Abstract
Summary
Osteoporosis is a well recognized complication of ankylosing spondylitis (AS). This study indicates that increased bone turnover, inflammation, and low vitamin D levels are important in the pathophysiology of AS-related osteoporosis, and that bone turnover markers (BTM) are valuable markers to detect bone loss in AS.
Introduction
The aim of this study was to elucidate the pathophysiology of AS-related osteoporosis by investigating the relation between bone mineral density (BMD), BTM, vitamin D, and clinical assessments of disease activity and physical function, as well as to identify parameters that are related to low BMD (osteopenia or osteoporosis) in AS patients with active disease.
Methods
One hundred twenty-eight consecutive Dutch AS outpatients were included in this cross-sectional study. Bath AS Disease Activity Index (BASDAI), erythrocyte sedimentation rate (ESR), C-reactive protein, ASAS-endorsed disease activity score (ASDAS), Bath AS Functional Index (BASFI), bone formation markers procollagen type 1 N-terminal peptide (PINP) and osteocalcin (OC), bone resorption marker serum C-telopeptides of type I collagen (sCTX), 25-hydroxyvitamin D (25OHvitD), lumbar spine and hip BMD, and vertebral fractures were assessed. Z-scores of BTM were calculated using matched 10-year cohorts of a Dutch reference group to correct for the normal influence that age and gender have on bone turnover.
Results
sCTX Z-score, OC Z-score, BASDAI, age, and gender were independently related to low BMD. In addition, PINP Z-score, ESR, 25OHvitD, age, and gender were independently related to sCTX and/or OC Z-score.
Conclusions
This study indicates that increased bone turnover, inflammation, and low vitamin D levels are important in the pathophysiology of AS-related osteoporosis. Furthermore, sCTX and OC Z-scores seem to be valuable markers to detect bone loss in AS patients in daily clinical practice where BMD of the lumbar spine, measured by DXA, may be overestimated due to osteoproliferation in patients with advanced AS.
Keywords: Ankylosing Spondylitis, Bone mineral density, Bone turnover markers, Inflammation, Vitamin D
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory disease that primarily affects the axial skeleton. The disease is characterized by new bone formation, which leads to the formation of syndesmophytes and ankylosis of the spine and sacroiliac joints. Osteoporosis is also a well-recognized complication of AS and is already observed in early stages of the disease. Early vertebral bone loss can be accompanied by severe complications. Previous studies have shown that, in contrast to non-vertebral fractures, the risk of clinical vertebral fractures is increased in AS patients [1, 2] and that vertebral fractures are frequently present in AS [3].
Knowledge about the pathophysiology of AS-related osteoporosis is limited. Various studies have shown involvement of inflammatory processes in the complex pathophysiological mechanism of AS-related osteoporosis [4–9]. Furthermore, various other factors such as drug intake and decreased mobility in relation to pain and stiffness may contribute to the development of osteoporosis in AS patients [10]. In addition, recent studies in AS have suggested that alterations in vitamin D metabolism are associated with inflammatory activity and bone mineral density (BMD) [7, 11–13]. Non-invasive assessment of biochemical bone turnover markers (BTM) may provide more information about the pathophysiology of osteoporosis [14–16]. So far, conflicting data have been published about the relation between BTM, BMD, and disease activity in AS [4, 9, 14, 15, 17–21].
BMD is usually monitored with dual-energy x-ray absorptiometry (DXA) [22]. However, previous studies have shown that the anterior-posterior lumbar spine BMD in AS can be overestimated by the presence of syndesmophytes, ligament calcifications, and fusion of facet joints [23–25]. Furthermore, measuring only hip BMD by DXA may not be sufficient to identify all patients with AS-related osteoporosis since bone loss may primarily occur in the spine [23]. Currently, quantitative computed tomography (QCT) is considered to be the best technique to measure spinal BMD in patients with advanced AS, since this technique can measure only trabecular BMD [17, 24, 26]. However, QCT is expensive and has a high radiation dose compared to DXA [27].Therefore, an alternative method to monitor bone loss in AS patients is desirable.
The aim of the present study was to elucidate the pathophysiology of AS-related osteoporosis by investigating the relation between BMD, BTM, vitamin D, and clinical assessments of disease activity and physical function in a cross-sectional cohort of AS patients with active disease, and to identify parameters that are related to low BMD (osteopenia or osteoporosis) in these patients.
Methods
Patients
Between November 2004 and February 2009, 128 consecutive Dutch AS outpatients from the Medical Center Leeuwarden (MCL, n = 97) and the University Medical Center Groningen (UMCG, n = 31) were included in this cross-sectional study. All patients were over 18 years of age, fulfilled the modified New York criteria for AS [28], and fulfilled the criteria for anti-tumor necrosis factor alpha (anti-TNF-α) treatment according to the Assessments in Ankylosing Spondylitis (ASAS) consensus statement [29]. Data collected before start of anti-TNF-α treatment were used in this cross-sectional study. Excluded were patients with the concomitant presence of inflammatory bowel disease, chronic renal or hepatic disease, diabetes mellitus, parathyroid or thyroid disease, recent fractures, malnutrition, or drug intake affecting bone metabolism (bisphosphonates, glucocorticoids, anticonvulsants, coumarin derivatives, or diuretics). The study was approved by the local ethics committees of the MCL and UMCG, and all patients provided written informed consent to participate in this study.
Clinical and laboratory assessments
Disease activity was assessed using Bath Ankylosing Spondylitis Disease Activity Index (BASDAI; on a scale of 0–10) [30], erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and ASAS-endorsed disease activity score (ASDAS) calculated from BASDAI questions 2, 3, and 6, patient's global assessment of disease activity, and CRP [31, 32]. Physical function was assessed using Bath Ankylosing Spondylitis Functional Index (BASFI; on a scale of 0–10) [33].
Bone turnover was studied by assessment of bone formation markers procollagen type 1 N-terminal peptide (PINP) and osteocalcin (OC), and bone resorption marker serum C-telopeptides of type I collagen (sCTX) [14]. PINP was measured by radioimmunoassay (RIA; Orion Diagnostica, Espoo, Finland; inter-assay coefficient of variation (IE-CV) 9.0%). OC was measured by immunoradiometric assay (IRMA; BioSource Europe S.A; IE-CV 9.4%). sCTX was measured by electrochemiluminescence immunoassay (ECLIA; Elecsys 2010 Roche Mannheim, Germany; IE-CV 10.8%). Serum 25-hydroxyvitamin D (25OHvitD) levels were measured by RIA (DiaSorin, Stillwater, MN, USA; IE-CV 15%; UMCG and MCL until July 2008) or ECLIA (Modular Analytics E170, Roche Mannheim, Germany; IE-CV 7.1%; MCL since July 2008). 25OHvitD <50 nmol/L was defined as a poor vitamin D status. Serum samples were stored at −20°C until analysis.
Z-scores of BTM were used to correct for the normal influence that age and gender have on bone turnover. Z-scores, the number of standard deviations (SD) from the normal mean for age and gender, were calculated using matched 10-year cohorts of a Dutch reference group (150 men or 350 women), checked for serum 25OHvitD levels >50 nmol/L as well as for lumbar spine and hip BMD T-score >−2.5 after 50 years of age.
BMD measurement
BMD of lumbar spine (anterior-posterior projection at L1–L4) and hip (total proximal femur) were measured using DXA (Hologic QDR Discovery (UMCG) or Hologic QDR Delphi (MCL), Waltman, MA, USA). According to the World Health Organization (WHO) classification, osteopenia was defined as a T-score between −1 and −2.5 and osteoporosis as a T-score ≤−2.5 [34]. Patients were categorized by the lowest T-score of the lumbar spine or hip. T-scores, the number of SD from the normal mean obtained from young healthy adults, were calculated using the NHANES reference database. DXA measurements of lumbar spine and hip were available for 106 and 108 patients, respectively.
Vertebral assessment
Anterior, middle, and posterior heights of vertebrae T4 to L4 were measured on lateral radiographs by two independent observers using a ruler. According to the Genant classification, a vertebral fracture was defined based on reduction in anterior, middle, and/or posterior height: grade 1, 20–25% reduction; grade 2, 25–40% reduction; and grade 3, >40% reduction [35]. In case of discrepancy between the two observers, a third independent observer measured vertebral height in order to confirm the presence or absence of a vertebral fracture. Radiographs were available for 106 patients.
Statistical analysis
Statistical analysis was performed with SPSS 16.0 software (SPSS, Chicago, IL, USA). Results were expressed as mean ± SD or median (range) for parametric and nonparametric data, respectively. Pearson's and Spearman's correlation coefficients were used as appropriate to analyze the relationship between BMD, BTM, vitamin D, and clinical measures of disease activity and physical function. Predictor analysis for low BMD, defined as lumbar spine or hip BMD T-score ≤−1, was performed using univariate logistic regression and multivariate logistic regression with conditional stepwise backward inclusion of variables that had a p value ≤ 0.3 in univariate analysis, together with variables that significantly correlated with lumbar spine or hip BMD T-scores. The probability of p for stepwise removal was 0.10. Predictor analyses for sCTX and OC Z-scores were performed using univariate linear regression and multivariate linear regression with backward inclusion of variables that had a p value ≤ 0.3 in univariate analysis, together with variables that significantly correlated with sCTX or OC Z-scores. The probability of F for removal was 0.10. p values ≤ 0.05 were considered statistically significant.
Results
Mean age of the 128 AS patients was 41.0 years (SD ± 11.1), median disease duration was 14 years (range 1–53), and 73% were male. Of the patients, 89% had a BASDAI score ≥4, 74% had increased ESR levels, and 77% had increased CRP levels (Table 1).
Table 1.
Characteristics of the AS study population (n = 128)
| Age (years) | 41.0 ± 11.1 | ||
| Gender (male) (n, %) | 93 (73) | ||
| Disease duration (years) | 14 (1–53) | ||
| HLA-B27+ (n, %) | 102 (84) | ||
| NSAID use (n, %) | 100 (78) | ||
| DMARD use (n, %) | 18 (14) | ||
| BASDAI (range 0–10) | 6.0 ± 1.6 | BASDAI ≥ 4 (n, %) | 116 (89) |
| ESR (mm/h) | 20 (2–90) | Increased ESR (n, %) | 95 (74) |
| CRP (mg/L) | 14 (2–92) | Increased CRP (n, %) | 99 (77) |
| ASDAS | 3.7 ± 0.8 | ||
| BASFI (range 0–10) | 5.6 ± 2.0 | ||
| LS BMD T-score | −0.68 ± 1.41 | Osteopenia LS (n, %) | 41 (39) |
| Osteoporosis LS (n, %) | 9 (9) | ||
| Hip BMD T-score | −0.52 ± 1.06 | Osteopenia hip (n, %) | 42 (39) |
| Osteoporosis hip (n, %) | 2 (2) | ||
| VF (n, %) | 41 (39) | VF grade 1 (n, %) | 27 (25) |
| VF grade 2 (n, %) | 14 (13) | ||
| VF grade 3 (n, %) | 0 (0) | ||
| PINP (μg/L) | 42.7 (16.0–101.5) | ||
| PINP Z-score | 0.14 (−1.74–3.55) | ||
| sCTX (pg/ml) | 200.3 (13.4–780.9) | ||
| sCTX Z-score | −0.36 (−2.58–5.90) | ||
| OC (μg/L) | 12.7 (0.1–24.9) | ||
| OC Z-score | −0.28 (−2.86–2.52) | ||
| 25OHvitD (nmol/L) | 61.4 (13.8–186) | Poor vitamin D status (n, %) | 30 (26) |
Values are mean ± SD or median (range) unless otherwise indicated
AS Ankylosing Spondylitis, HLA-B27+ human leukocyte antigen B27 positive, NSAID non-steroidal anti-inflammatory drug, DMARD disease-modifying antirheumatic drug, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, ESR erythrocyte sedimentation rate, CRP C-reactive protein, ASDAS ASAS-endorsed disease activity score, BASFI Bath Ankylosing Spondylitis Functional Index, LS lumbar spine, BMD bone mineral density, VF vertebral fracture, PINP procollagen type 1 N-terminal peptide, sCTX serum C-telopeptides of type I collagen, OC osteocalcin, 25OHvitD 25-hydroxyvitamin D
Correlations between biochemical and clinical assessments
Correlations between BMD, BTM, vitamin D, and clinical assessments of disease activity and physical function were calculated to obtain more knowledge about the pathophysiology of AS-related osteoporosis (Table 2). There was a significant positive correlation between lumbar spine and hip BMD T-scores. Lumbar spine BMD T-score positively correlated with BASDAI (p < 0.05) and hip BMD T-score negatively correlated with OC and sCTX Z-scores (p < 0.05).There were significant positive correlations between all BTM Z-scores. PINP Z-score positively correlated with age (p < 0.05), and PINP and sCTX Z-scores positively correlated with disease duration (p < 0.05). Finally, ESR, CRP, ASDAS, or BASFI were not significantly correlated with any of the BMD T-scores or BTM Z-scores.
Table 2.
Correlations between clinical and biochemical assessments in AS patients with active disease (n = 128)
| Age | Disease duration | BASDAI | ESR | CRP | ASDAS | BASFI | PINP Z | sCTX Z | OC Z | LS BMD T | Hip BMD T | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease duration (years) | 0.600a | – | ||||||||||
| BASDAI (range 0–10) | NS | NS | – | |||||||||
| ESR (mm/h) | NS | NS | NS | – | ||||||||
| CRP (mg/L) | NS | NS | NS | 0.693a | – | |||||||
| ASDAS | NS | 0.187a | 0.651a | 0.374a | 0.668a | – | ||||||
| BASFI (range 0–10) | NS | 0.203a | 0.561a | NS | NS | 0.472a | – | |||||
| PINP Z-score | 0.362a | 0.266a | NS | NS | NS | NS | NS | – | ||||
| sCTX Z-score | NS | 0.200a | NS | NS | NS | NS | NS | 0.443a | – | |||
| OC Z-score | NS | NS | NS | NS | NS | NS | NS | 0.578a | 0.601a | – | ||
| LS BMD T-score | NS | NS | 0.205a | NS | NS | NS | NS | NS | NS | NS | – | |
| Hip BMD T-score | NS | NS | NS | NS | NS | NS | NS | NS | −0.380a | −0.272a | 0.626a | – |
| 25OHvitD (nmol/L) | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
aStatistically significant correlation (p < 0.05)
See Table 1 for definitions
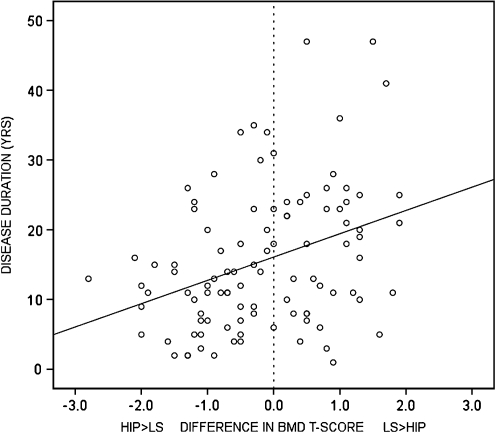
The difference between lumbar spine and hip BMD T-score positively correlated with disease duration (ρ = 0.340, p < 0.05). As shown in Fig. 1, patients with long disease duration never had a lumbar spine BMD T-score that was much lower than their hip BMD T-score, which indicates that osteoproliferation in the lumbar spine has resulted in an overestimation of the lumbar spine BMD T-score in patients with advanced AS.
Fig. 1.
The difference between lumbar spine and hip BMD T-score positively correlated with disease duration (ρ = 0.340, p < 0.05). Patients with long disease duration never had a lumbar spine BMD T-score that was much lower than their hip BMD T-score, which indicates that osteoproliferation in the lumbar spine has resulted in an overestimation of the lumbar spine BMD T-score in patients with advanced AS
Vertebral fractures
Of the patients, 39% had at least 20% reduction in anterior, middle, and/or posterior vertebral height, indicating vertebral fracture. In total, 74 vertebral fractures were detected; 59 wedge fractures, 14 biconcave fractures, and one crush fracture. No significant differences between patients with and without vertebral fractures were found in age (mean 43.1 years ± SD 11.1 vs. 39.9 years ± 11.0; p = 0.149), disease duration (median 15 years (range 1–47) vs. 12 years (1–53); p = 0.925), BMD T-scores (lumbar spine −0.70 ± 1.33 vs. −0.71 ± 1.51; p = 0.984, hip −0.47 ± 1.03 vs. −0.59 ± 1.10; p = 0.591), and BTM Z-scores (PINP 0.15 (−1.74–3.08) vs. 0.22 (−1.65–3.55); p = 0.493), sCTX −0.21 (−2.28–5.90) vs. −0.23 (−2.58–3.92); p = 0.778), OC −0.31 (−2.86–1.50) vs. −0.18 (−2.66–2.52); p = 0.460, respectively).
Predictors of low BMD
Predictor analysis was performed to identify parameters that are related to low BMD. In total, 57% of patients had a lumbar spine or hip BMD T-score of −1 or less, indicating low BMD. Male gender, lower BASDAI score, higher PINP Z-score, higher OC Z-score, and higher sCTX Z-score were significantly associated with low BMD in univariate regression analysis. Since male gender was significantly associated with low BMD, variables that significantly differed between men and women were included in multivariate analysis: age, ESR, OC Z-score, sCTX Z-score, and 25OHvitD. Multivariate regression analysis showed that older age (odds ratio (OR): 1.066, 95% confidence interval (CI): 1.008–1.129), lower BASDAI score (OR: 0.648, 0.445–0.923), higher ESR (OR: 1.025, 0.994–1.057), and higher sCTX Z-score (OR: 2.563, 1.370–4.794) were independently related to low BMD (Table 3). OC Z-score was not included in multivariate analysis, probably due to the strong correlation between sCTX Z-score and OC Z-score (ρ = 0.601, p = 0.000). However, higher OC Z-score was also independently related to low BMD in the presence of age, BASDAI, and ESR (OR: 2.255, 1.238–4.107), indicating that both sCTX Z-score and OC Z-score are important. The Nagelkerke R 2 of the multivariate models including sCTX Z-score and OC Z-score were 0.381 and 0.338, respectively.
Table 3.
Results of univariate and multivariate logistic regression analysis for low BMD
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age (years)a | 1.017 (0.981–1.055) | 0.353 | 1.066 (1.008–1.129) | 0.026 |
| Genderb | 4.368 (1.791–10.652) | 0.001 | –e | |
| Disease duration (years)a | 1.012 (0.974–1.052) | 0.539 | –e | |
| BASDAI (range 0–10)c | 0.728 (0.554–0.957) | 0.023 | 0.648 (0.455–0.923) | 0.016 |
| ESR (mm/h)c | 1.012 (0.980–1.034) | 0.287 | 1.025 (0.994–1.057) | 0.112 |
| CRP (mg/L)c | 1.018 (0.994–1.042) | 0.143 | –e | |
| ASDASc | 0.769 (0.461–1.283) | 0.315 | –f | |
| BASFI (range 0–10)c | 0.959 (0.790–1.165) | 0.674 | –f | |
| PINP Z-scorec | 1.602 (1.043–2.461) | 0.031 | –e | |
| sCTX Z-scorec | 1.878 (1.262–2.794) | 0.002 | 2.563 (1.370–4.794) | 0.003 |
| OC Z-scorec | 1.766 (1.135–2.749) | 0.012 | –e | |
| 25OHvitD (nmol/L)c | 0.998 (0.983–1.013) | 0.787 | –e | |
| VFd | 0.902 (0.385–2.109) | 0.811 | –f | |
See Table 1 for definitions
OR refers to the risk of low BMD (lumbar spine or hip BMD T-score ≤ −1)
aPer year
bIf gender is male (versus female)
cPer 1 grade or 1 point
dIf vertebral fracture is present (versus absent)
eThe variable was not selected during multivariate regression analysis
fThe variable was not tested in multivariate regression analysis because of a p value > 0.3 in univariate regression analysis, no significant correlation with lumbar spine or hip BMD T-scores, and no significant difference between men and women
Predictors of sCTX and OC Z-scores
Since sCTX and OC Z-scores seem to be valuable markers to detect bone loss, predictor analyses for these markers were performed to get more information about the pathophysiology of AS-related osteoporosis. Gender, PINP Z-score, OC Z-score, and 25OHvitD were significantly associated with sCTX Z-score in univariate regression analysis. Since gender was significantly associated with sCTX Z-score, the previous mentioned variables that significantly differed between men and women were included in multivariate analysis. Multivariate regression analysis showed that ESR (OR: 0.012, 0.000–0.025), PINP Z-score (OR: 0.292, 0.022–0.563), OC Z-score (OR: 0.505, 0.243–0.768), and 25OHvitD (OR: −0.009, −0.018–0.000) were independently related to sCTX Z-score (Table 4). The R 2 of this multivariate model was 0.424.
Table 4.
Results of univariate and multivariate linear regression analysis for sCTX Z-score
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| B (95% CI) | p value | B (95% CI) | p value | |
| Age (years)a | 0.018 (−0.004–0.041) | 0.112 | –d | |
| Genderb | −0.680 (−1.211–−0.148) | 0.013 | –d | |
| Disease duration (years)a | 0.018 (−0.005–0.041) | 0.114 | –d | |
| BASDAI (range 0-10)c | −0.060 (−0.213–0.092) | 0.436 | –e | |
| ESR(mm/h)c | 0.011 (−0.002–0.025) | 0.102 | 0.012 (0.000−0.025) | 0.069 |
| CRP(mg/L)c | 0.007 (−0.007–0.021) | 0.303 | –d | |
| ASDASc | 0.156 (−0.174–0.486) | 0.351 | –e | |
| BASFI (range 0–10)c | 0.004 (−0.124–0.132) | 0.953 | –e | |
| PINP Z-scorec | 0.581 (0.384–0.777) | 0.000 | 0.292 (0.022–0.563) | 0.035 |
| OC Z-scorec | 0.774 (0.577–0.971) | 0.000 | 0.505 (0.243–0.768) | 0.000 |
| 25OHvitD (nmol/L)c | −0.011 (−0.020–−0.002) | 0.020 | −0.009 (–0.018–0.000) | 0.041 |
See Table 1 for definitions
B refers to the influence on sCTX Z-score
aPer year
bIf gender is male (versus female)
cPer 1 grade or 1 point
dThe variable was not selected during multivariate regression analysis
eThe variable was not tested in multivariate regression analysis because of a p value>0.3 in univariate regression analysis, no significant correlation with sCTX Z-score, and no significant difference between men and women
Gender, PINP Z-score, and sCTX Z-score were significantly associated with OC Z-score in univariate regression analysis. Since gender was significantly associated with OC Z-score, the previous mentioned variables that significantly differed between men and women were included in multivariate analysis. Multivariate regression analysis showed that age (OR: −0.018, −0.034–−0.001), gender (OR: −0.607, −0.999 –−0.214), PINP Z-score (OR: 0.464, 0.282–0.646), and sCTX Z-score (OR: 0.243, 0.110–0.377) were independently related to OC Z-score (Table 5). The R 2 of this multivariate model was 0.509.
Table 5.
Results of univariate and multivariate linear regression analysis for OC Z-score
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| B (95% CI) | p value | B (95% CI) | p value | |
| Age (years)a | 0.008 (−0.011–0.027) | 0.409 | −0.018 (−0.034–−0.001) | 0.036 |
| Genderb | −0.687 (−1.129–−0.244) | 0.003 | −0.607 (−0.999–−0.214) | 0.003 |
| Disease duration (years)a | 0.007 (−0.012–0.026) | 0.460 | –e | |
| BASDAI (range 0–10)c | −0.029 (−0.155–0.098) | 0.655 | –e | |
| ESR (mm/h)c | 0.006 (−0.005–0.018) | 0.284 | –d | |
| CRP (mg/L)c | 0.009 (−0.003–0.022) | 0.130 | –d | |
| ASDASc | 0.052 (−0.222–0.326) | 0.708 | –e | |
| BASFI (range 0–10)c | 0.035 (−0.071–0.141) | 0.651 | –e | |
| PINP Z-scorec | 0.605 (0.453–0.756) | 0.000 | 0.464 (0.282–0.646) | 0.000 |
| sCTX Z-scorec | 0.464 (0.346–0.582) | 0.000 | 0.243 (0.110–0.377) | 0.000 |
| 25OHvitD (nmol/L)c | −0.007 (−0.016–0.001) | 0.076 | ||
See Table 1 for definitions
B refers to the influence on OC Z-score
aPer year
bIf gender is male (versus female)
cPer 1 grade or 1 point
dThe variable was not selected during multivariate regression analysis
eThe variable was not tested in multivariate regression analysis because of a p value>0.3 in univariate regression analysis, no significant correlation with OC Z-score, and no significant difference between men and women
Discussion
The present cross-sectional study in AS patients with active disease showed that sCTX and OC Z-scores are independently related to low BMD, which indicates that sCTX and OC Z-scores are valuable markers to detect bone loss in AS. An accurate and easily accessible marker of bone loss is needed in patients with advanced AS, since the anterior-posterior lumbar spine BMD measured by DXA can be overestimated by the presence of syndesmophytes, ligament calcifications, and fusion of facet joints in these patients [23–25]. Our finding that the difference between lumbar spine and hip BMD positively correlated with disease duration indicates that this overestimation also occurred in this study. Furthermore, our high prevalence of vertebral fractures and of low BMD (osteopenia or osteoporosis) underlines the importance of monitoring bone loss in AS.
In order to obtain more knowledge about the pathophysiology of AS-related osteoporosis, we investigated the relation between BMD, BTM, vitamin D, and clinical assessments. Our results demonstrate that increased bone turnover plays a significant role in the development of osteoporosis in AS patients. First, significant positive correlations were found between age or disease duration and PINP Z-score, a marker of bone formation, as well as between disease duration and sCTX Z-score, a marker of bone resorption. Since the use of Z-scores corrects for the normal influence that age and gender have on bone turnover, these correlations demonstrate that AS is characterized by both increased bone formation and increased bone resorption. Second, significant negative correlations were found between sCTX or OC Z-scores and hip BMD T-score, and a higher sCTX or OC Z-score was independently related to low BMD, which indicates that high bone turnover is associated with bone loss in AS. This finding is in agreement with the previous studies [4, 14, 15].
The results of this study also demonstrate involvement of inflammatory processes in the complex pathophysiological mechanism of AS-related osteoporosis. A higher ESR was independently related to low BMD. Furthermore, ESR had independent influence on sCTX Z-score. The importance of inflammatory processes was also shown in previous studies [4–9].
Finally, our finding that 25OHvitD level had an independent significant inverse influence on sCTX Z-score suggests that low vitamin D levels play a role in the development of AS-related osteoporosis. The importance of vitamin D was also suggested in previous studies [7, 11–13, 36]. Amento et al. reported that vitamin D is an endogenous modulator of the immune response, which may slow down the inflammatory process by suppressing active T cells and cell proliferation [36]. Lange et al. found negative correlations between serum levels of vitamin D and markers of disease activity or inflammation in AS patients. They also showed that AS patients with osteoporosis had significantly lower vitamin D levels compared to AS patients with normal BMD [7, 11]. Finally, Obermayer et al. suggested a close association of BMD, bone metabolism, and inflammatory activity with Fok1 polymorphisms of the vitamin D receptor gene in male AS patients [13].
Unexpectedly, a lower BASDAI score was independently related to low BMD in this study. A possible explanation for this finding may be that complaints related to new bone formation influence the BASDAI, a subjective measure of disease activity, in AS patients with active disease. The significant positive correlation between BASDAI and lumbar spine BMD T-score found in this study seems to confirm this suggestion. Another explanation may be that BMD, measured by DXA, reflects the influence of the disease on bone over time, while BASDAI reflects the current status of disease activity.
There are some strengths and limitations to this study. The main limitation is that the study is cross-sectional and that only AS patients with active disease were included. Further studies with longer follow-up are needed to confirm the usefulness of sCTX and OC Z-scores in monitoring bone loss in AS patients, as well as the importance of increased bone turnover, inflammation, and low vitamin D levels in the development of AS-related osteoporosis. Another limitation is that body mass index (BMI) was not assessed in this study. Therefore, it was not possible to correct for low BMI in multivariate analysis. Finally, it was not clear if the vertebral fractures occurred recently or if they were already present for many years. Therefore, analyses investigating the relation between BTM and vertebral fractures were difficult. The main strength is that Z-scores of BTM were calculated to correct for the influence that age and gender have on bone turnover in healthy persons. In this way, male and female patients of different age groups could be analyzed together.
In conclusion, this cross-sectional study in AS patients with active disease indicates that increased bone turnover, inflammation, and low vitamin D levels are important in the pathophysiology of AS-related osteoporosis. Furthermore, sCTX and OC Z-scores seem to be valuable markers to detect bone loss in AS. Combining biochemical BTM and BMD measurements may be useful to identify AS patients with osteoporosis in daily clinical practice where lumbar spine BMD, measured by DXA, may be overestimated due to osteoproliferation in patients with advanced AS.
Acknowledgements
This investigation was sponsored with an unrestricted grant from Wyeth pharmaceuticals. The authors thank Mrs. L. Bulstra, Mrs. A. Krol, Mrs. K. Rasing-Klein Goldewijk, and Mrs. J. Vierdag-Loth for their contribution to clinical data collection; Mr. J. Bijzet and Mrs. A. Weiland for their contribution to serum sample collection; Mrs. J. Hoving-Ensing, Mrs. M. Inia, Mrs. H. Kamminga-Rasker, Mrs. K. Koerts, and Mrs. L. Wagenmakers for their contribution to BTM and 25OHvitD assessments; and Mrs. M. Hofman for her contribution to vertebral fracture assessment.
Conflict of interests
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Cooper C, Carbone L, Michet CJ, et al. Fracture risk in patients with ankylosing spondylitis: a population based study. J Rheumatol. 1994;21(10):1877–1882. [PubMed] [Google Scholar]
- 2.Vosse D, Landewe R, van der Heijde D, et al. Ankylosing spondylitis and the risk of fracture: results from a large primary care-based nested case-control study. Ann Rheum Dis. 2009;68(12):1839–1842. doi: 10.1136/ard.2008.100503. [DOI] [PubMed] [Google Scholar]
- 3.Geusens P, Vosse D, van der Linden S. Osteoporosis and vertebral fractures in ankylosing spondylitis. Curr Opin Rheumatol. 2007;19(4):335–339. doi: 10.1097/BOR.0b013e328133f5b3. [DOI] [PubMed] [Google Scholar]
- 4.Franck H, Meurer T, Hofbauer LC. Evaluation of bone mineral density, hormones, biochemical markers of bone metabolism, and osteoprotegerin serum levels in patients with ankylosing spondylitis. J Rheumatol. 2004;31(11):2236–2241. [PubMed] [Google Scholar]
- 5.Ghozlani I, Ghazi M, Nouijai A, et al. Prevalence and risk factors of osteoporosis and vertebral fractures in patients with ankylosing spondylitis. Bone. 2009;44(5):772–776. doi: 10.1016/j.bone.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 6.Gratacos J, Collado A, Pons F, et al. Significant loss of bone mass in patients with early, active ankylosing spondylitis: a followup study. Arthritis Rheum. 1999;42(11):2319–2324. doi: 10.1002/1529-0131(199911)42:11<2319::AID-ANR9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 7.Lange U, Teichmann J, Strunk J, et al. Association of 1.25 vitamin D3 deficiency, disease activity and low bone mass in ankylosing spondylitis. Osteoporos Int. 2005;16(12):1999–2004. doi: 10.1007/s00198-005-1990-5. [DOI] [PubMed] [Google Scholar]
- 8.Maillefert JF, Aho LS, El Maghraoui A, et al. Changes in bone density in patients with ankylosing spondylitis: a two-year follow-up study. Osteoporos Int. 2001;12(7):605–609. doi: 10.1007/s001980170084. [DOI] [PubMed] [Google Scholar]
- 9.Toussirot E, Ricard-Blum S, Dumoulin G, et al. Relationship between urinary pyridinium cross-links, disease activity and disease subsets of ankylosing spondylitis. Rheumatology. 1999;38(1):21–27. doi: 10.1093/rheumatology/38.1.21. [DOI] [PubMed] [Google Scholar]
- 10.El Maghraoui A. Osteoporosis and ankylosing spondylitis. Joint Bone Spine. 2004;71(4):291–295. doi: 10.1016/j.jbspin.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Lange U, Jung O, Teichmann J, et al. Relationship between disease activity and serum levels of vitamin D metabolites and parathyroid hormone in ankylosing spondylitis. Osteoporos Int. 2001;12(12):1031–1035. doi: 10.1007/s001980170013. [DOI] [PubMed] [Google Scholar]
- 12.Mermerci Baskan B, Pekin Dogan Y, Sivas F, et al. The relation between osteoporosis and vitamin D levels and disease activity in ankylosing spondylitis. Rheumatol Int. 2009 doi: 10.1007/s00296-009-0975-7. [DOI] [PubMed] [Google Scholar]
- 13.Obermayer-Pietsch BM, Lange U, Tauber G, et al. Vitamin D receptor initiation codon polymorphism, bone density and inflammatory activity of patients with ankylosing spondylitis. Osteoporos Int. 2003;14(12):995–1000. doi: 10.1007/s00198-003-1501-5. [DOI] [PubMed] [Google Scholar]
- 14.Borman P, Bodur H, Bingol N, et al. Bone mineral density and bone turnover markers in a group of male ankylosing spondylitis patients: relationship to disease activity. J Clin Rheumatol. 2001;7(5):315–321. doi: 10.1097/00124743-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Park MC, Chung SJ, Park YB, et al. Bone and cartilage turnover markers, bone mineral density, and radiographic damage in men with ankylosing spondylitis. Yonsei Med J. 2008;49(2):288–294. doi: 10.3349/ymj.2008.49.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eastell R, Hannon RA. Biomarkers of bone health and osteoporosis risk. Proc Nutr Soc. 2008;67(2):157–162. doi: 10.1017/S002966510800699X. [DOI] [PubMed] [Google Scholar]
- 17.El Maghraoui A, Tellal S, Chaouir S, et al. Bone turnover markers, anterior pituitary and gonadal hormones, and bone mass evaluation using quantitative computed tomography in ankylosing spondylitis. Clin Rheumatol. 2005;24(4):346–351. doi: 10.1007/s10067-004-1039-8. [DOI] [PubMed] [Google Scholar]
- 18.Karberg K, Zochling J, Sieper J, et al. Bone loss is detected more frequently in patients with ankylosing spondylitis with syndesmophytes. J Rheumatol. 2005;32(7):1290–1298. [PubMed] [Google Scholar]
- 19.Sarikaya S, Basaran A, Tekin Y, et al. Is osteoporosis generalized or localized to central skeleton in ankylosing spondylitis? J Clin Rheumatol. 2007;13(1):20–24. doi: 10.1097/01.rhu.0000255688.83037.42. [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz N, Ozaslan J. Biochemical bone turnover markers in patients with ankylosing spondylitis. Clin Rheumatol. 2000;19(2):92–98. doi: 10.1007/s100670050024. [DOI] [PubMed] [Google Scholar]
- 21.Vosse D, Landewe R, Garnero P, et al. Association of markers of bone- and cartilage-degradation with radiological changes at baseline and after 2 years follow-up in patients with ankylosing spondylitis. Rheumatology. 2008;47(8):1219–1222. doi: 10.1093/rheumatology/ken148. [DOI] [PubMed] [Google Scholar]
- 22.Kanis JA, McCloskey EV, Johansson H, et al. A reference standard for the description of osteoporosis. Bone. 2008;42(3):467–475. doi: 10.1016/j.bone.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Baek HJ, Kang SW, Lee YJ, et al. Osteopenia in men with mild and severe ankylosing spondylitis. Rheumatol Int. 2005;26(1):30–34. doi: 10.1007/s00296-004-0516-3. [DOI] [PubMed] [Google Scholar]
- 24.Lee YS, Schlotzhauer T, Ott SM, et al. Skeletal status of men with early and late ankylosing spondylitis. Am J Med. 1997;103(3):233–241. doi: 10.1016/S0002-9343(97)00143-5. [DOI] [PubMed] [Google Scholar]
- 25.Meirelles ES, Borelli A, Camargo OP. Influence of disease activity and chronicity on ankylosing spondylitis bone mass loss. Clin Rheumatol. 1999;18(5):364–368. doi: 10.1007/s100670050120. [DOI] [PubMed] [Google Scholar]
- 26.Lange U, Kluge A, Strunk J, et al. Ankylosing spondylitis and bone mineral density—what is the ideal tool for measurement? Rheumatol Int. 2005;26(2):115–120. doi: 10.1007/s00296-004-0515-4. [DOI] [PubMed] [Google Scholar]
- 27.Bessant R, Keat A. How should clinicians manage osteoporosis in ankylosing spondylitis? J Rheumatol. 2002;29(7):1511–1519. [PubMed] [Google Scholar]
- 28.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27(4):361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 29.Braun J, Davis J, Dougados M, et al. First update of the international ASAS consensus statement for the use of anti-TNF agents in patients with ankylosing spondylitis. Ann Rheum Dis. 2006;65(3):316–320. doi: 10.1136/ard.2005.040758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garrett S, Jenkinson T, Kennedy LG, et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994;21(12):2286–2291. [PubMed] [Google Scholar]
- 31.Lukas C, Landewe R, Sieper J, et al. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis. 2009;68(1):18–24. doi: 10.1136/ard.2008.094870. [DOI] [PubMed] [Google Scholar]
- 32.van der Heijde D, Lie E, Kvien TK, et al. ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis. 2009;68(12):1811–1818. doi: 10.1136/ard.2008.100826. [DOI] [PubMed] [Google Scholar]
- 33.Calin A, Garrett S, Whitelock H, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol. 1994;21(12):2281–2285. [PubMed] [Google Scholar]
- 34.Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994;4(6):368–381. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 35.Genant HK, Wu CY, van Kuijk C, et al. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8(9):1137–1148. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 36.Amento EP. Vitamin D and the immune system. Steroids. 1987;49(1–3):55–72. doi: 10.1016/0039-128X(87)90079-1. [DOI] [PubMed] [Google Scholar]