Abstract
Our goal was to extend our understanding of the neural changes behind motor recovery with treadmill exercise in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned mouse. We determined the extent of dopamine (DA) terminal changes using western immunoblotting [striatal dopamine transporter (DAT) and tyrosine hydroxylase (TH)] and alterations in the mean number of DA cells/section by immunohistochemistry and Nissl staining [TH-labeled cells and thionin-stained cells in the substantia nigra pars compacta (SN-PC)]. We measured recovery of gait performance and amount of spontaneous physical activity using the parallel rod activity chamber (PRAC). We hypothesized that the decrease in TH-labeled neurons in the SN-PC due to MPTP will be partially reversed by treadmill exercise, leading to recovery of motor behavior as measured by the PRAC. Following MPTP or vehicle administration, mice ran on the treadmill for 1 hour per day at 18 cm/s, 5 days per week. Results showed that treadmill exercise improves gait performance and increases physical activity while promoting increased protein expression of striatal DAT and TH. Exercise was effective for all mice, however effects of early treadmill-based intervention appear to have an additional and unique benefit in mice who received MPTP. We are the first to show that, even following a nearly 50% decrease in the mean number of TH-labeled neurons/section in the SN-PC following MPTP, treadmill exercise leads to an increase of neurons in the SN-PC and improved motor behavior.
Keywords: tyrosine hydroxylase, dopamine, dopamine transporter, MPTP, Parkinson’s disease, exercise
1 INTRODUCTION
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is a systemic neurotoxin commonly used to produce loss of at least the dopamine (DA) input from the substantia nigra to the striatum. This also alters the glutamate input from the motor cortex to the striatum, consistent with the model of basal ganglia function described for Parkinson’s disease (Robinson, et al., 2003). The imbalance between DA and glutamate within the striatum plays an important role in the movement problems associated with Parkinson’s disease, and is known to be partially reversed following regular treadmill endurance exercise (Fisher, et al., 2004). Further studies and reviews have concluded that there are beneficial effects of exercise on motor control and balance in persons with Parkinson's disease, but the underlying molecular mechanisms responsible for these effects are not well understood (Dibble, et al., 2009, Fisher, et al., 2008, Petzinger, et al., 2007, Sasco, et al., 1992). In addition, it has been reported that forced versus voluntary exercise is more beneficial for people with Parkinson’s disease (Ridgel, et al., 2009), suggesting that more intense training may lead to more efficiency within the motor cortex, as measured by changes in regional cerebral blood flow-related tissue radioactivity in a recent animal study (Holschneider, et al., 2007).
In mice, treadmill exercise led to increased motor performance when the exercise was started 5 days after acute MPTP administration (i.e., 4 × 20 mg/kg every 2 hours), which allowed cell death to occur before exercise was initiated (Fisher, et al., 2004, Jackson-Lewis, et al., 1995). Results showed a downregulation of the DA transporter, a protein important in regulating dopamine uptake, and upregulation of the DA D2 receptor, a receptor important in motor behavior (Fisher, et al., 2004). Another study found no difference in striatal DA levels between MPTP-treated mice with or without exercise, but they did find increased striatal DA in saline-treated mice undergoing exercise (Petzinger, et al., 2007). Additionally, immunohistochemical staining for striatal tyrosine hydroxylase and DA transporter proteins demonstrated decreased expression in MPTP-treated mice that exercised as compared to MPTP-treated mice that did not exercise (Petzinger, et al., 2007). This suggests that the benefits of treadmill exercise, while beneficial for all mice, are different in MPTP-lesioned vs. non-MPTP-treated mice.
In addition to continued efforts to understand the neural mechanisms behind motor recovery with treadmill exercise in mice, there is interest in finding sensitive and task-specific measures of motor performance in MPTP-lesioned mice. Current tests include the rotarod (Petzinger, et al., 2007, Tillerson, et al., 2002), treadmill running speed and endurance (Fisher, et al., 2004), gait characteristics such as stride length (Amende, et al., 2005, Tillerson, et al., 2002, Tillerson, et al., 2003), stepping tests (Blume, et al., 2009), open field activity (Fredriksson, et al., 1999, Tillerson, et al., 2002, Tomac, et al., 1995), grid (Tillerson, et al., 2002, Tillerson, et al., 2003) and the grid hangtest (Tillerson, et al., 2002). Results from these tests are inconsistent, and although many of the tests show differences in performance between mice it is difficult to determine what aspect of motor performance they are testing, and/or the aspect of motor performance being tested does not match what is expected to change with training or lesioning (Guillot, et al., 2008, Kamens, et al., 2005, Meredith and Kang, 2006, Sedelis, et al., 2001).
The parallel rod activity chamber (PRAC) is a behavioral test that is particularly task-specific to treadmill exercise and able to detect subtle changes in gait (Kamens and Crabbe, 2007, Kamens, et al., 2005). As a result of the positive balance, strength and cardiovascular effects of treadmill exercise, we expect that mice will have improved gait ability and demonstrate greater amounts of overall physical activity following treadmill exercise. The PRAC quantifies the number of foot slips off of the separated parallel rods that create the floor and the level of spontaneous locomotor activity and has been used successfully to differentiate mice with ethanol impairment from healthy mice (Kamens and Crabbe, 2007, Kamens, et al., 2005). In this study we tested the hypothesis that the effects of MPTP on striatal DA terminal loss and TH- and thionin-labeled neurons in the SN-PC will be partially reversed by treadmill-based exercise, leading to increase of motor behavior as measured by the modified PRAC.
2 RESULTS
2.1 Behavioral Testing: Pre- vs. Post-Intervention
For spontaneous activity, the time by intervention interaction (F[1,35]= 22.96, p < 0.01, partial eta squared = 0.40) was significant, as was the group effect (F[1,35]= 6.62, p = 0.01, partial eta squared = 0.16). Post-hoc analyses revealed that the significant interaction was due to the fact that mice receiving exercise (i.e., both vehicle/exercise and MPTP/exercise; post-intervention) significantly increased activity following exercise (p < 0.01) while the non-exercising mice (i.e., both vehicle/no exercise and MPTP/no exercise; post-intervention) significantly decreased activity over the intervention period (p = 0.02) (see Figure 2).
Figure 2.
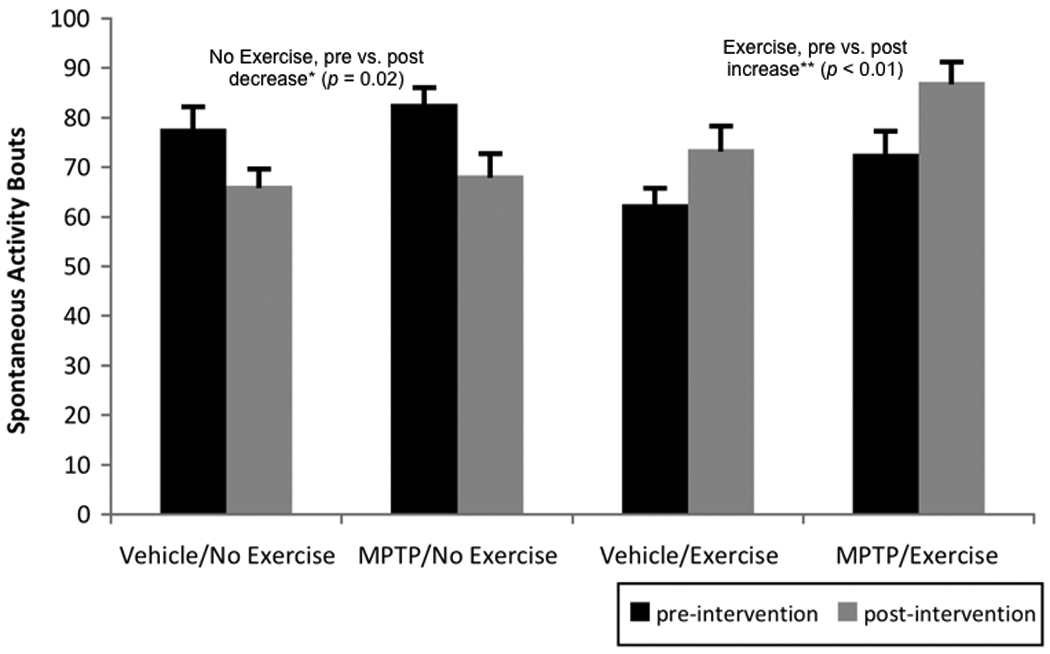
Pre- and post-intervention (i.e., before and after any exercise) spontaneous activity bouts (i.e., # beam breaks/5 minutes). Post-hoc analysis of the significant time by intervention interaction (p < 0.01) revealed that non-exercised mice became significantly less active over the time course of the intervention, while exercised mice became significantly more active. Values are means ± standard error of the mean. * = p < 0.05 and ** = p < 0.01.
For normalized foot faults, the time by intervention interaction was significant (F[1,35]= 6.22, p = 0.02, partial eta squared = 0.15). As shown in Figure 3, post-hoc analyses revealed that the significant interaction was due to the fact that the mice who exercised (i.e. post-intervention groups) significantly decreased normalized foot faults following exercise (p = 0.04) while the non-exercising mice did not significantly change number of normalized foot faults over the intervention period (p = 0.19).
Figure 3.
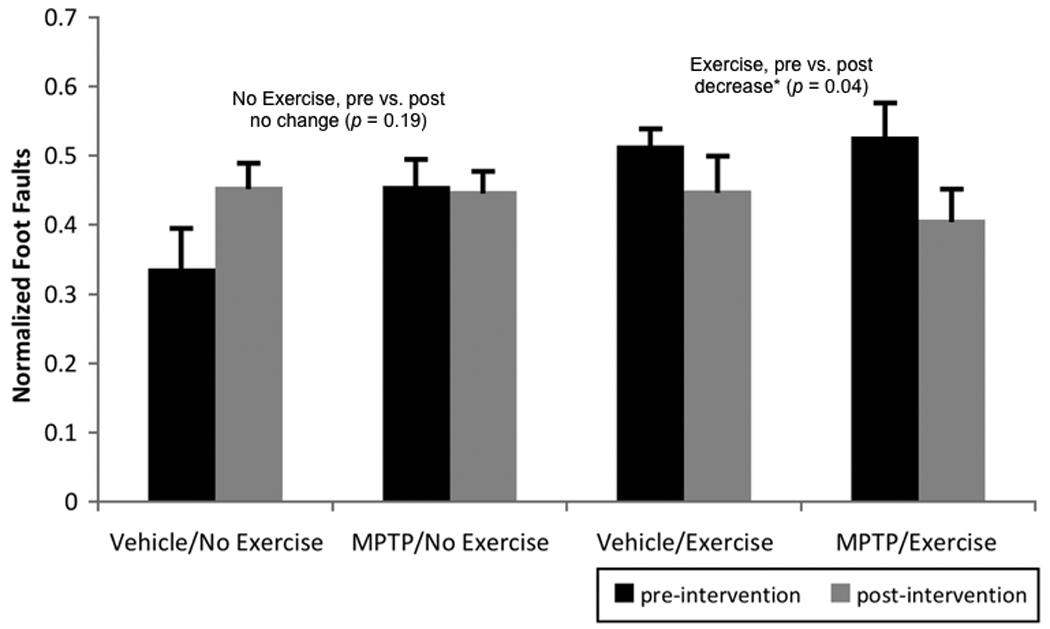
Pre- and post-intervention (i.e., before and after any exercise) foot faults, normalized to amount of activity (i.e., footfaults/beam breaks). Post-hoc analysis of the significant time by intervention interaction (p = 0.02) revealed that the mice who exercised significantly decreased normalized foot faults following exercise while the non-exercising mice did not significantly change the number of normalized foot faults over the intervention period. Values are means ± standard error of the mean. * = p < 0.05.
2.2 Immunohistochemistry and Nissl staining of the SN-PC
For TH-labeled neurons in the SN-PC (Figures 4a and 4b), the group by intervention interaction was significant (F[1,17]= 6.32, p = 0.02, partial eta squared = 0.27), as was the group effect (F[1,17]= 84.89, p < 0.01, partial eta squared = 0.84). Post-hoc analyses, as shown in Figure 4b, revealed that the MPTP mice significantly increased TH-labeled neurons 40% following exercise (p = 0.04), while vehicle mice were not significantly different following exercise (p = 0.36). MPTP mice had 50% less TH-labeled cells in the SN-PC/section as compared to the vehicle-treated mice. The decrease observed was not different between rostral, medial and caudal sections (data not shown), which is consistent with what we have previously reported using this method (Goldberg, et al., in press).
Figure 4.
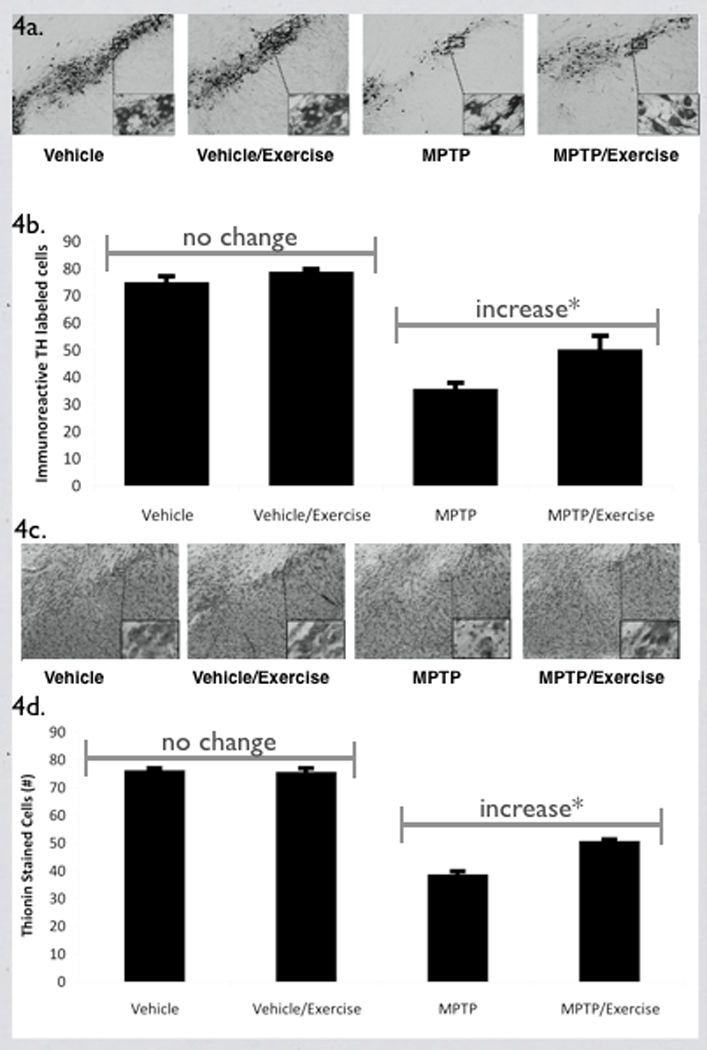
A) Example of TH-labeled neurons in the SN-PC for all four groups. Higher magnification of the SN-PC shows the TH-labeled cells in more detail in the inset. B) Effect of MPTP and exercise on the mean number of TH-labeled neurons/section in the SN-PC. The group by intervention interaction was significant (*p = 0.02) and post-hoc analysis showed that mean number of TH-labeled neurons/section in the vehicle-treated mice was not significantly different with and without exercise, while the MPTP mice with exercise had a significantly higher mean number of TH-labeled neurons/section compared to the MPTP mice without exercise. Values are the mean ± standard error of the mean. * = p < 0.05. C) Example of thionin-stained neurons in the SN-PC. Higher magnification of the SN-PC show the thionin-stained cells in more detail in the inset. D) Quantification of the effect of exercise following MPTP administration in the number of thionin-stained cells in the SN-PC. The group by intervention interaction was significant (*p = 0.02) and post-hoc analysis showed that the mean number of thionin-stained neurons/section in the vehicle-treated mice was not significantly different with and without exercise, while the MPTP mice with exercise had a significantly higher mean number of thionin-stained SN-PC neurons/section compared to the MPTP mice without exercise. Values are means ± standard error of the mean. . * = p < 0.05.
For thionin-stained cells in the SN-PC (Figures 4c and 4d), the group by intervention interaction was significant (F[1,13]= 7.77, p = 0.02, partial eta squared = 0.37). The group (F[1,13]= 292.90, p < 0.01, partial eta squared = 0.96) and intervention (F[1,15]= 6.71, p = 0.02, partial eta squared = 0.31) effects were also significant (Figure 4d). Post-hoc analyses revealed that the mean number of thionin-stained neurons/section in the vehicle-treated mice was not significantly different with and without exercise (p = 0.70), while the MPTP mice with exercise had a significant 31% increase in thionin-stained SN-PC neurons/section compared to the MPTP mice without exercise (p = 0.01; see Figure 4d). Of note, there was nearly a 50% decrease in the mean number of thionin-stained neurons/section in the MPTP compared to the vehicle group.
2.3 Western Immunoblotting
Immunoblots for striatal TH and DAT are illustrated in Figure 5a. For statistical testing with striatal TH as the dependent variable, the group effect was significant (F[1,15]= 24.13, p < 0.01, partial eta squared = 0.62), as was the intervention effect (F[1,15]= 6.71, p = 0.02, partial eta squared = 0.31). Follow up inspection of the means revealed that the MPTP-treated group had significantly less TH protein expression (38% less) in the striatum as compared to the vehicle-treated group. In addition, the no-exercise group had significantly less TH protein expression in the striatum as compared to the exercise mice (see Figure 5b). MPTP/exercise mice had a 32% increase in striatal TH compared to the MPTP/no exercise group, while vehicle/exercise mice had a 25% increase in striatal TH compared to the vehicle/no exercise group.
Figure 5.
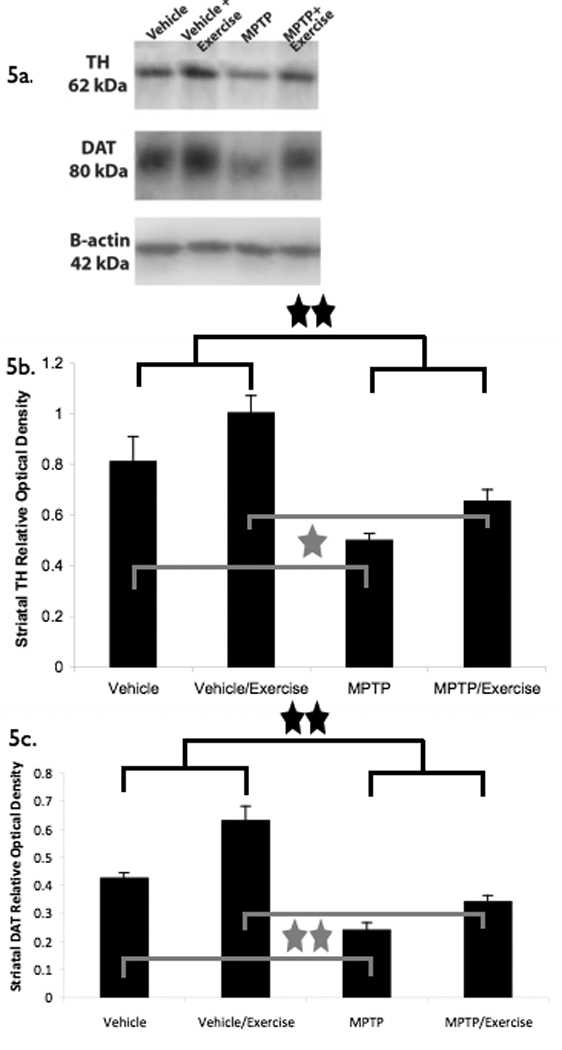
Effect of MPTP and exercise on striatal protein expression for TH and DAT. A.) Western immunoblots for striatal TH, DAT, and actin. B.) For TH protein, group and intervention effects were significant. MPTP led to significantly less TH expression in the striatum as compared to the vehicle-treated group (p < 0.01, MPTP vs. vehicle). In addition, the no-exercise group had significantly less TH protein expression in the striatum as compared to the exercise mice (p = 0.02, exercise vs. no exercise). * = p < 0.05 and ** = p < 0.01. C.) For DAT protein, group and intervention effects were significant. MPTP led to significantly less DAT expression in the striatum as compared to the vehicle-treated group (p < 0.01, MPTP vs. vehicle). In addition, the no-exercise group had significantly less DAT protein expression in the striatum as compared to the exercise mice (p < 0.01, exercise vs. no exercise). 10 µg of each sample was loaded. Values are means of the relative optical density ± the standard error of the mean and were normalized against β-actin protein in their respective groups. * = p < 0.05 and ** = p < 0.01.
With striatal DAT as the dependent variable, there was a significant group effect (F[1,15]= 57.03, p < 0.01, partial eta squared = 0.79) and intervention effect (F[1,15]= 23.56, p < 0.01, partial eta squared = 0.61). Follow up inspection of the means revealed that there was significantly less DAT protein expression (44% less) in the striatum of the MPTP group as compared to the vehicle group. In addition, the no-exercise group had significantly less DAT protein expression in the striatum as compared to the exercised mice (see Figure 5c). MPTP/exercise mice had a 42% increase in striatal DAT compared to the MPTP/no exercise group, while the vehicle/exercise group had a 47% increase in striatal DAT compared to the vehicle/no exercise group.
3 DISCUSSION
Our study shows that treadmill exercise is effective in increasing gait performance (i.e., footfaults) and amount of physical activity. We are the first to report a partial increase of both TH and DAT protein following exercise in pre-treated MPTP mice. We have previously reported that there is a 50% decrease in the mean number of TH-labeled and a 40% decrease in the mean number of thionin-stained neurons/section in the SN-PC in mice following 7 days of MPTP treatment (30 mg/kg)(i.e. day 8) (Schang, 2010). In the current study, exercise was initiated on day 8, with no further treatment with MPTP. Exercise was effective for both the mice who received MPTP and those who did not in terms of decreasing footfaults/beam breaks and increasing spontaneous activity. Further, the effects of early treadmill-based intervention appears to be unique in mice who received MPTP, since we are the first to report that treadmill exercise led to an increase in thionin-stained cells in SN-PC. The percent decrease of both TH-labeled and thionin-stained neurons in the SN-PC following MPTP was about 50%, with exercise leading to either a 40% or 31% increase in the mean number of SN-PC neurons/section, respectively.
The 50% decrease in the mean number of TH-labeled and thionin-stained cells/section in the SN-PC following MPTP would be considered a moderate lesion compared to the 60% to 70% loss of dopamine neurons as seen in patients with clinically-apparent Parkinson’s disease (Bernheimer, et al., 1973, Mori, et al., 2006). With a severe loss of TH-labeled neurons (> 60%), exercise does not result in an increase in the number of dopamine neurons (Al-Jarrah, et al., 2007, Fisher, et al., 2004, Petzinger, et al., 2007, Pothakos, et al., 2009), although exercise does promote locomotor recovery. Even in the non-lesioned groups, exercise resulted in an increase in both TH and DAT protein levels and improvement in motor function. It is possible that in the MPTP-treated group, the increase in motor function may not be directly related to changes in striatal dopamine markers. With the more severe loss of dopamine neurons, functional recovery is most likely due to changes in other neurotransmitter systems, such as glutamate (Fisher, et al., 2004). We reported that acute MPTP administration resulted in an increased density of nerve terminal glutamate immuno-gold labeling within the striatum and treadmill exercise reversed levels back to baseline (Fisher, et al., 2004). One possibility is that 60% loss of dopamine neurons represents a point at which dopamine terminal sprouting is no longer possible (Finkelstein, et al., 2000) and exercise can no longer induce an increase in dopamine cells. In the current study we used a moderate loss of dopamine cells to determine if exercise could reverse this effect.
Although PD is generally considered a disease of aging, studying the effects of exercise in an MPTP model using younger rodents with a moderate lesion equates to studying humans who are most likely mildly symptomatic and undiagnosed. There has been recent progress in identifying pre-motor symptoms in patients who eventually develop the classical motor deficits. Some of these pre-motor indications can develop as early as 20 years prior to the manifestations of the motor signs (Hawkes, et al., 2009). For this reason we studied the effects of exercise on reversing dopamine cell loss in young adult mice with moderate lesions.
3.1 Effect of exercise on motor behavior
Exercise led to increased levels of spontaneous activity in both MPTP- and vehicle-treated mice. Although the exercised mice were more active, they did not show a proportional increase in foot faults. The number of foot faults, normalized to the level of activity, actually decreased in mice who exercised as compared to those who did not, suggesting exercise improved their motor performance.
We recognize that following sub-acute administration of MPTP there is an approximately 50% decrease in TH-labeled neurons/section in the SN-PC (Schang, 2010), below the 60%–70% loss of TH-labeled neurons seen in patients with Parkinson’s disease who demonstrate typical, clinically-apparent impaired motor function (Bernheimer, et al., 1973, Mori, et al., 2006). The PRAC, however, is a sensitive measure of motor activity and we expected it would be able to measure subtle deficits in motor activity due to MPTP and exercise. Other studies, using less sensitive outcome measures, have had mixed outcomes in observing behavioral changes. Studies using high dose acute MPTP and subchronic MPTP/probenecid models show a greater loss of TH-labeled neurons compared to what we observed and a significant decrease in rotorod performance and an increase in the number of footfaults (Meredith, et al., 2009, Petroske, et al., 2001, Tillerson and Miller, 2003). Following a 6-OHDA lesion of the medial forebrain bundle, resulting in a nearly complete loss of striatal dopamine, it was reported that subsequent treadmill exercise started soon after the lesion results in neurochemical recovery of striatal dopamine but no motor recovery (Poulton and Muir, 2005). Just the opposite was reported following intrastriatal injections of 6-OHDA, in which there was behavioral recovery but no rescuing of the SN-PC dopamine neurons (O'Dell, et al., 2007).
Using the PRAC we were able to measure differences in motor performance between the exercise and non-exercise groups, but not between the MPTP and vehicle groups. It is possible that the novelty of the environment encouraged the MPTP mice to be more active than they otherwise would have. This finding could also be attributed to the fact that exercise had a much larger effect on physical performance of the mice than MPTP did. The PRAC appears to be a good outcome measure for intervention or lesions that are expected to affect gait performance and overall physical activity levels as it represents a task-specific match between training or lesions and the outcome. In fact, lower activity levels are associated with Parkinson’s disease; persons recently diagnosed with Parkinson’s disease have lower physical activity levels than their healthy peers (Fertl, et al., 1993, Thacker, et al., 2008).
3.2 Effect of MPTP on Tyrosine Hydroxylase Labeled and Thionin-stained Neurons
We may have underestimated the mean number of TH-labeled and thionin-stained neurons/section in the SN-PC due to our immunohistochemical methodology. We analyzed sections throughout the entire rostral/caudal extent of the SN-PC and believe this technique is appropriate as it has been reported that there is not a statistical distinction between a 2 or 3-dimentional approach for determining differences in the density of dopamine neurons within the SN-PC (Baquet, et al., 2009, Benes and Lange, 2001). In addition, we applied the Abercrombie algorithm to correct for fragmented nuclei and tissue thickness (Clarke, 1992, Smolen, et al., 1983).
In regard to the differential effects of exercise in MPTP vs. vehicle group, we did find a difference in the mean number of thionin-stained cells/section in the SN-PC. Vehicle-treated mice with and without exercise were not different, while the MPTP mice exposed to exercise had significantly more thionin-stained cells/section in the SN-PC compared to MPTP/no exercise mice. Our reported increase in TH-labeled and thionin-stained cells is in contrast to that reported by others (Al-Jarrah, et al., 2007, Fisher, et al., 2004, Petzinger, et al., 2007, Pothakos, et al., 2009). In those studies, the MPTP was given either acutely (20 mg/kg × 4 doses every 2 hours) or subchronically using probencid (25 mg/kg every 3 days + Probenecid). In each of these protocols there is a much higher loss of TH-labeled cells compared to the current study.
As opposed to studying the neuroprotective effects of exercise, we tested the effects of exercise on neuronal recovery following a moderate lesion. As a neuroprotective effect, it has been reported that running for 3 months prior to acute MPTP administration was partially protective of TH cell loss (Gerecke, et al., 2010). In terms of recovery, exercise two weeks after intrastriatal administration of 6-hydroxydopamine (6-OHDA) resulted in partial recovery of TH labeling and axonal fiber projections to the striatum (Yoon, et al., 2007). The loss of TH labeled SN-PC neurons was similar to the findings in our current study. Additionally, voluntary exercise following a partial lesion (i.e. 70% loss) of the nigrostriatal pathway through a low dose of 6-OHDA into the medial forebrain resulted in about a 30% recovery of TH-labeled neurons (Mabandla and Russell, 2010).
In the current study, we do not use a progressive animal model of Parkinson’s disease; levels of TH-labeled neurons do not continue to decrease during the exercise intervention period. We have reported that after the last dose of MPTP (on Day 8, when exercise is started), there is a 50% decrease in the mean number of TH-labeled neurons/section and there is no further decrease in TH-labeled neurons in the SN-PC for the following 3 weeks (Schang, 2010). Therefore, the effect of exercise in increasing the mean number of TH labeled and thionin-stained neurons/section in the SN-PC is likely not due to neuroprotection. In addition, the increase in TH-labeled and thionin-stained neurons following exercise is not due to stress. We have preliminary data that if animals are stressed just after administration of MPTP, there is an additional decline in the mean number of TH-labeled cells/section in the SN-PC (Goldberg and Meshul, unpublished findings). Therefore, if treadmill exercise were stressful to the mice we would have seen a decrease in TH-labeled or thionin-stained neurons in the SN-PC.
Overall, there is a loss of thionin-stained neurons in the SN-PC following administration of MPTP, and exercise results in some increase. It is not clear whether this subacute dosing regimen for MPTP leads to cell damage or actual cell loss. Because we used thionin to stain alternate SN-PC tissue sections and did not counter-stain with thionin or cresyl violet, it is possible that not all of the thionin-stained neurons were accounted for by TH-expressing cells. It has been reported that a sub-population of SN-PC neurons are gamma-aminobutyric acid (GABA)-ergic (Nagai, et al., 1983, Nair-Roberts, et al., 2008), which suggests that the changes in the mean number of thionin-stained neurons/section may have been due to changes in GABA neurons. Further, it has been suggested that the decrease in immunoreactive neurons in the SN-PC could be due to either dysfunction or degeneration in MPTP models of Parkinson’s disease (Jackson-Lewis, et al., 1995, Unal-Cevik, et al., 2004). Our results support degeneration, as we found that a nearly identical partial increase (50%) in the mean number of TH-labeled and thionin-stained cells/section in the SN-PC was associated with a similar increase in both DAT (42%) and TH (32%) striatal protein expression. If the neurons were merely dysfunctional and could be rescued following exercise, we would have expected to find many more thionin-stained neurons in the MPTP only group. Since this was not the case, it appears that there must be another mechanism behind the exercised-induced increase in both TH and thionin cells.
Although it is well established that exercise enhances adult hippocampal synaptogenesis and neurogenesis (van Praag, et al., 1999, van Praag, et al., 1999), there is little evidence that a partial lesion of the nigrostriatal pathway results in neurogenesis in the SN-PC (Aponso, et al., 2008, Frielingsdorf, et al., 2004, Peng, et al., 2008). There is only one report of neurogenesis in the SN-PC following exposure to an enriched environment in animals previously injected with 6-OHDA into the striatum (Steiner, et al., 2006), which has not been replicated by others. Although we have no evidence to support that neurogenesis occurs in the SN-PC, our results suggest this is a possible mechanism. There is also the potential that neurons may be migrating from the subventricular zone to the SN-PC. Additionally, it has been suggested that new nigrostriatal DA neurons observed in MPTP-treated macaques may result from a phenotypic shift of GABAergic interneurons (Tande, et al., 2006). In future studies we will focus on labeling neurons to differentiate these possibilities.
3.3 Effect of MPTP on the relative striatal protein levels of TH and DAT
Exercise led to higher protein expression levels of TH and DAT in the striatum compared to the non-exercise groups, perhaps due the to sprouting of new dopamine terminals. The increase in TH and DAT protein expression that we observed is in contrast to previous reports that found exercise results in a decrease in DAT striatal protein and no change in TH protein levels compared to the non-exercise group (Fisher, et al., 2004, Petzinger, et al., 2007). In our current study, mice were exercised continuously for 60 minutes. Fisher et al (2004) exercised their animals for a similar length of time but in two 30-minute sessions, with 30 minutes of rest between the two sessions. It is possible that the treadmill exercise in the current study was more rigorous, resulting in an increase in both TH and DAT protein (Fisher, et al., 2004, Petzinger, et al., 2007). Although an increase in DAT protein could conceivably have an effect on extracellular dopamine levels, the fact we observed an increase in TH protein suggests this might help offset the decrease in dopamine levels following MPTP. Indeed, in the MPTP/exercise mice, both TH and DAT protein expression is increased compared to the MPTP only group. Other differences that could have led to our different results were MPTP administration and time of exercise. In the current study MPTP was administered daily for 7 days and mice exercised for 15 days, in contrast to the high dose acute administration (i.e. 20 mg/kg × 4 doses every 2 hours) and 30 days of exercise reported by others (Fisher, et al., 2004, Petzinger, et al., 2007). It is also possible that with longer-term exercise DAT levels might decrease, in contrast to the increase observed in the current short-term study. Although we analyzed TH and DAT protein only within the striatum, we are aware that alterations in DA levels in the SN play an important role in terms of modulating locomotor activity. Using an aging model and investigating alterations in DA levels in the SN, it was recently reported that locomotor activity correlated with the DA content only in the SN and not the striatum (Salvatore, et al., 2009). However, whether such a correlation exists following the loss of DA neurons in the SN-PC is yet to be determined.
4 METHODS AND MATERIALS
4.1 Animals
Forty young adult (8 –10 weeks old) male C57BL/6J mice were obtained from Jackson Laboratory (Bar Harbor, ME). Animals were housed four to a cage and acclimated to a 12-hr shift in light/dark cycle. They had ad libitum access to food and water. Handling and care of mice was consistent with federal guidelines of the Public Health Service Policy on the Humane Care and Use of Laboratory Animals and protocols were approved by the Portland VA IACUC. Mice were randomized into four groups: 10 mice in the vehicle /no exercise group, 10 mice in the vehicle/exercise group, 10 mice in the MPTP/exercise group and 10 mice in the MPTP/no exercise group. The protocol was as follows:
4.2 Protocol
Days 1–7, at 7–8 am: Injected 20 mice with MPTP (Sigma-Aldrich, St. Louis, MO) dissolved in normal saline (0.9% sodium chloride) by intraperitoneal injection at a dose of 30 mg/kg (as the base; 0.1 mls/10 grams body weight). The other 20 mice received a similar dose of normal saline (0.2 mls) only.
Day 8: Measured post-injection/pre-intervention behavioral measures (spontaneous activity and foot faults, see Behavioral Testing (4.3) below).
Days 8–27: Ten of the MPTP mice and 10 of the control mice exercised on a treadmill for 1 hour per day at a treadmill speed of 18 cm/s, 5 days per week (Fisher, et al., 2004, Guillot, et al., 2008). The treadmill (Weslo brand) had 6 lanes created by a clear acrylic plastic divider. Each lane was 13 cm wide and 38 cm long and contained 2 mice.
Day 28: Measured post-intervention behavioral measures (spontaneous activity and foot faults).
Day 29: Euthanized mice for Immunohistochemistry/thionin staining, Western Immunoblotting.
4.3 Behavioral Testing: Foot-fault and Spontaneous Activity Tests
The Parallel Rod Activity Chamber (PRAC) consists of four clear acrylic plastic boxes (15×15×20-cm each) with four photocell beams to measure movement of the mouse (see Figure 1A). The steel rods that make up the floor are 1.6 mm in diameter, separated from each other by 6 mm. A stainless steel plate is set 1 cm below the rod floor. When the paw of the mouse slips through the parallel rods and contacts the metal plate, a circuit is closed and an error recorded by the computer. The sensitivity of the software was designed such that slips of the tail or feces through the rods are not recorded, nor does it distinguish between forepaw and hindpaw slips (see Figure 1B). A significant bout of activity was defined as a move from one quadrant of the cage to another breaking the beam (Kamens and Crabbe, 2007, Kamens, et al., 2005). Mice were acclimated to the PRAC for 5 minutes before foot faults and spontaneous activity bouts were recorded for 5 minutes. This allowed us to assess an active and reliable period for all mice (Crabbe, et al., 2003, Kamens and Crabbe, 2007). For foot fault results and statistics, the absolute number of foot faults was expressed relative to spontaneous activity count for the same period, to provide a normalized measure of foot faults per unit of spontaneous activity. This allowed us to evaluate whether changes in foot faults were due to changes in total activity or to changes in motor control (Kamens, et al., 2005).
Figure 1.


The modified parallel rods activity chamber. A) shows a view of the device from above. There are four laser beams, creating four quadrants in the device. Mice typically block two lasers at any point in time, although it is possible to block only one. The mouse in the figure is blocking two beams, the top one on the left side and the left one on the bottom of the photograph. B) demonstrates a foot fault. The mouse’s rear paw has slipped off of the rod and made contact with the floor, which is counted as a foot fault. The tail is also in contact with the floor, but the system does not count that as a foot fault. Only paw contacts are counted as footfaults.
4.4 Immunohistochemistry
One day following the last day of exercise, 5 mice per group were anesthetized with 1% ketamine/0.1% mg xylazine (2.0ml/0.1kg, I.P; Butler Schein, Dublin, OH USA), and euthanized by transcardial perfusion with 2% paraformaldehyde, 1% acrolein in 0.1M phosphate buffer (pH 7.4). Brains were removed and washed for 24 h in 0.1 M phosphate buffer, then the entire rostral/caudal extent of the substantia nigra (SN-PC) cut into 70 µm thick sections (Bregma −2.92 through −3.52; Paxinos and Franklin 2004) using a vibratome (Ted Pella Inc., Redding, CA). Two alternate sections from the rostral, middle and caudal SN-PC were used for TH immunohistochemistry or thionin staining. Free-floating sections were pre-incubated with 10 mM sodium citrate (pH 6) for 5 minutes using a microwave oven [PELCO BioWave® Pro microwave (Ted Pella Inc.)] at 500 Watts for antigen retrieval. Sections were incubated with 1% sodium borohydride for 30 min, then for 1 h in blocking solution [10% goat serum/0.5% Triton-X 100/ 0.1 M phosphate buffer, pH 7.4] at room temperature, followed by anti-tyrosine hydroxylase antibody (monoclonal, 1:20,000; Diasorin, Stillwater, MN) at 4°C overnight. The following incubations were carried out in the PELCO microwave: sections were washed for 2 min in 0.1M phosphate buffer at 150 Watts, incubated with biotinylated goat anti-mouse 2° antibody at 200 Watts (1:250; Vector, Burlingame, CA) for 12 minutes under cycled vacuum, and with avidin-biotin complex solution at 150 Watts (ABC; diluted according to manufacturer instruction; Vector) for 12 minutes under cycled vacuum. Sections were then incubated with diaminobenzidine (DAB kit with nickel enhancement, Vector) for 3 minutes. Sections were mounted on gel-coated slides and cover-slipped in Pro-Texx® medium (Lerner Laboratories, Pittsburgh, PA). Alternate sections for thionin staining were also mounted on slides, let to dehydrate at room temperature over night. Tissue sections were then stained in 0.1% thionin for 1 minute; followed by 1-minute washes in 50%, 70% and 100% ethanol, sequentially. Finally, slides were incubated in xylene for two consecutive 1-minute intervals and then coverslipped (Electron Microscopic Sciences, Fort Washington, PA).
4.5 Cell Counting Analysis
TH-labeled neurons at the surface of immunolabeled SN-PC tissue were counted using light microscopy (40× magnification). The number of TH-labeled neurons from the left and right SN-PC sections was averaged for each animal for all six sections analyzed since both sides of the SN-PC are affected following systemic administration of this neurotoxin. The same procedure was carried out for six sections from each animal. A mean was determined for each mouse, and then a grand mean calculated for all 5 animals and expressed as the mean number of TH-labeled SN-PC neurons/section. The mean number of TH-labeled neurons/section was used because approximating the total number of neurons in the SN-PC would require stereological methods. Based on recent findings, a 2-dimensional approached was used in terms of cell counting (Baquet, et al., 2009, Benes and Lange, 2001). This same cell-counting procedure was carried out on the alternate thionin-stained SN-PC sections. The SN-PC was defined in TH-labeled sections by the dense strip of large DAB-stained neurons that were lateral to the VTA. Neither TH-labeled nor thionin-stained neurons displayed features of necrosis, such as pyknotic-looking cells. TH-labeled neurons in the substantia nigra pars reticulata (SN-R) were not counted. This area was mapped onto digital images of thionin-stained sections in order to count neurons localized to the SN-PC. For thionin-stained sections, only larger cells with diameter in the range of TH-labeled cells were counted. In order to correct for fragmented nuclei and tissue thickness exceeding average soma diameter by 50%, the Abercrombie algorithm was applied to neuron counts (Clarke, 1992, Smolen, et al., 1983).
4.6 Western Immunoblotting
Mice (n = 5) were euthanized by cervical dislocation, and the dorsolateral striatum from both the left and right sides were dissected and frozen at −80°C. Protein was extracted from the tissue by sonication in lysis buffer [1 M Tris/ 0.5 M EDTA/1% Triton-X 100/0.5% Protease Inhibitor Cocktail III (Calbiochem)]. Protein concentrations were measured using the BCA Protein Assay Kit (Thermo Scientific), and 10µg of each sample was loaded into wells. Samples were mixed with β-mercaptoethanol Laemmli Buffer (1:1; Sigma, St. Louis, MO) and electrophoresed on a 7% Tris-HCL polyacrylamide gel (Bio-Rad Laboratories, Hercules, CA USA). Separated proteins were transferred to a nitrocellulose membrane, which was blocked in 5% non-fat dry milk in Tris-buffered saline with Tween-20 for 45 minutes. Membranes were then probed with primary antibodies for TH (monoclonal, 1:40,000; Diasorin, Stillwater, MN), dopamine transporter (DAT; Chemicon, 1:6000, rat monoclonal) or β-actin (1:6500, mouse monoclonal, Sigma Chemical Co, St Louis, MO). Secondary antibodies were goat-anti-mouse and goat-anti-rat, respectively. Visualization of the antigen-antibody binding density was performed using Omega 12iC Molecular Imaging System (Ultra Lum, Inc.). The optical density of the protein bands was quantified encompassing the entire band (ImagePro 6.3, Media Cybernetics) in unaltered scanning images, and the relative optical density of either TH (band at 62 kDa) or DAT (at ~80 kDa) was normalized to β-actin (band at ~42 kDa; detected on the same membrane as TH and DAT), and presented as a ratio. Each DAT band was analyzed as a single wider band as typically seen in DAT tissue samples (Chu et al 2008; Salahpour et al 2008). The normalized optical density was determined for each animal and a grand mean calculated by experimental group.
4.7 Statistics
We used SPSS version 17.0 for all statistical analysis with an alpha level set at 0.05. Analyses of variance (ANOVA) were used to compare groups, as further described below. Post-hoc ANOVAs were used to follow up on significant interaction effects. We tested for an effect of MPTP (group effect), an effect of any treadmill exercise (intervention effect) and an interaction between MPTP and exercise (group by intervention interaction). A significant group effect indicates that all mice who received MPTP (regardless of whether they exercised or not) were different than mice who received vehicle (regardless of whether they exercised or not). A significant exercise effect indicates that all mice who received exercise (regardless of whether they were MPTP or vehicle) were different than mice who did not exercise (regardless of whether they were MPTP or vehicle). A significant interaction indicates that the mice who received MPTP responded differently to the intervention than mice who received vehicle. We hypothesized that we would find a significant interaction effect, indicating exercise had a larger positive effect in mice who received MPTP than in mice who received vehicle.
We used separate ANOVA with repeated measures on time (pre to post exercise) to look for group differences in behavioral measures, with normalized foot fault and spontaneous activity bouts as the dependent variables. Measurements from day 8 (after injection/before any exercise) and day 28 (following any exercise) were compared to look for an effect of MPTP administration (group effect), an effect of any treadmill exercise (intervention effect) and an interaction between MPTP and exercise (group by intervention interaction).
Separate ANOVA were used to look for group differences in TH and thionin cell counts of the SN-PC and protein optical density for TH and DAT. We tested for an effect of MPTP administration (group effect), an effect of treadmill exercise (intervention effect) and an interaction between MPTP and exercise (group by intervention interaction).
5 CONCLUSIONS
Treadmill exercise was beneficial to both MPTP and vehicle groups, leading to increased physical activity and improved gait performance (i.e., less foot faults). In addition, we are the first to report that in mice receiving MPTP, treadmill exercise led to an increase of TH-labeled and thionin-stained cells in SN-PC. Treadmill exercise also led to higher expression of striatal TH and DAT levels in mice who exercised.
Exercise has been shown to improve motor control and balance in persons with Parkinson's disease (Dibble, et al., 2009, Fisher, et al., 2008, Sasco, et al., 1992). Our study provides insight into the neural basis of this benefit. In the current MPTP mouse model of Parkinson’s disease, exercise is associated with increased striatal TH and DAT protein expression and partial rescuing of DA neurons in the SN-PC, perhaps due to formation or migration of DA neurons. These data suggest that exercise in individuals showing mild or very early symptoms of Parkinson’s disease may result in partial recovery of dopamine neurons and potentially delay the progression of the disease, however this needs to be tested in a progressive rodent model of Parkinson’s disease.
ACKNOWLEDGEMENTS
None.
Funding Sources:
Beth A. Smith is currently supported on NIA 2T32AG023477-06 (H.Urbanski, PI). This work was further supported by the Department of Veterans Affairs Merit Review Program to Charles K. Meshul.
The funding sources provided funds only and did not have any role in the research.
Abbreviations used
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- DA
dopamine
- DAT
dopamine transporter
- TH
tyrosine hydroxylase
- SN-PC
substantia nigra pars compacta
- PRAC
parallel rod activity chamber
- ANOVA
analysis of variance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Al-Jarrah M, Pothakos K, Novikova L, Smirnova IV, Kurz MJ, Stehno-Bittel L, Lau YS. Endurance exercise promotes cardiorespiratory rehabilitation without neurorestoration in the chronic mouse model of parkinsonism with severe neurodegeneration. Neuroscience. 2007;149:28–37. doi: 10.1016/j.neuroscience.2007.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amende I, Kale A, McCue S, Glazier S, Morgan JP, Hampton TG. Gait dynamics in mouse models of Parkinson's disease and Huntington's disease. J Neuroeng Rehabil. 2005;2:20. doi: 10.1186/1743-0003-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aponso PM, Faull RL, Connor B. Increased progenitor cell proliferation and astrogenesis in the partial progressive 6-hydroxydopamine model of Parkinson's disease. Neuroscience. 2008;151:1142–1153. doi: 10.1016/j.neuroscience.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 4.Baquet ZC, Williams D, Brody J, Smeyne RJ. A comparison of model-based (2D) and design-based (3D) stereological methods for estimating cell number in the substantia nigra pars compacta (SNpc) of the C57BL/6J mouse. Neuroscience. 2009;161:1082–1090. doi: 10.1016/j.neuroscience.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benes FM, Lange N. Two-dimensional versus three-dimensional cell counting: a practical perspective. Trends Neurosci. 2001;24:11–17. doi: 10.1016/s0166-2236(00)01660-x. [DOI] [PubMed] [Google Scholar]
- 6.Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- 7.Blume SR, Cass DK, Tseng KY. Stepping test in mice: A reliable approach in determining forelimb akinesia in MPTP-induced Parkinsonism. Exp Neurol. 2009;19:19. doi: 10.1016/j.expneurol.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Clarke PG. How inaccurate is the Abercrombie correction factor for cell counts? Trends Neurosci. 1992;15:211–212. doi: 10.1016/0166-2236(92)90036-8. [DOI] [PubMed] [Google Scholar]
- 9.Crabbe JC, Metten P, Yu CH, Schlumbohm JP, Cameron AJ, Wahlsten D. Genotypic differences in ethanol sensitivity in two tests of motor incoordination. J Appl Physiol. 2003;95:1338–1351. doi: 10.1152/japplphysiol.00132.2003. [DOI] [PubMed] [Google Scholar]
- 10.Dibble LE, Addison O, Papa E. The effects of exercise on balance in persons with Parkinson's disease: a systematic review across the disability spectrum. J Neurol Phys Ther. 2009;33:14–26. doi: 10.1097/NPT.0b013e3181990fcc. [DOI] [PubMed] [Google Scholar]
- 11.Fertl E, Doppelbauer A, Auff E. Physical activity and sports in patients suffering from Parkinson's disease in comparison with healthy seniors. J Neural Transm Park Dis Dement Sect. 1993;5:157–161. doi: 10.1007/BF02251206. [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein DI, Stanic D, Parish CL, Tomas D, Dickson K, Horne MK. Axonal sprouting following lesions of the rat substantia nigra. Neuroscience. 2000;97:99–112. doi: 10.1016/s0306-4522(00)00009-9. [DOI] [PubMed] [Google Scholar]
- 13.Fisher BE, Petzinger GM, Nixon K, Hogg E, Bremmer S, Meshul CK, Jakowec MW. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. J Neurosci Res. 2004;77:378–390. doi: 10.1002/jnr.20162. [DOI] [PubMed] [Google Scholar]
- 14.Fisher BE, Wu AD, Salem GJ, Song J, Lin CH, Yip J, Cen S, Gordon J, Jakowec M, Petzinger G. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson's disease. Arch Phys Med Rehabil. 2008;89:1221–1229. doi: 10.1016/j.apmr.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fredriksson A, Palomo T, Chase T, Archer T. Tolerance to a suprathreshold dose of L-Dopa in MPTP mice: effects of glutamate antagonists. J Neural Transm. 1999;106:283–300. doi: 10.1007/s007020050158. [DOI] [PubMed] [Google Scholar]
- 16.Frielingsdorf H, Schwarz K, Brundin P, Mohapel P. No evidence for new dopaminergic neurons in the adult mammalian substantia nigra. Proc Natl Acad Sci U S A. 2004;101:10177–10182. doi: 10.1073/pnas.0401229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerecke KM, Jiao Y, Pani A, Pagala V, Smeyne RJ. Exercise protects against MPTP-induced neurotoxicity in mice. Brain Res. 2010;1341:72–83. doi: 10.1016/j.brainres.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberg NRS, Haack AK, Meshul CK. Enriched environment promotes similar neuronal and behavioral recovery in a young and aged mouse model of Parkinson's disease. Neuroscience. 2010:8. doi: 10.1016/j.neuroscience.2010.09.062. in press. [DOI] [PubMed] [Google Scholar]
- 19.Guillot TS, Asress SA, Richardson JR, Glass JD, Miller GW. Treadmill gait analysis does not detect motor deficits in animal models of Parkinson's disease or amyotrophic lateral sclerosis. J Mot Behav. 2008;40:568–577. doi: 10.3200/JMBR.40.6.568-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawkes CH, Del Tredici K, Braak H. A timeline for Parkinson's disease. Parkinsonism Relat Disord. 2009;16:79–84. doi: 10.1016/j.parkreldis.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Holschneider DP, Yang J, Guo Y, Maarek JM. Reorganization of functional brain maps after exercise training: Importance of cerebellar-thalamic-cortical pathway. Brain Res. 2007;1184:96–107. doi: 10.1016/j.brainres.2007.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995;4:257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 23.Kamens HM, Crabbe JC. The parallel rod floor test: a measure of ataxia in mice. Nat Protoc. 2007;2:277–281. doi: 10.1038/nprot.2007.19. [DOI] [PubMed] [Google Scholar]
- 24.Kamens HM, Phillips TJ, Holstein SE, Crabbe JC. Characterization of the parallel rod floor apparatus to test motor incoordination in mice. Genes Brain Behav. 2005;4:253–266. doi: 10.1111/j.1601-183X.2004.00100.x. [DOI] [PubMed] [Google Scholar]
- 25.Mabandla MV, Russell VA. Voluntary exercise reduces the neurotoxic effects of 6-hydroxydopamine in maternally separated rats. Behav Brain Res. 2010;211:16–22. doi: 10.1016/j.bbr.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meredith GE, Kang UJ. Behavioral models of Parkinson's disease in rodents: a new look at an old problem. Mov Disord. 2006;21:1595–1606. doi: 10.1002/mds.21010. [DOI] [PubMed] [Google Scholar]
- 27.Meredith GE, Totterdell S, Beales M, Meshul CK. Impaired glutamate homeostasis and programmed cell death in a chronic MPTP mouse model of Parkinson's disease. Exp Neurol. 2009;219:334–340. doi: 10.1016/j.expneurol.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mori F, Nishie M, Kakita A, Yoshimoto M, Takahashi H, Wakabayashi K. Relationship among alpha-synuclein accumulation, dopamine synthesis, and neurodegeneration in Parkinson disease substantia nigra. J Neuropathol Exp Neurol. 2006;65:808–815. doi: 10.1097/01.jnen.0000230520.47768.1a. [DOI] [PubMed] [Google Scholar]
- 29.Nagai T, McGeer PL, McGeer EG. Distribution of GABA-T-intensive neurons in the rat forebrain and midbrain. J Comp Neurol. 1983;218:220–238. doi: 10.1002/cne.902180209. [DOI] [PubMed] [Google Scholar]
- 30.Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152:1024–1031. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Dell SJ, Gross NB, Fricks AN, Casiano BD, Nguyen TB, Marshall JF. Running wheel exercise enhances recovery from nigrostriatal dopamine injury without inducing neuroprotection. Neuroscience. 2007;144:1141–1151. doi: 10.1016/j.neuroscience.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 32.Peng J, Xie L, Jin K, Greenberg DA, Andersen JK. Fibroblast growth factor 2 enhances striatal and nigral neurogenesis in the acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. Neuroscience. 2008;153:664–670. doi: 10.1016/j.neuroscience.2008.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petroske E, Meredith GE, Callen S, Totterdell S, Lau YS. Mouse model of Parkinsonism: a comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neuroscience. 2001;106:589–601. doi: 10.1016/s0306-4522(01)00295-0. [DOI] [PubMed] [Google Scholar]
- 34.Petzinger GM, Walsh JP, Akopian G, Hogg E, Abernathy A, Arevalo P, Turnquist P, Vuckovic M, Fisher BE, Togasaki DM, Jakowec MW. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci. 2007;27:5291–5300. doi: 10.1523/JNEUROSCI.1069-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pothakos K, Kurz MJ, Lau YS. Restorative effect of endurance exercise on behavioral deficits in the chronic mouse model of Parkinson's disease with severe neurodegeneration. BMC Neurosci. 2009;10:6. doi: 10.1186/1471-2202-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poulton NP, Muir GD. Treadmill training ameliorates dopamine loss but not behavioral deficits in hemi-parkinsonian rats. Exp Neurol. 2005;193:181–197. doi: 10.1016/j.expneurol.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Ridgel AL, Vitek JL, Alberts JL. Forced, not voluntary, exercise improves motor function in Parkinson's disease patients. Neurorehabil Neural Repair. 2009;23:600–608. doi: 10.1177/1545968308328726. [DOI] [PubMed] [Google Scholar]
- 38.Robinson S, Freeman P, Moore C, Touchon JC, Krentz L, Meshul CK. Acute and subchronic MPTP administration differentially affects striatal glutamate synaptic function. Exp Neurol. 2003;180:74–87. doi: 10.1016/s0014-4886(02)00050-x. [DOI] [PubMed] [Google Scholar]
- 39.Salvatore MF, Pruett BS, Spann SL, Dempsey C. Aging reveals a role for nigral tyrosine hydroxylase ser31 phosphorylation in locomotor activity generation. PLoS One. 2009;4:e8466. doi: 10.1371/journal.pone.0008466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasco AJ, Paffenbarger RS, Jr, Gendre I, Wing AL. The role of physical exercise in the occurrence of Parkinson's disease. Arch Neurol. 1992;49:360–365. doi: 10.1001/archneur.1992.00530280040020. [DOI] [PubMed] [Google Scholar]
- 41.Schang AY, Fisher BE, Sashkin NR, Moore C, Dirling LB, Petzinger GM, Jakowec MW, Meshul CK. Correlates and Analysis of Motor Function in Humans and Animal Models of Parkinson's Disease. In: Raber J, editor. Neuromethods: Behavioral Analysis. Totawa, Japan: Humana Press, Inc; 2010. pp. 55–90. [Google Scholar]
- 42.Sedelis M, Schwarting RK, Huston JP. Behavioral phenotyping of the MPTP mouse model of Parkinson's disease. Behav Brain Res. 2001;125:109–125. doi: 10.1016/s0166-4328(01)00309-6. [DOI] [PubMed] [Google Scholar]
- 43.Smolen AJ, Wright LL, Cunningham TJ. Neuron numbers in the superior cervical sympathetic ganglion of the rat: a critical comparison of methods for cell counting. J Neurocytol. 1983;12:739–750. doi: 10.1007/BF01258148. [DOI] [PubMed] [Google Scholar]
- 44.Steiner B, Winter C, Hosman K, Siebert E, Kempermann G, Petrus DS, Kupsch A. Enriched environment induces cellular plasticity in the adult substantia nigra and improves motor behavior function in the 6-OHDA rat model of Parkinson's disease. Exp Neurol. 2006;199:291–300. doi: 10.1016/j.expneurol.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Tande D, Hoglinger G, Debeir T, Freundlieb N, Hirsch EC, Francois C. New striatal dopamine neurons in MPTP-treated macaques result from a phenotypic shift and not neurogenesis. Brain. 2006;129:1194–1200. doi: 10.1093/brain/awl041. [DOI] [PubMed] [Google Scholar]
- 46.Thacker EL, Chen H, Patel AV, McCullough ML, Calle EE, Thun MJ, Schwarzschild MA, Ascherio A. Recreational physical activity and risk of Parkinson's disease. Mov Disord. 2008;23:69–74. doi: 10.1002/mds.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tillerson JL, Caudle WM, Reveron ME, Miller GW. Detection of behavioral impairments correlated to neurochemical deficits in mice treated with moderate doses of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Exp Neurol. 2002;178:80–90. doi: 10.1006/exnr.2002.8021. [DOI] [PubMed] [Google Scholar]
- 48.Tillerson JL, Caudle WM, Reveron ME, Miller GW. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson's disease. Neuroscience. 2003;119:899–911. doi: 10.1016/s0306-4522(03)00096-4. [DOI] [PubMed] [Google Scholar]
- 49.Tillerson JL, Miller GW. Grid performance test to measure behavioral impairment in the MPTP-treated-mouse model of parkinsonism. J Neurosci Methods. 2003;123:189–200. doi: 10.1016/s0165-0270(02)00360-6. [DOI] [PubMed] [Google Scholar]
- 50.Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ, Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- 51.Unal-Cevik I, Kilinc M, Gursoy-Ozdemir Y, Gurer G, Dalkara T. Loss of NeuN immunoreactivity after cerebral ischemia does not indicate neuronal cell loss: a cautionary note. Brain Res. 2004;1015:169–174. doi: 10.1016/j.brainres.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 52.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 54.Yoon MC, Shin MS, Kim TS, Kim BK, Ko IG, Sung YH, Kim SE, Lee HH, Kim YP, Kim CJ. Treadmill exercise suppresses nigrostriatal dopaminergic neuronal loss in 6-hydroxydopamine-induced Parkinson's rats. Neurosci Lett. 2007;423:12–17. doi: 10.1016/j.neulet.2007.06.031. [DOI] [PubMed] [Google Scholar]


