Abstract
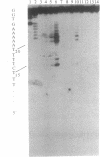
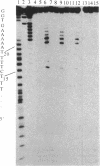
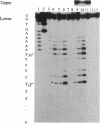
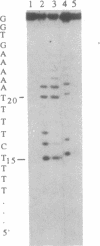
Manganese porphyrin-linker-triple-helix-forming oligonucleotide molecules were prepared and their ability to cleave in vitro a double-stranded DNA target present in the HIV-1 genome was studied. The nature of the linker is a determining factor of the cleavage efficiency. Cleavage yields as high as 80% were observed when the linker was a spermine residue and in the absence of a large excess of free spermine known to stabilize triplex structures. The hydrophobic nature of aliphatic diamine linker modified the cleaver-DNA interactions and reduced the efficiency of DNA cleavage.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beal P. A., Dervan P. B. Second structural motif for recognition of DNA by oligonucleotide-directed triple-helix formation. Science. 1991 Mar 15;251(4999):1360–1363. doi: 10.1126/science.2003222. [DOI] [PubMed] [Google Scholar]
- Casas C., Lacey C. J., Meunier B. Preparation of hybrid "DNA cleaver-oligonucleotide" molecules based on a metallotris(methylpyridiniumyl)porphyrin motif. Bioconjug Chem. 1993 Sep-Oct;4(5):366–371. doi: 10.1021/bc00023a011. [DOI] [PubMed] [Google Scholar]
- Dervan P. B. Design of sequence-specific DNA-binding molecules. Science. 1986 Apr 25;232(4749):464–471. doi: 10.1126/science.2421408. [DOI] [PubMed] [Google Scholar]
- Dervan P. B. Reagents for the site-specific cleavage of megabase DNA. Nature. 1992 Sep 3;359(6390):87–88. doi: 10.1038/359087a0. [DOI] [PubMed] [Google Scholar]
- Giovannangéli C., Rougée M., Garestier T., Thuong N. T., Hélène C. Triple-helix formation by oligonucleotides containing the three bases thymine, cytosine, and guanine. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8631–8635. doi: 10.1073/pnas.89.18.8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibler-Herzog L., Kell B., Zon G., Shinozuka K., Mizan S., Wilson W. D. Sequence dependent effects in methylphosphonate deoxyribonucleotide double and triple helical complexes. Nucleic Acids Res. 1990 Jun 25;18(12):3545–3555. doi: 10.1093/nar/18.12.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Doan T., Praseuth D., Perrouault L., Chassignol M., Thuong N. T., Hélène C. Sequence-targeted photochemical modifications of nucleic acids by complementary oligonucleotides covalently linked to porphyrins. Bioconjug Chem. 1990 Mar-Apr;1(2):108–113. doi: 10.1021/bc00002a004. [DOI] [PubMed] [Google Scholar]
- McShan W. M., Rossen R. D., Laughter A. H., Trial J., Kessler D. J., Zendegui J. G., Hogan M. E., Orson F. M. Inhibition of transcription of HIV-1 in infected human cells by oligodeoxynucleotides designed to form DNA triple helices. J Biol Chem. 1992 Mar 15;267(8):5712–5721. [PubMed] [Google Scholar]
- Moser H. E., Dervan P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science. 1987 Oct 30;238(4827):645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- Olivas W. M., Maher L. J., 3rd Competitive triplex/quadruplex equilibria involving guanine-rich oligonucleotides. Biochemistry. 1995 Jan 10;34(1):278–284. doi: 10.1021/bi00001a034. [DOI] [PubMed] [Google Scholar]
- Perrouault L., Asseline U., Rivalle C., Thuong N. T., Bisagni E., Giovannangeli C., Le Doan T., Hélène C. Sequence-specific artificial photo-induced endonucleases based on triple helix-forming oligonucleotides. Nature. 1990 Mar 22;344(6264):358–360. doi: 10.1038/344358a0. [DOI] [PubMed] [Google Scholar]
- Pitié M., Pratviel G., Bernadou J., Meunier B. Preferential hydroxylation by the chemical nuclease meso-tetrakis-(4-N-methylpyridiniumyl)porphyrinatomanganeseIII pentaacetate/KHSO5 at the 5' carbon of deoxyriboses on both 3' sides of three contiguous A.T base pairs in short double-stranded oligonucleotides. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3967–3971. doi: 10.1073/pnas.89.9.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praseuth D., Perrouault L., Le Doan T., Chassignol M., Thuong N., Hélène C. Sequence-specific binding and photocrosslinking of alpha and beta oligodeoxynucleotides to the major groove of DNA via triple-helix formation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1349–1353. doi: 10.1073/pnas.85.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen D., Gilbert W. A sodium-potassium switch in the formation of four-stranded G4-DNA. Nature. 1990 Mar 29;344(6265):410–414. doi: 10.1038/344410a0. [DOI] [PubMed] [Google Scholar]
- Sen D., Gilbert W. Novel DNA superstructures formed by telomere-like oligomers. Biochemistry. 1992 Jan 14;31(1):65–70. doi: 10.1021/bi00116a011. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Inoue H., Ohtsuka E. Detailed study of sequence-specific DNA cleavage of triplex-forming oligonucleotides linked to 1,10-phenanthroline. Biochemistry. 1994 Jan 18;33(2):606–613. doi: 10.1021/bi00168a028. [DOI] [PubMed] [Google Scholar]
- Tung C. H., Breslauer K. J., Stein S. Polyamine-linked oligonucleotides for DNA triple helix formation. Nucleic Acids Res. 1993 Nov 25;21(23):5489–5494. doi: 10.1093/nar/21.23.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann S., Jendis J., Frauendorf A., Moelling K. Inhibition of HIV-1 reverse transcription by triple-helix forming oligonucleotides with viral RNA. Nucleic Acids Res. 1995 Apr 11;23(7):1204–1212. doi: 10.1093/nar/23.7.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter L., Jablonski J. A., Ramachandran K. L. A simple and efficient procedure for the synthesis of 5'-aminoalkyl oligodeoxynucleotides. Nucleic Acids Res. 1986 Oct 24;14(20):7985–7994. doi: 10.1093/nar/14.20.7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]