6:876–885. doi: 10.1038/nrc1953. [DOI] [PubMed] [Google Scholar]
- 3.Gallo O, Chiarelli I, Boddi V, Bocciolini C, Bruschini L, Porfirio B. Cumulative prognostic value of p53 mutations and bcl-2 protein expression in head-and-neck cancer treated by radiotherapy. Int J Cancer. 1999;84:573–579. doi: 10.1002/(sici)1097-0215(19991222)84:6<573::aid-ijc6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 4.Roberg K, Jonsson AC, Grenman R, Norberg-Spaak L. Radiotherapy response in oral squamous carcinoma cell lines: evaluation of apoptotic proteins as prognostic factors. Head Neck. 2007;29:325–334. doi: 10.1002/hed.20520. [DOI] [PubMed] [Google Scholar]
- 5.Uno M, Otsuki T, Yata K, Fujii T, Sakaguchi H, Akisada T, et al. Participation of Fas-mediated apoptotic pathway in KB, a human head and neck squamous cell carcinoma cell line, after irradiation. Int J Oncol. 2002;20:617–622. [PubMed] [Google Scholar]
- 6.Kato T, Duffey DC, Ondrey FG, Dong G, Chen Z, Cook JA, et al. Cisplatin and radiation sensitivity in human head and neck squamous carcinomas are independently modulated by glutathione and transcription factor NF-kappaB. Head Neck. 2000;22:748–759. doi: 10.1002/1097-0347(200012)22:8<748::aid-hed2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Hermsen M, Alonso Guervos M, Meijer G, van Diest P, Suarez Nieto C, Marcos CA, et al. Chromosomal changes in relation to clinical outcome in larynx and pharynx squamous cell carcinoma. Cell Oncol. 2005;27:191–198. doi: 10.1155/2005/407216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roman E, Meza-Zepeda LA, Kresse SH, Myklebost O, Vasstrand EN, Ibrahim SO. Chromosomal aberrations in head and neck squamous cell carcinomas in Norwegian and Sudanese populations by array comparative genomic hybridization. Oncol Rep. 2008;20:825–843. [PubMed] [Google Scholar]
- 9.Gibcus JH, Kok K, Menkema L, Hermsen MA, Mastik M, Kluin PM, et al. High-resolution mapping identifies a commonly amplified 11q13.3 region containing multiple genes flanked by segmental duplications. Hum Genet. 2007;121:187–201. doi: 10.1007/s00439-006-0299-6. [DOI] [PubMed] [Google Scholar]
- 10.Tanimoto T, Tsuda H, Imazeki N, Ohno Y, Imoto I, Inazawa J, et al. Nuclear expression of cIAP-1, an apoptosis inhibiting protein, predicts lymph node metastasis and poor patient prognosis in head and neck squamous cell carcinomas. Cancer Lett. 2005;224:141–151. doi: 10.1016/j.canlet.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 11.Deveraux QL, Reed JC. IAP family proteins--suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 12.Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell. 2002;9:459–470. doi: 10.1016/s1097-2765(02)00482-3. [DOI] [PubMed] [Google Scholar]
- 13.Shu HB, Takeuchi M, Goeddel DV. The tumor necrosis factor receptor 2 signal transducers TRAF2 and c-IAP1 are components of the tumor necrosis factor receptor 1 signaling complex. Proc Natl Acad Sci U S A. 1996;93:13973–13978. doi: 10.1073/pnas.93.24.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 16.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 17.Devin A, Cook A, Lin Y, Rodriguez Y, Kelliher M, Liu Z. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity. 2000;12:419–429. doi: 10.1016/s1074-7613(00)80194-6. [DOI] [PubMed] [Google Scholar]
- 18.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 19.Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 21.Gaither A, Porter D, Yao Y, Borawski J, Yang G, Donovan J, et al. A Smac mimetic rescue screen reveals roles for inhibitor of apoptosis proteins in tumor necrosis factor-alpha signaling. Cancer Res. 2007;67:11493–11498. doi: 10.1158/0008-5472.CAN-07-5173. [DOI] [PubMed] [Google Scholar]
- 22.Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 24.Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Du F, Wang X. TNF-α induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 26.Brach MA, Hass R, Sherman ML, Gunji H, Weichselbaum R, Kufe D. Ionizing radiation induces expression and binding activity of the nuclear factor kappa B. J Clin Invest. 1991;88:691–695. doi: 10.1172/JCI115354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell JS, Tofilon PJ. Radiation-induced activation of nuclear factor-kappaB involves selective degradation of plasma membrane-associated I(kappa)B(alpha) Mol Biol Cell. 2002;13:3431–3440. doi: 10.1091/mbc.E02-05-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed KM, Li JJ. NF-kappa B-mediated adaptive resistance to ionizing radiation. Free Radic Biol Med. 2008;44:1–13. doi: 10.1016/j.freeradbiomed.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 30.Lobrich M, Jeggo PA. The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nat Rev Cancer. 2007;7:861–869. doi: 10.1038/nrc2248. [DOI] [PubMed] [Google Scholar]
- 31.Lu J, Bai L, Sun H, Nikolovska-Coleska Z, McEachern D, Qiu S, et al. SM-164: a novel, bivalent Smac mimetic that induces apoptosis and tumor regression by concurrent removal of the blockade of cIAP-1/2 and XIAP. Cancer Res. 2008;68:9384–9393. doi: 10.1158/0008-5472.CAN-08-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun H, Nikolovska-Coleska Z, Lu J, Meagher JL, Yang CY, Qiu S, et al. Design, synthesis, and characterization of a potent, nonpeptide, cell-permeable, bivalent Smac mimetic that concurrently targets both the BIR2 and BIR3 domains in XIAP. J Am Chem Soc. 2007;129:15279–15294. doi: 10.1021/ja074725f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradford CR, Zhu S, Ogawa H, Ogawa T, Ubell M, Narayan A, et al. P53 mutation correlates with cisplatin sensitivity in head and neck squamous cell carcinoma lines. Head Neck. 2003;25:654–661. doi: 10.1002/hed.10274. [DOI] [PubMed] [Google Scholar]
- 34.Brenner JC, Graham MP, Kumar B, Saunders LM, Kupfer R, Lyons RH, et al. Genotyping of 73 UM-SCC head and neck squamous cell carcinoma cell lines. Head Neck. 2010;32:417–426. doi: 10.1002/hed.21198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fertil B, Dertinger H, Courdi A, Malaise EP. Mean inactivation dose: a useful concept for intercomparison of human cell survival curves. Radiat Res. 1984;99:73–84. [PubMed] [Google Scholar]
- 36.Zheng M, Morgan-Lappe SE, Yang J, Bockbrader KM, Pamarthy D, Thomas D, et al. Growth inhibition and radiosensitization of glioblastoma and lung cancer cells by small interfering RNA silencing of tumor necrosis factor receptor-associated factor 2. Cancer Res. 2008;68:7570–7578. doi: 10.1158/0008-5472.CAN-08-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bockbrader KM, Tan M, Sun Y. A small molecule Smac-mimic compound induces apoptosis and sensitizes TRAIL- and etoposide-induced apoptosis in breast cancer cells. Oncogene. 2005;24:7381–7388. doi: 10.1038/sj.onc.1208888. [DOI] [PubMed] [Google Scholar]
- 38.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 39.Giagkousiklidis S, Vogler M, Westhoff MA, Kasperczyk H, Debatin KM, Fulda S. Sensitization for gamma-irradiation-induced apoptosis by second mitochondria-derived activator of caspase. Cancer Res. 2005;65:10502–10513. doi: 10.1158/0008-5472.CAN-05-0866. [DOI] [PubMed] [Google Scholar]
- 40.Hamakawa H, Nakashiro K, Sumida T, Shintani S, Myers JN, Takes RP, et al. Basic evidence of molecular targeted therapy for oral cancer and salivary gland cancer. Head Neck. 2008;30:800–809. doi: 10.1002/hed.20830. [DOI] [PubMed] [Google Scholar]
- 41.Allen CT, Ricker JL, Chen Z, Van Waes C. Role of activated nuclear factor-kappaB in the pathogenesis and therapy of squamous cell carcinoma of the head and neck. Head Neck. 2007;29:959–971. doi: 10.1002/hed.20615. [DOI] [PubMed] [Google Scholar]
- 42.Gyrd-Hansen M, Meier P. IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat Rev Cancer. 2010;10:561–574. doi: 10.1038/nrc2889. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.