Abstract
Propionic acidemia (PA) is an autosomal recessive disorder of metabolism caused by a deficiency of propionyl-coenzyme A carboxylase (PCC). Despite optimal dietary and cofactor therapy, PA patients still suffer from lethal metabolic instability and experience multisystemic complications. A murine model of PA (Pcca–/–) of animals that uniformly die within the first 48 hr of life was used to determine the efficacy of adeno-associated viral (AAV) gene transfer as a potential therapy for PA. An AAV serotype 8 (AAV8) vector was engineered to express the human PCCA cDNA and delivered to newborn mice via an intrahepatic injection. Greater than 64% of the Pcca–/– mice were rescued after AAV8-mediated gene transfer and survived until day of life 16 or beyond. Western analysis of liver extracts showed that PCC was completely absent from Pcca–/– mice but was restored to greater than wild-type levels after AAV gene therapy. The treated Pcca–/– mice also exhibited markedly reduced plasma levels of 2-methylcitrate compared with the untreated Pcca–/– mice, which indicates significant PCC enzymatic activity was provided by gene transfer. At the time of this report, the oldest treated Pcca–/– mice are over 6 months of age. In summary, AAV gene delivery of PCCA effectively rescues Pcca–/– mice from neonatal lethality and substantially ameliorates metabolic markers of the disease. These experiments demonstrate a gene transfer approach using AAV8 that might be used as a treatment for PA, a devastating and often lethal disorder desperately in need of new therapeutic options.
Propionic acidemia (PA) is an organic acidemia caused by mutations in either the PCCA or PCCB gene. The disease is observed primarily in neonates and presents as hyperammonemia, vomiting, p. feeding, and hypotonia and progresses into a life-threatening metabolic crisis. In this report, Chandler and colleagues demonstrate that AAV8-mediated gene delivery to Pcca−/− mice can provide long-term rescue of the neonatal lethal phenotype and significantly reduce the levels of propionyl-CoA-derived metabolites.
Introduction
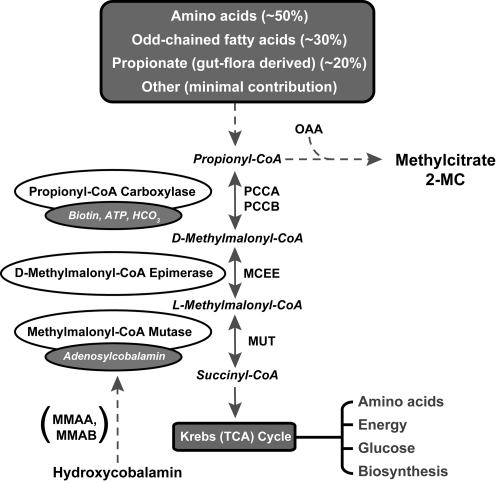
Propionic acidemia (PA) is an organic acidemia caused by mutations in either the PCCA or the PCCB genes, which encode for the mitochondrial enzyme propionyl-coenzyme A (CoA) carboxylase (PCC) (Fenton et al., 2001). PCC is composed of PCCA and PCCB subunits and is involved in the catabolism of propiogenic amino acids, particularly isoleucine, valine, methionine, and threonine, as well as odd-chain fatty acids. This biotin-dependent enzyme catalyzes the first step in the conversion of propionyl-CoA to d-methylmalonyl-CoA in the pathway of propionyl-CoA oxidation (Fig. 1).
FIG. 1.
The pathway of propionyl-CoA to succinyl-CoA metabolism is displayed. PA results from deficiency of the enzyme PCC and is caused by mutations in the PCCA or PCCB gene. Isolated MMA can be caused by defects in any of several downstream steps in the pathway, including d-methylmalonyl-CoA epimerase (MCEE), MUT, or enzymes that impair the synthesis of adenosylcobalamin (MMAA and MMAB). OAA, oxaloacetic acid; TCA, tricarboxylic acid.
Most frequently, PA presents in the neonatal period with hyperammonemia, vomiting, poor feeding, and hypotonia and progresses into a life-threatening metabolic crisis. Patients who survive suffer from recurrent metabolic instability and can develop multisystemic complications including neurological impairment (Haas et al., 1995; Nyhan et al., 1999), cardiomyopathy (Massoud and Leonard, 1993; Bhan and Brody, 2001; Mardach et al., 2005), and pancreatitis (Kahler et al., 1994). Typical laboratory abnormalities include severe metabolic acidosis, hyperglycinemia, ketosis, and marked hyperammonemia that may require dialysis. In addition to propionic acid (Hommes et al., 1968), 3-hydroxypropionate (Ando et al., 1972a), 2-methylcitrate (Ando et al., 1972b), and propionylcarnitine (Roe et al., 1984) are propionyl-CoA-derived metabolites that are present in an increased concentration in the blood and urine of affected patients. Although the precise roles that specific metabolite(s) have in the pathophysiology of PA remains uncertain, these compounds are clinical biomarkers of metabolism in PA patients.
The standard treatment for PA is dietary restriction of amino acid precursors, l-carnitine supplementation to replenish diminished carnitine levels, and administration of metronidazole to reduce the generation of propionic acid by intestinal bacteria. Liver transplantation has been used to relieve the metabolic decompensations of PA in circumstances where the patient cannot be managed by standard treatment (Saudubray et al., 1999; Yorifuji et al., 2000). However, despite the aggressive nutritional and cofactor therapy, the long-term prognosis for patients with PA remains poor (van Der Meer et al., 1996; Saudubray et al., 1999; Perez-Cerda et al., 2000). The inclusion of this disorder in expanded newborn screening programs highlights the need for new therapies to provide better long-term prognosis for patients with PA.
A murine model of PA, created by deleting a portion of the Pcca gene, has been established. The targeted allele does not produce Pcca RNA and behaves as a null mutation (Miyazaki et al., 2001). Pcca–/– mice recapitulate the severe neonatal manifestations of PA and uniformly die within the first 48 hr of life. A recent effort to test adenoviral gene delivery of the human PCCA cDNA to Pcca–/– mice resulted in a significant but modest increase in life expectancy of 38 hr or less (Hofherr et al., 2009). The same article also reported that adeno-associated virus (AAV) serotype 8 (AAV8)-mediated gene transfer of PCCA to Pcca–/– mice resulted in a life expectancy increase of only 10 hr or less. Although these experiments demonstrate theoretical potential for gene therapy in PA, a method of gene transfer with a sustained therapeutic effect needs to be established before considering gene transfer as a practical therapeutic option for patients with PA.
We have previously demonstrated rescue of a neonatal lethal murine model of methylmalonic acidemia (MMA) for over 18 months using AAV8 gene transfer (Carrillo-Carrasco et al., 2010; Chandler and Venditti, 2010). MMA is an organic acidemia caused by deficient activity of methylmalonyl-CoA mutase (MUT), an enzyme also needed for the conversion of propionyl-CoA to succinyl-CoA, and has a neonatal phenotype very similar to PA, in both patients and knock-out mice. To investigate the potential of viral gene transfer as a treatment for PA, we used the same methodology and vector design previously used in our successful AAV8 MMA gene therapy studies (Chandler and Venditti, 2010). In this report, we establish that AAV8-mediated gene delivery to Pcca–/– mice can provide long-term rescue of the neonatal lethal phenotype, result in hepatic PCCA expression, and significantly reduce the levels of propionyl-CoA-derived metabolites. These results unambiguously demonstrate AAV8-mediated gene transfer as a potential treatment for PA.
Materials and Methods
Animal studies
Murine experiments were approved and performed according to the regulations and standards of the National Human Genome Research Institute Animal Care and Use Committee (Bethesda, MD). Pcca knock-out mice harbor a targeted mutation in the Pcca gene that removes a 2-kbp region of exon 4 and its intron (Miyazaki et al., 2001; Hofherr et al., 2009). Pcca+/– mice on a mixed C57/BL6/129 background (Hofherr et al., 2009) were re-derived onto FVB/N at our facility. The triply-mixed Pcca+/– progeny have no disease manifestations and were intercrossed to generate Pcca–/– mice, which uniformly die in the neonatal period as previously described.
Virus production and delivery
The viral construct, AAV2/8.CB7.CI.RBG, was graciously provided by the University of Pennsylvania Vector Core (Philadelphia, PA). This construct, into which the full-length human PCCA cDNA was cloned, contains transcriptional control elements from the cytomegalovirus enhancer/chicken β-actin promoter, cloning sites for the insertion of a cDNA, and the rabbit β-globin polyA signal. Inverted terminal repeats from AAV serotype 2 flank the expression cassette. The genome was packaged into an AAV8 capsid, purified by cesium chloride centrifugation, and titrated by quantitative polymerase chain reaction. AAV2/8.CB7.CI.EGFP.RBG, also provided by the University of Pennsylvania Vector Core, substitutes the green fluorescent protein (GFP) gene for PCCA and was used as a negative control. Vector genome copies (GC) of AAV8-PCCA (1 × 1010) in a total volume of 10 μl were delivered into the liver parenchyma of neonatal Pcca–/– mice and control littermates using a 32-gauge needle during the first 8 hr of life.
Western blotting
Liver tissue samples were homogenized with a 2-ml Tenbroeck tissue grinder (Wheaton, Millville, NJ) in T-PER (Pierce Biotechnology, Rockford, IL) tissue protein extraction buffer in the presence of Halt (Pierce) protease inhibitor cocktail. Thirty micrograms of clarified extract was used in western blot analysis and were probed with PCCA MaxPab polyclonal antibody (catalog number H00005095-B01P, Abnova, Walnut, CA) at a dilution of 1:1,000 with 2% bovine serum albumin and mouse monoclonal anti-OxPhos Complex III core II antibody (catalog number A-11143, Invitrogen, Carlsbad, CA) at a dilution of 1:3,000. Horseradish peroxidase–conjugated sheep anti-mouse antibody (catalog number NA931, GE Healthcare Life Sciences, Piscataway, NJ) was used as the secondary antibody at a dilution of 1:20,000 for PCCA detection and 1:30,000 for Complex III detection. Signal was visualized with West Pico chemiluminescent substrate (catalog number 34080, Pierce).
Metabolite levels
Blood samples were taken from sacrificed animals on day of life 1 or by retro-orbital sinus plexus sampling on day of life 28. Samples were centrifuged immediately after collection, diluted in water, and stored at −80°C in a screw-top tube for later analysis. Plasma 2-methylcitrate levels were measured by gas chromatography–mass spectrometry with stable isotopic internal calibration as previously described (Marcell et al., 1985; Allen et al., 1993).
Results
AAV8-PCCA gene transfer rescues the neonatal lethal phenotype
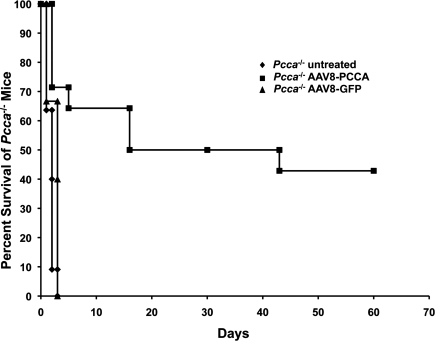
A total of 14 Pcca–/– mice received an intrahepatic injection of 1 × 1010 GC of AAV8 carrying the human PCCA cDNA AAV8-PCCA under the control of the ubiquitous chicken β-actin promoter during the first 8 hr of life. We chose a dose of 1 × 1010 GC because this dose is capable of rescuing the murine model of MMA (authors' unpublished data). Nine of the treated Pcca–/– mice (64%) survived the neonatal period and lived until day 16 of life (Fig. 2). Untreated Pcca–/– mice (n = 11) and Pcca–/– mice that received AAV8-GFP (n = 3) died by the second day of life (Fig. 2). Unfortunately, on day of life 16 two treated Pcca–/– mice received severe wounds as a result of fighting and were sacrificed. These mice as well as an additional treated Pcca–/– mouse that was sacrificed on day of life 43 were evaluated for hepatic PCCA protein expression. At the time of this report six of the AAV8-treated Pcca–/– mice were over 2 months old, with the oldest surviving Pcca–/– mice now 9 months of age.
FIG. 2.
Survival of Pcca–/– mice: Pcca–/– untreated mice (n = 11), Pcca–/– mice injected with 1 × 1010 GC of AAV8 carrying the human PCCA cDNA (n = 14), and Pcca–/– mice injected with 1 × 1010 GC of AAV8 carrying GFP cDNA (n = 3).
PCCA protein is expressed in Pcca–/– mice following gene transfer
To determine if PCCA protein was being expressed in Pcca–/– mice following AAV8-PCCA gene delivery, an immunoblot of 30 μg of total liver protein was probed with a murine PCC polyclonal antibody that cross-reacts against both the murine (Pcca) and human (PCCA) orthologs. Significant levels of PCCA protein were detected on an immunoblot of liver extracts from the treated Pcca–/– mice at both 16 and 43 days after birth (Fig. 3). Pcca protein was detected in the liver extracts from Pcca+/+ mice but not in the liver extracts from Pcca–/– mice.
FIG. 3.
Immunoblots of 30 μg of murine liver protein extract stained for Pcca and ubiquinol-cytochrome c reductase core protein II (Uqcrc2), which was used as a loading control. Lane 1 (Pcca+/+) and Lane 2 (Pcca–/–) are liver extracts from the respective untreated mice at day 1 of life. Lanes 3–5 are liver extracts from mice treated with 1 × 1010 GC of AAV8-PCCA at birth.
PCC activity detected in Pcca–/– mice following gene transfer
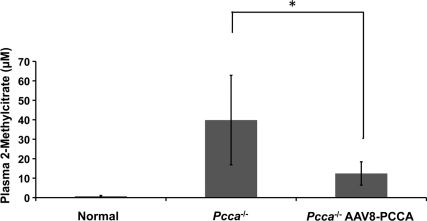
In order to generate functional PCC enzyme, the human PCCA subunit expressed from the transgene must be able to form a heterocopolymer with the endogenous murine Pccb subunits. To determine if there was functional PCC enzymatic activity following AAV8-PCCA treatment, we measured plasma 2-methylcitrate levels in both treated and untreated Pcca–/– mice (Fig. 4). 2-Methylcitrate is increased in PA because deficient PCC enzymatic activity causes accretion of the PCC substrate, propionyl-CoA (Fig. 1). The accumulating propionyl-CoA then condenses with oxaloacetate via the action of citrate synthase to form 2-methylcitrate. Therefore, increasing the amount of PCC enzymatic activity in the Pcca–/– mice by gene transfer should diminish the concentration of propionyl-CoA and cause a corresponding drop in 2-methylcitrate levels. We observed a significant (p = 0.01) decrease of plasma 2-methylcitrate (12.4 μM) levels in the treated Pcca–/– mice (n = 9) at 21–28 days post-treatment in comparison with plasma 2-methylcitrate (39.8 μM) levels in the untreated Pcca–/– mice (n = 5) at 1 day of life (Fig. 4), verifying that an increase in PCC enzymatic activity occurred after AAV8-PCCA gene transfer. However, the plasma 2-methylcitrate levels of the treated Pcca–/– mice did not normalize to the levels (709 nM) seen in control mice (Pcca+/+ and Pcca+/–). In addition, these treated Pcca–/– mice exhibit an increased ability to oxidize propionate in comparison to Mut–/– mice that also have a block in the same pathway (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com < http://www.liebertonline.com/hum>). The ability to oxidize propionate depends upon the presence of PCC activity (Fig. 1).
FIG. 4.
Mean plasma methylcitrate levels. Normal plasma (n = 9) is from Pcca+/+ and Pcca+/– mice at day of life 1. Because of neonatal lethality, Pcca+/+ plasma (n = 4) was collected at day of life 1 from untreated Pcca–/– mice (n = 5). Pcca–/– AAV8-PCCA plasma (n = 7) was collected from Pcca–/– mice that received an injection of AAV8 carrying the human PCCA cDNA between day of life 22 and 28. Error bars represent ± 1 SD. *p = 0.01 (t test).
Discussion
Our previous gene therapy studies on MMA provided a rational framework for the experimental approaches undertaken here to establish a viable method of gene transfer for patients with PA. We were able to rescue greater than 64% of the Pcca–/– mice from neonatal death with a single intrahepatic injection of 1 × 1010 GC of AAV8-PCCA at birth. More than 40% of the treated Pcca–/– mice have survived for more than 2 months. The oldest AAV8-PCCA-treated Pcca–/– mice were 6 months old at the time of this report. The treated Pcca–/– mice demonstrate robust expression of the PCCA protein and show significant reduction in the circulating levels of a disease-related biomarker, 2-methylcitrate. Our experiments demonstrate an AAV gene delivery approach that could be used as a possible treatment for PA, a severe organic acidemia that is frequently recalcitrant to medical management.
The murine models of both MMA and PA display neonatal lethality, and it is likely that other animal models lacking proteins required for propionyl-CoA oxidation will have a similar phenotype. Although AAV gene transfer affords a dramatic rescue of the lethal phenotype in murine models of MMA and PA, elevated levels of metabolites characteristic of the disorders in humans persist in the corrected mutant mice after gene transfer. Prolonged exposure to these metabolites is believed to contribute to the multisystemic ailments that afflict the patients. Thus, minimal AAV-mediated rescue could provide a means to study the pathophysiology of the chronic human conditions, which would not otherwise be possible using a neonatal lethal animal model.
Propionyl-CoA oxidation involves several proteins, including PCCA, PCCB, MUT, MMAA and MMAB, and d-methylmalonyl-CoA epimerase (Fig. 1). A deficiency in any one of these enzymes impedes the conversion of propionyl-CoA to succinyl-CoA and results in a severe metabolic disease in humans. The successful long-term rescue of both murine models of MMA (Mut knock-out) and PA (Pcca knock-out) with AAV indicates that gene transfer for all genetic defects in the conversion of propionyl-CoA to succinyl-CoA would also be amenable to gene therapy.
Supplementary Material
Acknowledgments
R.J.C., S.C., N.C.C., J.S.S., and C.P.V. were supported, in part, by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health and also graciously acknowledge research support and encouragement from the MMA Research Foundation/Angels for Alyssa Fund. Thanks to the National Human Genome Research Institute mouse core for mouse care and technical assistance. S.H. and M.A.B. were supported, in part, by the Propionic Acidemia Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- Allen R.H. Stabler S.P. Savage D.G. Lindenbaum J. Elevation of 2-methylcitric acid I and II levels in serum, urine, and cerebrospinal fluid of patients with cobalamin deficiency. Metabolism. 1993;42:978–988. doi: 10.1016/0026-0495(93)90010-l. [DOI] [PubMed] [Google Scholar]
- Ando T. Rasmussen K. Nyhan W.L. Hull D. 3-Hydroxypropionate: Significance of β-oxidation of propionate in patients with propionic acidemia and methylmalonic acidemia. Proc. Natl. Acad. Sci. U.S.A. 1972a;69:2807–2811. doi: 10.1073/pnas.69.10.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando T. Rasmussen K. Wright J.M. Nyhan W.L. Isolation and identification of methylcitrate, a major metabolic product of propionate in patients with propionic acidemia. J. Biol. Chem. 1972b;247:2200–2204. [PubMed] [Google Scholar]
- Bhan A.K. Brody C. Propionic acidemia: A rare cause of cardiomyopathy. Congest. Heart Fail. 2001;7:218–219. doi: 10.1111/j.1527-5299.2001.01011.x. [DOI] [PubMed] [Google Scholar]
- Carrillo-Carrasco N. Chandler R.J. Chandrasekaran S. Venditti C.P. Liver-directed recombinant adeno-associated viral gene delivery rescues a lethal mouse model of methylmalonic acidemia and provides long-term phenotypic correction. Hum. Gene Ther. 2010;21:1147–1154. doi: 10.1089/hum.2010.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler R.J. Venditti C.P. Long-term rescue of a lethal murine model of methylmalonic acidemia using adeno-associated viral gene therapy. Mol. Ther. 2010;18:11–16. doi: 10.1038/mt.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton W.A. Gravel R.A. Rosenblatt D.S. Disorders of propionate and methylmalonate metabolism. In: Scriver C.R., editor; Sly W.S., editor; Valle D., editor. The Metabolic & Molecular Bases of Inherited Disease. 8th. McGraw-Hill; New York: 2001. pp. 2165–2193. [Google Scholar]
- Haas R.H. Marsden D.L. Capistrano-Estrada S., et al. Acute basal ganglia infarction in propionic acidemia. J. Child Neurol. 1995;10:18–22. doi: 10.1177/088307389501000104. [DOI] [PubMed] [Google Scholar]
- Hofherr S. Senac J.S. Chen C.Y., et al. Short-term rescue of neonatal lethality in a mouse model of propionic acidemia by gene therapy. Hum. Gene Ther. 2009;20:169–180. doi: 10.1089/hum.2008.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommes F.A. Kuipers J.R. Elema J.D., et al. Propionicacidemia, a new inborn error of metabolism. Pediatr. Res. 1968;2:519–24. doi: 10.1203/00006450-196811000-00010. [DOI] [PubMed] [Google Scholar]
- Kahler S.G. Sherwood W.G. Woolf D., et al. Pancreatitis in patients with organic acidemias. J. Pediatr. 1994;124:239–243. doi: 10.1016/s0022-3476(94)70311-6. [DOI] [PubMed] [Google Scholar]
- Marcell P.D. Stabler S.P. Podell E.R. Allen R.H. Quantitation of methylmalonic acid and other dicarboxylic acids in normal serum and urine using capillary gas chromatography-mass spectrometry. Anal. Biochem. 1985;150:58–66. doi: 10.1016/0003-2697(85)90440-3. [DOI] [PubMed] [Google Scholar]
- Mardach R. Verity M.A. Cederbaum S.D. Clinical, pathological, and biochemical studies in a patient with propionic acidemia and fatal cardiomyopathy. Mol. Genet. Metab. 2005;85:286–290. doi: 10.1016/j.ymgme.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Massoud A.F. Leonard J.V. Cardiomyopathy in propionic acidaemia. Eur. J. Pediatr. 1993;152:441–445. doi: 10.1007/BF01955907. [DOI] [PubMed] [Google Scholar]
- Miyazaki T. Ohura T. Kobayashi M., et al. Fatal propionic acidemia in mice lacking propionyl-CoA carboxylase and its rescue by postnatal, liver-specific supplementation via a transgene. J. Biol. Chem. 2001;276:35995–35999. doi: 10.1074/jbc.M105467200. [DOI] [PubMed] [Google Scholar]
- Nyhan W.L. Bay C. Beyer E.W. Mazi M. Neurologic nonmetabolic presentation of propionic acidemia. Arch. Neurol. 1999;56:1143–1147. doi: 10.1001/archneur.56.9.1143. [DOI] [PubMed] [Google Scholar]
- Perez-Cerda C. Merinero B. Rodriguez-Pombo P., et al. Potential relationship between genotype and clinical outcome in propionic acidaemia patients. Eur. J. Hum. Genet. 2000;8:187–194. doi: 10.1038/sj.ejhg.5200442. [DOI] [PubMed] [Google Scholar]
- Roe C.R. Millington D.S. Maltby D.A., et al. l-Carnitine enhances excretion of propionyl coenzyme A as propionylcarnitine in propionic acidemia. J. Clin. Invest. 1984;73:1785–1788. doi: 10.1172/JCI111387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudubray J.M. Touati G. Delonlay P., et al. Liver transplantation in propionic acidaemia. Eur. J. Pediatr. 1999;158(Suppl 2):S65–S69. doi: 10.1007/pl00014325. [DOI] [PubMed] [Google Scholar]
- van der Meer S.B. Poggi F. Spada M., et al. Clinical outcome and long-term management of 17 patients with propionic acidaemia. Eur. J. Pediatr. 1996;155:205–210. doi: 10.1007/BF01953939. [DOI] [PubMed] [Google Scholar]
- Yorifuji T. Muroi J. Uematsu A., et al. Living-related liver transplantation for neonatal-onset propionic acidemia. J. Pediatr. 2000;137:572–574. doi: 10.1067/mpd.2000.108391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.