Abstract
The incidence and prevalence of atrial fibrillation (AF) are increasing worldwide. AF is of public health importance as it accounts for substantial morbidity, mortality, and health-care costs. AF may be transient initially, but many patients have progressive disease marked by increasing frequency and duration of episodes. Various classification schemes for AF have been proposed, although current guidelines are based on temporal rhythm-based patterns. We discuss existing schemes for the classification of AF, focusing on the advantages and limitations of the pattern-based scheme, in the context of new knowledge about AF pathophysiology, AF patterns, and clinical outcomes. Furthermore, we address gaps in knowledge that present opportunities to re-examine the current pattern-based classification of AF. A future classification scheme should ideally combine elements such as the risk of stroke, an assessment of symptoms, and the impairment of the atrial substrate.
Introduction
The incidence and prevalence of atrial fibrillation (AF) are increasing worldwide.1–7 This arrhythmia is of public health importance, as it accounts for substantial morbidity, mortality, and health-care costs.8–10 AF is marked by the loss of coordinated atrial electrical and mechanical function, and may be transient or permanent.11 The disease may progress in severity from a transient to a permanent state in some patients. Variability in the duration and frequency of AF episodes, and observations of disease progression within a given individual, have prompted the proposal of a classification scheme for AF that relies on rhythm-based patterns.12 This scheme for AF classification is currently endorsed by the American Heart Association (AHA), American College of Cardiology (ACC) and European Society of Cardiology (ESC).
Knowledge of AF patterns gathered through the use of continuous rhythm monitoring technologies, and advances in our understanding of the pathogenesis and natural history of AF present an opportunity to re-examine rhythm-based classification schemes for AF. We sought to address the following questions about the current rhythm-based classification scheme: Is it accurate and reproducible? Do patterns reflect distinct etiologic disease subtypes? Are patterns associated with AF-related morbidity or mortality? Are AF pattern-based classes associated with patient symptoms or well-being? In this Review, we will discuss pattern-based classification of AF in the context of new knowledge about AF pathophysiology, AF rhythm-based patterns, and clinical outcomes. Furthermore, we will discuss gaps in our current knowledge that provide an opportunity to revise the pattern-based classification of AF.
The ideal classification scheme
Disease-based classification schemes are used to inform clinical management decisions or describe the longitudinal evolution of the disease. Whether for clinical or research purposes, an optimal categorization must accurately reflect and distinguish one class of disease from another, assuming distinct classes exist. A reliable classification system must be applicable and reproducible across diverse patient populations with the disease. The relevance of a clinical classification scheme is demonstrated by its ability to discriminate between patients on the basis of the stage and severity of the underlying disease, prognosis, clinically meaningful symptoms, or indications for therapeutic intervention. A research classification scheme should distinguish the pathophysiological mechanisms or etiological subtypes of a disease. Clinical and research classification schemes need not overlap, although it is convenient if they do.
Existing classification scheme for AF
A variety of different classification schemes for AF, with a basis on etiology, electrophysiological characteristics, temporal pattern, symptoms, and quality of life have been proposed.12–21 The scheme currently recommended by the American and European cardiology societies emphasizes temporal rhythm-based patterns of AF (Figure 1).12 AF is classified as ‘first-detected’ in patients who have no history of this arrhythmia. AF that recurs after the first-detected episode is considered ‘paroxysmal’ if it self-terminates within 1 week, ‘persistent’ if it continues beyond this period and is not self-terminating, or ‘permanent’ if efforts to terminate the rhythm fail or are not attempted12 At the individual level, patients are generally considered to have the class of AF that corresponds to the rhythm that has occurred most often for the patient up to the time of assessment.
Figure 1.
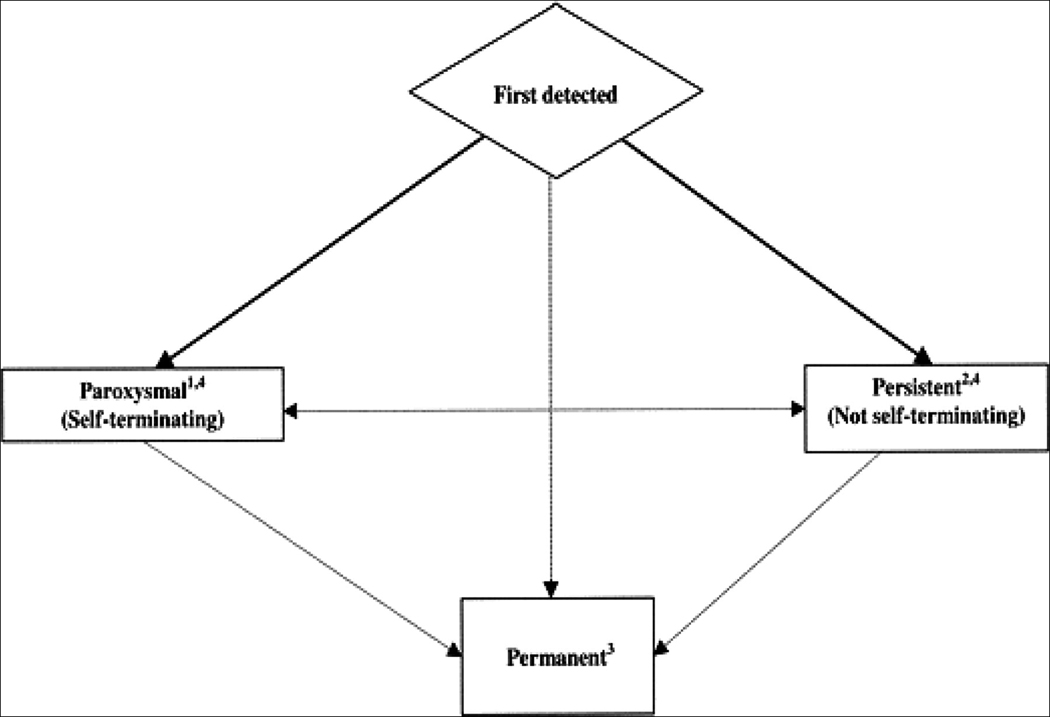
Rhythm-based patterns of atrial fibrillation (AF), as defined in the 2006 AHA, ACC and European Society of Cardiology guidelines for the management of patients with AF. Paroxysmal AF is characterized by episodes that generally last 7 days or less, with most lasting less than 24 h. Persistent AF is characterized by episodes that usually last longer than 7 days. When cardioversion failed or is not attempted, AF is classified as permanent. Both paroxysmal and persistent AF may be recurrent. Permission obtained from the American Heart Association.12
The current AF classification scheme acknowledges that an individual may experience periods of both paroxysmal and persistent AF, as some episodes may self-terminate whereas others may not. An important concept within the scheme is the notion that longstanding paroxysmal or persistent AF may progress and become permanent.12 However, the current guidelines do not explicitly define AF patterns as stages, likely in part due to inconsistent patterns of progression and recurrence. With the advent of catheter ablation interventions for AF, patients with persistent AF for longer than 1 year that are considered for ablation have been referred to as having ‘longstanding, persistent disease’ by the Heart Rhythm Society, European Heart Rhythm Association, and European Cardiac Arrhythmia Society in collaboration with other major international cardiovascular societies.22 Thus, patients with ‘longstanding, persistent disease’ are distinguished from those with permanent AF in whom attempts to restore sinus rhythm are unsuccessful or not attempted.
In addition to rhythm-based patterns, guidelines acknowledge that AF may be referred to as ‘lone’ if it occurs in young individuals in the absence of cardiopulmonary or other acute illness that may precipitate the arrhythmia.12,23 Although the appropriateness of distinguishing lone AF as a distinct subset is debatable, we utilize this nomenclature to provide a succinct summary of published reports. Individuals with lone AF may have all of the rhythm-based patterns defined within the current classification.
Secondary forms of AF, which are attributable to an underlying disorder (such as cardiac surgery or pericarditis), are excluded from the rhythm-based scheme proposed in the existing guidelines. Such forms of AF may resolve with proper treatment of the underlying disorder and recurrence is thought to be unlikely, unless it occurs independently of the treated disorder.12
Advantages of the pattern-based scheme
The ACC, AHA, and ESC originally endorsed the existing pattern-based classification scheme for AF in 2001.24 Standardization of nomenclature and definitions for classes of AF are major advantages of this scheme that led to its endorsement. Before this declaration, the labels used to describe AF were inconsistent and included additional terms such as ‘intermittent’, and ‘chronic’. Labels such as paroxysmal, persistent, and permanent were used with different definitions. The endorsed system was adopted by the ACC and AHA committees on data standards for AF in 2004,25 has become widely used in the clinical literature since its introduction, and has gradually led to the disuse of terms such as ‘chronic’ AF. An important strength of the scheme is its simplicity.
An additional strength of the current classification scheme is the fact that pattern-based classes correlate with the degree of atrial substrate impairment or remodeling. This observation has led to the recommendation of different approaches for catheter ablation for AF based, in part, on AF pattern.22 Generally, more extensive ablation beyond just isolation of the pulmonary veins is advocated for those with longstanding persistent AF. Pattern-based classes of AF also correlate with patient well-being, a primary determinant of medical therapy, as will be discussed further below.26
The distinction of secondary AF is another advantage, as it connotes to clinicians that the diagnosis of AF in the setting of a reversible disorder does not carry the same risks of recurrence of AF or AF-related morbidity that are attributable to non-secondary forms the arrhythmia.
Challenges to the existing scheme
Challenges to the existing pattern-based classification scheme include several shortcomings as well as gaps in knowledge about the nature of AF.
Accuracy and reproducibility
As existing AF pattern-based classes are empiric, the accuracy and reliability of these labels are uncertain. The often transient and variable nature of AF poses limits to clinician-guided, pattern-based categorization and is a challenge to AF classification. Evidence suggests that AF episodes are frequently asymptomatic, that symptoms do not reliably identify AF episodes, and that prolonged monitoring reveals otherwise undetected episodes of AF.27–31 Therefore, classification of the predominant AF pattern in patients on the basis of clinical encounters and office-based electrocardiograms, as is typically done in both practice and research, may result in misclassification by substantially underestimating the actual burden of AF.
Accuracy is also threatened by the ambiguity that exists in differentiating between paroxysmal and persistent AF. Patients that are cardioverted within 1 week of initiation of an AF episode may be misclassified as having persistent AF when they otherwise would have converted spontaneously to sinus rhythm within that week, whereas those with persistent AF lasting longer than 1 week may have self-terminating episodes. Similarly, the distinction between permanent and persistent AF may depend on a provider’s preference to restore sinus rhythm or not, or on the aggressiveness with which they pursue restoration of sinus rhythm. Thus, classification of AF may be heterogeneous across providers or in different settings.
An additional prerequisite for the clinical utility of a classification scheme is that it be reproducible both across providers and populations. We are unaware of any investigations that specifically address the intraobserver or interobserver variability and reliability of AF classification as outlined in existing guidelines, or the applicability of patterns in different populations.
Longitudinal history of AF
In several studies, attempts have been made to characterize the natural history of AF (Table 1).23,32–45 Direct comparison of studies is challenging owing to differences in study design, follow-up, and definitions of AF categories. Nonetheless, existing data suggest that recurrent episodes of AF are detected in most patients.23,32,33,35,36,43,46
Table 1.
Progression of first-detected AF according to various pattern-based classification schemes
| Study | Classification* |
n(n followed from first-detected episode) |
Follow-up, years‡ | Recurrence after first-detected episode |
Transition to permanent AF§ |
|---|---|---|---|---|---|
| Takahashi et al., 198132 |
Paroxysmal or permanent | 94 (94)‖, ¶ |
9 (study period) | 27% | 20% |
| Petersen et al., 198645 |
Paroxysmal or chronic | 426 (426)‖ |
3.5 (median) | NR | 33% |
| Kopecky et al., 198723 |
Isolated, recurrent or chronic |
97 (97)# |
14.8 (mean) | 21% | NR |
| Kato et al., 200444 | Paroxysmal or chronic | 171 (171)‖ ** |
14.1 (mean) | NA | 77%, incidence rate of 5.5% per year |
| Jahangir et al., 200737 |
Paroxysmal, persistent or permanent |
76 (76)#,‡‡ |
25.2 (mean) | NR | 29% cumulative incidence of permanent AF at 30 years |
| Davidson et al., 198943 |
Paroxysmal or chronic | 32 (NR)# |
4.9 (mean) | 44% | 0% |
| Scardi et al., 199933 | Isolated, recurrent or chronic |
145 (145)# |
10.4 (mean) | 17% | 23% |
| Levy et al., 199935 | Paroxysmal, recent onset or chronic |
756 (163)§§ |
8.6 (mean) | 60.7% | 8% |
| Al-Khatib et al., 200034 |
Intermittent or permanent | 231 (NR)‖‖ |
19 (study period) | NR | 18% cumulative incidence of permanent at 4 years |
| Kerr et al., 200536 | Paroxysmal or chronic | 757 (757)‖ |
8 (median) | 16% | 25% cumulative incidence of chronic AF at 5 years |
| Pappone et al., 200838 |
Paroxysmal, persistent or permanent |
106 (106)‖, ¶¶ |
5 (study period) | 47% | 15% |
| Nieuwlaat et al., 200839 |
First detected, paroxysmal, persistent or permanent |
4192 (708) |
1 (study period) | 46% | 20% |
| Keating et al., 200541 |
Paroxysmal, persistent or permanent |
270 (270) |
2.9 (mean) | 0%## | 34% cumulative incidence of permanent AF at 4 years |
| Ruigomez et al., 200542 |
Paroxysmal or chronic | 525 (525)‖ |
2.7 (mean) | 30% | 22% cumulative incidence of chronic AF at 4.5 years |
| Tsang et al., 200840 | Paroxysmal, persistent or permanent |
3,248 (3,248)‖ |
5.1 (median) | NR | 17% |
| de Vos et al., 201048 |
First-detected, paroxysmal, persistent or permanent |
1219 (165)*** |
1 (study period) | NR | 14% |
AF types had different definitions across classifications schemes despite similar labels.
The mean, median, or duration of the study period is reported.
Or most chronic form per respective classification scheme. Calculation refers only to those with first-detected AF in whom progression to a more chronic form was possible.
All paroxysmal at baseline.
Data do not account for censoring.
Lone AF only.
All had recurrent AF.
Follow-up subset from Kopecky et al.23
Refers to paroxysmal group only.
All intermittent at baseline.
51% individuals had lone AF and 10% of the overall sample underwent catheter ablation for AF and experienced no recurrent disease.
Only individuals with recurrent AF were included.
Subset from Nieuwlaat et al.39 with sinus rhythm after first-detected AF.
Abbreviations: AF, atrial fibrillation; NA, not applicable; NR, not reported.
AF not only recurs, but progresses from paroxysmal and persistent to permanent AF in a considerable proportion of individuals. For example, the cumulative incidence of permanent AF after an initial diagnosis of either paroxysmal or persistent AF has been reported to be as high as 34% at 4 years of follow-up.34,36,41,42 Among individuals with paroxysmal disease, the cumulative incidence of persistent disease was 20%, at 4 years after initial diagnosis,41 although as paroxysmal and persistent AF can coexist, ‘progression’ might not be an appropriate term for comparing the frequencies of these patterns over time. In a study of individuals with paroxysmal AF and bradyarrhythmias necessitating pacemaker implantation, the daily burden of AF did not increase, which demonstrates that AF patterns may be static in some individuals.47
Risk factors for progression include advanced age,34,36,38,39,44,48 valvular heart disease,36,39,42,44 cardiomyopathy, heart failure,36,38,39,48 increased left atrial size or volume,39,40,44,49 and prior occurrence of stroke or systemic thromboembolism45,48 (Box 1). AF stability and recurrence seem to be mediated by both electrical50,51 and structural52 remodeling. Such remodeling may manifest as calcium overload with a reduction in L-type calcium currents,53 intracellular glycogen accumulation accompanied by sarcomere loss,54 and a variety of characteristic proarrhythmic electrophysiological changes such as shortening of the atrial electrical refractory period.50,53 Hence the commonly cited adage, “atrial fibrillation begets atrial fibrillation.”50
Box 1 Risk factors for the progression of AF.
Prior occurrence of myocardial infarction44
Obesity40
Chronic obstructive pulmonary disease48
Hypertension48
Diabetes mellitus38
Moderate or high alcohol consumption42
Impaired chemoreflex sensitivity49
Increased daily AF burden47
Decreased heart rate during AF36
Abbreviation: AF, atrial fibrillation.
Although AF progresses in a substantial number of individuals, it is unclear whether progression can be prevented, or even if a clinical imperative to prevent progression exists. Areas of uncertainty include the exact incidence of progression, whether progression itself is associated with morbidity independent of other comorbid conditions, and whether an optimal time exists to target the prevention of AF progression. If AF progression is independently associated with morbidity, efforts to interrupt progression might be clinically relevant. Comparative studies in patients at high risk for progression that assess the efficacy of therapies for preventing AF progression, or that of intermediate outcomes such as atrial remodeling, might be worthwhile. Observations from secondary prevention trials have demonstrated a reduction in recurrent AF burden with antiarrhythmic drugs,55 statin therapy,56 and pulmonary vein isolation,38,57 which all may be of relevance for preventing AF progression. Some data suggest that inhibition of the renin–angiotensin–aldosterone axis is associated with a reduction in recurrent AF,58 although a randomized controlled trial comparing valsartan to placebo found no difference in the time to recurrent AF.59 We are unaware of any studies that tested the primary hypothesis that an intervention prevents AF progression. Future studies targeting risk factors for AF progression (Box 1) may be warranted.
The heterogeneous nature of recurrence and progression of AF poses a challenge to rhythm-based classification of this arrhythmia. The degree to which a pattern on one occasion is associated with the pattern on future occasions is variable and, therefore to classify individuals followed over a period of time as only having paroxysmal, persistent, or permanent AF, as is commonly done in the research setting, becomes problematic.
Relevance to prognosis
An additional challenge to the existing pattern-based classification scheme is the lack of evident relevance to clinical prognosis. Few studies have attempted to clarify whether current pattern-based classes of AF are associated with differences in AF-related morbidity or mortality. A notable limitation of comparing reported associations between patterns and prognosis, however, is the fact that many studies were published prior to standardization of pattern definitions.
Among AF-related morbidities that have been assessed according to pattern-based classes of AF, stroke is the best studied. Stroke occurs approximately three to five times more often in individuals with AF than in those without.60,61 Risk stratification schemes applied to patients with AF have evolved that help identify individuals at increased risk of stroke, but none rely on the distinction between rhythm-based classes of AF.62 Indeed, stroke risk does not seem to differ according to pattern in most,35,63–70 though not all,45,71 reports. In 1994, a pooled analysis including 3,706 individuals from five randomized controlled trials comparing either warfarin or aspirin to placebo for the prevention of stroke in individuals with AF demonstrated that stroke risk was independent of AF pattern.69 Although some data suggest that the temporal burden of AF may help discriminate individuals at risk of stroke,72,73 existing pattern-based AF classification schemes have not proven useful for the prediction of stroke.
Heart failure is another major AF-related morbidity.74 However, little research has been done to assess the relation between AF classification schemes and heart-failure outcomes. Data from the Euro Heart Survey, a prospective cohort study of 4,192 individuals with prevalent AF at the time of enrollment and followed for 1 year, demonstrated similar rates of new-onset heart failure in individuals with paroxysmal, persistent, and permanent AF, although the rate of hospitalization for existing heart failure was highest for those with permanent AF.39
Several studies have examined the association between AF pattern and mortality.39,41,42,75 In general, unadjusted analyses indicate a greater risk of mortality among those with permanent AF than among those with other patterns of AF, although differences were either attenuated or absent after adjustment for multiple potentially confounding factors. Some,41,75 but not all, 42 studies have reported lower mortality rates among those with persistent AF than among those with paroxysmal or permanent AF after adjustment for potential confounding factors. Whether the increased risk of mortality observed in individuals with paroxysmal AF relative to those with persistent AF is explained by residual confounding by variables that were not accounted for in the analyses, misclassification of AF pattern, or other forms of bias remains unclear.
Lone AF
AF-related morbidity has also been assessed specifically in individuals with lone AF.23,33,76 Among 97 patients with lone AF identified at the Mayo Clinic, the probability of survival following diagnosis of AF was 94% after 15 years, and did not differ from that of the general population.23 Survival was similar among those with initial, paroxysmal, and what was referred to as chronic AF. Rates of stroke were low (incidence rate 0.35 per 100 person-years), and did not differ between AF patterns. In a separate analysis of 157 individuals from Italy, neither survival nor rates of thromboembolism differed between those with paroxysmal and chronic lone AF.33
Relevance to quality of life
The clinical relevance of the existing pattern-based classification scheme is further challenged by gaps in knowledge about the relations between AF patterns and quality of life, a major driver of medical therapies in AF. The impairment in quality of life among some patients with AF has been increasingly recognized.77–80 The importance of patient well-being, as it relates to AF, is underscored by the attention that this outcome has received in clinical trials of therapeutic interventions, which generally report improved quality of life achieved with a broad array of interventions.78 Although improvements in quality of life may differ with specific therapies,20,21 management decisions are heavily influenced by patient symptoms.12
Numerous instruments have been implemented in the research setting to assess quality of life or symptoms, although only some have been designed specifically for the assessment of patients with AF.20,21,81,82 The reproducibility of such instruments within individuals, and their validity in diverse races and/or ethnicities and across clinical samples or cohorts, has not been consistently examined. In addition, little is known about quality of life as it relates to the long-term course of AF, or whether patient well-being differs according to rhythm-based patterns or frequency of AF episodes.78 Moreover, it is unclear to what extent current instruments distinguish between a reduction in symptoms and improvement in quality of life. Distinguishing between symptom burden and quality of life may have important implications for measuring effectiveness of therapies, particularly since many patients may have nonspecific symptoms or have asymptomatic episodes of AF.
In one recent example in which AF-related symptoms were related to rhythm-based patterns and quality of life, Dorian et al. developed the Canadian Cardiovascular Society Severity of Atrial Fibrillation (CCS–SAF) scale, modeled after the CCS angina and NYHA heart failure classifications.82 The CCS-SAF is a 0–4 scale that characterizes patient symptoms, the association of symptoms with AF, and the functional consequences of these symptoms. In a validation study, the CCS–SAF class differed significantly according to the pattern of AF and quality of life.26 Only 26% of patients with paroxysmal AF were categorized as having class 0 (asymptomatic) AF, as compared with 74% of patients with persistent or permanent AF when pooled together. After multivariable adjustment, both AF pattern and CCS-SAF class were significantly associated with patient quality of life. In each CCS–SAF class, patients with persistent or permanent AF scored lower on measures of quality of life than those with paroxysmal AF.
Evaluation of the clinical utility of parsimonious schemes that assess quality of life is warranted. Assessment of novel schemes should focus on determining whether instruments help to identify indications for therapy and predict therapeutic response, morbidity, or prognosis. Despite the widespread use of symptom scores and quality-of-life measures in clinical trials of AF, nonuniformity across trials is common, making standardized cross-trial comparisons of the accuracy and predictive value of these measures difficult. Furthermore, a pressing need exists for a simple and practical disease-specific symptom and quality-of-life scale that can be used in routine clinical practice. While provider-based assessments, such as the CCS–SAF, may ultimately fill this need it is possible that a scale based on objective patient-derived data that captures and quantifies a combination of AF-related symptoms, quality of life, functional status, and AF burden may explain a substantial proportion of variance in patient well-being. As demonstrated by Dorian et al.,26 rhythm-based patterns and patient symptoms are additively associated with quality of life. Instruments of this type are expected to emerge and evolve over the near term. A consensus among professional groups and specialty organizations will be required to achieve adoption and widespread use of a standardized and validated symptom scale in clinical practice.
Relevance to clinical management
An additional challenge to the existing classification scheme is that clinical management recommendations for the treatment of AF generally do not depend on rhythm-based patterns of disease. Indeed, only the decision to restore sinus rhythm clearly depends on the pattern of AF.12,22 However, expert opinion indicates that individuals with persistent AF may require more extensive tissue ablation than individuals with paroxysmal AF when undergoing catheter ablation for AF, presumably as a result of more advanced atrial remodeling.22 As such, rhythm-based patterns may be a surrogate for the degree of atrial substrate impairment or remodeling in patients with AF. The designation ‘longstanding persistent AF’ seems to be biologically and clinically arbitrary, and may not distinguish a precise threshold after which ablation success rates decline significantly and the disease is thus classified as permanent.
Nonetheless, evidence that rhythm-based patterns are associated with different rates of success after pulmonary vein isolation for AF was demonstrated in a report of 1,104 prospectively enrolled patients with paroxysmal, persistent, or longstanding persistent disease referred for ablation.83 In addition to targeting AF triggers, the investigators performed additional left atrial ablation in those with nonparoxysmal AF. The rates of freedom from recurrent atrial tachyarrhythmia after a single ablation procedure over follow-up periods up to 7 years were nearly identical among patients with either paroxysmal or persistent disease (78% versus 76%), though they were substantially lower for those with longstanding persistent disease (61%). Cautious interpretation is warranted, as success rates may depend on the mode of surveillance following ablation, particularly when surveillance is conditional on the presence of symptoms.84 The value of AF patterns for predicting long-term success with other forms of rhythm control therapy, such as electrical cardioversion, is not explicitly known. However, cardioversion success seems to be more durable in patients when performed in the initial weeks to months following AF onset than when performed later.85
Thus, rhythm-based patterns alone rarely inform clinical management decisions. Rhythm-based patterns are most applicable to clinical management when used in conjunction with other metrics. For example, AF patterns may help guide general catheter ablation approaches in a given individual, once a symptom or quality of life assessment indicates that ablation is appropriate.
Relevance to etiology and mechanism of AF
As discussed, substantial heterogeneity in AF patterns exists across and within individuals. Some heterogeneity may be accounted for by external factors, such as physician preference to restore sinus rhythm, development of comorbid conditions, or environmental factors that promote recurrence and progression. A current gap in knowledge exists as to whether factors intrinsic to a particular individual promote specific AF patterns. In other words, are AF patterns heritable? Do certain genetic variants or patient-specific processes increase the propensity to develop permanent AF, whereas others manifest exclusively as paroxysmal AF? To what degree do potential intrinsic factors act jointly with environmental factors to affect AF patterns?
The recognition that pathophysiological processes culminating in AF may differ between individuals,54,86 paired with knowledge that specific genetic mutations are associated with distinct electrophysiological AF mechanisms that lead to AF,87,88 raise the possibility that individuals may have an intrinsic propensity to develop a particular AF pattern. Although investigators have sought to determine whether features measured by medical history,89 physical examination,90 or molecular,91 circulating biochemical,92–96 and/or electrophysiological metrics90,97–99 distinguish between pattern-based classes or mechanism of AF, results have been mixed. Most studies are single-center and cross-sectional, and have not been replicated. Therefore, observed associations between the studied variables and AF patterns may merely serve as markers of the progression of AF, and may not signify distinct AF subtypes. Furthermore, most study samples are comprised of patients of European ancestry, which limits the ability to generalize findings to individuals of other races or ethnicities. Little knowledge exists to relate patterns and either AF etiology or mechanism although, as noted above, patterns may distinguish degrees of substrate impairment or remodeling. Understanding the mechanisms of AF may facilitate attempts to determine whether a propensity to develop specific rhythm-based patterns of AF exists, and if so, may provide further justification for rhythm-based classification of AF.
Future directions
Areas of research
An ideal clinical classification system is one that stratifies prognosis and informs patient management. Currently available data do not consistently demonstrate associations between existing pattern-based classes of AF and either clinical outcomes or indicated therapies. Gaps in knowledge exist, creating opportunities for future research with the aim of enhancing both our understanding of AF and the management of patients (Table 2).
Table 2.
Future directions to improve AF classification
| Potential questions | Examples of study measures that can be performed to address the questions |
|---|---|
| Are existing pattern-based AF classes valid and reliable? |
|
| Are existing pattern-based AF classes or AF progression associated with differences in health outcomes that are independent of comorbid conditions? |
|
| Do biological subtypes of disease manifest as different pattern-based classes of AF? |
|
| Can individuals at risk of new-onset, recurrent, or progressive AF be identified? |
|
| Can new-onset, recurrent, or progressive AF be prevented? |
|
| How does AF class change over time in each individual? |
|
| Is there utility in preventing the progression of AF? |
|
| What accounts for variability in AF patterns and progression? |
|
| Does classification of patient well-being inform prognosis and clinical management? |
|
| Can novel classification schemes explain variability in clinical outcomes better than existing pattern-based classification? |
|
Abbreviation: AF, atrial fibrillation
The identification of biological mechanisms through which genetic variation at common susceptibility loci mediates AF100–102 will improve our understanding of AF, and has the potential to explain some of the variability in the patterns and natural history of AF. Given that the majority of the epidemiological and pathophysiological AF data are derived from the study of individuals of European ancestry,103 the longitudinal course, characteristics of recurrence, and progression of AF in individuals of other racial and ethnic groups warrant further investigation. Other promising areas for future research include determining whether biological subtypes of disease predispose to specific AF patterns, and testing whether patterns associate with clinically relevant outcomes or indicate the appropriateness of different therapies.
The increasing availability of continuous ambulatory electrocardiographic monitoring may facilitate efforts to more precisely define pattern-based classes of AF. Electrophysiological measurements, coupled with genetic, imaging, and biomarker data, may identify features that correlate with the tendency for AF to self-terminate, recur, or progress, and replace arbitrary distinctions between AF classes based on time or duration of rhythm.
Unbiased approaches aimed at organizing or clustering AF into different categories on the basis of features such as clinical symptoms, genetic variation, biomarker patterns, imaging characteristics, electrophysiological parameters, and clinical outcomes may help better explain the biological basis of AF and identify distinct AF subtypes. Such insights might facilitate the discovery of therapeutic interventions that reduce morbidity attributable to AF.
Unifying disparate elements
As clinical management is often dependent on consideration of disparate elements, it may not be possible for any single scale to comprehensively capture all clinically relevant aspects of AF. However, stroke risk, patient symptoms or well-being, and the degree of atrial substrate remodeling all exist on continuums and factor prominently in the management of patients with AF (Figure 2). With the exception of stroke, the severity of symptoms or atrial substrate impairment that merit specific clinical interventions are not clear.
Figure 2.
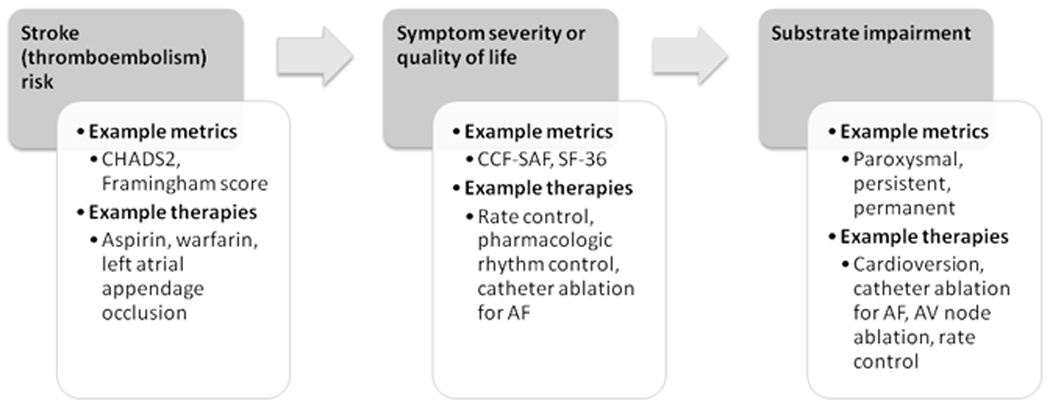
Proposed elements for inclusion in a unifying clinical classification scheme for AF. Elements with established or potential roles in guiding management of patients with AF are displayed. Examples of metrics that measure these elements are shown, along with potential management decisions that might depend on the degree of severity on each scale. Elements and decisions might be inter-related. Arrows indicate a proposed sequential flow of assessments. Abbreviations: AF, atrial fibrillation; AV, atrial-ventricular; CCF–SAF, Canadian Cardiovascular Society Severity of Atrial Fibrillation; CHADS2, where “C” denotes congestive heart failure, “H” denotes hypertension, “A” denotes age > 75 years, “D” denotes diabetes mellitus, and “S” denotes stroke or transient ischemic attack; SF-36, 36-item short-form survey for health and quality of life.
Adoption of an approach that considers each of three relevant ‘S’s’—stroke risk, symptoms, and substrate impairment—might allow for standardization and hence convenient comparison of patients in both the clinical and research arenas. Assessment of severity for each of the elements in the scheme could involve either established or new metrics, although acceptance of common standards by an expert consensus panel would be required in order to compare patients. Stroke risk stratification, for example, could be adopted by using any of several existing schemes.12 Perhaps an even more parsimonious scale than the CCS–SAF could be used to effectively stratify individuals with AF into meaningful prognostic and treatment classes on the basis of symptoms. Symptom assessment might follow the model of the NYHA classification scheme for heart failure. The NYHA scheme is simple, reproducible, and informs treatments and outcomes. Substrate impairment could be defined using the current pattern-based designation for AF, given its widespread acceptance.
A scheme that combines elements of stroke risk, symptoms, and substrate impairment has some limitations; for example, the scheme would not capture AF etiology, mechanisms, or other clinically relevant features. Confidently ascribing an etiology to AF in any individual may not be possible, however, considering the multitude of factors that increase one’s propensity to develop AF. Consideration of specific AF mechanisms may become increasingly important in the event that efficacy of therapies, such as pharmacologic agents, depend on specific mechanisms of AF onset or maintenance. Each of the individual domains of the composite scale for clinical classification of AF might be improved upon. Indeed, poor discrimination and calibration of established stroke risk stratification schemes has been reported,104 existing symptom scales might be too onerous to be clinically adopted, and pattern-based designation as a surrogate for substrate impairment is subject to ambiguity, arbitrary temporal thresholds, and misclassification.
Any future classification instrument must be evaluated for reproducibility and broad applicability. The prognostic value and clinical utility of new classification instruments should be established by their ability to discriminate between individuals, adequately calibrate observed risk, and improve classification of individuals into accurate categories of risk compared with that of preexisting classification schemes. Moreover, the validity of any instrument should be demonstrated in both sexes, different age strata, and across races and/or ethnicities.
Conclusions
Pattern-based classification schemes for AF have standardized definitions of AF patterns, are parsimonious, and are widely accepted. Yet the validity, reliability, and clinical utility of these patterns are largely untested. Moreover, a large proportion of the variability in the natural history of AF is unexplained, and whether distinct patterns of AF can be predicted or prevented remains unclear. Whether biological subtypes of AF predispose to existing pattern-based classes is also unknown. These gaps in knowledge, in conjunction with advances in our understanding of AF, present unique opportunities to re-examine the classification of AF.
Acknowledgments
S. A. Lubitz is supported by a training grant in the epidemiology of cardiovascular disease from the NIH (T32HL007575). This work was supported by NIH grants HL092577 to P. T. Ellinor and E. J. Benjamin, AG028321 and RC1-HL01056 to E. J. Benjamin, and DA027021 to P. T. Ellinor.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Go AS, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Miyasaka Y, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 3.Stewart S, et al. Trends in hospital activity, morbidity and case fatality related to atrial fibrillation in Scotland, 1986--1996. Eur Heart J. 2001;22:693–701. doi: 10.1053/euhj.2000.2511. [DOI] [PubMed] [Google Scholar]
- 4.Frost L, Vestergaard P, Mosekilde L, Mortensen LS. Trends in incidence and mortality in the hospital diagnosis of atrial fibrillation or flutter in Denmark, 1980–1999. Int J Cardiol. 2005;103:78–84. doi: 10.1016/j.ijcard.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 5.Tsang TS, et al. The prevalence of atrial fibrillation in incident stroke cases and matched population controls in Rochester, Minnesota: changes over three decades. J Am Coll Cardiol. 2003;42:93–100. doi: 10.1016/s0735-1097(03)00500-x. [DOI] [PubMed] [Google Scholar]
- 6.Humphries KH, et al. Population rates of hospitalization for atrial fibrillation/flutter in Canada. Can J Cardiol. 2004;20:869–876. [PubMed] [Google Scholar]
- 7.Wolf PA, et al. Secular trends in the prevalence of atrial fibrillation: The Framingham Study. Am Heart J. 1996;131:790–795. doi: 10.1016/s0002-8703(96)90288-4. [DOI] [PubMed] [Google Scholar]
- 8.Wolf PA, Mitchell JB, Baker CS, Kannel WB, D'Agostino RB. Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch Intern Med. 1998;158:229–234. doi: 10.1001/archinte.158.3.229. [DOI] [PubMed] [Google Scholar]
- 9.Coyne KS, et al. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006;9:348–356. doi: 10.1111/j.1524-4733.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee WC, et al. Direct treatment cost of atrial fibrillation in the elderly American population: a Medicare perspective. J Med Econ. 2008;11:281–298. doi: 10.3111/13696990802063425. [DOI] [PubMed] [Google Scholar]
- 11.Garrey WE. The nature of fibrillary contraction of the heart; its relation to tissue mass and form. Am J Physiol. 1914;33:397–414. [Google Scholar]
- 12.Fuster V, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2006;48:854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Wells JL, Jr, et al. Characterization of atrial fibrillation in man: studies following open heart surgery. Pacing Clin Electrophysiol. 1978;1:426–438. doi: 10.1111/j.1540-8159.1978.tb03504.x. [DOI] [PubMed] [Google Scholar]
- 14.Konings KT, et al. High-density mapping of electrically induced atrial fibrillation in humans. Circulation. 1994;89:1665–1680. doi: 10.1161/01.cir.89.4.1665. [DOI] [PubMed] [Google Scholar]
- 15.Sopher SM, Camm AJ. Therapy for atrial fibrillation: control of the ventricular response and prevention of recurrence. Coron Artery Dis. 1995;6:106–114. [PubMed] [Google Scholar]
- 16.Levy S, Novella P, Ricard P, Paganelli F. Paroxysmal atrial fibrillation: a need for classification. J Cardiovasc Electrophysiol. 1995;6:69–74. doi: 10.1111/j.1540-8167.1995.tb00758.x. [DOI] [PubMed] [Google Scholar]
- 17.Gallagher MM, Camm AJ. Classification of atrial fibrillation. Pacing Clin Electrophysiol. 1997;20:1603–1605. doi: 10.1111/j.1540-8159.1997.tb03527.x. [DOI] [PubMed] [Google Scholar]
- 18.Levy S, et al. Atrial fibrillation: current knowledge and recommendations for management. Working Group on Arrhythmias of the European Society of Cardiology. Eur Heart J. 1998;19:1294–1320. doi: 10.1053/euhj.1998.1050. [DOI] [PubMed] [Google Scholar]
- 19.Levy S. Classification system of atrial fibrillation. Curr Opin Cardiol. 2000;15:54–57. doi: 10.1097/00001573-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds MR, Ellis E, Zimetbaum P. Quality of life in atrial fibrillation: measurement tools and impact of interventions. J Cardiovasc Electrophysiol. 2008;19:762–768. doi: 10.1111/j.1540-8167.2007.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lane DA, Lip GY. Quality of life in older people with atrial fibrillation. J Interv Card Electrophysiol. 2009;25:37–42. doi: 10.1007/s10840-008-9318-y. [DOI] [PubMed] [Google Scholar]
- 22.Calkins H, et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816–861. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Kopecky SL, et al. The natural history of lone atrial fibrillation. A population-based study over three decades. N Engl J Med. 1987;317:669–674. doi: 10.1056/NEJM198709103171104. [DOI] [PubMed] [Google Scholar]
- 24.Fuster V, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to develop guidelines for the management of patients with atrial fibrillation) developed in collaboration with the North American Society of Pacing and Electrophysiology. Eur Heart J. 2001;22:1852–1923. doi: 10.1053/euhj.2001.2983. [DOI] [PubMed] [Google Scholar]
- 25.McNamara RL, et al. ACC/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Data Standards on Atrial Fibrillation) Circulation. 2004;109:3223–3243. doi: 10.1161/01.CIR.0000131893.41821.D1. [DOI] [PubMed] [Google Scholar]
- 26.Dorian P, et al. Validation of a new simple scale to measure symptoms in atrial fibrillation: the Canadian Cardiovascular Society Severity in Atrial Fibrillation scale. Circ Arrhythm Electrophysiol. 2009;2:218–224. doi: 10.1161/CIRCEP.108.812347. [DOI] [PubMed] [Google Scholar]
- 27.Kempster PA, Gerraty RP, Gates PC. Asymptomatic cerebral infarction in patients with chronic atrial fibrillation. Stroke. 1988;19:955–957. doi: 10.1161/01.str.19.8.955. [DOI] [PubMed] [Google Scholar]
- 28.Strickberger SA, et al. Relationship between atrial tachyarrhythmias and symptoms. Heart Rhythm. 2005;2:125–131. doi: 10.1016/j.hrthm.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 29.Israel CW, Gronefeld G, Ehrlich JR, Li YG, Hohnloser SH. Long-term risk of recurrent atrial fibrillation as documented by an implantable monitoring device: implications for optimal patient care. J Am Coll Cardiol. 2004;43:47–52. doi: 10.1016/j.jacc.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 30.Roche F, et al. Frequent and prolonged asymptomatic episodes of paroxysmal atrial fibrillation revealed by automatic long-term event recorders in patients with a negative 24-hour Holter. Pacing Clin Electrophysiol. 2002;25:1587–1593. doi: 10.1046/j.1460-9592.2002.01587.x. [DOI] [PubMed] [Google Scholar]
- 31.Page RL, Wilkinson WE, Clair WK, McCarthy EA, Pritchett EL. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation. 1994;89:224–227. doi: 10.1161/01.cir.89.1.224. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi N, Seki A, Imataka K, Fujii J. Clinical features of paroxysmal atrial fibrillation. An observation of 94 patients. Jpn Heart J. 1981;22:143–149. doi: 10.1536/ihj.22.143. [DOI] [PubMed] [Google Scholar]
- 33.Scardi S, et al. Lone atrial fibrillation: prognostic differences between paroxysmal and chronic forms after 10 years of follow-up. Am Heart J. 1999;137:686–691. doi: 10.1016/s0002-8703(99)70224-3. [DOI] [PubMed] [Google Scholar]
- 34.Al-Khatib SM, Wilkinson WE, Sanders LL, McCarthy EA, Pritchett EL. Observations on the transition from intermittent to permanent atrial fibrillation. Am Heart J. 2000;140:142–145. doi: 10.1067/mhj.2000.107547. [DOI] [PubMed] [Google Scholar]
- 35.Levy S, et al. Characterization of different subsets of atrial fibrillation in general practice in France: the ALFA study. The College of French Cardiologists. Circulation. 1999;99:3028–3035. doi: 10.1161/01.cir.99.23.3028. [DOI] [PubMed] [Google Scholar]
- 36.Kerr CR, et al. Progression to chronic atrial fibrillation after the initial diagnosis of paroxysmal atrial fibrillation: results from the Canadian Registry of Atrial Fibrillation. Am Heart J. 2005;149:489–496. doi: 10.1016/j.ahj.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 37.Jahangir A, et al. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: a 30-year follow-up study. Circulation. 2007;115:3050–3056. doi: 10.1161/CIRCULATIONAHA.106.644484. [DOI] [PubMed] [Google Scholar]
- 38.Pappone C, et al. Atrial fibrillation progression and management: A 5-year prospective follow-up study. Heart Rhythm. 2008 doi: 10.1016/j.hrthm.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Nieuwlaat R, et al. Prognosis, disease progression, and treatment of atrial fibrillation patients during 1 year: follow-up of the Euro Heart Survey on atrial fibrillation. Eur Heart J. 2008;29:1181–1189. doi: 10.1093/eurheartj/ehn139. [DOI] [PubMed] [Google Scholar]
- 40.Tsang TS, et al. Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: a longitudinal cohort study of 21 years. Eur Heart J. 2008;29:2227–2233. doi: 10.1093/eurheartj/ehn324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keating RJ, et al. Effect of atrial fibrillation pattern on survival in a community-based cohort. Am J Cardiol. 2005;96:1420–1424. doi: 10.1016/j.amjcard.2005.07.050. [DOI] [PubMed] [Google Scholar]
- 42.Ruigomez A, Johansson S, Wallander MA, Garcia Rodriguez LA. Predictors and prognosis of paroxysmal atrial fibrillation in general practice in the UK. BMC Cardiovasc Disord. 2005;5:20. doi: 10.1186/1471-2261-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davidson E, Rotenberg Z, Weinberger I, Fuchs J, Agmon J. Diagnosis and characteristics of lone atrial fibrillation. Chest. 1989;95:1048–1050. doi: 10.1378/chest.95.5.1048. [DOI] [PubMed] [Google Scholar]
- 44.Kato T, Yamashita T, Sagara K, Iinuma H, Fu LT. Progressive nature of paroxysmal atrial fibrillation. Observations from a 14-year follow-up study. Circ J. 2004;68:568–572. doi: 10.1253/circj.68.568. [DOI] [PubMed] [Google Scholar]
- 45.Petersen P, Godtfredsen J. Embolic complications in paroxysmal atrial fibrillation. Stroke. 1986;17:622–626. doi: 10.1161/01.str.17.4.622. [DOI] [PubMed] [Google Scholar]
- 46.Flaker GC, Fletcher KA, Rothbart RM, Halperin JL, Hart RG. Clinical and echocardiographic features of intermittent atrial fibrillation that predict recurrent atrial fibrillation. Stroke Prevention in Atrial Fibrillation (SPAF) Investigators. Am J Cardiol. 1995;76:355–358. doi: 10.1016/s0002-9149(99)80100-3. [DOI] [PubMed] [Google Scholar]
- 47.Saksena S, Hettrick DA, Koehler JL, Grammatico A, Padeletti L. Progression of paroxysmal atrial fibrillation to persistent atrial fibrillation in patients with bradyarrhythmias. Am Heart J. 2007;154:884–892. doi: 10.1016/j.ahj.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 48.de Vos CB, et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725–731. doi: 10.1016/j.jacc.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 49.Budeus M, et al. Prediction of conversion from paroxysmal to permanent atrial fibrillation. Pacing Clin Electrophysiol. 2007;30:243–252. doi: 10.1111/j.1540-8159.2007.00656.x. [DOI] [PubMed] [Google Scholar]
- 50.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 51.Todd DM, et al. Repetitive 4-week periods of atrial electrical remodeling promote stability of atrial fibrillation: time course of a second factor involved in the self-perpetuation of atrial fibrillation. Circulation. 2004;109:1434–1439. doi: 10.1161/01.CIR.0000124006.84596.D9. [DOI] [PubMed] [Google Scholar]
- 52.Ausma J, et al. Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation. 1997;96:3157–3163. doi: 10.1161/01.cir.96.9.3157. [DOI] [PubMed] [Google Scholar]
- 53.Van Wagoner DR, et al. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res. 1999;85:428–436. doi: 10.1161/01.res.85.5.428. [DOI] [PubMed] [Google Scholar]
- 54.Allessie MA, et al. Pathophysiology and prevention of atrial fibrillation. Circulation. 2001;103:769–777. doi: 10.1161/01.cir.103.5.769. [DOI] [PubMed] [Google Scholar]
- 55.Lafuente-Lafuente C, Mouly S, Longas-Tejero MA, Bergmann JF. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD005049.pub2. CD005049. [DOI] [PubMed] [Google Scholar]
- 56.Fauchier L, et al. Antiarrhythmic effect of statin therapy and atrial fibrillation a meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2008;51:828–835. doi: 10.1016/j.jacc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 57.Noheria A, Kumar A, Wylie JV, Jr, Josephson ME. Catheter ablation vs antiarrhythmic drug therapy for atrial fibrillation: a systematic review. Arch Intern Med. 2008;168:581–586. doi: 10.1001/archinte.168.6.581. [DOI] [PubMed] [Google Scholar]
- 58.Healey JS. et al. Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: a meta-analysis. J Am Coll Cardiol. 2005;45:1832–1839. doi: 10.1016/j.jacc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 59.Disertori M, et al. Valsartan for prevention of recurrent atrial fibrillation. N Engl J Med. 2009;360:1606–1617. doi: 10.1056/NEJMoa0805710. [DOI] [PubMed] [Google Scholar]
- 60.Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 61.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 62.Comparison of 12 risk stratification schemes to predict stroke in patients with nonvalvular atrial fibrillation. Stroke. 2008;39:1901–1910. doi: 10.1161/STROKEAHA.107.501825. [DOI] [PubMed] [Google Scholar]
- 63.Hart RG, et al. Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy. Stroke Prevention in Atrial Fibrillation Investigators. J Am Coll Cardiol. 2000;35:183–187. doi: 10.1016/s0735-1097(99)00489-1. [DOI] [PubMed] [Google Scholar]
- 64.Hohnloser SH, et al. Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W Substudy. J Am Coll Cardiol. 2007;50:2156–2161. doi: 10.1016/j.jacc.2007.07.076. [DOI] [PubMed] [Google Scholar]
- 65.Sage JI, Van Uitert RL. Risk of recurrent stroke in patients with atrial fibrillation and non-valvular heart disease. Stroke. 1983;14:537–540. doi: 10.1161/01.str.14.4.537. [DOI] [PubMed] [Google Scholar]
- 66.Roy D, Marchand E, Gagne P, Chabot M, Cartier R. Usefulness of anticoagulant therapy in the prevention of embolic complications of atrial fibrillation. Am Heart J. 1986;112:1039–1043. doi: 10.1016/0002-8703(86)90318-2. [DOI] [PubMed] [Google Scholar]
- 67.Cabin HS, Clubb KS, Hall C, Perlmutter RA, Feinstein AR. Risk for systemic embolization of atrial fibrillation without mitral stenosis. Am J Cardiol. 1990;65:1112–1116. doi: 10.1016/0002-9149(90)90323-s. [DOI] [PubMed] [Google Scholar]
- 68.Moulton AW, Singer DE, Haas JS. Risk factors for stroke in patients with nonrheumatic atrial fibrillation: a case-control study. Am J Med. 1991;91:156–161. doi: 10.1016/0002-9343(91)90008-l. [DOI] [PubMed] [Google Scholar]
- 69.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–1457. [PubMed] [Google Scholar]
- 70.Friberg L, Hammar N, Rosenqvist M. Stroke in paroxysmal atrial fibrillation: report from the Stockholm Cohort of Atrial Fibrillation. Eur Heart J. 2010;31:967–975. doi: 10.1093/eurheartj/ehn599. [DOI] [PubMed] [Google Scholar]
- 71.Treseder AS, Sastry BS, Thomas TP, Yates MA, Pathy MS. Atrial fibrillation and stroke in elderly hospitalized patients. Age Ageing. 1986;15:89–92. doi: 10.1093/ageing/15.2.89. [DOI] [PubMed] [Google Scholar]
- 72.Botto GL, et al. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol. 2009;20:241–248. doi: 10.1111/j.1540-8167.2008.01320.x. [DOI] [PubMed] [Google Scholar]
- 73.Glotzer TV, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythmia Electrophysiol. 2009;2:474–480. doi: 10.1161/CIRCEP.109.849638. [DOI] [PubMed] [Google Scholar]
- 74.Wang TJ, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 75.Friberg L, Hammar N, Pettersson H, Rosenqvist M. Increased mortality in paroxysmal atrial fibrillation: report from the Stockholm Cohort-Study of Atrial Fibrillation (SCAF) Eur Heart J. 2007;28:2346–2353. doi: 10.1093/eurheartj/ehm308. [DOI] [PubMed] [Google Scholar]
- 76.Brand FN, Abbott RD, Kannel WB, Wolf PA. Characteristics and prognosis of lone atrial fibrillation. 30-year follow-up in the Framingham Study. JAMA. 1985;254:3449–3453. [PubMed] [Google Scholar]
- 77.Luderitz B, Jung W. Quality of life in patients with atrial fibrillation. Arch Intern Med. 2000;160:1749–1757. doi: 10.1001/archinte.160.12.1749. [DOI] [PubMed] [Google Scholar]
- 78.Thrall G, Lane D, Carroll D, Lip GY. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med. 2006;119:448, e1–e19. doi: 10.1016/j.amjmed.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 79.Thrall G, Lip GY, Carroll D, Lane D. Depression, anxiety, and quality of life in patients with atrial fibrillation. Chest. 2007;132:1259–1264. doi: 10.1378/chest.07-0036. [DOI] [PubMed] [Google Scholar]
- 80.Lane DA, Langman CM, Lip GY, Nouwen A. Illness perceptions, affective response, and health-related quality of life in patients with atrial fibrillation. J Psychosom Res. 2009;66:203–210. doi: 10.1016/j.jpsychores.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 81.Harden M, et al. Validity and reliability of a new, short symptom rating scale in patients with persistent atrial fibrillation. Health Qual Life Outcomes. 2009;7:65. doi: 10.1186/1477-7525-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dorian P, et al. A novel, simple scale for assessing the symptom severity of atrial fibrillation at the bedside: the CCS-SAF scale. Can J Cardiol. 2006;22:383–386. doi: 10.1016/s0828-282x(06)70922-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bhargava M, et al. Impact of type of atrial fibrillation and repeat catheter ablation on long-term freedom from atrial fibrillation: Results from a multicenter study. Heart Rhythm. 2009 doi: 10.1016/j.hrthm.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 84.Hindricks G, et al. Perception of atrial fibrillation before and after radiofrequency catheter ablation: relevance of asymptomatic arrhythmia recurrence. Circulation. 2005;112:307–313. doi: 10.1161/CIRCULATIONAHA.104.518837. [DOI] [PubMed] [Google Scholar]
- 85.Dittrich HC, et al. Echocardiographic and clinical predictors for outcome of elective cardioversion of atrial fibrillation. Am J Cardiol. 1989;63:193–197. doi: 10.1016/0002-9149(89)90284-1. [DOI] [PubMed] [Google Scholar]
- 86.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 87.Roberts JD, Gollob MH. Impact of Genetic Discoveries on the Classification of Lone Atrial Fibrillation. J Am Coll Cardiol. 2010;55:705–712. doi: 10.1016/j.jacc.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 88.Hong K, Bjerregaard P, Gussak I, Brugada R. Short QT syndrome and atrial fibrillation caused by mutation in KCNH2. J Cardiovasc Electrophysiol. 2005;16:394–396. doi: 10.1046/j.1540-8167.2005.40621.x. [DOI] [PubMed] [Google Scholar]
- 89.Coumel P. Paroxysmal atrial fibrillation: a disorder of autonomic tone? Eur Heart J. 1994;(15 Suppl A):9–16. doi: 10.1093/eurheartj/15.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- 90.Nabauer M, et al. The Registry of the German Competence NETwork on Atrial Fibrillation: patient characteristics and initial management. Europace. 2009;11:423–434. doi: 10.1093/europace/eun369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gramley F, et al. Decreased plasminogen activator inhibitor and tissue metalloproteinase inhibitor expression may promote increased metalloproteinase activity with increasing duration of human atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:1076–1082. doi: 10.1111/j.1540-8167.2007.00906.x. [DOI] [PubMed] [Google Scholar]
- 92.Chung MK, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 93.Li-Saw-Hee FL, Blann AD, Gurney D, Lip GY. Plasma von Willebrand factor, fibrinogen and soluble P-selectin levels in paroxysmal, persistent and permanent atrial fibrillation. Effects of cardioversion and return of left atrial function. Eur Heart J. 2001;22:1741–1747. doi: 10.1053/euhj.2000.2531. [DOI] [PubMed] [Google Scholar]
- 94.Kamath S, Chin BS, Blann AD, Lip GY. A study of platelet activation in paroxysmal, persistent and permanent atrial fibrillation. Blood Coagul Fibrinolysis. 2002;13:627–636. doi: 10.1097/00001721-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 95.Tziakas DN, et al. Circulating levels of collagen type I degradation marker depend on the type of atrial fibrillation. Europace. 2007;9:589–596. doi: 10.1093/europace/eum072. [DOI] [PubMed] [Google Scholar]
- 96.Freestone B, Chong AY, Nuttall S, Blann AD, Lip GY. Soluble E-selectin, von Willebrand factor, soluble thrombomodulin, and total body nitrate/nitrite product as indices of endothelial damage/dysfunction in paroxysmal, persistent, and permanent atrial fibrillation. Chest. 2007;132:1253–1258. doi: 10.1378/chest.07-1185. [DOI] [PubMed] [Google Scholar]
- 97.Gaita F, et al. Different patterns of atrial activation in idiopathic atrial fibrillation: simultaneous multisite atrial mapping in patients with paroxysmal and chronic atrial fibrillation. J Am Coll Cardiol. 2001;37:534–541. doi: 10.1016/s0735-1097(00)01120-7. [DOI] [PubMed] [Google Scholar]
- 98.Tada H, et al. Prevalence and characteristics of continuous electrical activity in patients with paroxysmal and persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2008;19:606–612. doi: 10.1111/j.1540-8167.2008.01148.x. [DOI] [PubMed] [Google Scholar]
- 99.Xi Q, Sahakian AV, Frohlich TG, Ng J, Swiryn S. Relationship between pattern of occurrence of atrial fibrillation and surface electrocardiographic fibrillatory wave characteristics. Heart Rhythm. 2004;1:656–663. doi: 10.1016/j.hrthm.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 100.Benjamin EJ, et al. Variants in ZFHX3 are associated with atrial fibrillation in individuals of European ancestry. Nat Genet. 2009;41:879–881. doi: 10.1038/ng.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gudbjartsson DF, et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007;448:353–357. doi: 10.1038/nature06007. [DOI] [PubMed] [Google Scholar]
- 102.Gudbjartsson DF, et al. A sequence variant in ZFHX3 on 16q22 associates with atrial fibrillation and ischemic stroke. Nat Genet. 2009;41:876–878. doi: 10.1038/ng.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Benjamin EJ, et al. Prevention of atrial fibrillation: report from a National Heart, Lung, and Blood Institute workshop. Circulation. 2009;119:606–618. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fang MC, et al. Comparison of risk stratification schemes to predict thromboembolism in people with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2008;51:810–815. doi: 10.1016/j.jacc.2007.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koide Y, et al. Usefulness of P-wave dispersion in standard twelve-lead electrocardiography to predict transition from paroxysmal to persistent atrial fibrillation. Am J Cardiol. 2008;102:573–577. doi: 10.1016/j.amjcard.2008.04.065. [DOI] [PubMed] [Google Scholar]


