Abstract
This communication reports a new theranostic system with a combination of capabilities to both enhance the contrast of photoacoustic (PA) imaging and control the release of a chemical or biological effecter by high-intensity focused ultrasound (HIFU). The fabrication of this system simply involves filling the hollow interiors of gold nanocages with a phase-change material (PCM) such as 1-tetradecanol that has a melting point of 38–39 °C. The PCM can be pre-mixed and thus loaded with a dye, as well as other chemical or biological effecters. When exposed to direct heating or HIFU, the PCM will melt and escape from the interiors of nanocages through small pores on the surface, concurrently releasing the encapsulated molecules into the surrounding medium. We can control the release profile by varying the power of HIFU, the duration of exposure to HIFU, or both.
Hollow nanostructures have been extensively explored as drug delivery systems for biomedical applications due to their unique capability to hold and release drugs.1 Most of these structures are composed of lipids, including liposomes (uni- and multi-lamella vesicles) and multi-channeled cubo- or hexosomes.2 Polymeric nanoparticles with various compositions, structures, and porosities have also been investigated for similar applications.3 Typically, the interiors of these hollow nanostructures are loaded with drugs (hydrophilic or hydrophobic) in different formulations that are able to escape via diffusion or degradation-triggered release. In addition, the release can be triggered and regulated in response to environmental changes such as pH and temperature. Recently, inorganic and composite hollow nanostructures have also received attention for drug delivery applications. A number of groups have reported delivery systems based on mesoporous silica particles containing water-insoluble drugs,4 and metal-organic framework (MOF) particles incorporated with drugs.5 Although the inclusion of inorganic components such as metal ions may cause potential toxicity issues, it offers advantages such as multi-functionality, a feature required for future theranostic applications. For example, the metal ions can serve as contrast agents for magnetic resonance imaging (MRI), adding a unique capability to resolve and track the in vivo location of a drug delivery system.6
Hollow nanostructures of noble metals, such as Au nanocages (AuNCs), represent another attractive platform for theranostic applications. Thanks to their strong, highly tunable scattering and absorption in the near-infrared (NIR) from 700–900 nm, AuNCs are wonderful contrast agents for a variety of imaging modalities including photoacoustic tomography (PAT) and optical coherence tomography (OCT).7 They have also been used to control the release of hydrophilic drugs with NIR laser or HIFU by coating their surfaces with smart polymers.8 While the NIR laser-triggered release has to rely on the photothermal effect of AuNCs, HIFU can directly deposit acoustic energy in the focal volume to rapidly raise the temperature.8b The availability of AuNCs loaded with therapeutic drugs offers a great benefit to theranostic applications because the AuNCs can be monitored with an optical imaging technique while the drug is released at the targeted site in a controllable fashion. As expected, the released drug can also greatly enhance the efficacy of photothermal cancer treatment, which has been demonstrated with success in the absence of an anticancer drug.9 While the system based on AuNCs and smart polymers has been successfully used for controlled release under the irradiation of NIR laser or HIFU, the loaded drugs can escape from the hollow interiors of AuNCs through a slow diffusion process in the absence of any thermal stimulus. In addition, this system only works for hydrophilic drugs because the as-prepared nanocages are typically coated with a hydrophilic polymer such as poly(vinyl pyrrolidone), making it difficult for a hydrophobic species to diffuse into the hydrophilic interior of a AuNC. Since more than 40% of the active compounds identified by the combinatorial screening programs are poorly soluble in water,11 finding a system well-suited for encapsulation of both hydrophilic and hydrophobic drugs is expected to greatly extend their scope of use in theranostic applications. In addition to the features needed for imaging purpose, the new system should show negligible release until it is triggered by an external stimulus.
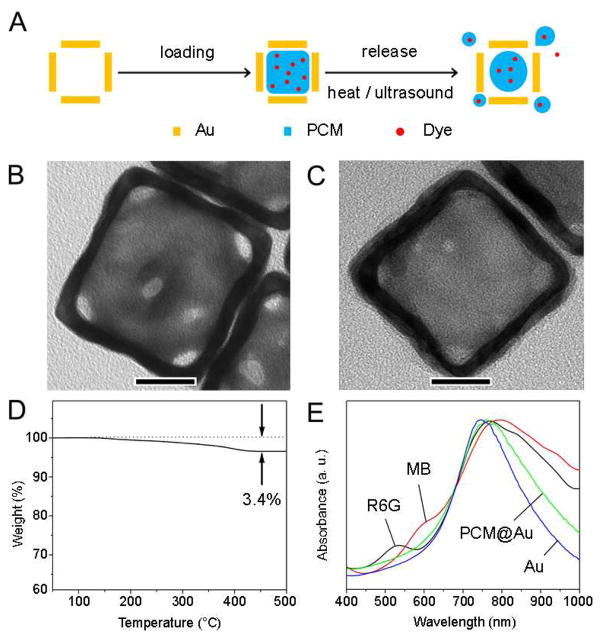
Herein, we present a facile and versatile strategy for loading either hydrophobic or hydrophilic drugs into the hollow interiors of AuNCs. We use a phase-change material (PCM) as the medium to help load the drug, which can also serve as a “gate-keeper” to control the release of drug in response to temperature increase.11 In principle, the encapsulated drug should not be released until the PCM has been melted due to heating by a thermal, photothermal, or ultrasonic means. Figure 1A shows a schematic diagram of the encapsulation and release mechanisms. Since a PCM reversibly changes its physical states between solid and liquid over a narrow temperature range, it can perfectly confine drug molecules inside the AuNCs at a temperature below its melting point (the solid state). When the local temperature is raised beyond the melting point of the PCM, it will begin to melt (the liquid state) and the drug will be released from the melted PCM through diffusion. Taken together, this new system should allow one to regulate the release of a drug and the profile by manipulating the temperature. As long as the drug is miscible with the PCM phase, it can be conveniently loaded into the hollow interiors of AuNCs as the PCM diffuses into the nanocages. This requirement can be readily met by choosing PCMs with the surfactant-like behavior, such as those containing both long hydrophobic tails and hydrophilic heads. In this work, we chose to focus on 1-tetradecanol, a fatty alcohol characterized by attractive features such as immiscibility with water, good biocompatibility, and a melting point (38–39 °C) slightly higher than the normal human body temperature (37.0 °C). It can also be mixed with a range of hydrophilic and hydrophobic substances. Furthermore, 1-tetradecanol is an ingredient widely used in cosmetics due to its low toxicity (oral, rat LD50 >5 g/kg).12
Figure 1.
(A) Schematic illustrating how to load the hollow interior of a AuNC with a dye-doped PCM and then have it released from the AuNC by direct or ultrasonic heating. (B, C) Typical TEM images of a AuNC before and after its hollow interior had been loaded with a mixture of 1-tetradecanol (the PCM) and R6G (the drug). The scale bars are 20 nm. (D) TGA data of the pristine AuNCs (dotted) and the AuNCs containing PCM/R6G (solid). (E) UV-Vis absorption spectra of the AuNCs before and after loading with the PCM/dye mixtures.
The AuNCs used in this study were prepared by means of a galvanic replacement reaction between Ag nanocubes (44 ± 6.7 nm in edge length) and HAuCl4 in an aqueous solution under refluxing condition.13 The resultant AuNCs had an outer edge length of 60 ± 11 nm, together with a wall thickness of 7.5 ± 2.8 nm. Essentially all of them contained at least one pores (~10 nm in size) on the surface (Fig. S1). The as-prepared nanocages were then re-dispersed in methanol after repeated washing with deionized water and centrifugation 4 times. For encapsulation, we used Rhodamine 6G (R6G) and methylene blue (MB) as two examples of drugs with different solubilities in water. As a major advantage over most real drugs, the release of these two dyes can be easily monitored and quantified through the use of UV-vis absorption spectroscopy.
In a typical experiment, the PCM was added to a glass vial and placed in an oil bath set to 90 °C to melt the PCM into a liquid, followed by the addition of a dye. After the dye and PCM had been thoroughly mixed, we introduced the AuNCs as a suspension in methanol. Even after the methanol had been evaporated due to stirring and heating, the AuNCs were still well dispersed in the liquid PCM. During this process, the mixture of PCM and dye molecules slowly entered the hollow interior of each nanocage by diffusion through the pores on the surface. After the system had been continued with heating at 90 °C for 2 h, a small amount of hot water was added to generate two separated phases, one being the PCM/dye mixture and the other containing the loaded AuNCs and water, as a result of immiscibility between the PCM and water. The AuNCs were preferentially extracted into the water phase due to their hydrophilic surfaces. Interestingly, both PCM and dye molecules inside the nanocages were effectively retained because it was difficult for the PCM molecules to quickly diffuse into water due to their immiscibility. Finally, the AuNCs encapsulated with the PCM/dye mixture were collected by centrifugation and then re-dispersed in deionized water under brief sonication (for 10 sec). In the course of re-dispersion, a very small portion of the encapsulated PCM/dye could escape from the AuNCs owning to the heat generated by sonication (Fig. S2).
Figure 1, B and C, shows typical transmission electron microscopy (TEM) images of the nanocages before and after loading of the PCM/dye mixture. Compared with the pristine sample, nanocages loaded with the PCM/dye mixture showed a conspicuous difference in contrast. It should be pointed out that the PCM-dye mixture inside the nanocage was often smaller in volume than the actual void of the nanocage. To quantify the amount of PCM/dye loaded inside the AuNCs, we obtained thermal gravimetric analysis (TGA) data in the range of 50–500 °C (Fig. 1D). The TGA results show a weight loss of 3.4%, which is lower than the loading of 4.0% calculated from the density of PCM and physical dimensions of the nanocages (Supporting Information). This data suggests that, on average, only 84% of the void space inside each AuNC was actually occupied by the PCM/dye mixture.
Figure 1E shows UV-vis absorption spectra recorded from aqueous suspensions of pristine AuNCs, AuNCs containing the PCM, and AuNCs containing both PCM and the dye (R6G or MB). It is worth pointing out that the AuNCs loaded with a PCM/dye mixture also exhibited characteristic peak of the dye, confirming successful encapsulation of the dye inside the AuNCs. The major localized surface plasmon resonance (LSPR) peak of the nanocages was found to be broadened and slightly red-shifted, most likely caused by some minor aggregation during sample preparation.
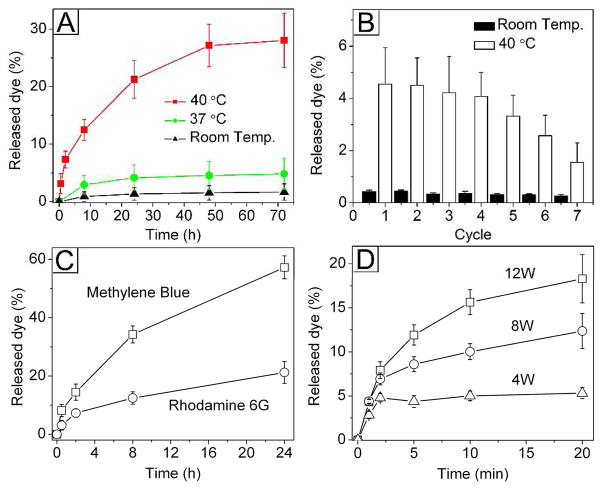
Figure 2 shows the release of R6G from the AuNCs by direct heating and HIFU heating, respectively. As shown in Figure 2A, the amount of R6G released was negligible at room temperature because the dye was entrapped by solid PCM inside the nanocages, which makes it difficult for the dye molecules to diffuse into the surrounding medium. At 37 °C, there was a slight increase in the release of R6G (still below 5% over a period of 3 days) although the melting point of 1-tetradecanol (38–39 °C) was still slightly higher than this temperature. In comparison, 28% of the encapsulated R6G was released from the AuNCs in 3 days when the sample was held at 40 °C. As an important feature, the PCM-based release could be easily regulated by controlling the temperature. We confirmed this feature by investigating the release behaviors during repeated heating-cooling cycles at temperatures below and above the melting point of 1-tetradecanol (Fig. 2B). Note that the release of dye was only observed when the sample was heated to 40 °C and held for 2 h.
Figure 2.
Release profiles of (A) R6G under direct heating to various temperatures for different periods of time, (B) R6G through different cycles of heating (40 °C, for 2 h) and cooling (to room temperature), (C) R6G and MB by direct heating to 40 °C for different periods of time, and (D) R6G by HIFU at different applied powers.
Due to its good compatibility with both hydrophobic and hydrophilic substances, we found that the PCM could also be used as a matrix for the encapsulation and release of a drug more soluble in water than R6G. We demonstrated this capability by switching to MB, a dye more hydrophilic than R6G. In this case, we found that the loading capacity of MB was much lower than that of R6G (57% vs. 84% for the MB and R6G, respectively, see Supporting Information). As a result, the cumulative release of MB was three times higher than that of R6G (Fig. 2C). Since MB was quickly photo-bleached (Fig. S3), the cumulative release percentage corrected for bleaching showed that 80% of the loaded MB was released in 24 h (Fig. S4). These results clearly demonstrate that the PCM works for chemical species with different solubilities in water, making this new system a useful platform for a wide variety of drugs.
Figure 2D shows the release profiles of R6G as a function of the power of HIFU. HIFU has been used as a promising clinical technology for tumor treatment due to its deep penetration and non-invasive nature.14 Most recently, we have also adapted this technique for triggering the release of dye molecules from AuNCs whose surfaces were covered with smart polymer brushes.8b As a major advantage over the smart polymers, the PCM-based system showed better encapsulation efficacy and much lower background release (data not shown). In the present work, we also found that the focused ultrasound wave could rapidly increase the overall temperature of a suspension of AuNCs loaded with PCM and dye (Fig. S5). As clearly shown in Figure 2D, the quick increase in temperature caused the R6G to release from the hollow interiors of AuNCs. As expected, the release profile also displayed a strong dependence on the power of HIFU, making it possible to control the release dosage on demand.
In conclusion, we have demonstrated a nanoscale, temperature-regulated drug release system by combining the unique features of AuNCs and PCMs. As a deeply penetrating energy source, HIFU can be used to trigger the release and to control the release profile by adjusting the duration of exposure and/or the power applied. The new hybrid system described in this work can also be further developed into a theranostic system with an array of functions, including the capabilities for in vivo molecular imaging, as well as chemo- and photothermal therapy.
Supplementary Material
Acknowledgments
This work was supported in part by a 2006 NIH Director’s Pioneer Award (DP1 OD000798), startup funds from Washington University in St. Louis, and the World Class University (WCU) program at Yonsei University. Part of the work was done at the Nano Research Facility (NRF), a member of the National Nanotechnology Infrastructure Network (NINN), which is supported by the NSF under award no. ECS-0335765.
Footnotes
Supporting Information Available: Synthesis procedures and other supplementary data. The information in available free of charge via the internet at http://pubs.acs.org.
References
- 1.(a) Lou XW, Archer LA, Yang Z. Adv Mater. 2008;20:3987. [Google Scholar]; (b) Li Z-Z, Wen L-X, Shao L, Chen J-F. J Control Release. 2004;98:245. doi: 10.1016/j.jconrel.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 2.(a) Lasic DD. Trends Biotechnol. 1998;16:307. doi: 10.1016/s0167-7799(98)01220-7. [DOI] [PubMed] [Google Scholar]; (b) Drummond DC, Meyer O, Hong K, Kirpotin DB, Paapahadjopoulos D. Pharmacol Rev. 1999;51:691. [PubMed] [Google Scholar]; (c) Zamboni WC. Oncologist. 2008;13:248. doi: 10.1634/theoncologist.2007-0180. [DOI] [PubMed] [Google Scholar]
- 3.(a) Nasongkla N, Be E, Ren J, Ai H, Khemtong C, Guthi JS, Chin SF, Sherry AD, Bothman DA, Gao J. Nano Lett. 2006;6:2427. doi: 10.1021/nl061412u. [DOI] [PubMed] [Google Scholar]; (b) Medina SH, El-Sayed MEH. Chem Rev. 2009;109:3141. doi: 10.1021/cr900174j. [DOI] [PubMed] [Google Scholar]; (c) De Cock LJ, De Koker S, De Geest BG, Grooten J, Vervaet C, Remon JP, Sjihorukov GB, Antipina MN. Angew Chem Int Ed. 2010;49:2. doi: 10.1002/anie.200906266. [DOI] [PubMed] [Google Scholar]; (d) Tong R, Christian DA, Tang L, Cabral H, Baker JR, Jr, Kataoka K, Discher D, Cheng J. MRS Bulletin. 2009;34:422. [Google Scholar]
- 4.(a) Rosenholm JM, Peuhu E, Eriksson JE, Sahlgren C, Linden M. Nano Lett. 2009;9:3308. doi: 10.1021/nl901589y. [DOI] [PubMed] [Google Scholar]; (b) Wang Y, Yan Y, Cui J, Hosta-Rigau L, Heath JL, Nnice EC, Caruso F. Adv Mater. 2010;22:4293. doi: 10.1002/adma.201001497. [DOI] [PubMed] [Google Scholar]
- 5.(a) Horcajada P, Serre C, Vallet-Regi M, Sebban M, Taulelle F, Ferey G. Angew Chem Int Ed. 2006;45:5974. doi: 10.1002/anie.200601878. [DOI] [PubMed] [Google Scholar]; (b) Uemura K, Kitagawa S, Kondo M, Fukui K, Kitaura R, Chang H-C, Mizutani T. Chem-Eu J. 2002;8:3586. doi: 10.1002/1521-3765(20020816)8:16<3586::AID-CHEM3586>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 6.(a) Lin W, Hyeon T, Lanza GM, Zhang M, Meade TJ. MRS Bulletin. 2009;34:441. doi: 10.1557/mrs2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Que EL, Chang CJ. Chem Soc Rev. 2010;39:51. doi: 10.1039/b914348n. [DOI] [PubMed] [Google Scholar]; (c) Rocca JD, Lin W. Eur J Inorg Chem. 2010:3725. [Google Scholar]
- 7.(a) Kim C, Cho EC, Chen J, Song KH, Au L, Favazza C, Zhang Q, Cobley CM, Gao F, Xia Y, Wang LV. ACS Nano. 2010;4:4559. doi: 10.1021/nn100736c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yang X, Skrabalak S, Li Z, Xia Y, Wang LV. Nano Lett. 2007;7:3798. doi: 10.1021/nl072349r. [DOI] [PubMed] [Google Scholar]; (c) Song KH, Kim C, Cobley CM, Xia Y, Wang LV. Nano Lett. 2009;9:183. doi: 10.1021/nl802746w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Chang H, Sun T, Li ZY, Chen J, Wiley bJ, Xia Y, Li X. Opt Lett. 2005;30:3048. doi: 10.1364/ol.30.003048. [DOI] [PubMed] [Google Scholar]
- 8.(a) Mustafa SY, Cheng Y, Chen J, Cobley CM, Zhang Q, Rycenga M, Xie J, Kim C, Song KH, Schwartz AG, Wang LV, Xia Y. Nat Mater. 2009;8:935. doi: 10.1038/nmat2564. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Li W, Cai X, Kim C, Sun G, Zhang Y, Deng R, Yang M, Chen J, Achilefu S, Wang LV, Xia Y. Nanoscale. 2011 doi: 10.1039/C0NR00932F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Skrabalak SE, Chen J, Au L, Lu X, Li X, Xia Y. Adv Mater. 2007;19:3177. doi: 10.1002/adma.200701972. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chen J, Wang D, Xi J, Au L, Siekkinen A, Warsen A, Li ZY, Zhang H, Xia Y, Li X. Nano Lett. 2007;7:1318. doi: 10.1021/nl070345g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chen J, Glaus C, Laforest R, Zhang Q, Yang M, Gidding M, Welch MJ, Xia Y. Small. 2010;6:811. doi: 10.1002/smll.200902216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Lipinski CA. J Pharmacol Toxicol Methods. 2000;44:235. doi: 10.1016/s1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]; (b) Merisko-Liversidge EM, Liversidge GG. Toxicol Pathol. 2008;36:43. doi: 10.1177/0192623307310946. [DOI] [PubMed] [Google Scholar]
- 11.(a) Mondal S. Appl Therm Eng. 2007;28:1536. [Google Scholar]; (b) Choi SW, Zhang Y, Xia Y. Angew Chem Int Ed. 2010;49:7904. doi: 10.1002/anie.201004057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gosselin RE, Hodge HC, Smith RP, Gleason MN. Clinical Toxicology of Commercial Products. 4. Baltimore: Williams and Wilkins; 1976. pp. II–118.(b) MSDS ALCH 414-1.
- 13.(a) Zhang Q, Li W, Wen L-P, Chen J, Xia Y. Chemistry: A European Journal. 2010;16:10234. doi: 10.1002/chem.201000341. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Skrabalak SE, Chen J, Sun Y, Lu X, Au L, Cobley CM, Xia Y. Acc Chem Res. 2008;41:1587. doi: 10.1021/ar800018v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy JE, ter Haar GR, Cranston D. Brit J Radiol. 2003;76:590. doi: 10.1259/bjr/17150274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.